ABSTRACT
Directing cell trafficking toward a target site of interest is critical for advancing stem cell therapy in clinical theranostic applications. In this study, we investigated the effects of inflammatory and/or hypoxic stimuli on the migration of bone marrow mesenchymal stem cells (BMMSCs) during in vitro culture and after in vivo implantation. Using tablet scratch experiments and observations from a transwell system, we found that both inflammatory and hypoxic stimuli significantly enhanced cell migration. However, the combination of inflammatory and hypoxic stimuli did not result in a synergistic effect. The presence of stromal cell-derived factor-1 (SDF-1) significantly enhanced cell migration irrespective of the incubation conditions, and these positive effects could be blocked by treatment with AMD3100. Based on a time course experiment, we found that preconditioning cells with either inflammatory or hypoxic stimuli for 24 h or with both stimuli for 12 h led to high levels of chemokine receptor type 4 (CXCR4) expression. In vivo studies further demonstrated that pretreatment of BMMSCs with inflammatory and/or hypoxic stimuli resulted in an increased number of systemically injected cells migrating toward skin injuries, and local SDF-1 administration significantly increased cell migration. These findings suggest that in vitro control of either inflammatory or hypoxic stimuli has significant potential to enhance SDF-1-directed BMMSC migration via the upregulation of CXCR4 expression. Although combining the stimuli did not necessarily lead to a synergistic effect, the potential to reduce the dose and time required for cell preconditioning indicates that combinations of various strategies warrant further exploration.
KEYWORDS: cell migration, chemokine receptor type 4, hypoxia; inflammation, mesenchymal stem cells; stromal cell-derived factor-1
Introduction
Stem cell therapies promise to produce cures for a wide variety of diseases and disorders either by serving as external, integrated participants in target tissues of interest or by acting as secretory vehicles that deliver extracellular signals necessary for enhancing the healing cascade.1 Many cell populations, particularly those derived from adult human tissues, have been used as powerful tools in regenerative medicine. In particular, bone marrow-derived mesenchymal stem cells (MSCs), commonly referred to as BMMSCs, have demonstrated substantial potential for clinical use.2 Although the major focus of stem cell-based therapy in past years has been the development of optimized cell populations to serve as therapeutics under given scenarios, it is now clear that developing techniques to foster and deliver cells that enhance in vivo cell survival and augment engraftment efficiency is an equally important task.3 Knowledge of how ex vivo-expanded cells respond to their surrounding environment during in vitro cultivation and subsequent in vivo implantation is crucial for guiding the design of cell-based therapeutics. Although localized administration of BMMSCs is a potentially effective strategy in certain ideal scenarios, the potential for minimally invasive infusion of these cells via the circulatory system is of particular interest. For example, systemic infusion of cells is more frequently employed than direct injection of cells into the ischemic myocardium because it simplifies clinical administration technique and facilitates greater ease in repeated dosing. In turn, this enables a greater number of patients to receive cell therapy. This technique also aids in ensuring that the injected cells remain in close proximity to nutrient- and oxygen-rich vessels and permits the cells to reach the ischemic myocardium by mimicking natural cell trafficking processes.4,5 However, a significant obstacle to the use of stem cells as therapeutics is the inability of current techniques to target exogenously infused cells to therapeutic sites of interest with high levels of engraftment and efficiency. This observation is particularly true when cells are systemically administered because the efficacy of this approach is limited by the quantity of viable cells that reach an injured tissue.5-7 Unfortunately, previous investigations have uniformly demonstrated poor engraftment of transplanted cells. In the absence of ex vivo pretreatment or modification, less than 3% of transplanted cells typically engraft following their introduction into the body.3 Research on techniques that enhance stem cell trafficking and migration is therefore clinically relevant for, but not limited to, minimally invasive cell therapy.8
The migratory capacity of BMMSCs is under the control of a large range of soluble factors and tyrosine kinase receptors. This complex regulatory network implies that BMMSC homing to injured tissues is influenced by systemic and local inflammatory status.5,9 Tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) are major inflammatory mediators present in damaged tissues and can used to establish an inflammatory condition wherein cells exhibit enhanced migration capacity.10 Prestimulation of adipose tissue-derived MSCs with TNF-α alone can induce these cells to home to injured sites in vivo following intravenous administration, thereby improving their therapeutic potential.11 When adipose tissue-derived MSCs were preincubated with various chemokines or growth factors at a concentration of 100 ng/mL, the cells treated with TNF-α showed the most effective chemoattractant activity.12 In contrast, pretreatment with IL-1β enhanced the efficacy of MSC transplantation in treating dextran sulfate sodium (DSS)-induced colitis, which at least partially depended on an enhancement in cell migration ability.13 Moreover, short-term stimulation of BMMSCs with a cocktail of cytokines resulted in upregulation of both cell surface and intracellular CXC chemokine receptor 4 (CXCR4). These changes increased the cells' in vitro migration capacity toward stromal cell-derived factor-1 (SDF-1) as well as their homing behavior to bone marrow following intravenous infusion into sublethally irradiated NOD/SCID mice.14 Although the mechanisms underlying the pathways that are relevant to these changes are incompletely understood, the incubation of cells under inflammatory conditions prior to transplantation has been extensively used to modulate cell migration in a variety of preclinical situations.15-17
Oxygen tension also plays an important role in various cell behaviors, such as cell migration.18 In cell culture, hypoxia can modulate cell proliferation and differentiation, and hypoxic preconditioning of cells before transplantation might increase survival capacity and engraftment efficiency in target tissues.19,20 In addition, hypoxic modulation improves the capacity of satellite cells to promote angiogenesis, which involves a reduction in satellite cell hepatocyte growth factor expression.21 Hypoxia also regulates the paracrine activity of stem cells, causing the upregulation of a broad spectrum of secretory factors, including various angiogenic factors that are important for therapeutic angiogenesis in ischemic tissue.22,23 Given the evidence that the hypoxia-HIF-1α-CXCR4 pathway plays a crucial role during human osteosarcoma cell migration, targeting of this pathway may serve as a novel approach for enhancing stem cell homing.24 Indeed, hypoxic preconditioning of BMMSCs was demonstrated to enhance the efficacy of cell therapy for the treatment of ischemic acute kidney injury by improving cell migration to injured kidney tissue.25 Currently, substantial evidence indicates that hypoxia plays an important role in the mobilization and homing of MSCs, primarily via its ability to induce the expression of both SDF-1 and its receptor CXCR4.19,26,27
Although data from in vitro and in vivo studies suggest that either an inflammatory or a hypoxic stimulus can positively impact cell migration, an optimized strategy for cell preconditioning before transplantation has not been established. In comparing MSCs stimulated with TNF-α alone using different dose and time parameters, the highest level of CXCR4 expression (up to 512-fold greater than in untreated cells) was found after treatment with 10 ng/mL of TNF-α for 24 h.11 However, substantially higher concentrations of cytokines and growth factors have also been used in research. For example, a combination of 10 ng/mL IL-3; 50 ng/mL each of stem cell factor (SCF), IL-6 and hepatocyte growth factor (HGF); and 300 ng/mL Flt-3 ligand still resulted in a positive outcome.14 For hypoxic preconditioning, previously reported concentrations of oxygen and durations of incubation have been highly diverse; for example, the following conditions have been reported: 1% oxygen for 10 h,21 24 h23 or 2 d or more; 20,22 2% oxygen for the entire length of an experiment;24 3% oxygen for 6 h;19 and 5% oxygen for 24 h.23 Occasionally, cobalt chloride has been used to mimic hypoxic preconditioning.25 These data imply that in vitro control of both dose and time factors with regard to either inflammatory or hypoxic stimuli might be paramount in modulating stem cell migration capacity. The aforementioned studies are also complicated by the diverse methods used for the in vitro cultivation and characterization of cell migration activity and by the variety of methods used to transplant and assess in vivo cell-homing events. In the current study, the effects of inflammatory and/or hypoxic stimuli on BMMSC migration both in culture and after implantation were investigated using well-established in vitro and in vivo models. We attempted to control prestimulation parameters to enhance cell expression of CXCR4, which is believed to augment cell migration toward SDF-1. Moreover, we sought to determine whether a combination of inflammatory and hypoxic stimuli might generate a synergistic effect in activating cell migration.
Results
Isolation and characterization of human BMMSCs
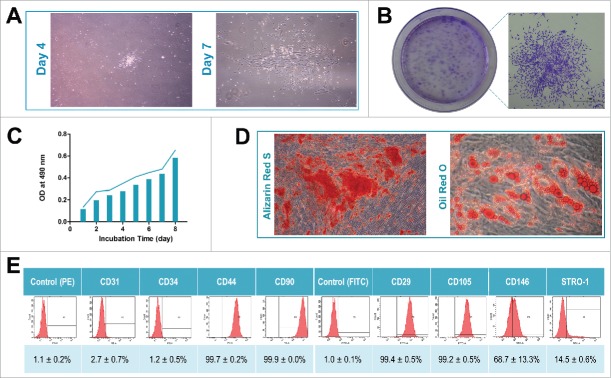
Primary cells were successfully extracted from the bone marrow of 4 donors. When the primary cells reached 80% confluence, a limited dilution method was used to purify BMMSCs. Generally, cells derived from different individuals exhibited a similar elongated spindle shape at days 4 and 7 (Fig. 1A). All of the isolated BMMSCs displayed the ability to form colonies (Fig. 1B) and could proliferate in vitro (Fig. 1C). After either 4 weeks of osteogenic induction or 3 weeks of adipogenic induction, Alizarin Red staining (for mineralized cell nodules) and Oil Red O staining (for lipid droplets) illustrated the significant differentiation potential of the isolated BMMSCs (Fig. 1D). In addition, flow cytometry data demonstrated that the BMMSCs were positive for various mesenchymal-associated markers, including CD29, CD44, CD90, CD105, CD146 and STRO-1, and negative for haematopoietic markers, including CD31 and CD34 (Fig. 1E).
Figure 1.
Isolation and characterization of human BMMSCs. (A) Representative images of primary cells derived from bone marrow on days 4 and 7 (40×). (B) Representative images of colony-forming units and a random single-cell clone on day 12. (C) Proliferative capacity of BMMSCs evaluated by MTT assay during an 8-day incubation. (D) Representative images of mineralized cell nodules (histochemically stained with Alizarin Red, 40×) following a 4-week osteogenic induction and of lipid droplets (stained with Oil Red O, 40×) following a 3-week adipogenic induction. (E) Representative images of cell surface marker expression and data obtained from the 4 cell lines shown as the mean ± SD.
Inflammatory and/or hypoxic stimuli enhance BMMSC migration in vitro
A tablet scratch experiment was used for preliminary exploration of how inflammatory and/or hypoxic stimuli affected cell migration. A greater number of cells migrated into a cell-free area after exposure to inflammatory or hypoxic stimuli relative to unstimulated cells (P < 0.05); however, exposure to inflammatory and hypoxic stimuli in combination did not synergistically affect cell migration (Fig. 2A-B). Interestingly, the number of migrated cells in the dual-stimuli group was lower than that in either the inflammation group or the hypoxia group, although no significant differences were found (P > 0.05). Additionally, no significant difference was observed in cell migration between the no-stimulus group and the dual-stimuli group (P > 0.05). Quantitatively, however, exposure to inflammatory and hypoxic stimuli in combination resulted in an increased number of migrated cells (Fig. 2A-B).
Figure 2.

Investigation of cell migration in a culture without inflammatory or hypoxic stimuli (no-stimulus) or in cultures exposed to an inflammatory stimulus (inflammation), hypoxic conditions (hypoxia) or both stimuli (dual-stimuli) using a tablet scratch experiment in vitro. (A) Representative images showing BMMSC migration at 0 h and 15 h after scratching (40×). (B) Statistical analysis of the number of migrated cells. The data are shown as the mean ± SD. *P < 0.05 represents significant differences compared with the no-stimulus group.
To investigate the effects of inflammatory and/or hypoxic stimuli on cell migration toward SDF-1, we first had to determine an SDF-1 concentration that induces effective cell migration in our transwell membrane system in the absence of inflammatory or hypoxic stimuli. The presence of SDF-1 (either 100 ng/mL or 200 ng/mL) in the lower chamber of the system led to an increased number of migrated cells relative to those cultured in the absence of SDF-1 (P < 0.05; Fig. 3A-B). Because both of the tested SDF-1 concentrations (i.e., 100 ng/mL and 200 ng/mL) produced similar outcomes with respect to cell migration (P > 0.05; Fig. 3B), we used 100 ng/mL SDF-1 for subsequent assays.
Figure 3.

Investigation of cell migration using a transwell membrane system in vitro. (A) Representative images showing BMMSC migration in response to SDF-1. Culture medium with different concentrations of SDF-1 (0 ng/mL, 100 ng/mL or 200 ng/mL) was applied to the lower chambers of the transwell system (100×). (B) Statistical analysis of the number of migrated cells under SDF-1-induced chemotaxis. (C) Representative images showing BMMSC migration in conditions without inflammatory or hypoxic stimuli (no-stimulus) or under inflammatory conditions (inflammation), hypoxic conditions (hypoxia) or both (dual-stimuli; experimental design shown in Table 1, 100×). (D) Statistical analysis of the number of migrated cells under different conditions. All data are shown as the mean ± SD. **P < 0.01 and ***P < 0.001 represent significant differences compared with the system without SDF-1.###P < 0.001 represents significant differences compared with the system with 100 ng/mL SDF-1 in the lower chamber.ΔP < 0.05,ΔΔP < 0.01 andΔΔΔP < 0.001 represent significant differences between the indicated groups.
Based on the design shown in Table 1, cell migration toward SDF-1 under inflammatory and/or hypoxic conditions was studied using a transwell membrane system. When SDF-1 was absent from the lower chambers of the system, both inflammatory and hypoxic stimuli led to increased cell migration from the upper to the lower chambers compared with cells without any stimulation (no-stimulus group); these differences were significant (P < 0.05 or P < 0.01; Fig. 3C-D). Similarly to the data obtained in the tablet scratch experiment, cells exposed to inflammatory and hypoxic stimuli in combination (dual-stimuli group) did not exhibit enhanced cell migration compared with cells without stimulation (no-stimulus group; P > 0.05; Fig. 3C-D). When the lower chambers of the system were supplemented with 100 ng/mL SDF-1, almost twofold more cells migrated compared to those under SDF-1-free conditions (P < 0.01 or P < 0.001); this trend was true in all 4 cell groups (no-stimulus, inflammation, hypoxia and dual-stimuli). However, when cells were stimulated with inflammatory and/or hypoxic stimuli, similar cell migration patterns emerged regardless of whether SDF-1 was present or absent. Unexpectedly, the number of migrated cells in the dual-stimuli group was lower than that in the inflammation group (Fig. 3C-D). As expected, the positive effects of inflammatory and/or hypoxic stimuli on cell migration toward SDF-1 could be blocked by the addition of 5 µg/ml of the CXCR4 antagonist AMD3100 in the upper chamber. There was no significant difference between the number of migrated cells in the system treated with both AMD3100 and SDF-1 and that in the system without SDF-1 (100 ng/mL), regardless of whether inflammatory and/or hypoxic stimuli were present (P > 0.05; Fig. 3C-D).
Table 1.
Study design for cell migration under inflammatory and/or hypoxic stimuli toward SDF-1 (100 ng/mL).
| Group | Lower chamber |
Upper chamber |
Incubator atmosphere | ||
|---|---|---|---|---|---|
| |
Medium |
SDF-1 (ng/mL) |
Medium |
AMD3100 (μg/mL) |
|
| No-stimulus | Normal | 0 | Normal | 0 | Normal (20% O2) |
| 100 | 0 | ||||
| 100 | 5 | ||||
| Inflammation | Inflammatory | 0 | Inflammatory | 0 | Normal(20% O2) |
| 100 | 0 | ||||
| 100 | 5 | ||||
| Hypoxia | Normal | 0 | Normal | 0 | Hypoxic(2% O2) |
| 100 | 0 | ||||
| 100 | 5 | ||||
| Dual-stimuli | Inflammatory | 0 | Inflammatory | 0 | Hypoxic(2% O2) |
| 100 | 0 | ||||
| 100 | 5 | ||||
Normal medium: α-MEM supplemented with 5% FBS;
Inflammatory medium: α-MEM supplemented with 5% FBS containing tumor necrosis factor (TNF)-α (10 ng/mL) and interleukin (IL)-1β (5 ng/mL);
Hypoxic condition: cell culture in a humidified atmosphere containing 2% O2 (instead of 20% O2).
Effects of inflammatory and/or hypoxic stimuli on CXCR4 expression in BMMSCs
Temporal changes in CXCR4 expression under inflammatory and/or hypoxic stimuli
To investigate the effects of inflammatory and/or hypoxic stimuli on CXCR4 expression in BMMSCs, CXCR4 mRNA expression was analyzed via real-time PCR, and CXCR4 protein levels were analyzed using Western blot. Under inflammatory conditions, CXCR4 mRNA and protein expression levels both showed time-dependent increases that peaked at levels almost 4-fold higher than those of the control after 24 h of incubation. Further incubation led to a gradual decrease in CXCR4 expression at both the gene and protein levels (Fig. 4A-B). However, for cells exposed to hypoxic conditions, CXCR4 gene expression reached a peak that was 3-fold higher than that of the control after 12 h of incubation; a similarly high level was maintained through 24 h (the levels at 12 h and 24 h showed no significant differences; P > 0.05; Fig. 4C). Furthermore, Western blotting showed that CXCR4 protein expression in these cells reached a peak that was 4-fold higher than that in the controls after 24 h of incubation (Fig. 4D). When cells were exposed to inflammatory and hypoxic stimuli in combination, CXCR4 expression peaked after 6 h of incubation and was over 3-fold higher than the control; no significant differences were found in CXCR4 expression levels between 6 h and 12 h of incubation (P > 0.05; Fig. 4E). However, CXCR4 protein levels peaked at 12 h of incubation and were almost 3-fold higher than in the control (Fig. 4F). Based on the high levels of CXCR4 expression obtained via very brief stimulation, for subsequent assays, we used a 24-h exposure time for inflammatory stimuli, a 24-h exposure time for hypoxic stimuli and a 12-h exposure time for inflammatory and hypoxic stimuli in combination.
Figure 4.
Effects of inflammatory and/or hypoxic stimuli on CXCR4 expression. (A) and (B) Incubation under inflammatory conditions (inflammation). (C) and (D) Incubation under hypoxic conditions (hypoxia). (E) and (F) Incubation under inflammatory plus hypoxic conditions (dual-stimuli). Relative CXCR4 expression under (A) inflammatory conditions, (C) hypoxic conditions or (E) both at different time points. Representative images and quantitative analysis of CXCR4 protein expression via Western blotting under (B) inflammatory conditions, (D) hypoxic conditions or (F) both at different time points. All data are shown as the mean ± SD. NS represents no significant differences between the indicated columns.
Effects of inflammatory and/or hypoxic stimuli on CXCR4 expression in BMMSCs after varying exposure lengths
CXCR4 expression in BMMSCs was analyzed at the selected timepoints noted in the preceding section. The cells were incubated in medium containing inflammatory stimuli (inflammation group) for 24 h, under hypoxic conditions (hypoxia group) for 24 h, or in an environment that included both conditions (dual-stimuli group) for 12 h. As a control, a subset of cells was cultured in the absence of both types of stimuli (no-stimulus group). Real-time PCR analysis indicated that both inflammatory stimuli and inflammatory plus hypoxic stimuli significantly increased CXCR4 expression (P < 0.05 or P < 0.001). However, no significant increase was found under hypoxic conditions alone (Fig. 5A), although the cells grown under this condition tended to present quantitatively higher levels of CXCR4 expression relative to cells grown in the absence of inflammatory or hypoxic stimuli. It is worth emphasizing that cells exposed to inflammatory stimuli alone exhibited the highest CXCR4 expression among all 4 groups (Fig. 5A). Western blot analysis showed that exposing cells to any of the evaluated stimuli individually significantly increased CXCR4 protein expression (P < 0.001), while exposure to inflammatory and hypoxic stimuli in combination caused a relative decrease in CXCR4 expression (P < 0.01 or P < 0.001; Fig. 5B). Intramembrane CXCR4 proteins on the surfaces of BMMSCs from all 4 groups were subjected to immunofluorescence staining, and the most obvious CXCR4 signal was observed in the cells exposed to inflammatory stimuli (Fig. 5C). Quantitative analysis of fluorescence intensity showed that exposure to either inflammatory or hypoxic stimuli led to increased fluorescence intensity on the membrane surfaces of BMMSCs compared with cells not exposed to either stimulus (P < 0.01; Fig. 5D). Similarly, exposing cells to inflammatory and hypoxic stimuli in combination did not produce a synergistic effect relative to exposing cells to either stimulus individually; in this case, fluorescence intensity decreased instead of increased (Fig. 5D).
Figure 5.

Effect of inflammatory and/or hypoxic conditions on CXCR4 expression in BMMSCs after varying lengths of time. To compare CXCR4 expression among the 4 groups, the cells were induced under inflammatory conditions for 24 h, under hypoxic conditions for 24 h, or under both conditions for 12 h. A culture free from inflammatory and hypoxic stimuli served as a control. CXCR4 expression was analyzed by (A) real-time PCR and (B) Western blotting. (C) Representative immunofluorescent images of CXCR4 proteins on BMMSC membranes (600×). (D) Quantitative analysis of CXCR4 fluorescence intensity. All data are shown as the mean ± SD. *P < 0.05, **P < 0.01 and ***P < 0.001 represent significant differences compared with the control (no-stimulus).##P < 0.01 represents significant differences compared to the cells cultured under hypoxic conditions (hypoxia).ΔP < 0.05,ΔΔP < 0.01 and ΔΔΔP < 0.001 represent significant differences compared to the cells grown under inflammatory plus hypoxic conditions (dual-stimuli).
Effects of different pretreatments on in vivo cell migration toward injury
To investigate the effects of various pretreatments with different stimuli on cell migration toward sites of injury, BMMSCs expressing green fluorescent protein (GFP) were administered to rats through the caudal vein. Prior to the administration of BMMSCs, the rats underwent surgery to generate 2 longitudinally aligned, full-thickness defects on the dorsal side. On the second day after wounding, the rats were subjected to intravenous injection of GFP-BMMSCs. Whether a rat received GFP-BMMSCs pretreated for 24 h with either inflammatory or hypoxic stimuli or GFP-BMMSCs treated for 12 h with both stimuli was randomly decided. Cells without pretreatment served as a control (no-stimulus group). Recombinant SDF-1 was applied to a randomly selected defect on each rat to evaluate its potential chemotactic effect. On day 7, skin samples were collected to count the number of migrated cells via immunofluorescent staining of GFP. Regardless of whether SDF-1 was present, the BMMSCs that were pretreated with inflammatory and/or hypoxic stimuli exhibited increased migration relative to the control (both autofluorescence and immunofluorescence were evident; Fig. 6A). This enhanced migratory effect was further demonstrated by quantitatively analyzing the numbers of migrated cells in each of the 4 groups (Fig. 6B). We found that cells exposed to inflammatory or hypoxic stimuli showed substantially increased migration toward sites of injury compared with cells without any stimulation, irrespective of local SDF-1 administration to injury sites (P < 0.001). Furthermore, pretreating BMMSCs with inflammatory stimuli induced greater cell migration than pretreatment under hypoxic conditions (P < 0.05; Fig. 6B). Again, pretreatment of cells with both stimuli (inflammatory plus hypoxic) did not create a synergistic effect, and no significant difference was observed in cell migration between the no-stimulus group and the dual-stimuli group (P > 0.05; Fig. 6B). Interestingly, local administration of SDF-1 significantly increased cell migration, regardless of whether BMMSCs were pretreated with inflammatory or hypoxic stimuli (P < 0.05), whereas no significant increases were noted in either the no-stimulus group or the dual-stimuli group. However, higher numbers of migrated cells might be obtained at an injury site following local SDF-1 administration (Fig. 6B).
Figure 6.

Effect of pretreatments on in vivo cell migration toward an injury in a skin defect model. Rats were systemically injected with GFP-BMMSCs pretreated with an inflammatory stimulus for 24 h (inflammation), a hypoxic stimulus for 24 h (hypoxia), or both stimuli for 12 h (dual-stimuli). Rats in the no-stimulus group received injections of cells without any pretreatment. Injuries with and without local administration of SDF-1 (100 ng/mL) were used to investigate the effects of the local presence of SDF-1 on cell recruitment. (A) Representative images obtained with a confocal laser scanning microscope system. Autofluorescence and immunofluorescent staining were both used to track transplanted GFP-BMMSCs (200×). (B) Analysis of the number of migrated cells in the 4 groups with or without local SDF-1 administration. All data are shown as the mean ± SD.ΔP < 0.05,ΔΔP < 0.01, and ΔΔΔP < 0.001 represent significant differences between the indicated columns. *P < 0.05 represents significant differences compared with the absence of local SDF-1 administration.
Discussion
Stem cells are emerging as fundamental underpinnings for the physiological maintenance of tissue and the development of disease states. In the context of injury, BMMSCs contribute to wound healing and regeneration, largely through the secretion of trophic factors that confer paracrine support to reparative cells and regulate local extracellular matrix remodeling.28 Furthermore, within the in vivo milieu, BMMSCs are thought to exert beneficial functions via direct cellular differentiation and/or fusion to generate other tissue-specific cell populations that directly participate in tissue regeneration.3 These multi-factorial roles of BMMSCs at sites of injury make them especially appealing for cell-based therapeutics, as illustrated by their ability to promote the regeneration of various tissues and organs.2 Further highlighting their beneficial functions for a variety of autoimmune diseases, the administration of BMMSCs has been demonstrated to regulate immune responses in animal models, primarily via cytokine-mediated activation of Tregs and/or upregulation of regulatory T cells.29-31
Although mounting evidence has demonstrated the beneficial effects and therapeutic potential of BMMSCs for tissue regeneration and recovery of organ function, an obvious obstacle to the effective implementation of MSC-based therapies is the inability to target large quantities of viable cells to tissues of interest; indeed, notably few MSCs migrate to target tissues, especially after systemic infusion.4 Many studies have explored strategies for enhancing cell migration and the corresponding underlying mechanisms.32,33 The current generally accepted theory is that the chemokine SDF-1 (also known as CXCL12) is critical for cell chemotaxis and tissue-specific recruitment of reparative cells to sites of injury through its interactions with its cognate receptor, CXCR4, which is located on the surfaces of these cells.34 SDF-1 was demonstrated to be significantly upregulated in nearly all tissues suffering from injury or disease, indicating that alterations in SDF-1 and CXCR4 expression regulate cell migration and homing.35,36 Indeed, targeting the SDF-1/CXCR4 axis has offered a promising novel therapeutic approach for improving cell therapy by attracting circulating stem cells that are either exogenously infused or endogenously induced to migrate and home to diseased areas.37-40
Given the finding that preconditioning cells prior to their in vivo delivery might enhance their targeted migration, we systemically investigated the effects of inflammatory and/or hypoxic stimuli on the migration of BMMSCs in culture and after implantation using in vitro and in vivo models. In particular, we aimed to identify whether a combination of inflammatory and hypoxic stimuli might be more efficacious than a single, focused pretreatment. In our previous study, we utilized treatment with TNF-α at a concentration of 10 ng/mL and IL-1β at a concentration of 5 ng/mL to create an environment to evaluate the effects of inflammation on cell behavior.10 At the same time, a humidified atmosphere containing 2% O2 (instead of the normal oxygen concentration of 20% O2) was applied to create a hypoxic environment, based on various previous studies.19-24 The duration needed for conditioning was determined based on CXCR4 expression levels under different stimuli. Before preconditioning, robust stem cell properties were found in 4 cell lines, which were independently characterized via colony-forming unit and MTT assays, osteogenic/adipogenic potentials and MSC surface marker expression (Fig. 1). Using a tablet scratch assay, we found that exposure to either an inflammatory or hypoxic stimulus alone significantly enhanced cell migration (Fig. 2). This effect may have occurred because BMMSCs themselves can produce SDF-1 to induce cell migration. However, an analysis of the data published to date suggests that SDF-1 produced by tissues in response to injury plays a more powerful role in cell migration than does SDF-1 secreted from BMMSCs. This outcome could also be explained by previous studies that have demonstrated that certain inflammatory cytokines (e.g., IL-1β) or hypoxic stimuli can activate CXCR4 downstream of the PI3K/AKT signaling pathway,19,41 leading to cell migration.42 In our tablet scratch experiment, however, no synergistic effect was observed as a result of exposure to a combination of inflammatory and hypoxic stimuli. Indeed, the dual-stimuli group had smaller numbers of migrated cells than either of the individual stimulus groups. Given that MSCs have previously been shown to migrate to sites of injury and inflammation in response to SDF-1,43,44 a transwell membrane system was also used to evaluate the chemotactic effect of SDF-1 in directing cell migration.
Based on the concentration of recombinant SDF-1 used in a previous study44,45 and the data obtained in the current study, a concentration of 100 ng/mL was selected to determine cell response to SDF-1 under inflammatory and/or hypoxic conditions (Fig. 3A). Our in vitro results confirmed that inflammatory and/or hypoxic stimuli tended to enhance BMMSC migration toward SDF-1. Additionally, the presence of SDF-1 in the lower chamber of the system showed a remarkable effect on cell recruitment (Fig. 3B-C), suggesting that BMMSC movement was largely based on SDF-1-directed cell migration. As shown in this study, the positive effects of inflammatory and/or hypoxic stimuli on cell migration could be blocked by treatment with AMD3100, a CXCR4-specific small-molecule antagonist (Fig. 3C). This protein molecule has been found to potently inhibit CXCR4-mediated calcium signaling and chemotaxis in different cell populations in a concentration-dependent manner. In addition, AMD3100 inhibits SDF-1-induced endocytosis of CXCR4 without affecting phorbol ester-induced receptor internalization. Of particular importance, AMD3100 alone is unable to induce chemotaxis, elicit intracellular calcium fluxes, or trigger CXCR4 internalization, suggesting that it does not act as a CXCR4 agonist.46 Therefore, AMD3100 has been widely used as a research tool for studying stem cell migration that is dependent on SDF-1/CXCR4 interactions.45 Interestingly, cell migration in our transwell system in the presence of both AMD3100 and SDF-1 was reduced compared with that in the system without SDF-1, regardless of whether the cells were incubated with inflammatory/hypoxic stimuli (Fig. 3B-C). This result is likely because AMD3100 could block not only exogenous SDF-1 but also a portion of the cell-secreted protein. These findings suggest that CXCR4 plays a crucial role in regulating MSC migration.
In general, CXCR4 has been widely accepted as the key chemokine and receptor involved in MSC migration. Although CXCR4 is highly expressed in BMMSCs within their in vivo niche, its expression has been found to decrease markedly during ex vivo cell expansion, which would likely decrease cell migration in response to homing signals (e.g.,, SDF-1) emanating from injured sites.35,47 Indeed, mounting evidence confirms that culture-expanded MSCs show downregulation of surface CXCR4 expression;48,49 as a result, their ability to respond to homing signals is remarkably decreased.43 Therefore, many attempts have been made to develop alternative approaches of optimizing MSC migration efficiency. These efforts include exploring the potential of constitutively active CXCR4 mutants (CXCR4-CAMs)50 or creating an economical and effective method of upregulating CXCR4 expression on MSC surfaces.
Encouragingly, selected studies have reported that cell migration toward sites of injury can be enhanced by pretreating MSCs with a hypoxic stimulus prior to systemic injection.19 This positive effect of hypoxia on MSC mobilization and homing operates primarily via upregulation of CXCR4 expression on the cell membrane.18 Additionally, it was found that pretreatment with IL-1β and/or TNF-α can enhance cell migration by upregulating CXCR4 expression.13,51 In addition to the growing evidence that stem cells can be isolated from clinically inflamed human tissue, there are also indications that these ‘inflamed’ cells have a stronger potential to migrate.52,53 All of these findings suggest that inflammatory conditions can enhance stem cell migration potential. In the current study, the data from our transwell assays demonstrated more obvious increases in cell migration in the inflammation and hypoxia groups compared to the controls. This phenomenon occurred because CXCR4 expression on the surfaces of the tested BMMSCs was notably increased when the cells were exposed to inflammatory or hypoxic stimuli. Following the addition of AMD3100, the numbers of migrated cells were no longer significantly different between the 4 groups. This outcome further confirms that inflammation and hypoxia promote cell migration by upregulating CXCR4 expression. Furthermore, we also used CXCR4 expression as a key parameter to evaluate the effects of different stimuli on cell migration. To accomplish this, CXCR4 mRNA expression was analyzed by real-time PCR and CXCR4 protein expression was analyzed by Western blotting. In both cases, there were significant increases in CXCR4 expression in cells following exposure to inflammatory and/or hypoxic stimuli (Fig. 4). Based on our analysis, the highest levels of CXCR4 gene and protein expression were obtained after incubating cells with each individual stimulus for 24 h (Fig. 4A-D). When the cells were exposed to both inflammatory and hypoxic stimuli in combination, however, the highest level of CXCR4 expression was obtained after 12 h of incubation (Fig. 4E-F). These findings suggest that a combination of different stimuli might shorten the necessary length of exposure, although exposure to dual stimuli resulted in a lower level of CXCR4 expression than exposure to a single stimulus (Fig. 5). The temporal changes in CXCR4 expression in BMMSCs exposed to inflammatory and/or hypoxic stimuli and the synergistic effect of both stimuli on cell migration remain elusive, in part because the stimulus parameters used in this study were largely based on data reported in the literature. Perhaps not surprisingly, if a combination of different stimuli is used for cell pretreatment, a lower dose of inflammatory cytokines and/or less severe hypoxia (e.g., a humidified atmosphere containing between 2% and 20% O2) than applied in this study would be more suitable for BMMSCs. In addition, the preconditioning protocol should be re-optimized based on overall cell fate because the therapeutic outcome resulting from cell administration depends not only on cell migration but also on stem cell properties, at least in terms of differentiation and immunomodulation. Therefore, the current research can only provide evidence that a simple combination of different pretreatments would not readily lead to additional benefits, and substantially more work must still be performed before a well-established protocol can be generated for various clinical situations.
Under physiological conditions, BMMSCs are maintained within their niche via tightly regulated interactions between cytokines, chemokines and other signaling factors and cellular receptors, in addition to specific adhesion and extracellular matrix molecules present in the in vivo milieu.54 Following injury, this homeostasis is altered by locally upregulated growth factors and cytokines released from activated platelets and the vascular endothelium, thus offering a signal gradient that triggers BMMSCs to mobilize and exit the bone marrow.55 During this process, SDF-1 is released at sites of injury, creating a chemokine gradient that is thought to promote CXCR4-mediated BMMSC recruitment.56,57 Therefore, the presence of SDF-1 at a destination targeted for cell migration can result in an increased number of migrated cells both in vitro and in vivo. In addition, immobilized SDF-1 found on or around ischemic blood vessels can eliminate CXCR4 desensitization to facilitate tissue-specific localization and adhesion.56,58 However, even in acute injuries, the expression of SDF-1 and other factors might occur over a notably short duration, which in part explains why self-healing is generally restricted by the intrinsically low regenerative potential of many tissues. Therefore, the localized administration of exogenous signals is generally necessary for both endogenous regenerative technology and cell transplantation approaches.54,59 Although no biomaterials were used for delivery in this study, the presence of exogenous SDF-1 in the created skin wounds significantly enhanced the recruitment of infused BMMSCs, thus indicating that the migration of circulating cells to sites of injury is based on SDF-1-directed cell homing.(Fig. 6)
An appealing approach for enhancing stem cell movement following injury is to bolster cellular migration following transplantation. To this end, a variety of cellular or wounding microenvironment modifications using growth factors and/or biomaterials have shown beneficial effects.55,60 Similar to studies focused on the recruitment of endogenously mobilized stem cells, the enhancement of SDF-1 signaling within and around injured tissue is also used to augment cellular transplantation.59 Based on the in vivo data obtained from our study (Fig. 6), we conclude that a combination of injury-environment modifications to achieve ex vivo modulation of cells prior to transplantation might be even more efficacious than single, focused therapies. This finding s also illustrated by a previous study showing the synergistic beneficial effects of a combination of locally released SDF-1 and systemic delivery of substance P from an implant.61 However, small-molecule modulation and gene transfer are commonly used techniques for cell manipulation.62 Taking advantage of the presumably shorter modulatory effect of small molecules or the use of viral vectors, which are clinically unappealing, would require careful risk-benefit analysis prior to translational work. In contrast, stem cell preconditioning is thought to be an important step that not only enhances cell survival, migration and engraftment but also has great potential to modulate cellular expansion, paracrine activity and differentiation.18,19,26,27
In a clinical setting, cell-based therapies are typically administered via injections of cells that are suspended in an appropriate medium. However, previous studies have uniformly demonstrated that the administered cells exhibit notably poor engraftment, regardless of whether they are injected directly into a tissue of interest or infused into systemic circulation. The spread of the cells from an injection site and the restricted amount of recruited cells are likely jointly responsible for the limited clinical success of this approach.3 Fortunately, our accumulating knowledge of stem cell biology and cell-environment interactions continues to offer valuable insights into and technologies for advanced cell preconditioning as well as transplantation design. Based on the synergy observed in the parallel utilization of multiple manipulation techniques,61,63-65 we believe that further research in this field will demonstrate that a combination of strategies, such as exposure to inflammatory and/or hypoxic stimuli coupled with manipulation of the in vivo injury environment, will maximize the therapeutic effect of stem cells and facilitate the translation of many clinically relevant therapies from bench to bedside.
Conclusions
Current stem cell therapy designs hold much room for improvement. In addition to detailed insights into the transdifferentiation potential and functional coupling of stem cells, the efficacy of cell therapy in combating ischemic and degenerative diseases is underscored by the need to induce appropriate cell migration and homing to injury sites of interest. Such induction is necessary regardless of whether it involves the stimulation of endogenous cells or the transplantation of cell populations that have been expanded ex vivo. Techniques that attempt to augment cell migration in response to injury and/or following transplantation, together with techniques designed to increase cell survival, signaling and function, have gained increasing attention in stem cell science and have become a major focus of research on improving the overall success rates for cell therapy. Pretreatment of cells with inflammatory or hypoxic stimuli showed positive outcomes in both situations established in this study; however, no synergistic effect was found when combining the stimuli in our test design. Nevertheless, we believe that optimization of the treatment parameters described here might significantly increase the therapeutic potential of MSCs, including the migration ability of BMMSCs, and will therefore contribute to the development of multiple clinically relevant cell-based therapies.
Materials and methods
Isolation and culture of human BMMSCs
The use of human bone marrow (BM) samples for cell isolation and the research in this study was approved by the Institutional Review Board (IRB) of the Fourth Military Medical University (FMMU) School of Stomatology, which abides by the Helsinki Declaration. The BM samples were harvested from 4 systemically healthy donors. Density gradient centrifugation was used to separate BMMSCs from whole BM. Briefly, the BM sample (4–5 mL) collected from each donor was diluted to 7 mL with phosphate-buffered saline (PBS) and then carefully layered on the surface of 7 mL of lymphocyte separation medium (LSM; MP Biomedicals, Santa Ana, CA, USA) in a centrifuge tube. After centrifugation at 2000 rpm (rpm) for 20 min, the buffy coat containing the BMMSCs in the middle fraction was transferred into a new centrifuge tube. The tube was further centrifuged at 1000 rpm for 8 min, and the pellets were rinsed twice with PBS. Next, the resulting cell pellets were resuspended in complete medium containing α-minimum essential medium (α-MEM; Gibco BRL, Gaithersburg, MD, USA), 10% fetal bovine serum (FBS; Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Zhejiang, China), 0.292 mg/mL glutamine (Invitrogen Life Technologies, Carlsbad, CA, USA), 100 U/mL penicillin (Gibco BRL) and 100 mg/mL streptomycin (Gibco BRL). Finally, the primary cells were cultured in 6-well dishes (Corning, Lowell, MA, USA) at 37°C in a humidified atmosphere containing 5% CO2, and the medium was refreshed every 3 d When the cells reached 80% confluence, BMMSCs were purified using a limiting dilution technique, as previously described for the isolation of periodontal ligament stem cells.66 Cells from each donor were used independently in the following investigations at passages 1 to 4 (P1-P4).
Characterization of human BMMSCs
Colony-forming assay
For this assay, 1 × 103 cells (P3) were seeded into a 10-cm culture dish (Corning) and cultured in complete medium. The medium was refreshed every 3 d On day 12, the cells in the dishes were fixed in 4% paraformaldehyde for 20 min and stained with 0.2% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 1 h. Cell colonies were subsequently observed under a stereomicroscope.
Cell multiplication assay
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay was used to evaluate the proliferative capacity of the isolated BMMSCs. Briefly, cells (P4) were seeded in 96-well plates (Corning) at a density of 2 × 103 cells per well and cultured in complete medium for 8 d The medium was refreshed every 2 d From the second day on, the test wells were incubated with 20 µL of 5 mg/mL MTT solution for 4 h at 37°C. Next, the plates were shocked lightly for 5 min after the MTT solution was replaced with 150 µL of dimethyl sulfoxide (DMSO; Sigma-Aldrich). Finally, the absorbance was measured at 490 nm using a microplate reader (ELx800, BioTek, Instruments Inc.., Winooski, VT, USA).
Flow cytometry analysis
BMMSC (P2) surface antigens were detected by flow cytometry analysis. Briefly, 5 × 105 BMMSCs were washed in PBS containing 3% FBS and then transferred into EP tubes (300 µL per tube). Then, each sample was incubated with antibodies (2 µL) against human STRO-1, CD29, CD31, CD34, CD44, CD90, CD105 or CD146 (BD Bioscience, San Jose, CA, USA) at 4°C for 1 h in the dark. One tube without antibodies served as a negative control. Finally, the samples were washed twice with PBS containing 3% FBS and analyzed using a Beckman Coulter Epics XL flow cytometer (Beckman Coulter, Fullerton, CA, USA).
Differentiation assays
Osteogenic and adipogenic differentiation assays were used to confirm the multiple differentiation potentials of the isolated human BMMSCs. Briefly, cells (P4) were plated in 6-well culture plates at a density of 2 × 105 cells per well and cultured in complete medium until the cells reached 60% confluence. Then, the medium was changed to an osteogenic medium (complete medium containing 50 μg/mL vitamin C, 5 mM β-glycerophosphate and 0.1 mM dexamethasone) or adipogenic medium (complete medium containing 200 μM indomethacin, 10 μM insulin, 0.5 mM 1-methyl-3-isobutylxanthine (IBMX) and 1 μM dexamethasone) (all reagents from Sigma-Aldrich). The cells were incubated for 4 weeks for the osteogenic differentiation assay or 3 weeks for the adipogenic differentiation assay, and the medium was refreshed twice per week for both. Finally, the resultant mineralized cell nodules were characterized by Alizarin Red S staining, and the resultant lipid droplets were characterized by Oil Red O staining, using previously described methods.66
Cell migration assays in vitro
Establishment of cell culture environment with inflammatory and/or hypoxic stimuli
To determine how inflammatory and/or hypoxic stimuli affected BMMSC migration, we investigated changes in cell migration capacity under inflammatory conditions (inflammation group), hypoxic conditions (hypoxia group), or both (dual-stimuli group). A subset of cells was cultured without exposure to inflammatory or hypoxic stimuli to serve as a control (no-stimulus group). For these experiments, α-MEM containing 5% FBS supplemented with TNF-α (10 ng/mL) and IL-1β (5 ng/mL; both from Sigma-Aldrich) was used as the inflammatory stimulus medium, and cell culture in a humidified atmosphere containing 2% O2 (instead of 20% O2, which is the general oxygen concentration used for cell culture) was used to create a hypoxic condition.
Tablet scratch experiment
A tablet scratch experiment was used to evaluate cell migration. To accomplish this goal, BMMSCs (P4) were seeded into 6-well culture dishes at a density of 2 × 105 cells per well and cultured in complete medium until they reached 70% confluence. Then, the medium was changed to α-MEM without FBS (to starve the cells to avoid rapid cell proliferation that could influence counting of the migrated cells). After culturing for another 24 h, the cells were scratched using a 1 mL pipette tip to generate a cell-free strip. The baseline was recorded under an inverted microscope (Olympus Optical Co., Ltd, Tokyo, Japan), and the medium was changed. The experiment was performed for all 4 of the experimental groups (i.e., no-stimulus, inflammation, hypoxia and dual-stimuli). The migrated cells in different groups with respect to the baseline were counted under an inverted microscope following a 15-h incubation.
Cell migration toward SDF-1 under inflammatory and/or hypoxic conditions
The chemotactic effect of SDF-1 on cell migration was observed using a transwell membrane system45 with polycarbonate transwell culture inserts with an 8-µm pore size (Nest Biotechnology, Wuxi, China) in 24-well plates. To identify a desirable concentration of SDF-1 (PeproTech, NJ, USA) for cell migration, we assayed different SDF-1 concentrations in the lower chambers of the system (total volume of 600 μL per well). The medium used for this experiment was α-MEM (supplemented with 5% FBS) containing 0 ng/mL SDF-1, 100 ng/mL SDF-1 or 200 ng/mL SDF-1. A total of 1 × 105 BMMSCs (P4) were placed in each upper chamber after resuspension in 150 μL of α-MEM containing 5% FBS. The system was incubated for 6 h and fixed with 4% paraformaldehyde for 20 min. Each transwell membrane was removed after stripping to detach nonmigrated cells. Finally, the cells that migrated to the lower surface of each membrane were stained with 0.2% crystal violet (Sigma-Aldrich) for 1 h, and cells in 5 random fields (100×) were counted for statistical analysis. Based on the data obtained from the cell migration assay using this transwell membrane system, a concentration of 100 ng/mL SDF-1 was selected for all subsequent assays.
The design of the transwell membrane system used to evaluate cell migration toward SDF-1 (100 ng/mL) under inflammatory and/or hypoxic conditions is described in Table 1. To examine the effect of the presence of SDF-1 on cell migration, a CXCR4 antagonist (AMD3100, Sigma-Aldrich)46 was used in the upper chamber of the transwell system. This antagonist was able to inhibit the biological function of SDF-1 on the targeted cells at a concentration of 5 μg/mL.45
Effects of inflammatory and/or hypoxic stimuli on CXCR4 expression in BMMSCs
Duration of stimulation on CXCR4 expression under inflammatory and/or hypoxic stimuli
To investigate the effects of stimulation duration on CXCR4 expression in BMMSCs, cells were incubated for different lengths of time under inflammatory, hypoxic, or dual-stimuli conditions. A culture free of these stimuli was used as a control (no-stimulus group). The design of each culture setup was similar to the description above, but the FBS concentration was increased from 5% to 10%. After incubation for 6 h, 12 h, 18 h, 24 h, 36 h and 48 h, cell samples were collected for real-time PCR and Western blot analysis (noted in the following sections).
Analysis of CXCR4 expression in BMMSCs exposed to different stimuli for varying lengths of time
To quantify CXCR4 expression in BMMSCs at selected timepoints (noted in the section entitled “Temporal changes in CXCR4 expression under inflammatory and/or hypoxic conditions”), cells were incubated under inflammatory conditions for 24 h, hypoxic conditions for 24 h, or both for 12 h. Again, a culture not exposed to these stimuli served as a control (no-stimulus group). Cell samples were collected for real-time PCR and Western blotting (noted in the following sections). For immunofluorescent staining of CXCR4 in BMMSCs, 2 × 104 cells (P4) were seeded into a 35-mm dish designed for confocal microscopy (Nest Biotechnology). After culturing in complete medium for 1 day, the cells were incubated under 4 different conditions. The same design was used for real-time PCR and Western blotting (i.e., no-stimulus, inflammation, hypoxia and dual-stimuli). Following incubation for 12 h or 24 h, the cells were fixed with 4% paraformaldehyde for 10 min, rinsed twice with pre-chilled PBS (5 min per rinse) and blocked with 1% BSA (Sigma-Aldrich) for 30 min. The fixed cells were incubated with a primary antibody against human CXCR4 (Abcam Plc., Cambridge, UK) overnight at 4°C and subsequently with a secondary fluorescent rabbit antibody at 37°C for 1 h in the dark. After rinsing with PBS, the cells were stained with Hoechst 33342 to label nuclei. Finally, images were captured and analyzed using an Olympus Fluo-Viem™ FV1000 confocal laser scanning microscope system (Olympus, Tokyo, Japan).
Real-time PCR analysis
For real-time PCR analysis, cell samples were collected, and TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA, USA) was used to extract RNA. Then, cDNA samples were obtained via reverse transcription using a RevertAid First Strand cDNA Synthesis Kit (Takara, Bio, Otsu, Japan). CXCR4 expression was analyzed using a CFX Connect®Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) under the following conditions: denaturation at 95°C for 3 min, followed by 39 cycles of 95°C for 15 s and 60°C for 30 s. GAPDH was used as an internal control. The following primer sequences were used in this study: CXCR4 (forward: 5′-TCATCCTCATCCTGGCTTTC-3′, reverse: 5′-CAAACTCACACCCTTGCTTG-3′) and GAPDH (forward: 5′-GGTGAAGGTCGGAGTCAACGGA-3′, reverse: 5′-GAGGGATCTCGCTCCTGGAAGA-3′).
Western blot analysis
For Western blot analysis, total protein was extracted from collected cells using lysis buffer (Sigma-Aldrich) containing a proteinase inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined using a BCA Protein Assay (Beyotime, Shanghai, China). Then, the proteins (10 µg per sample) were loaded onto 12% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), followed by blocking in 5% bovine serum albumin (BSA; Sigma-Aldrich) for 2 h. Next, the membranes were incubated with primary antibodies against human CXCR4 and β-tubulin overnight at 4°C, followed by incubation with secondary rabbit and mouse antibodies at room temperature for 2 h. Finally, protein bands were visualized using a Western-Light Chemiluminescent Detection System (Peiqing, Shanghai, China) and measured using Image-Pro Plus 6.0 software.
Effects of pretreatments on cell migration toward tissue defects
We used a skin defect model to investigate the effects of different pretreatments on cell migration toward sites of injury. Twelve male Sprague-Dawley (SD) rats weighing 220 ± 20 g each were obtained from the laboratory animal research center of FMMU, Xi'an, China. The guidelines of the Animal Care Committee of FMMU were strictly followed for all animal procedures. Two longitudinally aligned, full-thickness defects (15 mm in diameter) were surgically generated on the dorsal side of each rat. On the second day, the rats were randomly implanted with cells pretreated as described above (i.e., the cells were incubated under inflammatory conditions (inflammation) for 24 h, hypoxic conditions (hypoxia) for 24 h, or both (dual-stimuli) for 12 h). Cells without pretreatment served as a control (no-stimulus). Briefly, GFP-labeled rat BMMSCs (P7) (GFP-BMMSCs; Cyagen Biosciences, Guangdong, China) were injected into the rats through the caudal vein. To evaluate the potential chemotactic effect of SDF-1, 100 μL of recombinant SDF-1 (100 ng/mL in PBS) was applied daily to a randomly selected defect in each rat, together with subcutaneous injection of 300 μL of SDF-1 under the injury every 3 d After 7 days, the rats were sacrificed via the administration of 1% pentobarbital (0.01 mL/g). Skin samples (2 mm larger than the diameter of the original defect) were collected and fixed with 4% paraformaldehyde for 1 day. After soaking in 30% sucrose solution for 12 h, the samples were cut into 12-μm sections on a freezing microtome for immunofluorescent staining of GFP. The sections were blocked with sheep serum for 30 min and subsequently incubated with a primary rat antibody against GFP overnight at 4°C. Then, the antibody was changed to a secondary red-fluorescent antibody, and incubation proceeded at 37°C for 1 h in the dark. Hoechst 33342 was used to stain nuclei. Finally, the stained specimens were observed under a confocal laser scanning microscope system (Olympus) to count both autofluorescent and immunofluorescent cells in 5 random fields (200×) for statistical analysis.
Statistical analyses
All numerical values are presented as the mean ± standard deviation (SD) from 3 independent experiments for 4 cell lines. GraphPad Prism 5 software was used to analyze the data using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test or 2-way ANOVA. P < 0.05 was considered significant.
Abbreviations
- α-MEM
α-minimum essential medium
- BMMSCs
bone marrow mesenchymal stem cells
- CXCR4
chemokine receptor type 4
- DMSO
dimethyl sulfoxide
- ECM
extracellular matrix
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- IL-1β
interleukin-1β
- LSM
lymphocyte separation medium
- PBS
phosphate-buffered saline
- SDF-1
stromal cell-derived factor-1
- TNF-α
tumor necrosis factor-α
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81530050, 81500853 and 81471791), the Program for Changjiang Scholars and Innovative Research Team in University (IRT13051) and the Program for New Century Excellent Talents in University (NCET-12-1005).
References
- [1].Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell 2008; 132:544-8; PMID:18295571; http://dx.doi.org/ 10.1016/j.cell.2008.02.009 [DOI] [PubMed] [Google Scholar]
- [2].Kassem M, Abdallah BM. Human bone-marrow-derived mesenchymal stem cells: biological characteristics and potential role in therapy of degenerative diseases. Cell Tissue Res 2008; 331:157-63; PMID:17896115; http://dx.doi.org/ 10.1007/s00441-007-0509-0 [DOI] [PubMed] [Google Scholar]
- [3].Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008; 2:205-13; PMID:18371446; http://dx.doi.org/ 10.1016/j.stem.2008.02.005 [DOI] [PubMed] [Google Scholar]
- [4].Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, et al.. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 2003; 108:863-8; PMID:12900340; http://dx.doi.org/ 10.1161/01.CIR.0000084828.50310.6A [DOI] [PubMed] [Google Scholar]
- [5].Smart N, Riley PR. The stem cell movement. Circ Res 2008; 102:1155-68; PMID:18497316; http://dx.doi.org/ 10.1161/CIRCRESAHA.108.175158 [DOI] [PubMed] [Google Scholar]
- [6].Kedziorek DA, Solaiyappan M, Walczak P, Ehtiati T, Fu Y, Bulte JW, Shea SM, Brost A, Wacker FK, Kraitchman DL. Transplantation of heterospheroids of islet cells and mesenchymal stem cells for effective angiogenesis and antiapoptosis. Tissue Eng Part A 2015; 21:1024-35; PMID:25344077; http://dx.doi.org/ 10.1089/ten.tea.2014.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials 2014; 35:5627-35; PMID:24731711; http://dx.doi.org/ 10.1016/j.biomaterials.2014.03.070 [DOI] [PubMed] [Google Scholar]
- [8].Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009; 4:206-16; PMID:19265660; http://dx.doi.org/ 10.1016/j.stem.2009.02.001 [DOI] [PubMed] [Google Scholar]
- [9].Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007; 25:1737-45; PMID:17395768; http://dx.doi.org/ 10.1634/stemcells.2007-0054 [DOI] [PubMed] [Google Scholar]
- [10].Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, Jin Y, Chen FM. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 2013; 34:7033-47; PMID:23768902; http://dx.doi.org/ 10.1016/j.biomaterials.2013.05.025 [DOI] [PubMed] [Google Scholar]
- [11].Ziaei R, Ayatollahi M, Yaghobi R, Sahraeian Z, Zarghami N. Involvement of TNF-α in differential gene expression pattern of CXCR4 on human marrow-derived mesenchymal stem cells. Mol Biol Rep 2014; 41:1059-66; PMID:24395293; http://dx.doi.org/ 10.1007/s11033-013-2951-2 [DOI] [PubMed] [Google Scholar]
- [12].Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med 2011; 43:596-603; PMID:21847008; http://dx.doi.org/ 10.3858/emm.2011.43.10.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1beta enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol 2012; 9:473-81; PMID:23085948; http://dx.doi.org/ 10.1038/cmi.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica 2007; 92:897-904; PMID:17606439; http://dx.doi.org/ 10.3324/haematol.10669 [DOI] [PubMed] [Google Scholar]
- [15].Park DH, Eve DJ, Musso J 3rd, Klasko SK, Cruz E, Borlongan CV, Sanberg PR. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev 2009; 18:693-702; PMID:19199787; http://dx.doi.org/ 10.1089/scd.2009.0008 [DOI] [PubMed] [Google Scholar]
- [16].DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm 2010; 2010:865601; PMID:20628526; http://dx.doi.org/ 10.1155/2010/865601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med 2012; 1:51-8; PMID:23197640; http://dx.doi.org/ 10.5966/sctm.2011-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 2010; 16:159-68; PMID:19698058; http://dx.doi.org/ 10.1089/ten.teb.2009.0296 [DOI] [PubMed] [Google Scholar]
- [19].Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H, Ding X, Tian P, Tian X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Commun 2010; 401:509-15; PMID:20869949; http://dx.doi.org/ 10.1016/j.bbrc.2010.09.076 [DOI] [PubMed] [Google Scholar]
- [20].Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival and promotes cell retention in vivo. Stem Cells 2015; 33:1818-28; PMID:25702874; http://dx.doi.org/ 10.1002/stem.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Flann KL, Rathbone CR, Cole LC, Liu X, Allen RE, Rhoads RP. Hypoxia simultaneously alters satellite cell-mediated angiogenesis and hepatocyte growth factor expression. J Cell Physiol 2014; 229:572-9; PMID:24122166; http://dx.doi.org/ 10.1002/jcp.24479 [DOI] [PubMed] [Google Scholar]
- [22].Muir C, Chung LW, Carson DD, Farach-Carson MC. Hypoxia increases VEGF-A production by prostate cancer and bone marrow stromal cells and initiates paracrine activation of bone marrow endothelial cells. Clin Exp Metastasis 2006; 23:75-86; PMID:16826426; http://dx.doi.org/ 10.1007/s10585-006-9021-2 [DOI] [PubMed] [Google Scholar]
- [23].Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells 2007; 25:1166-77; PMID:17289933; http://dx.doi.org/ 10.1634/stemcells.2006-0347 [DOI] [PubMed] [Google Scholar]
- [24].Guo M, Cai C, Zhao G, Qiu X, Zhao H, Ma Q, Tian L, Li X, Hu Y, Liao B, et al.. Hypoxia promotes migration and induces CXCR4 expression via HIF-1α activation in human osteosarcoma. PLoS One 2014; 9:e90518; http://dx.doi.org/ 10.1371/journal.pone.0090518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu X, Lu C, Liu H, Rao S, Cai J, Liu S, Kriegel AJ, Greene AS, Liang M, Ding X. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PloS one 2013; 8:e62703; http://dx.doi.org/ 10.1371/journal.pone.0062703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J, Béliveau R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells 2003; 21:337-47; PMID:12743328; http://dx.doi.org/ 10.1634/stemcells.21-3-337 [DOI] [PubMed] [Google Scholar]
- [27].Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 2006; 24:2202-8; PMID:16778152; http://dx.doi.org/ 10.1634/stemcells.2006-0164 [DOI] [PubMed] [Google Scholar]
- [28].Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 2008; 132:598-611; PMID:18295578; http://dx.doi.org/ 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012; 10:544-55; PMID:22542159; http://dx.doi.org/ 10.1016/j.stem.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res 2012; 91:1003-10; PMID:22988011; http://dx.doi.org/ 10.1177/0022034512460404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Y, Yang R, Shi S. Systemic infusion of mesenchymal stem cells improves cell-based bone regeneration via upregulation of regulatory T cells. Tissue Eng Part A 2015; 21:498-509; PMID:25159486; http://dx.doi.org/ 10.1089/ten.tea.2013.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Casati L, Celotti F, Negri-Cesi P, Sacchi MC, Castano P, Colciago A. Platelet derived growth factor (PDGF) contained in Platelet Rich Plasma (PRP) stimulates migration of osteoblasts by reorganizing actin cytoskeleton. Cell Adh Migr 2014; 8:595-602; PMID:25482626; http://dx.doi.org/ 10.4161/19336918.2014.972785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guan BZ, Yan RL, Huang JW, Li FL, Zhong YX, Chen Y, Liu FN, Hu B, Huang SB, Yin LH. Activation of G Protein Coupled Estrogen Receptor (GPER) promotes the migration of renal cell carcinoma via the PI3K/AKT/MMP-9 signals. Cell Adh Migr 2015; 14:0; http://dx.doi.org/ 10.4161/19336918.2014.990781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther 2011; 129:97-108; PMID:20965212; http://dx.doi.org/ 10.1016/j.pharmthera.2010.09.011 [DOI] [PubMed] [Google Scholar]
- [35].Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 2007; 28:299-307; PMID:17560169; http://dx.doi.org/ 10.1016/j.it.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zepeda-Moreno A, Saffrich R, Walenda T, Hoang VT, Wuchter P, Sánchez-Enríquez S, Corona-Rivera A, Wagner W, Ho AD. Modeling SDF-1-induced mobilization in leukemia cell lines. Exp Hematol 2012; 40:666-74; PMID:22613469; http://dx.doi.org/ 10.1016/j.exphem.2012.05.001 [DOI] [PubMed] [Google Scholar]
- [37].Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res 2012; 94:400-7; PMID:22451511; http://dx.doi.org/ 10.1093/cvr/cvs132 [DOI] [PubMed] [Google Scholar]
- [38].Day RB, Link DC. Regulation of neutrophil trafficking from the bone marrow. Cell Mol Life Sci 2012; 69:1415-23; PMID:22045556; http://dx.doi.org/ 10.1007/s00018-011-0870-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ho CY, Sanghani A, Hua J, Coathup M, Kalia P, Blunn G. Mesenchymal stem cells with increased stromal cell-derived factor 1 expression enhanced fracture healing. Tissue Eng Part A 2015; 21:594-602; PMID:25251779; http://dx.doi.org/ 10.1089/ten.tea.2013.0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].de Lourdes Perim A, Amarante MK, Guembarovski RL, de Oliveira CE, Watanabe MA. CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target. Cell Mol Life Sci 2015; 72:1715-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang L, Guo XG, Du CQ, Yang JX, Jiang DM, Li B, Zhou WJ, Zhang FR. Interleukin-1 β increases activity of human endothelial progenitor cells: involvement of PI3K-Akt signaling pathway. Inflammation 2012; 35:1242-50; PMID:22371121; http://dx.doi.org/ 10.1007/s10753-012-9434-9 [DOI] [PubMed] [Google Scholar]
- [42].Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer 2013; 49:219-30; PMID:22683307; http://dx.doi.org/ 10.1016/j.ejca.2012.05.005 [DOI] [PubMed] [Google Scholar]
- [43].Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int 2013; 2013:561098; PMID:24381939; http://dx.doi.org/ 10.1155/2013/561098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rice CM, Scolding NJ. Adult human mesenchymal cells proliferate and migrate in response to chemokines expressed in demyelination. Cell Adh Migr 2010; 4:235-40; PMID:20234187; http://dx.doi.org/ 10.4161/cam.4.2.11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhou SB, Wang J, Chiang CA, Sheng LL, Li QF. Mechanical stretch upregulates SDF-1alpha in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells 2013; 31:2703-13; PMID:23836581; http://dx.doi.org/ 10.1002/stem.1479 [DOI] [PubMed] [Google Scholar]
- [46].Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett 2002; 527:255-62; PMID:12220670; http://dx.doi.org/ 10.1016/S0014-5793(02)03143-5 [DOI] [PubMed] [Google Scholar]
- [47].Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 2006; 24:1254-64; PMID:16410389; http://dx.doi.org/ 10.1634/stemcells.2005-0271 [DOI] [PubMed] [Google Scholar]
- [48].Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006; 24:1030-41; PMID:16253981; http://dx.doi.org/ 10.1634/stemcells.2005-0319 [DOI] [PubMed] [Google Scholar]
- [49].Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004; 104:2643-5; PMID:15251986; http://dx.doi.org/ 10.1182/blood-2004-02-0526 [DOI] [PubMed] [Google Scholar]
- [50].Sharma M, Afrin F, Tripathi RP, Gangenahalli G. Transgene expression study of CXCR4 active mutants. Potential prospects in up-modulation of homing and engraftment efficiency of hematopoietic stem/progenitor cells. Cell Adh Migr 2014; 8:384-8; PMID:25482641; http://dx.doi.org/ 10.4161/cam.29285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhi Y, Lu H, Duan Y, Sun W, Guan G, Dong Q, Yang C. Involvement of the nuclear factor-κB signaling pathway in the regulation of CXC chemokine receptor-4 expression in neuroblastoma cells induced by tumor necrosis factor-α. Int J Mol Med 2015; 35:349-57; PMID:25503960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, Tuan RS, Huang GT. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 2010; 5:617-31; PMID:20465527; http://dx.doi.org/ 10.2217/rme.10.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sun HH, Chen B, Zhu QL, Kong H, Li QH, Gao LN, Xiao M, Chen FM, Yu Q. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials 2014; 35:9459-72; PMID:25172527; http://dx.doi.org/ 10.1016/j.biomaterials.2014.08.003 [DOI] [PubMed] [Google Scholar]
- [54].Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials 2011; 32:3189-209; PMID:21300401; http://dx.doi.org/ 10.1016/j.biomaterials.2010.12.032 [DOI] [PubMed] [Google Scholar]
- [55].Rennert RC, Sorkin M, Garg RK, Gurtner GC. Stem cell recruitment after injury: lessons for regenerative medicine. Regen Med 2012; 7:833-50; PMID:23164083; http://dx.doi.org/ 10.2217/rme.12.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004; 10:858-64; PMID:15235597; http://dx.doi.org/ 10.1038/nm1075 [DOI] [PubMed] [Google Scholar]
- [57].Massberg S, Konrad I, Schürzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, et al.. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med 2006; 203:1221-33; PMID:16618794; http://dx.doi.org/ 10.1084/jem.20051772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest 1999; 104:1199-211; PMID:10545519; http://dx.doi.org/ 10.1172/JCI7615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012; 33:6320-44; PMID:22695066; http://dx.doi.org/ 10.1016/j.biomaterials.2012.05.048 [DOI] [PubMed] [Google Scholar]
- [60].Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010; 31:7892-927; PMID:20684986; http://dx.doi.org/ 10.1016/j.biomaterials.2010.07.019 [DOI] [PubMed] [Google Scholar]
- [61].Ko IK, Ju YM, Chen T, Atala A, Yoo JJ, Lee SJ. Combined systemic and local delivery of stem cell inducing/recruiting factors for in situ tissue regeneration. FASEB J 2012; 26:158-68; PMID:21965595; http://dx.doi.org/ 10.1096/fj.11-182998 [DOI] [PubMed] [Google Scholar]
- [62].Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med 2007; 4:S110-3; PMID:17230207; http://dx.doi.org/ 10.1038/ncpcardio0734 [DOI] [PubMed] [Google Scholar]
- [63].Yu JX, Huang XF, Lv WM, Ye CS, Peng XZ, Zhang H, Xiao LB, Wang SM. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg 2009; 50:608-16; PMID:19595531; http://dx.doi.org/ 10.1016/j.jvs.2009.05.049 [DOI] [PubMed] [Google Scholar]
- [64].Hannoush EJ, Sifri ZC, Elhassan IO, Mohr AM, Alzate WD, Offin M, Livingston DH. Impact of enhanced mobilization of bone marrow derived cells to site of injury. J Trauma 2011; 71:283-9; PMID:21825928; http://dx.doi.org/ 10.1097/TA.0b013e318222f380 [DOI] [PubMed] [Google Scholar]
- [65].Shin JW, Lee JK, Lee JE, Min WK, Schuchman EH, Jin HK, Bae JS. Combined effects of hematopoietic progenitor cell mobilization from bone marrow by granulocyte colony stimulating factor and AMD3100 and chemotaxis into the brain using stromal cell-derived factor-1α in an Alzheimer disease mouse model. Stem Cells 2011; 29:1075-89; PMID:21608078; http://dx.doi.org/ 10.1002/stem.659 [DOI] [PubMed] [Google Scholar]
- [66].Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, Chen FM. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 2012; 33:6974-86; PMID:22789721; http://dx.doi.org/ 10.1016/j.biomaterials.2012.06.032 [DOI] [PubMed] [Google Scholar]