Highlight
Crop plants would benefit from deeper rooting if they can overcome challenges to root exploration of the subsoil
Key words: Calcium, carbon sequestration, crop breeding, nitrogen, phosphorus, root depth, soil impedance, subsoil, temperature water.
Abstract
Greater exploitation of subsoil resources by annual crops would afford multiple benefits, including greater water and N acquisition in most agroecosystems, and greater sequestration of atmospheric C. Constraints to root growth in the subsoil include soil acidity (an edaphic stress complex consisting of toxic levels of Al, inadequate levels of P and Ca, and often toxic levels of Mn), soil compaction, hypoxia, and suboptimal temperature. Multiple root phenes under genetic control are associated with adaptation to these constraints, opening up the possibility of breeding annual crops with root traits improving subsoil exploration. Adaptation to Al toxicity, hypoxia, and P deficiency are intensively researched, adaptation to soil hardness and suboptimal temperature less so, and adaptations to Ca deficiency and Mn toxicity are poorly understood. The utility of specific phene states may vary among soil taxa and management scenarios, interactions which in general are poorly understood. These traits and issues merit research because of their potential value in developing more productive, sustainable, benign, and resilient agricultural systems.
Introduction
Sustaining a human population of 10 billion with a degrading soil resource base in a changing climate is a pre-eminent challenge of the 21st century. An important element of this effort will be the development of crops able to yield well with limited availability of water and nitrogen (N). Suboptimal availability of water and N are primary limitations to plant growth in terrestrial environments, including natural ecosystems and the low-input agroecosystems characteristic of developing nations. Food insecurity is already a massive problem in developing nations, and is projected to worsen in coming decades due to population growth, resource degradation, and climate change. In rich nations, intensive N fertilization meets crop requirements at the cost of substantial pollution of air and water resources (Cassman et al., 2002; Ribaudo et al., 2011). Irrigation has limited potential to address expanding water needs in intensive agriculture because of ongoing degradation and exhaustion of freshwater resources (Wallace and Gregory, 2002; Rosegrant et al., 2009). Global climate change is projected to aggravate crop water limitation by increasing evaporative demand, accelerating soil degradation, and altering the distribution of precipitation in time and space (Wheeler and von Braun, 2013; IPCC, 2014). Crops with reduced requirements for water and N inputs would directly improve food production and therefore food security in developing nations, and would improve agricultural sustainability and productivity in rich nations (Lynch, 2007). Such crops would also benefit the global environment by converting more atmospheric CO2 to plant biomass and eventually soil organic carbon (Lal, 2004).
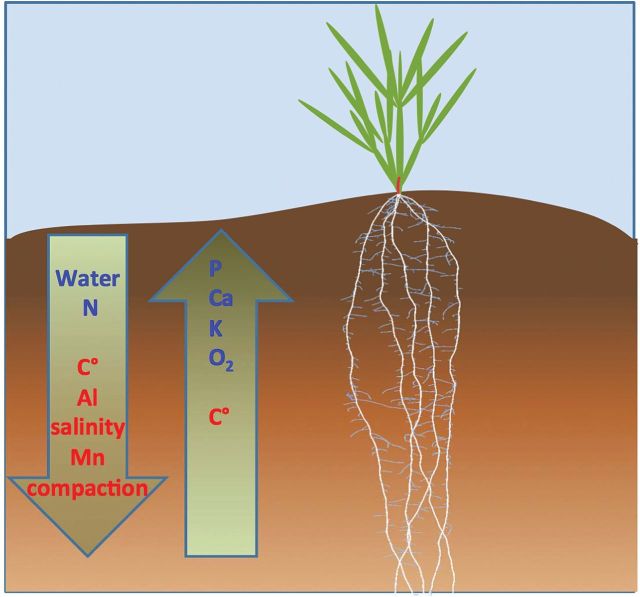
The development of crops with greater rooting depth addresses all three of these aims. Deeper rooting improves water and N capture in many agroecosystems, and increases the stability of plant-derived carbon (C) in the soil. This is generally recognized and has recently been the subject of several reviews (Manschadi et al., 2010; Kell, 2011; Wasson et al., 2012; Lynch, 2013). The purpose of this article is to summarize these potential benefits very briefly, and then consider several of the constraints to achieving greater rooting depth (Fig. 1). These will be considered in the context of annual crop production, with an emphasis on opportunities for genetic improvement of crop rooting depth, as these are more generally applicable, but also some consideration of general issues regarding soil management. We do not attempt a comprehensive review of these topics but, where possible, cite recent reviews. Our goal is to contribute to the development of crop ideotypes as breeding targets for improved rooting depth.
Fig. 1.
Depiction of nutrient and physical constraints occurring in deeper soils, as discussed in the text. The root image is a barley root system as simulated in the structural-functional plant model SimRoot, courtesy of Johannes A. Postma.
Deeper rooting affords several potential benefits
Rooting depth is related to water capture
There is good evidence that in many drought environments, rooting depth is positively related to soil exploration and greater acquisition of water from deep soil strata. The utility of rooting depth for drought tolerance is well documented in the case of dryland wheat, in which genotypes with greater rooting depth are better able to exploit moisture stored from summer rainfall (Manschadi et al., 2006; Wasson et al., 2012). Evidence exists that deep rooting is also important in terminal and intermittent drought scenarios that are more common in rainfed agriculture. For example, rooting depth was positively associated with yield under terminal or intermittent drought in, for example, wheat (Lopes and Reynolds, 2010) and common bean (Sponchiado et al., 1989). Steeper root growth angles increase rooting depth and drought tolerance in rice (Uga et al., 2013) and common bean (Ho et al., 2005). In maize, deeper rooting associated with genotypic variation for several root anatomical traits that reduce the metabolic cost of soil exploration substantially improves yield under terminal and intermittent drought (Zhu et al., 2010; Jaramillo et al., 2013; Chimungu et al., 2014a , b ; Lynch et al., 2014). In agroecosystems with limited in-season precipitation, vigorous growth and rapid water extraction from the subsoil can reduce yield under drought by exhausting available soil water before the crop can mature (Manschadi et al., 2006; Palta et al., 2011). In some drought environments, regular precipitation that is insufficient to meet evapotranspirative demand following a prolonged dry season may result in greater availability of water in shallow soil strata, but such cases are relatively rare in the rainfed agriculture that sustains the majority of global crop production. In general, the ability to exploit water in deep soil strata is clearly advantageous, and has been posited as a central element of ideotypes for breeding more drought-tolerant crops (Wasson et al., 2012; Lynch, 2013).
Rooting depth is related to N capture
The predominant form of plant-available nitrogen in most agroecosystems is nitrate, which is highly soluble in water and therefore leaches to deeper soil horizons with precipitation. Rapid nitrate leaching below the root zone can be a significant source of N effluents in high-input agroecosystems (Wiesler and Horst, 1993; Raun, 1999; Cassman et al., 2002; Chen et al., 2010). Rapid development of root foraging in deep soil strata would increase the capture of nitrate in such environments (Wiesler and Horst, 1994; Dunbabin et al., 2003), although surface soil strata can continue to be a source of N throughout the crop season (Wiesler and Horst, 1994), presumably as a result of mineralization, or fertility in excess of crop requirements. Mineralization of organic matter in the topsoil can also be a significant source of N in some systems, and is often the major source of N in low-input systems. In the case of low-input systems or systems in which N is provided in organic material (manure, crop residues, etc.), N availability may be greatest in shallow soil strata (Poudel et al., 2001). In such cases, deeper rooting will not directly improve N capture, although, by improving water acquisition and thereby reducing a nearly universal limitation to crop growth, deep root phenotypes would have indirect benefits for plant growth, soil exploration, and N capture. Cereal root systems continually form nodal roots which pass through the topsoil as they descend to deeper soil strata, and so may not be as susceptible to the opportunity cost of exploring shallow versus deep soil strata, in contrast to dicotyledonous root architectures, which are dominated by lateral roots that form at distinct soil depths (Lynch, 2013). The hypothesis that deeper root phenotypes will enhance N acquisition in the majority of agricultural systems, despite the fact that N availability may be greater in surface soils in some situations, is consistent with available evidence from modelling and empirical studies (Pedersen et al., 2009; Thorup-Kristensen et al., 2009; Dai et al., 2014; Dresbøll and Thorup-Kristensen, 2014). It is important to note that the effect of root traits on N capture will show strong interactions with environmental and management factors, especially as they affect N distribution and leaching rates (Dresbøll and Thorup-Kristensen, 2014). Accordingly, crop ideotypes for efficient N capture include deeper rooting itself (Mi et al., 2010), and in the ‘steep, cheap, and deep’ (SCD) ideotype, architectural and anatomical traits that increase rooting depth (Lynch, 2013). Recent reports support specific elements of the SCD ideotype for improved N capture in maize, including reduced nodal root number; longer, fewer lateral roots, and increased root cortical aerenchyma (Postma and Lynch, 2011; Postma et al., 2014; Saengwilai et al., 2014a, b ).
Rooting depth is related to C sequestration
A significant portion of plant photosynthate is deposited below-ground via roots, root symbionts, and root exudates, and by the senescence and mortality of plants and their organs (Lambers et al., 2002; Farrar et al., 2003; Jones et al., 2004). Globally, soil C is estimated to be twice as large as the pool of atmospheric C (Kell, 2011, 2012), and the capacity of soils to retain C has not yet been saturated. Since the depth of C deposited in the soil by root activity is related to its residence time, deeper crop rooting, achieved by genetic selection or agronomic management, has been proposed as a viable option to sequester atmospheric CO2 and partially mitigate global climate change (Kell, 2011, 2012).
Subsoil constraints to root growth
In this review, we use the generic terms ‘topsoil’ and ‘subsoil’. By ‘topsoil’ we refer to the epipedon, the surface or A horizon, generally 15–30cm in depth and highly influenced by plant growth and associated microbial activity, and therefore being more enriched in organic carbon, mineral nutrients, and generally characterized by more active nutrient transformations between organic and mineral forms. By ‘subsoil’ we refer to deeper soil strata, the E, B, and, in some cases, C horizons.
Soil sodicity/salinity and boron
In arid regions, subsoils can contain toxic levels of sodium (Na), boron (B), and salinity. Stress caused by saline and sodic soils has been intensively researched and recently reviewed, so will not be treated at length here (e.g. Munns and Tester, 2008). Substantial genetic differences in tolerance to these stresses exist among and within crop species, related generally to exclusion of toxic ions from the cytoplasm of active tissues, production of compatible solutes, and mechanisms to balance the competing osmotic needs for water acquisition and ion exclusion. Inhibition of calcium (Ca) transport and metabolism by Na is one aspect of salinity stress (Lynch and Läuchli, 1985) that may be ameliorated by reduced tissue Ca requirement (see below). B toxicity directly interferes with root growth (Aquea et al., 2012). Significant genotypic variation in B tolerance exists within species, and manipulation of B transporters has the potential to improve B tolerance further (Schnurbusch et al., 2010).
Soil acidity
A large proportion of terrestrial vegetation is located in moist environments with weathered soils characterized by acid subsoils (Table 1). Acid subsoils (generally defined as having a pH <5) present several direct challenges to root growth, mainly aluminium (Al) toxicity and Ca deficiency, as well as several indirect challenges to plant health, mainly phophorus (P) deficiency and manganese (Mn) toxicity. Crop adaptation to Al toxicity and P deficiency has been intensively researched, whereas crop adaptation to Ca deficiency and Mn toxicity has received less attention despite their global importance.
Table 1.
Principal characteristics and areas for the 12 orders of the USDA Soil Taxonomy system, with common subsoil constraints to root growth in representative suborders
| Order | Description | Area (%)a | Common subsoil constraints |
|---|---|---|---|
| Alfisols | Soils from semi-arid to humid regions, typically developed under hardwood forest cover, with subsurface accumulation of clay | 9.6 | Hypoxia, hardness, temperature |
| Andisols | Developed from volcanic ash | 0.7 | Mn toxicity, low P |
| Aridisols | Desert soils without water for plants | 12 | Hardness, salinity/sodicity, low P |
| Entisols | Soils with minimal evolution, as in eroded or accumulation regions. No subsurface horizons | 16.2 | – |
| Gelisols | Permafrost (frozen soil) within 100cm from the surface | 8.6 | Temperature, low P, Mn toxicity |
| Histosols | Organic rich, generally in cold latitudes | 1.2 | Hypoxia, low P, temperature, low Ca |
| Inceptisols | Soils with weakly developed subsurface horizons | 9.8 | – |
| Mollisols | Thick dark surface horizon | 6.9 | Hardness, hypoxia, temperature |
| Oxisols | Soils from tropical regions, highly weathered, deep, and uniform profiles | 7.5 | Acidity (low P, low Ca, K, Mg, Al, and Mn toxicity) |
| Spodosols | Bleached horizon over grey-brown (spodic) horizon | 2.5 | Hypoxia, acidity (low P, low Ca, Al, and Mn toxicity) |
| Ultisols | Weathered soils with low base saturation (low fertility) in subsurface | 8.5 | Acidity (low P, low Ca, K, Mg, Al, and Mn toxicity), hypoxia, hardness |
| Vertisols | Shrink and swell soils, that is soils that exhibit temporal variability in volume | 2.4 | Hypoxia, hardness |
a Percentage of the total land area (1.3×108 km2); rows sum to 86%—the remainder (~14%) is from rock and ice-covered regions (Wilding, 2000).
Aluminium toxicity
Al toxicity and tolerance are the subject of several recent reviews (Kochian et al., 2005; Ryan et al., 2011; Liu et al., 2014). Below a pH of ~4.8, Al solubility increases and trivalent Al causes direct injury to root apices, reducing root growth, soil exploration, and hence water and nutrient acquisition. High Al3+ activity in the soil solution also directly interferes with the uptake of Ca, magnesium (Mg), and potassium (K), and reduces P bioavailability. Al tolerance consists of both tolerance of tissue Al, presumably via mechanisms such as intracellular and tissue level compartmentation (Hiradate et al., 1997; Feng Ma et al., 1998), as well as exclusion of Al from the growing root tissues via the exudation of carboxylates, which complex and detoxify Al in the rhizosphere and apoplast. Al detoxification via carboxylate exudation, primarily as malate or citrate, accounts for substantial genotypic variation in Al tolerance in some crop species, and appears to have arisen through convergent evolution in multiple plant genera (Ryan and Delhaize, 2010), but does not account for genotypic variation in Al tolerance in other species (Kochian et al., 2004). Al tolerance of root hairs is important since hairs are critical for P acquisition and in the formation of rhizosheaths which create favourable microenvironments for growing roots (Delhaize et al., 2012).
Tolerance of trivalent Al is necessary for root exploration of acid subsoils. Inducible carboxylate exudation is an important mechanism of Al exclusion in several important crops, is well understood from genetic and physiological perspectives, and is already being deployed in crop breeding programmes in the tropics. However, additional research is needed to better understand mechanisms of Al tolerance in species for which carboxylates are not the main mechanism of tolerance, the genetic and physiological basis of Al detoxification or compartmentation in cells, tissues, and organs, and the role of rhizosphere communities in determining the fate of root exudates and in creating microenvironments with altered Al and nutrient bioavailability. This research should take into account the large spatiotemporal variation of root properties within and among root classes of mature plants, which might be different from the primary or seminal roots of seedlings that have received the majority of research attention.
Low calcium availability
Low Ca availability is another primary challenge to root growth in acid subsoils. It has long been recognized that Ca plays an integral role in Al toxicity (e.g. Foy et al., 1969). Trivalent Al interferes with Ca transport and metabolism (Rengel and Zhang, 2003), and reduces the activity of Ca at the surface of the plasma membrane, an effect which is associated with Al toxicity and tolerance (Kobayashi et al., 2013). Indeed, supplemental Ca ameliorates Al toxicity in moderately acid soils. This is especially important in acid subsoils, since Ca availability declines with depth in such soils, due to decreasing Ca content and increasing saturation of soil exchange sites with soluble Al (Table 1). Although other nutrients including K and Mg may also be limited in acid subsoils, low Ca availability is particularly problematic for root growth since Ca is poorly mobile in the phloem. Demands of root apices for Ca, primarily for cell wall biosynthesis and stability, must therefore be satisfied by Ca supplied directly by soil adjacent to the growing root, with only small amounts provided by the plant itself (Marschner, 1995). Tissue requirements for Ca are primarily in the apoplast, and vary among and within plant species, a large difference being evident between grasses and dicots, since pectins are more abundant in the cell walls of dicots, which consequently have a much greater Ca requirement (Marschner, 1995). Tissue Ca requirement also varies among genotypes of the same species (Spehar and Galwey, 1997), which may be due to differences in cell wall composition, since genotypes having less pectin or greater pectin methylation might have a reduced Ca requirement (Blamey et al., 2014). It is possible that genetic variation for anatomical traits that influence the amount of cell wall material per unit volume, such as cell size, also affect tissue Ca requirement. Selecting plants with reduced internal Ca requirement may be more fruitful than selecting plants with greater Ca acquisition, since Ca is primarily drawn to roots via transpiration-driven mass flow (Barber, 1995), and increasing transpiration would have significant trade-offs for water economy in most agroecosystems.
Crop genotypes with reduced Ca requirement would be better able to explore acid subsoils despite reduced Ca availability and Al toxicity. Other than the possibility of selecting for reduced pectin content or greater pectin methylation in dicot species, it is not clear how this might be achieved, since this research topic is neglected. Given the importance of soil acidity for crop production, and the importance of Ca for root growth in acid soil, further research is warranted.
Low phosphorus availability
Low P availability is an inherent challenge to root growth in acid subsoil. Unlike Ca, P moves readily in the phloem and can be provided to growing root tips by the plant. However, bioavailable P is concentrated in the topsoil because of the low mobility of P in soil and the accumulated effects of biomass deposition and greater microbiological activity in the topsoil (Lynch and Brown, 2001). Plant adaptations to low P availability consist of mechanisms to increase soil foraging, especially in the topsoil where P availability is greatest, mechanisms to increase the availability of P in the rhizosphere, and mechanisms to reduce internal P requirements (Vance et al., 2003; Lynch, 2011; Richardson et al., 2011).
Genotypic variation for plant P requirements through such mechanisms as prolonged phenology (Nord and Lynch, 2008, 2009; Nord et al., 2011), low tissue P requirement (Lambers et al., 2011), or reduced seed P content (Rose et al., 2010) are potential, largely unexploited breeding targets. One reason for this is that they may entail significant trade-offs. For example, extended phenology may incur fitness penalties when the length of the growing season is limited by temperature or water, and reducing seed P content may have fitness impacts on young seedlings growing in low P soil. Species adapted to extremely low P ecosystems with low tissue P content also have slow growth, a trait that is incompatible with annual crops. A priori, it is reasonable to expect that the evolution of land plants would have already optimized the cellular and tissue level utilization of P, which is a nearly universal limitation in terrestrial environments (Vance et al., 2003; Lynch, 2007).
The prospect of improving P acquisition by genetic modification of the production of P-solubilizing compounds has been intensively researched, but despite promising results in synthetic media, results in actual soil have been disappointing (Richardson et al., 2011). For example, a recent comparison of near-isogenic wheat lines found no benefit of citrate efflux for P uptake (Ryan et al., 2014). Transgenic Trifolium subterraneum lines expressing a fungal phytase had greater P uptake in only one of five soils tested (George et al., 2005b ). Such results highlight the need to confirm results from studies conducted in artificial media with studies in living soil, where complex interactions with soil chemistry and in particular with the microbial communities in the rhizosphere occur. Microorganisms can immobilize and metabolize plant exudates (Jones et al., 2004), and have their own P-solubilizing mechanisms, which can dramatically change fitness outcomes resulting from plant phenotypes (George et al., 2005a, b, 2007).
Several architectural, morphological, and anatomical traits improve P acquisition by increasing topsoil foraging. Since these have been recently reviewed (Lynch, 2011; Richardson et al., 2011), we focus here on the specific case of subsoil exploration. Root architectural traits that improve P acquisition by increasing topsoil foraging may incur trade-offs for subsoil resources such as water (Ho et al., 2005). The metabolic costs of soil exploration by plant roots and their symbionts are large (Lambers et al., 2002; Lynch and Ho, 2005), so the need to explore both deep and shallow soil domains without sacrificing yield is a challenge. One set of traits that may be useful in this context are those that reduce or optimize the metabolic costs of soil exploration. The formation of root cortical aerenchyma (RCA), which converts living cortical tissue to air space through programmed cell death, improves P acquisition by reducing the metabolic cost of soil exploration (Fan et al., 2003; Postma and Lynch, 2010, 2011; Lynch, 2014). Other anatomical traits that reduce the cortical burden of root tissue merit research in this context, including root etiolation, cortical senescence, variation in cortical cell file number, and cortical cell size. Root hairs are metabolically inexpensive and critically important for P acquisition by expanding the effective depletion zone around the root axis (Gahoonia et al., 1997; Bates and Lynch, 2000a , b; Ma et al., 2001; Zhu et al., 2009). Genotypic variation in root hair length and density is therefore an excellent avenue for breeding more P-efficient crops (Richardson et al., 2011). Arbuscular mycorrhizal (AM) symbioses are also important for P acquisition in most crops, but in the context of subsoil exploration it is noteworthy that AM fungal inoculum and colonization decline substantially with soil depth (Higo et al., 2013; Shukla et al., 2013). It is also not clear how much progress may be made by breeding for more effective AM symbioses, as AM plants are typically highly effective already, and associate with a variety of fungal symbionts already present in agricultural soils. Several root architectural traits may be useful in balancing the needs for topsoil and subsoil foraging. Lateral root branching density can be optimized to balance the needs for intensive soil foraging for P and more extensive, deeper soil foraging for mobile resources such as nitrate (Postma et al., 2014). Dimorphic root architectures exist, possessing axial roots with a greater range of growth angles, or combinations of traits for topsoil foraging such as hypocotyl-borne roots with traits for subsoil foraging such as steep axial growth angles (Miller et al., 2003; Miguel et al., 2013). The utility of such dimorphic root architectures merits validation.
Manganese toxicity
Mn toxicity is an important but widely ignored constraint to plant growth in weathered soils. This is partially because of the complexity of the processes regulating Mn bioavailabilty in the rhizosphere as well as Mn toxicity in leaves, both of which have substantial interactions with other environmental variables. Plant-available Mn is divalent, created by reduction of Mn oxides in the soil (Sparrow and Uren, 2014). Mn bioavailablity is therefore sensitive to soil redox potential (Eh/pe) as well as pH and soil Mn content. Mn toxicity often occurs at pH 5.3 and below (in contrast to pH 4.8 and below for Al toxicity), or even at a pH as high as 7 in hypoxic conditions (Schlichting and Sparrow, 1998). Mn bioavailability is also affected by the rhizosphere pH, which in turn is affected by the balance of anion and cation uptake by the root, usually governed by the ratio of nitrate and ammonium uptake (Elamin and Wilcox, 1986). Mn uptake is also greatly affected by competition with base cations such as Mg2+ and Ca2+, and the other transition metals, especially Fe (Marschner, 2002). The importance of redox status and therefore localized or temporary hypoxia is an important factor in the subsoil, which generally has low oxygen status and may be compacted (see below). Mn toxicity in the plant may be related to photo-oxidative stress in photosynthetic tissue (Gonzalez et al., 1998; St.Clair and Lynch, 2005). Substantial genotypic variation in Mn tolerance may be related to antioxidant mechanisms and subcellular compartmentation (Gonzalez et al., 1998; Gonzalez and Lynch, 1999).
Crop tolerance to Mn toxicity in the subsoil may be improved by RCA formation, which would increase the oxygen status of the rhizosphere, as shown in flooded soils (Jackson and Armstrong, 1999), as well as mechanisms to tolerate Mn in leaf tissue, through tissue and cellular level compartmentation as well as greater antioxidant capacity.
Deep rooting is limited by soil physical properties
Deep rooting is generally limited by abiotic factors such as temperature, aeration, and high mechanical soil impedance. For example, in a wet compacted soil the elongation of roots may be limited due to mechanical soil impedance and hypoxia, while in a compacted dry soil, root elongation may be limited by water deficit and soil strength as root elongation is significantly limited under the following conditions: penetrometer resistances >2MPa, air-filled volume of <10%, and a matric potential greater than –1.5MPa (Bengough et al., 2011).
Soil compaction
Soil compaction limits root elongation in deep soils (Materechera et al., 1991; Bengough et al., 2006). Soil drying also increases soil impedance in many soils, which reduces root growth (e.g. Bengough et al., 1997, 2006; Hinsinger et al., 2009; Whitmore and Whalley, 2009; Bingham et al., 2010). Large impedance is associated with strong soils of high soil bulk density or low water content. Bengough et al. (2006) reported that roots prefer to grow in looser soils than in denser soils but that this response depended on genotype and the spatial arrangement of the soil with respect to the main root axes. Impeded soils are known to restrict plant root growth, reduce water and nutrient uptake, and thereby restrict plant development (Ishaq et al., 2001). The extent of mechanical impedance of the soil depends on the physical properties of the soil including its bulk density, matric potential and soil structure, and volumetric water content (reviewed by Bengough et al., 2011; Valentine et al., 2012). A survey of a range of field soils suggested that root elongation was generally limited by mechanical impedance, even in relatively wet field soils (Bengough et al., 2011).
Previous research has focused on the biophysical relationships between cell wall extension and water potential (Watt et al., 2005; Bengough et al., 2006; Hinsinger et al., 2009). A strategy for annual plant roots to avoid soil hardening due to receding water tables, and, subsequently, increasing soil impedance, would be to adapt elongation rates accordingly, allowing the root tip to remain in moist and favourable soil conditions. This may be achieved through changes in the relative length of the elongation zone since the elongation rate and length of the elongation zone are correlated (e.g. Sharp et al., 2004; Bengough et al., 2006). The adaptations could be achieved through changes in the cell wall properties of the expanding cells in the elongation zone. Increased impedance reduces the rate of root elongation and increases radial thickening (Bengough and Mullins, 1990; Clark et al., 2003). Similarly, increased soil strength decreases the length of the elongation zone of the root, and can as much as double the root diameter (Bengough and Mullins, 1990; Watt et al., 2005; Hinsinger et al., 2009; Bengough et al., 2011). RCA formation reduces the radial transport of water and nutrients (Fan et al., 2007; Hu et al., 2014), but the location of RCA formation in older root segments might not have a large net impact on plant resource acquisition. Therefore, we suggest that RCA formation might be beneficial, preventing lateral water and nutrient loss along the root in soils with a low water potential, functioning as a barrier to water loss in drier topsoils. It may also be advantageous to avoid dessication of the root tip and surrounding soil by limiting water transport from root apices to mature xylem through such mechanisms as delayed xylem maturation, increased suberization of the endodermis, and hydraulic isolation of rhizosheaths (Lynch, 2014). Architectural traits that position roots in deeper soil domains may also aid in the avoidance of soil hardening in drying surface strata (Lynch, 2013).
Genetic diversity has potential for trait discovery to overcome the constraints of soil compaction. For example, wheat (e.g. Botwright Acuña and Wade, 2012) and lupin (e.g. Chen et al., 2014) genotypes differ in their response to mechanical soil impedance. In general, root systems responded to mechanical impedance as roots are repelled, reduce their elongation rate, and thicken radially on encountering the physical barrier (Materechera et al., 1992). Radial thickening makes roots more resistant to buckling in hard soils (Materechera et al., 1992; Bengough et al., 1997; Clark et al., 2008; Whalley et al., 2008), which increases soil penetration. Bengough et al. (2011) and Haling et al. (2013) hypothesized a further root trait—root anchorage—to facilitate root penetration into harder soils. The anchorage of roots might be attained by friction of soil particles and maturing tissues behind the elongation zone by producing root hairs and lateral roots. The anchoring of the root apex through root hair growth may support the turgor pressure to force the root tip further into the soil. Another root trait may be the ability to change trajectory as a reaction to soil compaction, using the soil matrix at the bend of the root as an anchor point which reflects the reaction force entering the compacted soil layer. Future research will have to address soil environments with multiple stresses such as limited water availability in compacted soils. A strategy for soil exploration for water in compacted soils would be to exploit paths of less resistance using cracks and biopores, and sensing water availability within soils. Studies showed that roots were found more frequently in artificially created pores in compacted soils than expected by chance alone (Stirzaker et al., 1996). Recently, Bao et al. (2014) indicated that root systems are able to respond to local hydraulic changes of the soil environment, which they called ‘hydropatterning’. This recent study reported that plants can distinguish between wet and drier soil domains, developing root hairs and lateral roots in the wet environments. Preferential root growth in wetter soil domains would result in reshaping of root architecture over time. In the common case in which the topsoil dries before the subsoil, this would result in deeper root architecture, which would be favourable for water acquisition. If genotypic differences in sensing such localized resources exist, this may be an opportunity to increase resource acquisition efficiency of crop roots in deeper soil profiles.
Hypoxia
Hypoxia or anoxia affects roots in soils if the air-filled pore space is limited (<10 %) due to the soil structure or the water saturation of the soil. Survival of roots in anaerobic soil conditions requires avoidance mechanisms (reviewed by Vartapetian and Jackson, 1997). This is achieved in some species by changing the growth trajectory of the roots, increasing root growth in oxygenated soil profiles or, in flooded environments, the formation of aerial roots. Internally interconnected RCA will also overcome the restriction of low oxygen levels in the soil. In well-adapted species, the aerenchyma can extend from the leaf stomata to the root tip (Vartapetian and Jackson, 1997). Plants are known to form RCA under waterlogged conditions (Jackson and Armstrong, 1999), which may occur during the rainy season or flooding as an agricultural practice in tropical regions. Oxygen transport in RCA may be facilitated by concentration gradients or through temperature-driven pressure gradients. Many wetland plants, including rice, develop a barrier to radial oxygen loss through suberization of the hypodermis (Colmer, 2003; Garthwaite et al., 2008). Temperate cereals may follow another strategy by removing the cortex through cortical senescence, subsequently reducing the requirement for oxygen. Other anatomical traits may also reduce the cortical burden (Jaramillo et al., 2013) in hypoxia and therefore conserve oxygen for the growing tip in deeper soils, including cell file numbers or cortical cell size (Chimungu et al., 2014; Lynch et al., 2014), which may facilitate root elongation under low oxygen conditions. However, these hypotheses need to be tested.
Temperature
Vertical temperature gradients, often of substantial magnitude, are common in natural soils (Table 1). Temperature effects on root growth have been studied for many years. Bauer and Bradow (1996) screened cotton genotypes that were impacted less by cold temperatures, finding that root length was genotype dependent under low temperature. Maize root growth and development decreased linearly with decreasing temperatures in a nutrient solution experiment using a temperature range from 22 °C to 13 °C (Nagel et al., 2009). A similar effect of temperature on root elongation and lateral root growth in other plant species such as canola, sunflower, barley, and cotton were reported in several studies (Cumbus and Nye, 1982; Abbas Al-ani and Hay, 1983; Macduff et al., 1986; Nagel et al., 2009). Low temperatures may inhibit deep rooting of plants, restricting apical root elongation as low temperature decreases metabolic activity and therefore sink strength of roots, suggesting feedback mechanisms on phloem loading and photosynthesis (Minchin, 2002). The effects of vertical temperature gradients on assimilate transport depend on the fraction of roots exposed to lower temperatures (Sowinski et al., 1998). Plant breeding for cold tolerance may increase deep rooting of crops, allowing root elongation in deeper and, subsequently, colder environments. For example, a chilling-sensitive maize variety had decreased root length and root surface area when compared with a chilling-tolerant variety in low temperature treatments (Richner et al., 1996). Hund et al. (2007) investigated the effects on root morphology of low temperature in 21 modern inbred maize lines under low temperature and found great variation of root morphology among the genotypes, concluding that variation of root traits accounted for early vigour under low temperatures and could be a target for future plant breeding. Furthermore, high temperature may also inhibit root growth as roots and the soil respire rapidly in warm temperatures, which may lead to anaerobic conditions in the root zone within a short period, and, subsequently, may decrease root growth into the deeper soil profile. The formation of RCA would help to provide oxygen under those growth conditions, facilitating deeper root growth along the vertical temperature gradient.
Agronomic practices facilitating deep rooting
Production system, timing, and frequency of cultivation affect soils; the impact depends on soil type, topography, and climate (Stoate et al., 2001). For example, increases in field size and increased use of heavy machinery have contributed to greater levels of soil erosion (Evans, 1996). Additionally, the use of heavier farm machinery may increase soil impedance in arable production systems (reviewed by Batey, 2009). Soil compaction is a particular problem on soils with low organic matter where earthworm abundance and activity are low (Makeschin, 1997). Mechanical soil impedance is a major limitation to agricultural production systems, unless a network of channels or biopores exists for roots to exploit (White and Kirkegaard, 2010), as roots are able to grow in continuous biopores or channels and therefore explore compacted soils for nutrients and water in deeper soils. For example, wheat root growth in hard subsoils is almost confined entirely to biopores in very hard Australian soils (White and Kirkegaard, 2010). Some no-tillage farms use rotations to encourage the formation of biopores/channels (Passioura, 2002). Alternatively, deep-ripping during cultivation (Delroy and Bowden, 1986; reviewed by Batey, 2009) or the application of gypsum to alter soil structure and aggregate stability, could be used to facilitate deeper root growth in compacted soils. Certain plant species could be used as pioneer species creating such biopores/channels. Materechera et al. (1992) found that dicotyledonous plants had larger diameters and penetrated harder soil layers better than monocotyledonous species. Therefore, dicots such as alfalfa and lupin may be used as pioneer species to avoid unsuitable topsoil conditions and to exploit nutrients and water in deeper subsoils, while creating root channels that can be exploited by subsequent cereal crops. For example, Lupinus albus can penetrate subsoil to depths of >100cm, avoiding unfavourable topsoils (Pennisi, 2008). However, White and Kirkegaard (2010) found that >85% of roots were clustered together using those channels and biopores, which may limit the exploration of those soils as roots are unable to explore the soils for nutrients and water except within the biopores and channels.
Frequent cultivation decreases soil organic matter content and, subsequently, impacts soil structure and composition (Stoate et al., 2001). The use of fertilizers (organic or inorganic), herbicides, and pesticides also influences soil structure directly or indirectly through impacts on the soil fauna (e.g. Seghers et al., 2005; reviewed by Bünemann et al., 2006). For example, earthworms may improve soil structure in soils that are not compacted and maintain high organic matter, improving aeration, crop root growth, and drainage (Marinissen and Bok, 1988; Makeschin, 1997). Other agricultural practices such as minimum tillage can increase soil organic matter (Logan et al., 1991; Fox, 2000; Bescansa et al., 2006; McCarthy et al., 2008), which would also facilitate deeper rooting and deeper C sequestration through greater water availability in the soil, as organic matter improves soil moisture retention (Benckiser, 1997).
Agronomic practices have substantial effects on soil properties, crop growth, and subsoil exploration by crop roots. The development of crop genotypes with root traits improving subsoil exploration will have to consider not only the target soil environment but also the target management environment. Such ‘G×E×M’ interactions are in general poorly understood. The complexity and scope of possible scenarios, including future climate scenarios, call for the use of multiscale mechanistic modelling approaches to inform and guide empirical research.
Updated ideotype for deep rooting
The ‘steep, cheap, and deep’ ideotype consists of architectural, anatomical, and physiological traits that could improve the capture of soil water and N by maize root systems by improving subsoil exploration (Lynch, 2013). In this review we propose several additional elements of this ideotype as adaptations to subsoil constraints to root growth, as summarized in Table 2.
Table 2.
Deep soil constraints, root traits/phenes, and example references
| Soil constraint | Root trait(s)/phene(s) | Reference |
|---|---|---|
| Al toxicity | Carboxylate exudation | Kochian et al. (2004) |
| Al tolerance of root hairs | Delhaize et al. (2012) | |
| Rhizosheaths | Delhaize et al. (2012) | |
| Tissue tolerance | Hiradate et al. (1997); Feng Ma et al. (1998) | |
| Low Ca availability | Reduced tissue Ca requirement | Spehar and Galwey (1997) |
| Larger cell size | Hypothetical | |
| Lower pectin content or greater pectin methylation | Blamey et al. (2014) | |
| Low P availability | RCA | Fan et al. (2003) |
| Cortical senescence, reduction of cortical cell file number, and cortical cell size | Hypothetical | |
| Root architecture | Richardson et al. (2011) | |
| Root hairs | Richardson et al. (2011) | |
| Mycorrhizal symbiosis | Lambers et al. (2002); Lynch and Ho (2005) | |
| Root exudates | Jones et al. (2004); George et al. (2005b ) | |
| Mn toxicity | RCA | Hypothetical |
| Antioxidant mechanisms | Gonzalez et al. (1998) | |
| Subcellular compartmentation | Gonzalez and Lynch (1999) | |
| Soil compaction | Adaptation of the elongation zone | Hypothetical |
| Reduction of water transport from the root tip | Hypothetical | |
| Radial thickening | Materechera et al. (1992); Bengough et al. (1997); Clark et al. (2008); Whalley et al. (2008) | |
| Root anchorage (root hairs and lateral roots) | Hypothetical | |
| ‘Hydropatterning’ | hypothetical | |
| Hypoxia | RCA | Jackson and Armstrong (1999); Colmer (2003); Garthwaite et al. (2008); Henry et al. (2012) |
| Suberization | Hypothetical | |
| Temperature | Breeding for cold tolerance | Hund et al. (2007) |
| RCA | Hypothetical |
In acid subsoils, Al tolerance is critically important, and low Ca requirement, P efficiency, and Mn tolerance would be useful. Soil impedance is a pervasive subsoil constraint to root growth. Traits improving root penetration in hard subsoil include ‘hydopatterning’ and the ability to find cracks and biopores which provide optimal deep soil exploration for nutrients and water. Root hairs and lateral root formation provide anchorage and reduce buckling of roots in compacted soils. Tolerance to low soil temperature is important for subsoil exploration in temperate soils. The formation of RCA and reduction of cortical cell file number and cortical cell size would facilitate growth by reducing metabolic burden and oxygen requirements, allowing deeper penetration of soils, and, potentially, greater tolerance of hypoxia or low temperature. Additionally, RCA could increase oxygen levels, thereby reducing Mn toxicity in low pH soils. In fact, any traits reducing the respiratory requirements of root tissue, including anatomical traits such as RCA, cortical cell file number, cortical senescence, and cortical cell size, should afford multiple benefits for subsoil exploration. Reduced respiratory requirements would result in greater soil exploration and therefore better acquisition of P and Ca, greater tolerance to hypoxia, greater tolerance to the increased respiratory demand of high soil temperature, greater tolerance of the challenge to maintain respiration under low soil temperature, and potentially greater tolerance of Mn toxicity by diminishing oxygen consumption in the rhizosphere, thereby maintaining Mn in a more oxidized state. Traits that slow dessication of soil surrounding root apices, such as delayed xylem maturation and suberization of the endodermis, may reduce resistance to root penetration. The utility of any of these traits will of course depend on the nature of the soil environment and crop management. We propose that many of these traits will exhibit synergism; that is, their value in combination will exceed their additive value. For example, alleviation of Al toxicity will improve plant root growth and therefore plant acquisition of Ca and P, other limiting resources. These traits and issues merit research because of their potential value in developing more productive, sustainable, benign, and resilient agricultural systems.
Acknowledgements
We thank Kathleen Brown, Alan Richardson, and Larry York for helpful comments. JPL acknowledges support from Agriculture and Food Research Initiative competitive grant number 2014-67013-2157 of the USDA National Institute of Food and Agriculture. Root biology research at IBG-2 Plant Sciences is institutionally funded by the Helmholtz Association.
References
- Abbas Al-ani MK, Hay RKM. 1983. The influence of growing temperature on the growth and morphology of cereal seedling root systems. Journal of Experimental Botany 34, 1720–1730. [Google Scholar]
- Aquea F, Federici F, Moscoso C, Vega A, Jullian P, Haseloff J, Arce-Johnson P. 2012. A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant, Cell and Environment 35, 719–734. [DOI] [PubMed] [Google Scholar]
- Bao Y, Aggarwal P, Robbins NE, et al. 2014. Plant roots use a patterning mechanism to position lateral root branches toward available water. Proceedings of the National Academy of Sciences, USA 111, 9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SA. 1995. Soil nutrient bioavailability: a mechanistic approach. New York: John Wiley & Sons, Inc. [Google Scholar]
- Bates TR, Lynch JP. 2000a. Plant growth and phosphorus accumulation of wild type and two root hair mutants of Arabidopsis thaliana (Brassicaceae). American Journal of Botany 87, 958–963. [PubMed] [Google Scholar]
- Bates TR, Lynch JP. 2000b. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. American Journal of Botany 87, 964–970. [PubMed] [Google Scholar]
- Batey T. 2009. Soil compaction and soil management—a review. Soil Use and Management 25, 335–345. [Google Scholar]
- Bauer PJ, Bradow JM. 1996. Cotton genotype response to early season cold temperatures. Crop Science 36, 1602–1606. [Google Scholar]
- Benckiser G. 1997. Organic inputs and soil metabolism. In: Benckiser G, ed. Fauna in soil ecosystems. New York: Dekker, 7–62. [Google Scholar]
- Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA. 2006. Root responses to soil physical conditions; growth dynamics from field to cell. Journal of Experimental Botany 57, 437–447. [DOI] [PubMed] [Google Scholar]
- Bengough A, Croser C, Pritchard J. 1997. A biophysical analysis of root growth under mechanical stress. Plant and Soil 189, 155–164. [Google Scholar]
- Bengough AG, Mckenzie BM, Hallett PD, Valentine TA. 2011. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany 62, 59–68. [DOI] [PubMed] [Google Scholar]
- Bengough AG, Mullins CE. 1990. Mechanical impedance to root growth: a review of experimental techniques and root growth responses. Journal of Soil Science 41, 341–358. [Google Scholar]
- Bescansa P, Imaz MJ, Virto I, Enrique A, Hoogmoed WB. 2006. Soil water retention as affected by tillage and residue management in semiarid Spain. Soil and Tillage Research 87, 19–27. [Google Scholar]
- Bingham IJ, Bengough AG, Rees RM. 2010. Soil compaction–N interactions in barley: root growth and tissue composition. Soil and Tillage Research 106, 241–246. [Google Scholar]
- Blamey FPC, Wehr JB, Wang P, Menzies NW, Kopittke PM. 2014. Kinetics and mechanisms of cowpea root adaptation to changes in solution calcium. Plant and Soil 379, 301–314. [Google Scholar]
- Botwright Acuña TL, Wade LJ. 2012. Genotype×environment interactions for root depth of wheat. Field Crops Research 137, 117–125. [Google Scholar]
- Bünemann EK, Schwenke GD, Van Zwieten L. 2006. Impact of agricultural inputs on soil organisms—a review. Australian Journal of Soil Research 44, 379. [Google Scholar]
- Cassman KG, Dobermann A, Walters DT. 2002. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31, 132–140. [DOI] [PubMed] [Google Scholar]
- Chen XP, Zhang FS, Cui ZL, Li F, Li JL. 2010. Optimizing soil nitrogen supply in the root zone to improve maize management. Soil Science Society of America Journal 74, 1367–1373. [Google Scholar]
- Chen YL, Palta J, Clements J, Buirchell B, Siddique KHM, Rengel Z. 2014. Root architecture alteration of narrow-leafed lupin and wheat in response to soil compaction. Field Crops Research 165, 61–70. [Google Scholar]
- Chimungu J, Brown K, Lynch J. 2014a. Large root cortical cell size improves drought tolerance in maize (Zea mays L.). Plant Physiology (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP. 2014b. Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LJ, Ferraris S, Price AH, Whalley WR. 2008. A gradual rather than abrupt increase in soil strength gives better root penetration of strong layers. Plant and Soil 307, 235–242. [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB. 2003. How do roots penetrate strong soil? Plant and Soil 255, 93–104. [Google Scholar]
- Colmer TD. 2003. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumbus IP, Nye PH. 1982. Root zone temperature effects on growth and nitrate absorption in rape (Brassica napus cv. Emerald). Journal of Experimental Botany 33, 1138–1146. [Google Scholar]
- Dai X, Xiao L, Jia D, Kong H, Wang Y, Li C. 2014. Increased plant density of winter wheat can enhance nitrogen–uptake from deep soil. Plant and Soil 384, 141–152. [Google Scholar]
- Delhaize E, James RA, Ryan PR. 2012. Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytologist 195, 609–619. [DOI] [PubMed] [Google Scholar]
- Delroy ND, Bowden JW. 1986. Effect of deep ripping, the previous crop, and applied nitrogen on the growth and yield of a wheat crop. Australian Journal of Experimental Agriculture 26, 469–479. [Google Scholar]
- Dresbøll DB, Thorup-Kristensen K. 2014. Will breeding for nitrogen use efficient crops lead to nitrogen use efficient cropping systems?: a simulation study of G×E×M interactions. Euphytica 199, 97–117. [Google Scholar]
- Dunbabin V, Diggle A, Rengel Z. 2003. Is there an optimal root architecture for nitrate capture in leaching environments? Plant, Cell and Environment 26, 835–844. [DOI] [PubMed] [Google Scholar]
- Elamin OM, Wilcox GE. 1986. Effect of magnesium and manganese nutrition on muskmelon growth and manganese toxicity. Journal of the American Society for Horticultural Science 111, 582–587. [Google Scholar]
- Evans R. 1996. Some soil factors influencing accelerated water erosion of arable land. Progress in Physical Geography 20, 205–215. [Google Scholar]
- Fan M, Bai R, Zhao X, Zhang J. 2007. Aerenchyma formed under phosphorus deficiency contributes to the reduced root hydraulic conductivity in maize roots. Journal of Integrative Plant Biology 49, 598–604. [Google Scholar]
- Fan M, Zhu J, Richards C, Brown KM., Lynch JP. 2003. Physiological roles for aerenchyma in phosphorus-stressed roots. Functional Plant Biology 30, 493–506. [DOI] [PubMed] [Google Scholar]
- Farrar J, Hawes M, Jones D, Lindow S. 2003. How roots control the flux of carbon to the rhizosphere. Ecology 84, 827–837. [Google Scholar]
- Feng Ma J, Hiradate S, Matsumoto H. 1998. High aluminum resistance in buckwheat. II. Oxalic acid detoxifies aluminum internally. Plant Physiology 117, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TR. 2000. Sustained productivity in intensively managed forest plantations. Forest Ecology and Management 138, 187–202. [Google Scholar]
- Foy CD, Fleming AL, Armiger WH. 1969. Aluminum tolerance of soybean varieties in relation to calcium nutrition. Agronomy Journal 61, 505–511. [Google Scholar]
- Gahoonia TS, Care D, Nielsen NE. 1997. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant and Soil 191, 181–188. [Google Scholar]
- Garthwaite AJ, Armstrong W, Colmer TD. 2008. Assessment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytologist 179, 405–416. [DOI] [PubMed] [Google Scholar]
- George TS, Richardson AE, Simpson RJ. 2005a. Behaviour of plant-derived extracellular phytase upon addition to soil. Soil Biology and Biochemistry 37, 977–988. [Google Scholar]
- George TS, Richardson AE, Smith JB, Hadobas PA, Simpson RJ. 2005b. Limitations to the potential of transgenic Trifolium subterraneum L. plants that exude phytase when grown in soils with a range of organic P content. Plant and Soil 278, 263–274. [Google Scholar]
- George TS, Simpson RJ, Gregory PJ, Richardson AE. 2007. Differential interaction of Aspergillus niger and Peniophora lycii phytases with soil particles affects the hydrolysis of inositol phosphates. Soil Biology and Biochemistry 39, 793–803. [Google Scholar]
- Gonzalez A, Lynch JP. 1999. Subcellular and tissue Mn compartmentation in bean leaves under Mn toxicity stress. Australian Journal of Plant Physiology 26, 811–822. [Google Scholar]
- Gonzalez A, Steffen KL, Lynch JP. 1998. Light and excess manganese—implications for oxidative stress in common bean. Plant Physiology 118, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS. 2013. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. Journal of Experimental Botany 64, 3711–3721. [DOI] [PubMed] [Google Scholar]
- Henry A, Cal AJ, Batoto TC, Torres RO, Serraj R. 2012. Root attributes affecting water uptake of rice (Oryza sativa) under drought. Journal of Experimental Botany 63, 4751–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo M, Isobe K, Yamaguchi M, Drijber RA, Jeske ES, Ishii R. 2013. Diversity and vertical distribution of indigenous arbuscular mycorrhizal fungi under two soybean rotational systems. Biology and Fertility of Soils 49, 1085–1096. [Google Scholar]
- Hinsinger P, Bengough AG, Vetterlein D, Young IM. 2009. Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant and Soil 321, 117–152. [Google Scholar]
- Hiradate S, Nomoto K, Iwashita T, Matsumoto H, Danchi TN. 1997. Internal detoxification mechanism of Al in Hydrangea. Plant Physiology 113, 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. 2005. Root architectural trade-offs for water and phosphorus acquisition. Functional Plant Biology 32, 737–748. [DOI] [PubMed] [Google Scholar]
- Hu B, Henry A, Brown KM, Lynch JP. 2014. Root cortical aerenchyma inhibits radial nutrient transport in maize (Zea mays). Annals of Botany 113, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund A, Richner W, Soldati A. 2007. Root morphology and photosynthetic performance of maize inbred lines at low temperature. European Journal of Agronomy 27, 52–61. [Google Scholar]
- IPCC. 2014. Climate change 2014: impacts, adaptation, and vulnerability. Cambridge: Cambridge Universtiity Press. [Google Scholar]
- Ishaq M, Hassan A, Saeed M, Ibrahim M, Lal R. 2001. Subsoil compaction effects on crops in Punjab, Pakistan I. Soil physical properties and crop yield. Soil and Tillage Research 59, 57–65. [Google Scholar]
- Jackson MB, Armstrong W. 1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1, 274–287. [Google Scholar]
- Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP. 2013. Root cortical burden influences drought tolerance in maize. Annals of Botany 112, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Hodge A, Kuzyakov Y. 2004. Plant and mycorrhizal regulation of rhizodeposition. New Phytologist 163, 459–480. [DOI] [PubMed] [Google Scholar]
- Kell DB. 2011. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany 108, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB. 2012. Large-scale sequestration of atmospheric carbon via plant roots in natural and agricultural ecosystems: why and how. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kobayashi Y, Watanabe T, Shaff JE, Ohta H, Kochian LV, Wagatsuma T, Kinraide TB, Koyama H. 2013. Molecular and physiological analysis of Al3+ and H+ rhizotoxicities at moderately acidic conditions. Plant Physiology 163, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Pineros MA. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annual Review of Plant Biology 55, 459–493. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Piñeros MA, Hoekenga OA. 2005. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant and Soil 274, 175–195. [Google Scholar]
- Lal R. 2004. Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627. [DOI] [PubMed] [Google Scholar]
- Lambers H, Atkin O, Millenaar FF. 2002. Respiratory patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Kafkaki K, eds. Plant roots: the hidden half. New York: Marcel Dekker, Inc, 521–552. [Google Scholar]
- Lambers H, Finnegan P, Laliberte E, Pearse SJ, Ryan MH, Shane MW, Veneklass EJ. 2011. Phosphorus nutrition of proteaceae in severely phosphorus-impoverished soils: are there lessons to be learned for future crops? Plant Physiology 156, 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Piñeros MA, Kochian LV. 2014. The role of aluminum sensing and signaling in plant aluminum resistance. Journal of Integrative Plant Biology 56, 221–230. [DOI] [PubMed] [Google Scholar]
- Logan T, Lal R, Dick W. 1991. Tillage systems and soil properties in North America. Soil and Tillage Research 20, 241–270. [Google Scholar]
- Lopes MS, Reynolds MP. 2010. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Functional Plant Biology 37, 147–156. [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55, 493–512. [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. 2014. Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant, Cell and Environment (in press). [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2001. Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant and Soil 237, 225–237. [Google Scholar]
- Lynch JP, Chimungu JG, Brown KM. 2014. Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. Journal of Experimental Botany 65, 6155–6166. [DOI] [PubMed] [Google Scholar]
- Lynch JP, Ho MD. 2005. Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil 269, 45–56. [Google Scholar]
- Lynch J, Läuchli A. 1985. Salt stress disturbs the Ca nutrition of barley (Hordeum vulgare L.). New Phytologist 99, 345–354. [Google Scholar]
- Ma Z, Walk TC, Marcus A, Lynch JP. 2001. Morphological synergism in root hair length, density, initiation and geometry for phosphorus acquisition in Arabidopsis thaliana: a modeling approach. Plant and Soil 236, 221–235. [Google Scholar]
- Macduff J, Wild A, Hopper M, Dhanoa M. 1986. Effects of temperature on parameters of root growth relevant to nutrient uptake: measurements on oilseed rape and barley grown in flowing nutrient solution. Plant and Soil 94, 321–332. [Google Scholar]
- Makeschin F. 1997. Earthworms (Lumbricidae: Oligochaeta): important promoters of soil development and soil fertility. In: Benckiser G, ed. Fauna in soil ecosystems. Dekker: New York, 173–223. [Google Scholar]
- Manschadi AM, Christopher J, deVoil P, Hammer GL. 2006. The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823. [DOI] [PubMed] [Google Scholar]
- Manschadi AM, Christopher JT, Hammer GL, Devoil P. 2010. Experimental and modelling studies of drought-adaptive root architectural traits in wheat (Triticum aestivum L.). Plant Biosystems 144, 458–462. [Google Scholar]
- Marinissen JCY, Bok J. 1988. Earthworm-amended soil structure: its influence on Collembola populations in grassland. Pedobiologia 32, 243–252. [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants. London: Academic Press. [Google Scholar]
- Materechera S, Alston A, Kirby J, Dexter A. 1992. Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant and Soil 144, 297–303. [Google Scholar]
- Materechera S, Dexter A, Alston A. 1991. Penetration of very strong soils by seedling roots of different plant species. Plant and Soil 135, 31–41. [Google Scholar]
- McCarthy JF, Ilavsky J, Jastrow JD, Mayer LM, Perfect E, Zhuang J. 2008. Protection of organic carbon in soil microaggregates via restructuring of aggregate porosity and filling of pores with accumulating organic matter. Geochimica et Cosmochimica Acta 72, 4725–4744. [Google Scholar]
- Mi G, Chen F, Wu Q, Lai N, Yuan L, Zhang F. 2010. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Science China Life Sciences 53, 1369–1373. [DOI] [PubMed] [Google Scholar]
- Miguel MA, Widrig A, Vieira RF, Brown KM, Lynch JP. 2013. Basal root whorl number: a modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Annals of Botany 112, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP. 2003. Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Functional Plant Biology 30, 973–985. [DOI] [PubMed] [Google Scholar]
- Minchin PEH. 2002. Source–sink coupling in young barley plants and control of phloem loading. Journal of Experimental Botany 53, 1671–1676. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681. [DOI] [PubMed] [Google Scholar]
- Nagel KA, Kastenholz B, Jahnke S, et al. 2009. Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping. Functional Plant Biology 36, 947. [DOI] [PubMed] [Google Scholar]
- Nord E, Lynch J. 2008. Delayed reproduction in Arabidopsis thaliana improves fitness in soil with suboptimal phosphorus availability. Plant, Cell, and Environment 31, 1432–1441. [DOI] [PubMed] [Google Scholar]
- Nord E a, Lynch JP. 2009. Plant phenology: a critical controller of soil resource acquisition. Journal of Experimental Botany 60, 1927–1937. [DOI] [PubMed] [Google Scholar]
- Nord EA, Shea K, Lynch JP. 2011. Optimizing reproductive phenology in a two-resource world: a dynamic allocation model of plant growth predicts later reproduction in phosphorus-limited plants. Annals of Botany 108, 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M. 2011. Large root systems: are they useful in adapting wheat to dry environments? Functional Plant Biology 38, 347–354. [DOI] [PubMed] [Google Scholar]
- Passioura JB. 2002. Soil conditions and plant growth. Plant, Cell and Environment 25, 311–318. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Zhang K, Thorup-Kristensen K, Jensen LS. 2009. Modelling diverse root density dynamics and deep nitrogen uptake—a simple approach. Plant and Soil 326, 493–510. [Google Scholar]
- Pennisi E. 2008. Getting to the root of drought responses. Science 320, 173. [DOI] [PubMed] [Google Scholar]
- Postma J, Dathe A, Lynch J. 2014. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiology 166, 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP. 2010. Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Annals of Botany 107, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Lynch JP. 2011. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiology 156, 1190–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel DD, Horwath WR, Mitchell JP, Temple SR. 2001. Impacts of cropping systems on soil nitrogen storage and loss. Agricultural Systems 68, 253–268. [Google Scholar]
- Raun GV, Johnson WR. 1999. Improving nitrogen use efficiency for cereal production. Agronomy Journal 91, 357–363. [Google Scholar]
- Rengel Z, Zhang W-H. 2003. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytologist 159, 295–314. [DOI] [PubMed] [Google Scholar]
- Ribaudo M, Delgado J, Hansen L, Livingston M, Mosheim R, Williamson J. 2011. Nitrogen in agricultural systems: implications for conservation policy. United States Department of Agriculture, Economic Research Service, Economic Research Report Number 127, Washington, DC. [Google Scholar]
- Richardson AE, Lynch JP, Ryan PR, et al. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349, 121–156. [Google Scholar]
- Richner W, Soldati A, Stamp P. 1996. Shoot-to-root relations in field grown maize seedlings. Agronomy Journal 88, 56–61. [Google Scholar]
- Rose TJ, Pariasca-Tanaka J, Rose MT, Fukuta Y, Wissuwa M. 2010. Genotypic variation in grain phosphorus concentration, and opportunities to improve P-use efficiency in rice. Field Crops Research 119, 154–160. [Google Scholar]
- Rosegrant MW, Ringler C, Zhu T. 2009. Water for agriculture: maintaining food security under growing scarcity. Annual Review of Environment and Resources 34, 205–222. [Google Scholar]
- Ryan PR, Delhaize E. 2010. The convergent evolution of aluminium resistance in plants exploits a convenient currency. Functional Plant Biology 37, 275. [Google Scholar]
- Ryan PR, James RA, Weligama K, et al. 2014. Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiologia Plantarum 151, 230–242. [DOI] [PubMed] [Google Scholar]
- Ryan PR, Tyerman SD, Sasaki T, Furuichi T, Yamamoto Y, Zhang WH, Delhaize E. 2011. The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. Journal of Experimental Botany 62, 9–20. [DOI] [PubMed] [Google Scholar]
- Saengwilai P, Nord EA, Brown KM, Lynch JP. 2014a. Root cortical aerenchyma enhances nitrogen acquisition from low nitrogen soils in maize. Plant Physiology 166, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch J. 2014b. Low crown root number enhances nitrogen acquisition from low nitrogen soils in maize. Plant Physiology 166, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting E, Sparrow LA. 1988. Distribution and amelioration of manganese toxic soils. In: Graham RD, Hannam J, Uren NC, eds. Manganese in soils and plants. Dordrecht: Kluwer Academic Publishers, 277–288. [Google Scholar]
- Schnurbusch T, Hayes J, Hrmova M, Baumann U, Ramesh S a, Tyerman SD, Langridge P, Sutton T. 2010. Boron toxicity tolerance in barley through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiology 153, 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghers D, Siciliano SD, Top EM, Verstraete W. 2005. Combined effect of fertilizer and herbicide applications on the abundance, community structure and performance of the soil methanotrophic community. Soil Biology and Biochemistry 37, 187–193. [Google Scholar]
- Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT. 2004. Root growth maintenance during water deficits: physiology to functional genomics. Journal of Experimental Botany 55, 2343–2351. [DOI] [PubMed] [Google Scholar]
- Shukla A, Vyas D, Jha A. 2013. Soil depth: an overriding factor for distribution of arbuscular mycorrhizal fungi. Journal of Soil Science and Plant Nutrition 13, 23–33. [Google Scholar]
- Sowinski P, Richner W, Soldati A, Stamp P. 1998. Assimilate transport in maize (Zea mays L.) seedlings at vertical low temperature gradients in the root zone. Journal of Experimental Botany 49, 747–752. [Google Scholar]
- Sparrow LA, Uren NC. 2014. Manganese oxidation and reduction in soils: effects of temperature, water potential, pH and their interactions. Soil Research 52, 483. [Google Scholar]
- Spehar CR, Galwey NW. 1997. Screening soya beans [Glycine max (L.) Merill] for calcium efficiency by root growth in low-Ca nutrient solution. Euphytica 94, 113–117. [Google Scholar]
- Sponchiado BN, White JW, Castillo JA, Jones PG. 1989. Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Experimental Agriculture 25, 249–257. [Google Scholar]
- St.Clair SB, Lynch JP. 2005. Differences in the success of sugar maple and red maple seedlings on acid soils are influenced by nutrient dynamics and light environment. Plant, Cell and Environment 28, 874–885. [Google Scholar]
- Stirzaker R, Passioura J, Wilms Y. 1996. Soil structure and plant growth: impact of bulk density and biopores. Plant and Soil 185, 151–162. [Google Scholar]
- Stoate C, Boatman N., Borralho R., Carvalho CR, de Snoo GR, Eden P. 2001. Ecological impacts of arable intensification in Europe. Journal of Environmental Management 63, 337–365. [DOI] [PubMed] [Google Scholar]
- Thorup-Kristensen K, Salmerón Cortasa M, Loges R. 2009. Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant and Soil 322, 101–114. [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, et al. 2013. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Valentine TA, Hallett PD, Binnie K, Young MW, Squire GR, Hawes C, Bengough AG. 2012. Soil strength and macropore volume limit root elongation rates in many UK agricultural soils. Annals of Botany 110, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157, 423–447. [DOI] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB. 1997. Plant adaptations to anaerobic stress. Annals of Botany 79, 3–20. [Google Scholar]
- Wallace JS, Gregory PJ. 2002. Water resources and their use in food production systems. Aquatic Sciences 64, 363–375. [Google Scholar]
- Wasson P, Richards R, Chatrath R, Misra SC, Prasad SVS, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M. 2012. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. Journal of Experimental Botany 63, 3485–3498. [DOI] [PubMed] [Google Scholar]
- Watt M, Kirkegaard J, Rebetzke G. 2005. A wheat genotype developed for rapid leaf growth copes well with the physical and biological constraints of unploughed soil. Functional Plant Biology 32, 695–706. [DOI] [PubMed] [Google Scholar]
- Whalley WR, Watts CW, Gregory AS, Mooney SJ, Clark LJ, Whitmore AP. 2008. The effect of soil strength on the yield of wheat. Plant and Soil 306, 237–247. [Google Scholar]
- Wheeler T, von Braun J. 2013. Climate change impacts on global food security. Science 341, 508–13. [DOI] [PubMed] [Google Scholar]
- White RG, Kirkegaard JA. 2010. The distribution and abundance of wheat roots in a dense, structured subsoil—implications for water uptake. Plant, Cell and Environment 33, 133–48. [DOI] [PubMed] [Google Scholar]
- Whitmore AP, Whalley WR. 2009. Physical effects of soil drying on roots and crop growth. Journal of Experimental Botany 60, 2845–2857. [DOI] [PubMed] [Google Scholar]
- Wiesler F, Horst WJ. 1993. Differences among maize cultivars in the utilization of soil nitrate and the related losses of nitrate through leaching. Plant and Soil 151, 193–203. [Google Scholar]
- Wiesler F, Horst W. 1994. Root growth and nitrate utilization of maize cultivars under field conditions. Plant and Soil 163, 267–277. [Google Scholar]
- Wilding LP. 2000. Classification of soils. In: Sumner ME, ed. Handbook of soil science. Boca Raton, FL: CRC Press, 175–183. [Google Scholar]
- Zhu JM, Brown KM, Lynch JP. 2010. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell and Environment 33, 740–749. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang C, Lynch JP. 2009. The utility of phenotypic plasticity for root hair length for phosphorus acquisition. Functional Plant Biology 37, 313–322. [Google Scholar]