Abstract
Purpose of review
The purpose of this article is to describe the function of the vascular cell adhesion and signaling molecule, PECAM-1, in endothelial cells, with special emphasis on its role in maintaining and restoring the vascular permeability barrier following disruption of the endothelial cell junction.
Recent findings
In addition to its role as an inhibitory receptor in circulating platelets and leukocytes, PECAM-1 is highly expressed at endothelial cell-cell junctions, where it functions as an adhesive stress-response protein to both maintain endothelial cell junctional integrity and speed restoration of the vascular permeability barrier following inflammatory or thrombotic challenge.
Summary
Due to the unique ability of antibodies that bind the membrane-proximal region of the extracellular domain to trigger conformational changes leading to affinity modulation and homophilic adhesion strengthening, PECAM-1 might be an attractive target for treating vascular permeability disorders.
Keywords: PECAM-1, CD31, endothelial cell junctions, vascular permeability
INTRODUCTION
Platelet/endothelial cell adhesion molecule-1 (PECAM-1) was originally described in the mid-1980’s as the CD31 differentiation antigen expressed on the surface of human granulocytes, monocytes and platelets [1–4]. At the same, several other groups independently reported the presence of an endothelial cell surface antigen - variously known as glycoprotein (GP) IIa [5], GPIIa’ [6], hec7 antigen [7], and EndoCAM [8] - that became highly enriched at cell-cell junctions. Screening of an endothelial cell cDNA expression library with an antibody specific for platelet integral membrane proteins led to the cloning of a 130 kDa protein having homology with recently cloned cell adhesion molecule members of the immunoglobulin gene (Ig) superfamily, and the protein was named platelet/endothelial cell adhesion molecule-1 (PECAM-1) to denote its cloning origins, its family membership, and its likely function [9]. Immunochemical and biochemical characterization, together with its subsequent cloning from two different leukocyte libraries [10,11] established that the endothelial cell junctional protein, the CD31 hematopoietic differentiation antigen, and platelet PECAM-1 were identical entities, facilitating investigation of the role that this cell adhesion and signaling molecule plays in the biology of blood and vascular cells. A number of excellent reviews exist on the function of PECAM-1 in platelet biology [12], in signal transduction [13,14], and on its role in leukocyte transendothelial migration and inflammation [15,16]. In contrast, this chapter will focus primarily on the role that PECAM-1 plays in endothelial cell biology, with a special emphasis on the homophilic adhesive properties of the PECAM-1 extracellular domain and how it functions to regulate the endothelial cell vascular permeability barrier.
STRUCTURAL FEATURES OF THE EXTRACELLULAR AND CYTOPLASMIC DOMAINS, AND TISSUE DISTRIBUTION
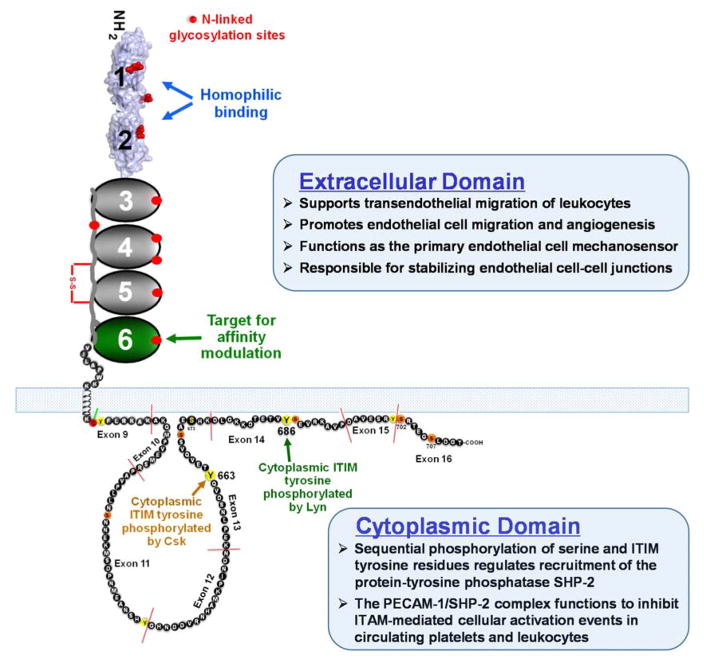
PECAM-1 (Figure 1) has a molecular mass of 130 kDa, approximately 40% of which is carbohydrate [9,19]. The 574 amino acid extracellular domain is comprised of six Ig homology domains of approximately 100 amino acids each, the amino terminal two of which belong to the I2 set of Ig-superfamily folds [17]. Extensive mutagenesis studies, coupled with both functional and structural information, have shown that IgD1 and IgD2 function in (1) mediating homophilic PECAM-1/PECAM-1 interactions between leukocytes and endothelial cells, and (2) concentrating PECAM-1 at endothelial cell-cell borders, where it functions as both a major endothelial mechanosensor [20–23], and as regulator of vascular permeability. The former will be covered extensively in an accompanying article by Tzima in this volume, while the role of PECAM-1 in maintaining endothelial cell barrier function will be described in detail below. Further down the molecule, membrane-proximal Ig domains 5 and 6 each contain calcium coordination sites [24], and antibodies that bind IgD6 have the interesting property of increasing the homophilic binding affinity of the receptor [25,26], a property with potential translational applications that will be examined in more detail below.
Figure 1.
Schematic diagram of PECAM-1. The extracellular domain is comprised of six Ig-like domains, the first two of which are shown as a space-filling model of the recently determined homophilic binding domain (reference 17) that is involved in cell adhesion. The structure of the cytoplasmic domain was determined by 2D NMR (reference 18), and is characterized by two lipid-associated regions separated by a large unstructured region. PECAM-1-mediated signaling is initiated by phosphorylation of serine 702, which releases ITIM tyrosine 686 from its association with the plasma membrane, facilitating its phosphorylation by the Src-family kinase, Lyn. Sequential phosphorylation of ITIM tyrosine 663 completes the process, and allows PECAM-1 to now recruit SH2 domain-containing proteins, the most notable of which is the protein-tyrosine phosphatase, SHP-2.
The cytoplasmic domain of PECAM-1 is comprised of eight separate exons that are subject to alternative splicing [27], yielding isoforms that are expressed in a tissue- and differentiation-specific manner [28,29], and that have the potential to differ in their functional properties [30–32]. Though largely unstructured, the cytoplasmic domain contains a single lipid-associated segment that is susceptible, upon cellular activation, to inducible, sequential phosphorylation [18,33]; first of serine residues that release a membrane-associated control region from the inner face of the plasma membrane (see Figure 1), and then of tyrosines 663 and 686, each of which exist within immunoreceptor tyrosine-based inhibitory motifs (ITIMs). PECAM-1 ITIMs, when phosphorylated, recruit the protein-tyrosine phosphatase, SHP-2 [34], resulting in formation of a PECAM-1/SHP-2 complex that functions in circulating blood cells to inhibit a plethora of tyrosine kinase-initiated cellular activation events [35]. Endothelial PECAM-1 is able to recruit cytosolic SHP-2 to the inner face of the plasma membrane in a phospho-ITIM-specific manner [36,37] to form a complex that functions to increase endothelial cell motility and migration in a process that will be discussed in Section 5 below.
THE CONTRIBUTION OF IgD1 AND IgD2 TO HOMOPHILIC INTERACTIONS
The adhesive properties of PECAM-1 largely depend on its ability to form PECAM-1/PECAM-1 homophilic interactions. Such interactions are essential for concentrating PECAM-1 at endothelial cell-cell borders [38] where it functions both to regulate the vascular permeability barrier (see below) and leukocyte trafficking [39]. Sun et al. were the first to demonstrate that PECAM-1 homophilic interactions require PECAM-1 IgD1 and IgD2 [25], however this interaction is species-specific, as substituting human PECAM-1 with murine IgD1, abrogates PECAM-1 homophilic interactions [25,40]. Studies by Newton et al. demonstrated that five residues (D11, D33, K50, D51, and K89) are required for homophilic binding [41]. Disruption of at least one of these, K89, results in loss of both endothelial cell border localization [38] and the ability of PECAM-1 to contribute to endothelial cell junctional integrity [42,43]. The structure of the homophilic binding domain of PECAM-1 has been recently been solved [17], and reveals that both IgD1 and IgD2 participate importantly in the formation of the trans homophilic binding interface, with a total buried interface area of more than 2300 Å2. Such extensive contacts likely enable PECAM-1 to maintain vascular integrity and to resist mechanical force under conditions of fluid shear stress. A space-filling model of IgD1/D2 based on the crystal structure is shown in Figure 1, while select residues participating in formation of the homophilic binding interface are listed in Table 1.
Table 1.
Amino acid interacting * pairs on the IgD1 and IgD2 inter-chain interfaces present in the crystal structure of the homophilic binding domain of human PECAM-1
| Interacting interfaces | Amino acid pairs |
|---|---|
| IgD1-IgD1 | V34-K13, T37-K24/P16, T36-P16, S38-P16/L15, K62-T64/A32/S63/F31, T64-F31, V40-L15, P42-L15, H39-L15/F68/T27/N25, F3-D11, K41-S66/Q29, F68-S35, T27-S35/N8, L15-N8, Q29-N8/S9/D33, S66-D33 |
| IgD1-IgD2 | Y49-R122/E165, D51-K154, D51-K154, D52-R122, K81-E165 |
| IgD2-IgD2 | R157-K131, D158-K131, A132-R157 |
Multiple amino acids interacting with a single amino acid are separated by a “/”
LECTIN-LIKE PROPERTIES OF PECAM-1
PECAM-1 is heavily glycosylated [19], with nine N-glycosylation sites within the extracellular domain, three of which are in IgD1 and IgD2 [9,17]. Kitazume et al. were the first to demonstrate a role for carbohydrate residues in mediating PECAM-1 homophilic interactions [44], and proposed that PECAM-1 possess lectin-like properties similar to the Siglec family of cell adhesion receptors [45]. Interestingly, homophilic binding ability was linked to the presence of α2,6-sialic acid modified glycan residues, which appeared to be necessary for the ability of PECAM-1 to traffic normally to the cell surface and confer a cell survival advantage to endothelial cells in culture [46]. α2,6-linked sialic acids were also shown to be necessary for endothelial cells to form tube-like structures in vitro [47]. An important caveat of each of these studies is that they were performed using primarily murine cells and murine PECAM-1, More recent studies [48] have identified important species-specific requirements for PECAM-1-mediated homophilic binding, as α2,3- but not α2,6-, sialylated glycans, appear to participate in PECAM-1/PECAM-1 interactions in humans. Structural and functional studies aimed at identifying and characterizing the specific role of glycans in human PECAM-1/PECAM-1 interactions are the subject of an ongoing investigation in our laboratory.
PECAM-1 AND ENDOTHELIAL MIGRATION AND CELL SURVIVAL
Cell migration
The first hint that PECAM-1 might be involved in cell migration and angiogenesis came from findings that anti-PECAM-1 antibodies inhibit the ability of endothelial cells grown on Matrigel to form tube-like structures [49–52]. This concept was supported shortly thereafter by the observation that antibodies specific for PECAM-1 inhibit tumor-induced angiogenesis in vivo in mice [53,54], and later by the observation that tumor angiogenesis is impaired in PECAM-1-null mice [55]. The mechanism by which PECAM-1 promotes cell migration appears to be due to the ability of the PECAM-1/SHP-2 complex to alter the cytoskeleton, both by dephosphorylating focal adhesion kinase [56,57], as well as by altering the activity of the small G-protein, RhoA [58,59]. Taken together, these findings provide strong rationale for targeting PECAM-1 in endothelialopathies such as tumor angiogenesis and the growth and development of hemangiomas.
Cell survival
Exposure of endothelial cells to a variety of apoptotic and/or inflammatory stimuli results in endothelial injury and dysfunction (reviewed in [60]), and their ability to resist programmed cell death is crucial for endothelial cells to maintain vascular homeostasis. PECAM-1 homophilic binding [61,62] and subsequent signaling through the PECAM-1 cytoplasmic domain [63,64] play important roles in endothelial cell cytoprotection. Interestingly, although PECAM-1 ITIMs are required to inhibit the intrinsic pathway of Bax-induced apoptosis [64], they appear to do so independent of their ability to recruit and activate SHP-2 [65] – at least in endothelial cells exposed to genotoxic chemotherapeutic drugs. PECAM-1 has also recently been reported to endow the vascular endothelium with the ability to maintain vascular integrity during inflammation-induced activation of the extrinsic pathway of apoptosis [66]. As in chemotherapy-induced endothelial cell death, PECAM-1 ITIM tyrosines appear to be required for cytoprotection. The distinct signaling pathways employed downstream from PECAM-1 ITIM tyrosine phosphorylation leading to protection of endothelial from pro-apoptotic stimuli remain to be fully elucidated.
ORGANIZATION OF THE ENDOTHELIAL CELL JUNCTION
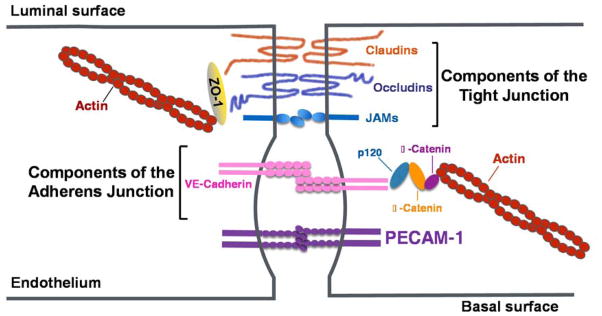
The vascular endothelium regulates the flow of fluids and cells via a number of mechanisms. Cell surface negatively-charged glycans located on the luminal surface of the endothelium form a charged repulsive surface that prevents platelets, red cells, and leukocytes from adhering to the endothelium under normal conditions [67], while membrane compartments like caveolae regulate transendothelial transport of soluble macromolecules [68]. Most trafficking, however, takes place at the endothelial cell-cell junction, the integrity of which is tightly regulated by the coordinated action of a series of cell surface receptors and cytoskeletal elements that work together to regulate fluid exchange with the underlying tissue while retaining blood cells within the vessel [69]. There are two types of junctional adhesive structures (Figure 2); Tight Junctions (TJ) and Adherens Junctions (AJ). Tight junctional components, comprised of claudins, occludins, and JAMs, are present to various degrees in different endothelial cell beds – especially those that require tight regulation of vascular permeability such as in the blood-brain barrier [70]. Adherens Junctions, on the other hand, are made up of the vascular-specific cadherin, VE cadherin, linked to the actin cytoskeleton via members of the catenin family, and play probably the most important role in regulating vascular permeability [71,72]. Finally, the most abundant component of the endothelial cell junction, PECAM-1, is present in neither tight nor adherens junctions [73], rather becoming concentrated deep within the junction as a consequence of “diffusion-trapping [38] – a process in which N-terminal IgD1 and IgD2 mediate trans homophilic interactions between PECAM-1 molecules on adjacent cells.
Figure 2.
Adhesive molecules of the endothelial cell-cell junction. The vascular permeability barrier is maintained by tight junctions comprised of claudins, occludins, and JAMs, followed by adherens junctions comprised primarily of vascular endothelial cadherin (VE-cadherin) associated with the actin cytoskeleton via members of the catenin family. Underneath these specialized compartments lies the most abundant endothelial cell surface receptor, PECAM-1, which is expressed at 1–2 x 106 molecules per cell. Figure adapted from E. Dejana, Nature Reviews Molecular Biology 5:261, 2004 (69).
PECAM-1 AND THE MAINTANENCE OF THE ENDOTHELIAL CELL PERMEABILITY BARRIER
A plethora of studies support the concept that PECAM-1 contributes importantly to the maintenance of the endothelial cell permeability barrier. Ferrero demonstrated twenty years ago that addition of anti-PECAM-1 antibodies to endothelial cell monolayers in culture increases the rate of albumin transit in transwells, that transfection of PECAM-1 into cultured fibroblasts reduces albumin transit, and that injection of the PECAM-1 mAbs into mice results in fluid leak into the hepatic and renal vasculature [74]. Though PECAM-1-deficient mice exhibit no vascular abnormalities while sitting quietly in a cage in an animal facility, they have a profound, easily observable phenotype when subjected to inflammatory [75–77] or hemostatic [78] challenge.
While signal transduction events initiated by phosphorylation of PECAM-1 cytoplasmic domain ITIM tyrosines dominate the function of PECAM-1 in circulating platelets and leukocytes, the homophilic binding properties of PECAM-1 appear to be critical for its “firewall” role supporting endothelial cell junctional integrity. Recent mechanistic studies employing Electric Cell-substrate Impedance Sensing technology found that, compared with PECAM-1-deficient endothelial cells, PECAM-1-expressing endothelial cell monolayers exhibit increased steady-state barrier function, as well as more rapid restoration of barrier integrity following thrombin-induced perturbation of the endothelial cell monolayer integrity [42]. This effect was found to be dependent upon the ability of PECAM-1 to interact homophilically and become localized to cell–cell junctions, because a homophilic binding-crippled mutant form of PECAM-1 that could not localize to cell-cell borders was unable to support efficient barrier function. In contrast, cells expressing ITIM-less forms of PECAM-1 exhibited normal to near-normal barrier integrity. Whether non-ITIM sequences with the cytoplasmic domain play a role in stabilizing endothelial cell-cell junctions is not known, nor is the role that PECAM-1-linked carbohydrate residues play in this process understood. Both are the subject of ongoing investigations.
Perhaps most intriguing from a translational point of view is the observation that the adhesive properties of PECAM-1 are subject to affinity modulation [25,26] – a property well known for members of the integrin family of adhesion receptors, but relatively rare for members of the Ig superfamily. In a process that is not yet understood mechanistically, addition of antibodies that bind membrane-proximal IgD6 are able not only to increase the homophilic binding affinity of PECAM-1, but are able to actually enhance the rate of endothelial cell migration and barrier restoration in endothelial cell monolayers subjected to physical or inflammatory challenge. The finding that the adhesive properties of PECAM-1 are regulatable may allow for the development of novel approaches and reagents that can enhance endothelial cell migration and restore barrier function in a wide variety of vascular permeability disorders.
CONCLUSION
There is growing appreciation that the fields of thrombosis and inflammation are inextricably and mechanistically linked. PECAM-1, via its ability to inhibit the activation of circulating platelets and leukocytes, while at the same time supporting the integrity of endothelial cell-cell junctions and providing protection of the vascular bed to apoptotic stimuli, appears to play a significant role in each of these interrelated processes. PECAM-1 has predictably been implicated in a number of clinically-relevant disorders, ranging from thrombosis and cardiovascular disease to inflammation and cancer. It is hoped that this brief review will spur additional efforts to improve our understanding of the structure and function of this novel cell adhesion and signaling molecule in the vascular cells in which it is expressed, and allow for translational opportunities to be exploited.
KEY POINTS.
PECAM-1 is enriched at endothelial cell intercellular junctions, where it regulates leukocyte trafficking, mechanotransduction, and vascular permeability.
Extensive homophilic contacts between amino acids located in amino terminal Ig homology domains 1 and 2 of the molecule enable PECAM-1 to maintain vascular integrity and to resist mechanical force under conditions of fluid shear stress.
The adhesive properties of PECAM-1 are subject to affinity modulation – a rather unique property for a member of the Ig-superfamily – and may be physiologically important in thrombosis, inflammation and the immune response.
Acknowledgments
The authors thank Dr. Jieqing Zhu, Blood Research Institute, BloodCenter of Wisconsin for providing the space-filling model of IgD1/D2 for Figure 1, and Dr. Debra Newman for critical reading of the manuscript.
Financial support and sponsorship: PECAM-1 research in the authors’ laboratory is supported by grant HL40926 from the Heart, Lung, and Blood Institute of the National Institutes of Health. DL is a visiting Ph.D. student from the Huazhong University of Science and Technology sponsored by the China Scholarship Council.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Ohto H, Maeda H, Shibata Y, Chen R-F, Qzaki Y, Higashihara M, Takeuchi A, Tohyama H. A novel leukocyte differentiation antigen: Two monoclonal antibodies TM2 and TM3 define a 120-kd molecule present on neutrophils, monocytes, platelets, and activated lymphoblasts. Blood. 1985;66:873–881. [PubMed] [Google Scholar]
- 2.Goyert SM, Ferrero EM, Seremetis SV, Winchester RJ, Silver J, Mattison AC. Biochemistry and expression of myelomonocytic antigens. J Immunol. 1986;137:3909–3914. [PubMed] [Google Scholar]
- 3.Lyons AB, Cooper SJ, Cole SR, Ashman LK. Human myeloid differentiation antigens identified by monoclonal antibodies to the myelomonocytic leukemia cell line RC-2A. Pathology. 1988;20:137–146. doi: 10.3109/00313028809066624. [DOI] [PubMed] [Google Scholar]
- 4.Cabanas C, Sanchez-Madrid F, Bellon T, Figdor CG, Te Velde AA, Fernandez JM, Acevedo A, Bernabeu C. Characterization of a novel myeloid antigen regulated during differentiation of monocytic cells. European Journal of Immunology. 1989;19:1373–1378. doi: 10.1002/eji.1830190804. [DOI] [PubMed] [Google Scholar]
- 5.van Mourik JA, Leeksma OC, Reinders JH, de Groot PG, Zandbergen-Spaargaren J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from platelet membrane glycoprotein IIa. J Biol Chem. 1985;260:11300–11306. [PubMed] [Google Scholar]
- 6.Giltay JC, Brinkman HJ, Modderman PW, von dem Borne AE, van Mourik JA. Human vascular endothelial cells express a membrane protein complex immunochemically indistinguishable from the platelet VLA- 2 (glycoprotein Ia–IIa) complex. Blood. 1989;73:1235–1241. [PubMed] [Google Scholar]
- 7.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: A novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990;110:1227–1237. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 10.Stockinger H, Gadd SJ, Eher R, Majdic O, Schreiber W, Kasinrerk W, Strass B, Schnabl E, Knapp W. Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol. 1990;145:3889–3897. [PubMed] [Google Scholar]
- 11.Simmons DL, Walker C, Power C, Pigott R. Molecular cloning of CD31, a putative intercellular adhesion molecule closely related to carcinoembryonic antigen. J Exp Med. 1990;171:2147–2152. doi: 10.1084/jem.171.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman PJ, Newman DK. PECAM-1. 3. Boston: Academic Press; 2013. [Google Scholar]
- 13.Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 14.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1. New roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 15.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 16.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Paddock C, Zhou D, Lertkiatmongkol P, Newman PJ, Zhu J. Structural basis for PECAM-1 homophilic binding. Blood. 2016 doi: 10.1182/blood-2015-07-660092. This study provides the first detailed look at the structure of the PECAM-1 homophilic binding domain and provides insights into how it forms part of the endothelial cell permeability barrier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paddock C, Lytle BL, Peterson FC, Holyst T, Newman PJ, Volkman BF, Newman DK. Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood. 2011;117:6012–6023. doi: 10.1182/blood-2010-11-317867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton JP, Hunter AP, Simmons DL, Buckley CD, Harvey DJ. CD31 (PECAM-1) exists as a dimer and is heavily N-glycosylated. Biochem Biophys Res Commun. 1999;261:283–291. doi: 10.1006/bbrc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 20.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 21.Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry AK, Wang N, Leckband DE. Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J Cell Sci. 2015;128:1341–1351. doi: 10.1242/jcs.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson DE, Loo RO, Holyst MT, Newman PJ. Identification and characterization of functional cation coordination sites in platelet endothelial cell adhesion molecule-1. Biochemistry. 1997;36:9395–9404. doi: 10.1021/bi970084x. [DOI] [PubMed] [Google Scholar]
- 25.Sun Q-H, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996;271:11090–11098. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 26*.Mei H, Campbell JM, Paddock CM, Lertkiatmongkol P, Mosesson MW, Albrecht R, Newman PJ. Regulation of endothelial cell barrier function by antibody-driven affinity modulation of platelet endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 2014;289:20836–20844. doi: 10.1074/jbc.M114.557454. This study demonstrates, using PECAM-1 nanodiscs, that the homophilic binding affinity of PECAM-1 can be regulated, providing future translational opportunities for treating vascular permeability disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldwin HS, Shen HM, Yan H-C, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum N, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): Alternatively spliced, functionally distinct isoforms expressed during early mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- 28.Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev Dyn. 1999;214:44–54. doi: 10.1002/(SICI)1097-0177(199901)214:1<44::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Su X, Sorenson CM, Sheibani N. Modulation of PECAM-1 expression and alternative splicing during differentiation and activation of hematopoietic cells. J Cell Biochem. 2003;88:1012–1024. doi: 10.1002/jcb.10451. [DOI] [PubMed] [Google Scholar]
- 30.Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol. 2007;292:C2070–C2083. doi: 10.1152/ajpcell.00489.2006. [DOI] [PubMed] [Google Scholar]
- 31.Bergom C, Paddock C, Gao C, Holyst T, Newman DK, Newman PJ. An alternatively spliced isoform of PECAM-1 is expressed at high levels in human and murine tissues, and suggests a novel role for the C-terminus of PECAM-1 in cytoprotective signaling. J Cell Sci. 2008;121:1235–1242. doi: 10.1242/jcs.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park S, Sorenson CM, Sheibani N. PECAM-1 isoforms, eNOS and endoglin axis in regulation of angiogenesis. Clin Sci (Lond) 2015;129:217–234. doi: 10.1042/CS20140714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tourdot BE, Brenner MK, Keough KC, Holyst T, Newman PJ, Newman DK. Immunoreceptor tyrosine-based inhibitory motif (ITIM)-mediated inhibitory signaling is regulated by sequential phosphorylation mediated by distinct nonreceptor tyrosine kinases: a case study involving PECAM-1. Biochemistry. 2013;52:2597–2608. doi: 10.1021/bi301461t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds PECAM-1 and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1 and integrin-mediated cellular signaling. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 35.Newman PJ. Switched at birth: a new family for PECAM-1. J Clin Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. Febs Letters. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- 37.Maas M, Wang R, Paddock C, Kotamraju S, Kalyanaraman B, Newman PJ, Newman DK. Reactive oxygen species induce reversible PECAM-1 tyrosine phosphorylation and SHP-2 binding. Am J Physiol Heart Circ Physiol. 2003;285:H2336–H2344. doi: 10.1152/ajpheart.00509.2003. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Paddock C, Shubert J, Zhang H, Amin K, Newman PJ, Albelda SM. Contributions of the extracellular and cytoplasmic domains of platelet- endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell- cell localization. J Cell Sci. 2000;113:1459–1469. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- 39.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Williams J, Yan H-C, Amin KM, Albelda SM, DeLisser HM. Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- 41.Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites on PECAM-1/CD31. JBC. 1997;272:20555–20563. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- 42.Privratsky JR, Paddock CM, Florey O, Newman DK, Muller WA, Newman PJ. Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J Cell Sci. 2011;124:1477–1485. doi: 10.1242/jcs.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Privratsky JR, Newman PJ. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res. 2014;355:607–619. doi: 10.1007/s00441-013-1779-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitazume S, Imamaki R, Ogawa K, Komi Y, Futakawa S, Kojima S, Hashimoto Y, Marth JD, Paulson JC, Taniguchi N. a2,6-sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J Biol Chem. 2010;285:6515–6521. doi: 10.1074/jbc.M109.073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varki A, Angata T. Siglecs--the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 46*.Kitazume S, Imamaki R, Kurimoto A, Ogawa K, Kato M, Yamaguchi Y, Tanaka K, Ishida H, Ando H, Kiso M, et al. Interaction of platelet endothelial cell adhesion molecule (PECAM) with alpha2,6-sialylated glycan regulates its cell surface residency and anti-apoptotic role. J Biol Chem. 2014;289:27604–27613. doi: 10.1074/jbc.M114.563585. This study, together with those reported in references 47 and 48, provide the first evidence that PECAM-1-linked glycans contribute to its ability to form homophilic interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee C, Liu A, Miranda-Ribera A, Hyun SW, Lillehoj EP, Cross AS, Passaniti A, Grimm PR, Kim BY, Welling PA, et al. NEU1 sialidase regulates the sialylation state of CD31 and disrupts CD31-driven capillary-like tube formation in human lung microvascular endothelia. J Biol Chem. 2014;289:9121–9135. doi: 10.1074/jbc.M114.555888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lertkiatmongkol P, Paddock C, Newman PJ. Species-specific roles for α2,3- and α2,6-linked sialic acid residues in PECAM-1-mediated homophilic interactions. Blood 2015. Blood. 2015;126:3450a. [Google Scholar]
- 49.Sheibani N, Newman PJ, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, regulates Platelet-Endothelial Cell Adhesion Molecule-1 expression and endothelial cell morphogenesis. Mol Biol Cell. 1997;8:1329–1341. doi: 10.1091/mbc.8.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. Journal of Immunology. 1997;158:3408–3416. [PubMed] [Google Scholar]
- 52.Yang S, Graham J, Kahn JW, Schwartz EA, Gerritsen ME. Functional roles for PECAM-1 (CD31) and VE-cadherin (CD144) in tube assembly and lumen formation in three-dimensional collagen gels. Am J Pathol. 1999;155:887–895. doi: 10.1016/S0002-9440(10)65188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Z, Christofidou-Solomidou M, Garlanda C, DeLisser HM. Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis. 1999;3:181–188. doi: 10.1023/a:1009092107382. [DOI] [PubMed] [Google Scholar]
- 54.Cao G, O’Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol. 2002;282:C1181–C1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- 55.Cao G, Fehrenbach ML, Williams JT, Finklestein JM, Zhu JX, DeLisser HM. Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am J Pathol. 2009;175:903–915. doi: 10.2353/ajpath.2009.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Brien CD, Cao G, Makrigiannakis A, DeLisser HM. Role of immunoreceptor tyrosine-based inhibitory motifs of PECAM-1 in PECAM-1-dependent cell migration. Am J Physiol Cell Physiol. 2004;287:C1103–C1113. doi: 10.1152/ajpcell.00573.2003. [DOI] [PubMed] [Google Scholar]
- 57.Zhu JX, Cao G, Williams JT, Delisser HM. SHP-2 phosphatase activity is required for PECAM-1-dependent cell motility. Am J Physiol Cell Physiol. 2010;299:C854–865. doi: 10.1152/ajpcell.00436.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoenwaelder SM, Petch LA, Williamson D, Shen R, Feng GS, Burridge K. The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr Biol. 2000;10:1523–1526. doi: 10.1016/s0960-9822(00)00831-9. [DOI] [PubMed] [Google Scholar]
- 59.Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. Faseb J. 2003;17:1458–1469. doi: 10.1096/fj.02-1040com. [DOI] [PubMed] [Google Scholar]
- 60.Winn RK, Harlan JM. The role of endothelial cell apoptosis in inflammatory and immune diseases. J Thromb Haemost. 2005;3:1815–1824. doi: 10.1111/j.1538-7836.2005.01378.x. [DOI] [PubMed] [Google Scholar]
- 61.Bird IN, Taylor V, Newton JP, Spragg JH, Simmons DL, Salmon M, Buckley CD. Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. J Cell Sci. 1999;112:1989–1997. doi: 10.1242/jcs.112.12.1989. [DOI] [PubMed] [Google Scholar]
- 62.Noble KE, Wickremasinghe RG, DeCornet C, Panayiotidis P, Yong KL. Monocytes stimulate expression of the Bcl-2 family member, A1, in endothelial cells and confer protection against apoptosis. J Immunol. 1999;162:1376–1383. [PubMed] [Google Scholar]
- 63.Evans PC, Taylor ER, Kilshaw PJ. Signaling through CD31 protects endothelial cells from apoptosis. Transplantation. 2001;71:457–460. doi: 10.1097/00007890-200102150-00020. [DOI] [PubMed] [Google Scholar]
- 64.Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169–179. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- 65.Bergom C, Goel R, Paddock C, Gao C, Newman DK, Matsuyama S, Newman PJ. The cell-adhesion and signaling molecule PECAM-1 is a molecular mediator of resistance to genotoxic chemotherapy. Cancer Biol Ther. 2006;5:1699–1707. doi: 10.4161/cbt.5.12.3467. [DOI] [PubMed] [Google Scholar]
- 66*.Cheung K, Ma L, Wang G, Coe D, Ferro R, Falasca M, Buckley CD, Mauro C, Marelli-Berg FM. CD31 signals confer immune privilege to the vascular endothelium. Proc Natl Acad Sci U S A. 2015;112:E5815–5824. doi: 10.1073/pnas.1509627112. This study builds importantly on the results of reference 65 to demonstrate that PECAM-1 ITIM signaling confers cytoprotection to the vascular endothelium exposed to pro-apoptotic stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- 69.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 70.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 71.Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest. 1996;100:S7–10. [PubMed] [Google Scholar]
- 72.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J Cell Biol. 1994;126:247–258. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrero E, Ferrero ME, Pardi R, Zocchi MR. The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. Febs Lett. 1995;374:323–326. doi: 10.1016/0014-5793(95)01110-z. [DOI] [PubMed] [Google Scholar]
- 75.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- 77.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, Madri JA. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]