Summary
Compared with wild-type plants, a cadmium-tolerant pea mutant showed different metal accumulation, root-pressure induced xylem exudation, and biomass depending on whether it was exposed to cadmium or mercury.
Key words: Aquaporin, cadmium, drought, mercury, pea, root sap flow, water deficit.
Abstract
Heavy metals have multiple effects on plant growth and physiology, including perturbation of plant water status. These effects were assessed by exposing the unique Cd-tolerant and Cd-accumulating pea (Pisum sativum L.) mutant SGECdt and its wild-type (WT) line SGE to either cadmium (1, 4 μM CdCl2) or mercury (0.5, 1, 2 μM HgCl2) in hydroponic culture for 12 days. When exposed to Cd, SGECdt accumulated more Cd in roots, xylem sap, and shoot, and had considerably more biomass than WT plants. WT plants lost circa 0.2 MPa turgor when grown in 4 μM CdCl2, despite massive decreases in whole-plant transpiration rate and stomatal conductance. In contrast, root Hg accumulation was similar in both genotypes, but WT plants accumulated more Hg in leaves and had a higher stomatal conductance, and root and shoot biomass compared with SGECdt. Shoot excision resulted in greater root-pressure induced xylem exudation of SGECdt in the absence of Cd or Hg and following Cd exposure, whereas the opposite response or no genotypic differences occurred following Hg exposure. Exposing plants that had not been treated with metal to 50 μM CdCl2 for 1h increased root xylem exudation of WT, whereas 50 μM HgCl2 inhibited and eliminated genotypic differences in root xylem exudation, suggesting differences between WT and SGECdt plants in aquaporin function. Thus, root water transport might be involved in mechanisms of increased tolerance and accumulation of Cd in the SGECdt mutant. However, the lack of cross-tolerance to Cd and Hg stress in the mutant indicates metal-specific mechanisms related to plant adaptation.
Introduction
Plant water status is determined by complex and interdependent processes such as root water absorption, water transport to the aerial organs, and transpiration, predominantly through the stomata. Heavy metals (such as Cd, Hg, Pb, Zn, Cu, and Ni) are widespread soil pollutants that are toxic to plants and can negatively affect all the processes related to plant water status (Poschenrieder and Barceló, 1999) through root growth inhibition that reduces total soil moisture availability to plants, decreased xylem vessel size (Kasim, 2007; De Silva et al., 2012) and number (Barceló et al., 1988; De Silva et al., 2012), decreased root and stem hydraulic conductivity (Barceló et al., 1988; Maggio and Joly, 1995; Nedjimi and Daoud, 2009), decreased stomatal density and conductance (Baryla et al., 2001), and inhibited activity of the molecular water channels aquaporins (AQPs), thereby reducing the permeability of water across the cell membrane (Tazawa et al., 1996; Savage and Stroud, 2007; Postaire et al., 2010). Negative effects on AQPs were observed mostly in plants treated with mercury (Hg); therefore this metal was often used as an inhibitor of AQPs. Nevertheless, not all AQPs are sensitive to Hg (Biela et al., 1999; Aroca et al., 2006; Verdoucq et al., 2008) and some aquaporin genes can be up-regulated by treatment with this metal (Tamas et al., 2010; Frick et al., 2013) including the pea AQP PsPIP-2 (Beaudette et al., 2007). Heavy-metal induced perturbation of aquaporin function may decrease both root and shoot hydraulic conductance, thereby decreasing leaf water potential and turgor, which may directly close the stomata. Decreased leaf and root water status caused by heavy metals can enhance biosynthesis of the phytohormone abscisic acid (ABA) (Poschenrieder et al., 1989), which acts to maintain plant water status. Root ABA biosynthesis can enhance root hydraulic conductivity (Thompson et al. 2007) and increase root-to-shoot ABA signalling thereby promoting stomatal closure (Davies and Zhang 1991; Dodd 2005). Indeed, decreased stomatal conductance observed in Cd-treated plants (Becerril et al., 1989; Poschenrieder et al., 1989; Zhu et al., 2005) could be due to increased ABA concentrations. Nevertheless, Cd-induced stomatal closure may be independent of ABA signalling as it also occurred in the ABA-insensitive Arabidopsis thaliana mutant abi1-1 (Perfus-Barbeoch et al., 2002). It was also proposed that stomatal closure may decrease heavy metal uptake from soil thereby decreasing metal accumulation in vivo and its toxic effects (Pascual et al., 2004; Lefevre et al., 2009; Disante et al., 2014).
The unique Cd-tolerant and Cd-accumulating multicellular plant mutant SGECdt was found in pea (Pisum sativum L.) line SGE (Tsyganov et al., 2007) and provides promising new genetic material to study the effects of heavy metals on plant water relations, and may be important for understanding responses of plants to combined stresses (e.g. metal toxicity and drought). SGECdt was obtained by chemical mutagenesis, and is characterized by a monogenic inheritance and a recessive phenotype, and physiological mechanisms allowing enhanced biomass production under cadmium stress. Biochemical studies showed that in the presence of toxic Cd (4 μM) ions, the mutant SGECdt had lower contents of non-protein thiols and free proline, and activities of catalase and peroxidase than wild-type (WT) plants. Although it had adequate nutrient status, it actively accumulated Cd in roots, leaves, and mesophyll protoplasts (Tsyganov et al., 2007), suggesting a cadmium-insensitive phenotype. To date, when grown under optimal conditions the only difference found between these pea genotypes was a 35% increase in the concentration of oxidized glutathione in SGECdt mutant roots (Tsyganov et al., 2007). However, it was concluded that Cd tolerance of SGECdt was not linked to glutathione and/or phytochelatin biosynthesis, as only marginal genotypic differences in their concentrations were found in Cd-treated plants (Tsyganov et al., 2007). Experiments with reciprocally grafted SGECdt and WT plants showed the crucial role of the root in the increased Cd-tolerance and Cd-accumulation of SGECdt (Malkov et al., 2007), but the impact of cadmium on plant water relations was not assessed in previous reports (Tsyganov et al. 2007; Malkov et al., 2007).
The present report aimed to characterize water relations (root hydraulic conductance, stomatal conductance, xylem vessel anatomy) of the pea mutant SGECdt in the absence and presence of both toxic Cd and Hg to elucidate the mechanisms underlying the effects of cadmium on plant water status. Mercury was included in some experiments as it inhibits AQP activity. Measurements of leaf water relations, xylem heavy metal concentrations, and root-to-shoot ABA signalling aimed to distinguish alternative mechanisms of stomatal closure.
Materials and methods
Plant culture
Seeds of wild-type (WT) pea (Pisum sativum L.) line SGE and its chemically induced mutant SGECdt characterized by increased Cd tolerance and Cd accumulation (Tsyganov et al., 2007) were surface sterilized and scarified by treatment with 98% H2SO4 for 30min, rinsed carefully with tap water, and germinated on filter paper in Petri dishes for 3 d at 25 °C in the dark. Seedlings were transferred to plastic pots (two pots with 10 seeds per genotype and treatment) containing 1500ml of nutrient solution, which contained (μM): KH2PO4, 400; KNO3, 1200; Ca(NO3)2, 60; MgSO4, 250; KCl, 300; CaCl2, 60; KCl, 250; Fe-tartrate, 12; H3BO3, 2; MnSO4, 1; ZnSO4, 3; NaCl, 6; Na2MoO4, 0.06; AlCl3, 1; CoCl2, 0,06; CuCl2, 0.06; KJ, 0.06; KBr, 0.06; NiCl2, 0,06; pH, 5.5. Plants were cultivated for 12 d in a naturally lit greenhouse (additional artificial lighting of 200 μmol m–2 s–1, a 12h photoperiod with minima/maxima temperatures of 18 °C/23 °C). In experiments with long exposure to heavy metals (12 d), one day after planting (DAP) the nutrient solution was supplemented with: (i) 1 μM or 4 μM CdCl2; (ii) 0.5, 1, or 2 μM HgCl2. In all experiments the nutrient solution was changed, and where necessary the supplements were added, at 5 and 9 DAP.
In experiments with short exposure to heavy metals (1h), the plants were cultivated in nutrient solution without metals for 11 d. On the 12th day the nutrient solution was supplemented or not with 50 μM CdCl2 or 50 μM HgCl2 (mercury was used to inhibit AQPs), the plants were treated for 1h and roots were briefly washed with deionized water for 20 s. Then the plants from each metal treatment were transferred to pots with fresh nutrient solution and xylem sap allowed to exude from de-topped roots as described below.
Anatomical and physiological measurements
To measure whole-plant transpiration rate on the day before harvest, pots were weighed at the beginning, middle, and end of the photoperiod and the time elapsed between weighing noted. Pots with and without plants were weighed to correct for evaporation from the surface of the nutrient solution and transpiration rate per plant was calculated. Stomatal conductance of young fully expanded leaves was measured with a transient time porometer (Model AP4, Delta-T Devices, Burwell UK) during the middle of the photoperiod. Before harvest, negative replicas of the lower leaf surfaces were taken using the Xantopren VL Plus (Heraeus Kulzer GmbH, Germany) and transferred as positive replicas to nail polish to measure stomatal density. At harvest, cross sections of roots (2–3cm from cotyledons) were prepared and stained with toluidine blue (water solution containing 0.05mg ml–1 toluidine blue, 0.30mg ml–1 Na-benzoate and 0.25mg ml–1 benzoic acid) for 1min to visualise xylem and phloem tissues in roots. Root cross sections and epidermal imprints were inspected and images were taken using a light microscope AxioVertA1 (Carl Zeiss, USA). Leaf images were analysed to count the number of stomata. Xylem and phloem area in roots was calculated using software OPTIMAS 6.1 (Optimas Corporation, Houston, USA).
Xylem sap exudation from de-topped roots
Shoots were cut 2cm above the cotyledons between 12.00h and 15.00h and cotton-filled 0.5ml Eppendorf tubes (of defined weight) placed on the cut stumps and wrapped with PARAFILM® to prevent evaporative losses of sap. The pots were covered with aluminium foil to avoid direct sunlight and sap was collected for 3h. Then tubes were collected, weighed, and root sap flow rate (Jv) calculated per plant (total Jv) or per 1mm2 of xylem area (specific Jv).
Shoot xylem sap collection
Shoot water potential was measured using a Scholander-type pressure chamber (Plant Moisture Systems, Santa Barbara, CA, USA). Xylem sap was then collected for 3min from detached whole shoots at 0.5MPa above balancing pressure (to gain enough sap for further analysis), frozen in liquid nitrogen, and stored at –20 °C. ABA concentration in xylem sap was determined through radioimmunoassay with the monoclonal antibody MAC252 (Quarrie et al., 1988).
Leaf water relations
Leaf water potential was measured in 8mm diameter discs punched from young fully expanded leaves at the top of the canopy. Discs were incubated for 3h in thermocouple psychrometers (C-52 chambers, Wescor Inc., Logan, UT, USA) before voltages were read with a microvoltmeter (HR-33T, Wescor Inc., Logan, UT, USA). Discs were then removed from the chambers, wrapped in aluminium foil, frozen in liquid nitrogen, and then re-inserted into the psychrometers and allowed to incubate for 30min before leaf osmotic potential was measured. Voltages were converted to water and osmotic potentials based on calibration curves (for each individual psychrometer) with salt solutions of known osmotic potential. Leaf turgor was calculated by subtracting osmotic potential from water potential.
Cd and Hg concentrations
Before harvest, roots were soaked in 5mM Pb-citrate solution (pH 11) for 10min and washed in deionized water for desorption of apoplastically bound Cd or Hg and then dried at room temperature. Then roots and leaves were dried at room temperature, ground, and digested in a mixture of concentrated HNO3 and 38% H2O2 at 70 °C. Concentrations of Cd or Hg in digested plant samples and in xylem sap were determined using atomic absorption spectrophotometers AA-7000 (Shimadzu, Japan) or Varian AA240 FS with flow vapour generation accessory VGA 77 (Agilent Technologies, USA), respectively.
Statistical analysis
Two way ANOVA along with an LSD test to discriminate differences between means was applied using the software STATISTICA version 10 (StatSoft Inc., USA).
Results
Effects of long-term cadmium exposure
Although 1 μM and 4 μM CdCl2 significantly inhibited root and shoot growth of both pea genotypes (Fig. 1), the SGECdt mutant was much more tolerant to Cd. At 4 μM CdCl2 the growth of WT plants was markedly decreased compared with plants grown in the absence of Cd, whereas the growth of the SGECdt mutant was similar to that of WT plants exposed to 1 μM CdCl2 (Fig. 1). Images of typical control and treated (4 μM CdCl2) plants are presented (Fig. 2E, F, G, and H).
Fig. 1.
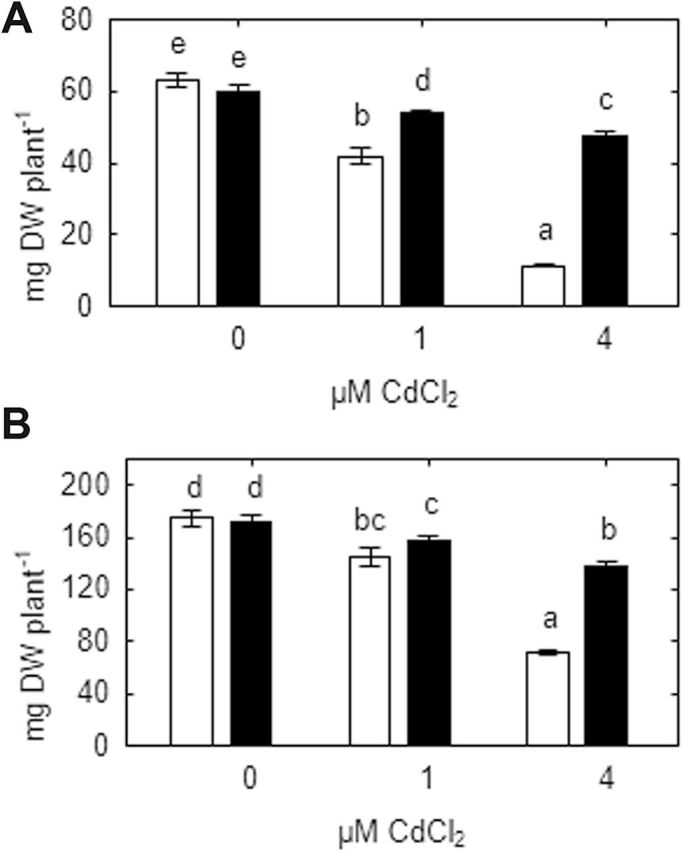
Root (A) and shoot (B) biomass of SGE (open bars) and SGECdt (filled bars) pea genotypes grown at different cadmium concentrations. Different letters show significant differences between treatments (LSD test; P<0.05). Means of three experiments with at least five determinations each.
Fig. 2.

Images of lower leaf surface (A–D) with stomatal complexes (sc) indicated, representative pea plants (E–H), and root cross sections (I–L) with xylem (xl) and phloem (ph) tissues indicated, from wild-type SGE plants untreated with cadmium (A, E, I), mutant SGECdt untreated with cadmium (B, F, J), wild type SGE plants treated with 4 μM CdCl2 (C, G, K), and mutant SGECdt treated with 4 μM CdCl2 (D, H, L).
In the presence of 1 μM CdCl2, no genotypic differences were found in root, leaf, or xylem cadmium concentrations (Table 1). In the presence of 4 μM CdCl2, root, xylem sap, and leaf cadmium concentrations of the mutant SGECdt were increased by 39%, 2.1-fold, and 11-fold, respectively, compared with WT plants. Xylem cadmium concentrations were similar to those in the nutrient solution, suggesting root-to-shoot Cd transport, particularly by the mutant plants treated with 4 μM CdCl2 (Table 1). No Cd was detected in Cd-untreated plants (data not shown).
Table 1.
Cadmium concentrations in pea plants grown in cadmium supplemented nutrient solution
Data are means±SE of three experiments with at least four determinations. Different letters show significant differences within columns (LSD test, P<0.05).
| Genotype | Root, μg Cd g–1 DW | Leaf, μg Cd g–1 DW | Xylem sap, μM |
|---|---|---|---|
| 1 μM CdCl 2 | |||
| SGE | 270±11 a | 20±2 ab | 1.3±0.08 a |
| SGECdt | 300±18 a | 30±5b | 0.9±0.05 a |
| 4 μM CdCl 2 | |||
| SGE | 763±63 b | 11±1 a | 2.6±0.4 b |
| SGECdt | 1060±45 c | 120±7 c | 5.3±0.4 c |
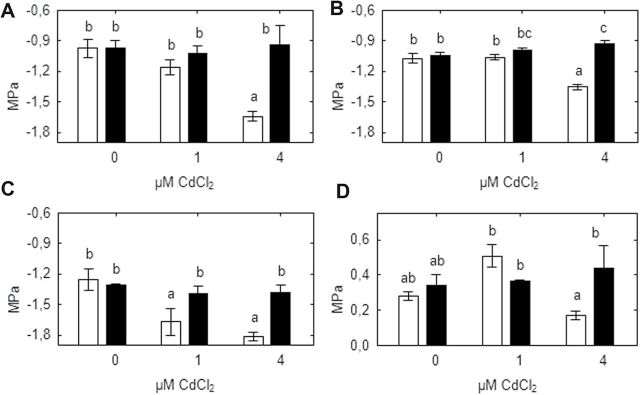
WT plants exposed to 4 μM CdCl2 had decreased leaf water potential (Fig. 3A) and shoot water potential (Fig. 3B) compared with other genotype/treatment combinations. Increasing cadmium concentrations did not change leaf osmotic potential in the SGECdt mutant, but exposure to 1 and 4 μM CdCl2 decreased it by 0.5–0.6MPa in WT plants (Fig. 3C). These changes allowed WT plants to maintain turgor when exposed to 1 μM CdCl2, but at 4 μM CdCl2 leaf turgor of WT plants was significantly less than in SGECdt mutant plants (Fig. 3D).
Fig. 3.
Effect of cadmium on leaf water potential (A), shoot water potential (B), leaf osmotic potential (C), and turgor (D) of SGE (open bars) and SGECdt (filled bars) pea genotypes. Different letters show significant differences between treatments (LSD test; P<0.05). Means of three experiments with at least five determinations.
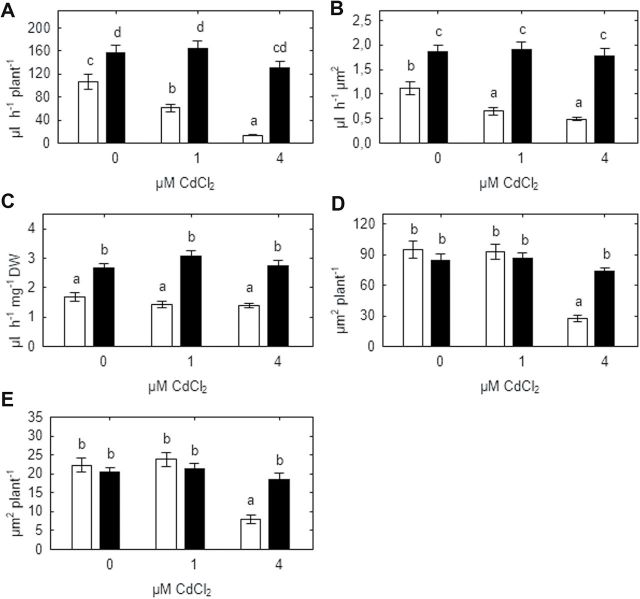
Xylem sap exudation from de-topped roots of SGECdt plants was significantly higher than from WT plants in the absence of cadmium (by 48–66% according to the basis of expression), and was insensitive to cadmium concentration irrespective of whether data were expressed on a per plant, per root xylem area, or per root weight basis (Fig 4A–C). Treating WT plants with 1 and 4 μM CdCl2 decreased sap exudation by 43% and 87%, respectively, on a whole-plant basis (Fig. 4A), and by 41% and 56%, respectively, on a per root xylem area basis (Fig. 4B) compared with untreated controls. However this effect was not observed when sap exudation was expressed per unit root weight (Fig. 4C).
Fig. 4.
Effect of cadmium on absolute root sap flow rate (A), and specific root sap flow rates expressed on a root xylem area (B) or root weight (C) basis, along with xylem (D) and phloem (E) area of SGE (open bars) and SGECdt (filled bars) pea genotypes. Different letters show significant differences between treatments (LSD test; P<0.05). Means of two experiments with at least three determinations.
Likewise, root xylem and phloem area of SGECdt plants was insensitive to cadmium concentration, but xylem and phloem area of WT plants treated with 4 μM CdCl2 was 2.5–3 times less than control treatments and SGECdt plants (Fig. 4D, E). Images of root cross sections from typical control and treated (4 μM CdCl2) plants are presented (Fig. 2I–L).
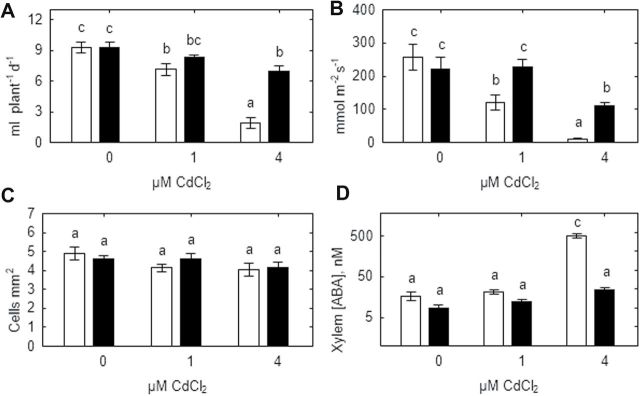
Whole-plant transpiration and stomatal conductance of SGECdt plants was unaffected by 1 μM CdCl2, but decreased by 26% and 50%, respectively, at 4 μM CdCl2 (Fig 5A, B). In contrast, cadmium significantly decreased whole-plant transpiration of WT plants by 28% and 75% at 1 and 4 μM CdCl2, respectively (Fig. 5A) and stomatal conductance by 52% and 95%, respectively. Differences in the percentage decrease between whole-plant transpiration and stomatal conductance probably reflect gradients in stomatal conductance according to the node at which the leaf is inserted, and the integrated effects of the treatments on stomatal conductance at all nodes affect whole-plant transpiration rate. Stomatal density did not vary with genotype or treatment (Fig. 5C). In control plants and those grown at 1 μM CdCl2, xylem ABA concentration of WT and SGECdt plants did not significantly differ and was 19±10nM and 11±5nM, respectively (pooled across both treatments). Treatment with 4 μM CdCl2 significantly increased xylem ABA concentration of WT plants by 30-fold, whereas the 3-fold increase observed in SGECdt was not statistically significant (Fig. 5D).
Fig. 5.
Effect of cadmium on whole-plant transpiration (A), stomatal conductance (B), stomatal density (C), and xylem ABA concentration (D) of SGE (open bars) and SGECdt (filled bars) pea genotypes. Note the logarithmic scale on (D). Different letters show significant differences between treatments (LSD test; P<0.05). Means of two experiments with at least five determinations.
Effects of long-term mercury exposure
All mercury concentrations applied (0.5, 1, 2 μM HgCl2) significantly inhibited root growth of both genotypes (Fig. 6A) and shoot growth of the SGECdt mutant even at 0.5 μM HgCl2. At all mercury concentrations tested, growth of the SGECdt mutant was significantly less than the WT plants, suggesting lower Hg tolerance. Root mercury concentrations were similar in both genotypes in plants exposed to 1 μM or 2 μM HgCl2 (Table 2). Generally, root mercury concentrations were three orders of magnitude higher than leaf mercury concentrations. Tissue mercury concentrations were not detected in Hg-untreated plants (data not shown) and not measured in plants treated with 0.5 μM HgCl2.
Fig. 6.
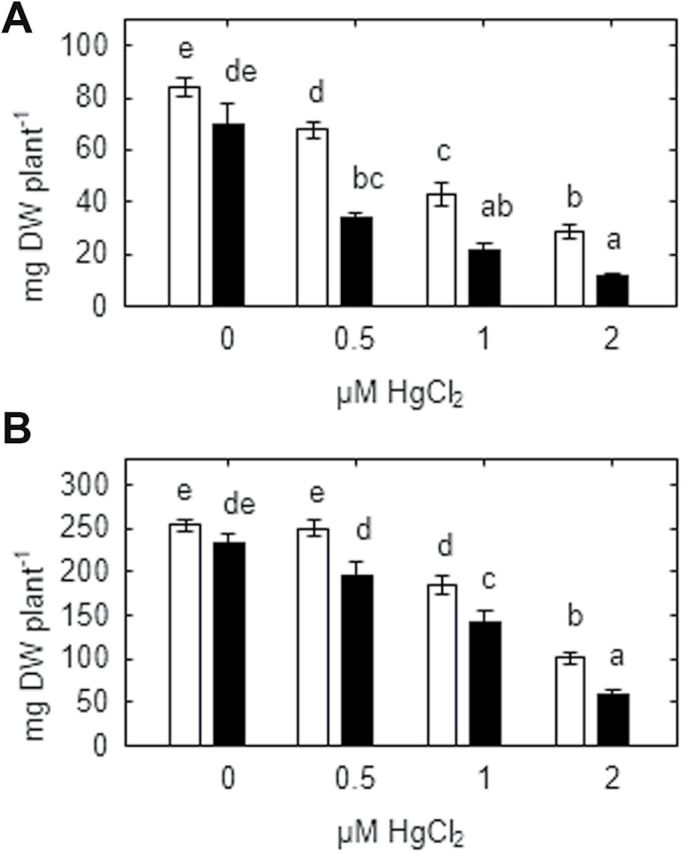
Root (A) and shoot (B) biomass of SGE (open bars) and SGECdt (filled bars) pea genotypes grown at different mercury concentrations. Different letters show significant differences between treatments (LSD test; P<0.05). Means of two experiments with at least five determinations.
Table 2.
Mercury concentrations in pea plants grown in mercury-supplemented nutrient solution
Data are means±SE of one experiment with four determinations. Different letters show significant differences within columns (LSD test, P<0.05).
| Genotype | Root,μg Hg g–1 DW | Leaf, μg Hg g–1 DW |
|---|---|---|
| 1 μM HgCl 2 | ||
| SGE | 2370±240 ab | 2.4±0.1 a |
| SGECdt | 1832±215 a | 1.9±0.02 a |
| 2 μM HgCl 2 | ||
| SGE | 2777±138 bc | 10.3±0.9 b |
| SGECdt | 3047±262 c | 2.9±0.7 a |
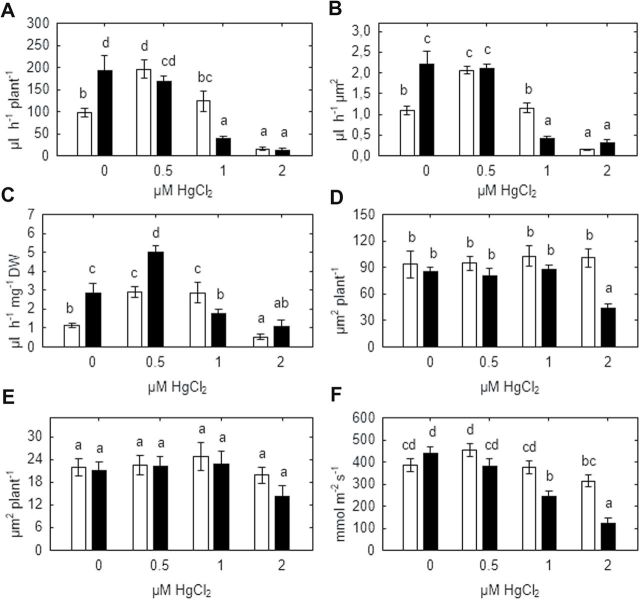
In the absence of HgCl2, xylem sap exudation of de-topped WT plants was approximately halved compared with SGECdt mutant plants, irrespective of whether data were expressed on an absolute, xylem-area, or root weight basis (Fig. 7A–C), consistent with previous results (Fig. 3A–C). Treatment with 0.5 μM HgCl2 increased xylem exudation of WT plants, thereby eliminating genotypic differences expressed on an absolute (Fig. 7A) or xylem-area specific (Fig. 7B) basis, but not on a root-weight specific basis (Fig. 7C). Treatment of SGECdt mutant plants with 0.5 μM HgCl2 had no effect on absolute xylem exudation, but approximately doubled exudation on a root weight specific basis (Fig. 7C). Irrespective of the basis of expression, at 1 μM HgCl2 xylem exudation of WT plants was significant higher than in SGECdt mutant plants but 2 μM HgCl2 markedly inhibited root exudation in both pea genotypes and eliminated any genotypic differences.
Fig. 7.
Effect of mercury on absolute root sap flow rate (A), and specific root sap flow rates expressed on a root xylem area (B) or root weight (C) basis, along with xylem (D) and phloem (E) area and stomatal conductance (F) of SGE (open bars) and SGECdt (filled bars) pea genotypes. Different letters show significant differences between treatments (LSD test; P<0.05). Means of two experiments with at least five determinations.
Genotype and Hg concentration generally had no statistically significant effect on either xylem (Fig. 7D) or phloem (Fig. 7E) area, but 2 μM HgCl2 significantly decreased xylem area of SGECdt plants by 50% (Fig. 7D). Stomatal conductance (gs) of WT plants was relatively insensitive to nutrient solution Hg concentration with 2 μM HgCl2 decreasing gs by 19%, but 1 and 2 μM HgCl2 decreased gs of SGECdt leaves by 43% and 74%, respectively (Fig. 7F).
Short-term effects of cadmium and mercury on xylem sap exudation
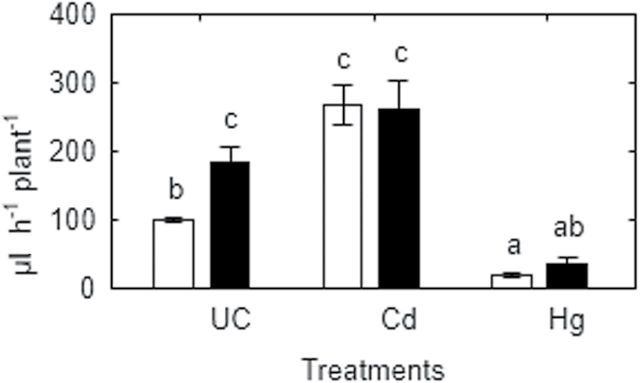
As all plants used in this experiment were pre-cultivated for 12 d without cadmium or mercury, their biomass and root xylem area was similar (data not shown). Therefore only absolute root xylem exudation is presented (Fig. 8). As in previous experiments, xylem exudation of metal-untreated SGECdt plants was higher (84%) than WT plants. These genotypic differences were eliminated following short (1h) exposure to cadmium (50 μM CdCl2), which significantly increased xylem sap exudation in WT, but not SGECdt mutant plants. In contrast, treatment with mercury (50 μM HgCl2) significantly reduced root xylem exudation of both genotypes and eliminated any genotypic differences between WT and mutant plants (Fig. 8).
Fig. 8.
Absolute root sap flow rate of SGE (open bars) and SGECdt (filled bars) pea genotypes after 1h exposure to 50 μM CdCl2 (Cd), 50 μM HgCl2 (Hg) or untreated controls (UC). Different letters show significant differences between treatments (LSD test; P<0.05). Means of two experiments with at least four determinations.
Discussion
Effects of Cd on water relations of the SGECdt mutant
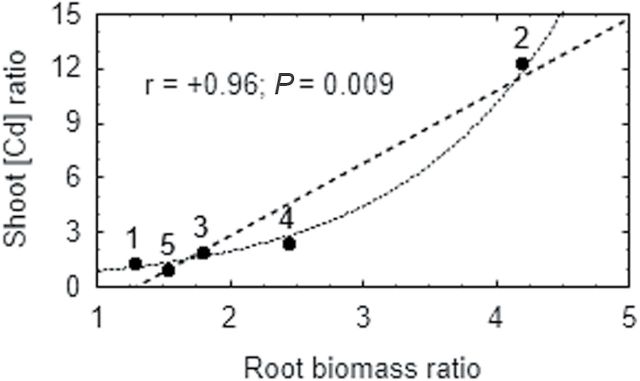
Consistent with previous work (Tsyganov et al., 2007), the pea mutant SGECdt grew better and accumulated higher Cd concentrations in roots and leaves than WT plants. By compiling all available data where these genotypes were treated with different Cd concentrations, a positive correlation (r=+0.96, P=0.009, n=5) was detected between genotypic differences (SGECdt/SGE ratio) in root biomass and in shoot Cd concentration (Fig. 9). This observation suggests that improved root growth (and probably root function) of the mutant compared with WT allows more active root-to-shoot xylem Cd transport and shoot Cd accumulation. Nevertheless, Cd-insensitivity allows the mutant to better maintain water (Fig. 3) and nutrient (Tsyganov et al., 2007) status, resulting in better shoot growth. Taken together it may be proposed that the mutant has affected some gene(s) related to regulation of water uptake or/and transport in roots, and most probably the same gene(s) are involved in response of the plant to Cd toxicity. Previously, using reciprocally grafted plants, the crucial role of the root in the increased Cd-tolerance and Cd-accumulation of SGECdt was shown (Malkov et al., 2007).
Fig. 9.
Pooled data on relationship between the effect of cadmium on genotypic differences (SGECdt/SGE ratio) in root biomass and shoot cadmium concentration. Black points show experimental data with cadmium concentrations in nutrient solution and reference: 1, 1 μM CdCl2 (this study); 2, 4 μM CdCl2 (this study); 3, 2.5 μM CdCl2 (Tsyganov et al., 2004); 4, 3 μM CdCl2 (Tsyganov et al., 2007); 5, 4 μM CdCl2 (Malkov et al., 2007). Dash and dotted lines show linear and exponential fitting, respectively.
Cadmium dose-response curves allowed genotypic differences in physiology to be compared in plants of the same developmental stage, as effects of Cd on WT plants grown at 1 μM were similar those of mutant plants grown at 4 μM (Fig. 1). Previously, under similar growth conditions, in the presence of 3 μM CdCl2, root and shoot (or leaf) Cd content of SGECdt was 19% and two times higher, respectively, than in the WT (Tsyganov et al., 2007). Here, in the presence of 4 μM CdCl2, such differences were much greater: 39% in roots and 11 times in leaves. When plants were treated with 1 μM CdCl2, there were no significant genotypic differences in root, leaf, or xylem sap Cd concentrations, suggesting these effects depend on Cd concentrations in the nutrient solution. At 4 μM CdCl2, xylem Cd concentration of the mutant was 2.1 times that of the WT and quite similar to that of the nutrient solution (5.3 μM). These results indicate that SGECdt actively transports Cd from root to shoot, and confirms significant root and shoot Cd accumulation within the SGECdt mutant, along with its high capacity to tolerate potentially toxic tissue Cd concentrations (Tsyganov et al., 2007).
Maintaining high root water uptake capacity is very important for tolerance of plants to abiotic stresses caused by drought, flooding, salt, and low temperatures (Aroca et al., 2012), but little is known about relationships between water uptake and heavy metal tolerance in different plant genotypes. An important finding was the greater absolute and specific (expressed per unit root weight or root xylem area) Jv of the mutant SGECdt in the absence of toxic cadmium (Figs 4A–C, 7 A–C, and 8). Absolute and specific Jv (expressed per unit root xylem area basis) significantly decreased with increasing Cd concentrations in WT plants, but not in the mutant SGECdt (Fig. 4A, B), and this correlated well with genotypic differences in plant growth (Fig. 1A). Exposing WT plants to 4 μM CdCl2 for 12 d caused marked degradation of root xylem tissue, expressed as decreased lumen area (Figs 2K and 4D) and this could contribute to decreased Jv. Further measurements are required to determine whether these anatomical changes affect root hydraulic conductance in vivo. Indeed, Cd decreased root hydraulic conductivity of the newly found Cd-hyperaccumulating plant Atriplex halimus (Nedjimi and Daoud, 2009). Previously, Cd treatment (4.5 μM CdCl2 for 21 d) reduced both the number and the size of xylem elements in bush bean stems (Barceló et al., 1988) although no changes in diameter and number of metaxylem elements were found in roots of maize seedlings exposed to 100 μM CdCl2 for 7 d (Maksimovic et al., 2007). Although exposing maize seedlings to 5 μM Cd(NO3)2 for 10 d did not visibly alter root xylem tissues, significant lignification of endodermal and xylem cells was detected and interpreted as a defence mechanism preventing loading cadmium into xylem (Lux et al. 2011). The decreased area of phloem tissue in WT plants treated with 4 μM CdCl2 (Fig. 2K) may also decrease the basipetal flow of photosynthates and thereby exacerbate negative effects of Cd on root growth and function. Paradoxically, expressing Jv on a root weight basis indicated no effect of different Cd concentrations in either genotype (Fig. 4C), indicating close coordination of root biomass allocation and water transport in these genotypes. Irrespective of how Jv is expressed, the effect of cadmium on shoot water relations may best indicate root function.
Treatment with 4 μM CdCl2 decreased leaf and shoot water potentials and leaf osmotic potential of only WT plants (Fig. 3A, B). Similarly, Cd exposure decreased leaf osmotic potential and water content in leaves and roots of the Cd-treated halophyte species Atriplex halimus (Lefevre et al., 2009). Although stomatal closure of plants exposed to 4 μM CdCl2 acts to maintain leaf water status (as in drought-induced stomatal closure of pea where plants exposed to soil drying had a higher Ψleaf; Belimov et al., 2009), the almost complete absence of root-pressure induced xylem exudation following long Cd-exposure (Fig. 4A) resulted in leaf water deficit (Fig. 3D). Cadmium-induced decreases in transpiration and stomatal conductance were repeatedly reported in different species, including legumes such as Medicago sativa (Becerril et al., 1989) and Phaseolus vulgaris (Poschenrieder et al., 1989). Although long-term exposure to Cd (47 d in soil supplemented with 100mg Cd kg–1) can decrease stomatal density (Baryla et al., 2001), in the experiments here there were no genotypic or Cd-dependent differences in stomatal density (Fig. 5C); thus transpiration rate was mostly controlled by stomatal apertures. Stomatal closure of WT plants exposed to 1 μM CdCl2 in the absence of changes in leaf or shoot water potential (Fig. 3A, B) implied that root-sourced chemical signals were controlling leaf water status.
Stomatal closure has been correlated with increased foliar ABA concentrations in Phaseolus vulgaris (Poschenrieder et al., 1989) and Hordeum vulgare (Hollenbach et al., 1997). Similarly, decreased whole-plant transpiration (Fig. 5A) and stomatal conductance (Figs 2C and 5B) of WT plants treated with 4 μM CdCl2 correlated with increased xylem ABA concentration (Fig. 5D), although it is not clear whether ABA was synthesized in the roots as a response to Cd toxicity or in the shoots as a result of shoot water deficit. Irrespective, xylem ABA concentrations were sufficiently high (0.5 μM in WT plants exposed to 4 μM CdCl2) to induce stomatal closure, as feeding detached pea leaves similar ABA concentrations via the petiole approximately halved transpiration (Dodd et al., 2008). An alternative explanation is that cadmium transported in the xylem (Table 1) enhances apoplastic Cd concentrations around the guard cells and closes stomata in an ABA-independent manner (Perfus-Barbeoch et al., 2002). Further work to measure the sensitivity of detached leaf transpiration to xylem-supplied Cd will be needed to discriminate these competing hypotheses.
The data confirm that in the mutant there is greater Cd tolerance and accumulation of Cd, and demonstrate that root water uptake may be important in regulating these processes. Determining whether impaired shoot water relations inhibits shoot growth of WT plants exposed to high rhizospheric Cd concentration requires that these plants are grown at high humidity to obviate any change in leaf water status.
Effects of Hg on growth and water relations of the SGECdt mutant
In contrast, the Cd tolerant SGECdt mutant was less tolerant to Hg, having lower root and shoot biomass, and stomatal conductance than WT plants under similar Hg treatments (Figs 6 and 7F). Decreased Hg tolerance of this mutant was associated with statistically equivalent root Hg concentrations, but substantially lower leaf Hg concentrations when plants were grown at 2 μM HgCl2 (Table 2). These differences were opposite of that observed with Cd treatments, where simultaneously lower Cd tolerance and uptake were found in WT plants. In both cases genotypic differences in root metal concentrations were several times lower than differences in shoot metal concentrations, suggesting that root to shoot heavy metal transport is associated with the mutant phenotype and plays an important role in metal tolerance. The decreased Hg tolerance of SGECdt was accompanied by decreased stomatal conductance, which may decrease root-to-shoot Hg transport. Information about co-tolerance of plants to Cd and Hg is quite limited. A cadmium-tolerant cell line of tomato was similarly sensitive to Hg (Huang et al., 1987). Comparing responses to Cd and Hg in Zea mays (Rellan-Alvarez et al., 2006) and Medicago sativa (Ortega-Villasante et al., 2005) revealed that biosynthesis of metal binding peptides phytochelatins was increased after treatments with Cd only, but not with Hg, suggesting different tolerance mechanisms to Cd and Hg. This study represents the first report of an inverse relationship between plant tolerance to these heavy metals, probably mediated by the same gene.
The molecular water channels AQPs, a numerous and multi-form family of proteins, can regulate root hydraulic conductance and water transport in various plant species, particularly under abiotic stress conditions (Maurel et al., 2008, Aroca et al., 2012). Although Cd had little effects on AQP activity of Arabidopsis halleri, it increased water permeability through the plasmalemma of individual leaf epidermal cells (Przedpelska et al., 2008). Inserting the AQP gene AtPIP2;1 from A. thaliana into the yeast Pichia pastoris showed that Cd but not Hg blocks this AQP (Verdoucq et al., 2008). However, up-regulation of AQP genes in barley root tips was detected after treatments with either Cd or Hg (Tamas et al., 2010). Here, a short treatment with a very high Cd concentration (50 μM CdCl2) rapidly increased Jv only in WT plants, indicating genotypic differences in response to different metal treatments (Fig. 8). Further experiments are required to determine whether increased Jv of WT roots is due to up-regulation of AQP gene expression or some Cd-induced disturbances in regulation of these channels. In contrast to the conflicting effects of Cd on AQP gene expression and activity, treatment with millimolar Hg concentrations was repeatedly used to inhibit AQPs in plants (Maggio and Joly, 1995; Aroca et al., 2001; Savage and Stroud, 2007; Postaire et al., 2010), including pea (Beaudette et al., 2007). Similarly, a short treatment with 50 μM HgCl2 not only inhibited Jv, but also eliminated genotypic differences in this parameter, suggesting that AQP regulation differs in SGECdt mutant and WT plants and that AQPs may be involved in the increased Jv of the mutant under optimal conditions (Fig. 8).
An unexpected result was that long exposure to relatively low Hg concentration (0.5 μM) significantly increased Jv of WT pea to the level of the SGECdt mutant, without affecting root xylem and phloem areas (Fig. 7). Up-regulation of AQP genes in response to chronic Hg concentrations may be involved (Tamas et al. 2010). Similarly, increased root hydraulic conductance under relatively long exposure to a mild stresses such as chilling and oxidative stress (Aroca et al., 2005) or drought and ABA treatment (Aroca et al., 2006) was a result of adaptive responses in expression of AQP genes and AQP protein abundance. Thus further investigations of the role of AQPs in the water relations of the studied plant genotypes is needed to elucidate the mechanisms involved.
Conclusion
When grown in Cd-supplemented solution, the Cd-tolerant and Cd-accumulating pea (Pisum sativum L.) mutant SGECdt had better water uptake and transport (moderated by morphology of root vascular tissues, and diminished xylem ABA concentration, but higher stomatal conductance, leaf water and osmotic potentials, leaf turgor, and whole-plant transpiration) than the WT SGE line. The mutant also had higher root sap flow rate than WT plants in both the presence and absence of toxic Cd ions. These observations suggest that root water transport might be involved in mechanisms of increased tolerance and accumulation of Cd in SGECdt, which has a Cd-insensitive phenotype. In contrast, SGECdt possessed decreased Hg-tolerance and foliar Hg-accumulation, and the negative effects of Hg on water relations parameters were more pronounced in the mutant. Such genotypic specificity in tolerance to different heavy metals has not been described previously and provides new evidence for the importance of water relations, particularly proper root function in water uptake and transport, in tolerance to and accumulation of heavy metals by plants.
Acknowledgements
We thank YV Homyakov for determination of mercury in plant samples. This work was supported by the Royal Society and the Russian Foundation of Basic Research (09-04-01614-a; 12-04-01501-а). The work on manuscript preparation was supported by the Russian Science Foundation (Grant 14-16-00137).
References
- Aroca R, Amodeo A, Fernandez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ. 2005. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiology 137, 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Ferrante A, Vernieri P, Chrispeels MJ. 2006. Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Annals of Botany 98, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R., Porcel R., Ruiz-Lozano J.M. 2012. Regulation of root water uptake under abiotic stress conditions. Journal of Experimental Botany 63, 43–57. [DOI] [PubMed] [Google Scholar]
- Aroca R, Tognoni F, Irigoyen JJ, Sanchez-Diaz M, Pardossi A. 2001. Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiology and Biochemistry 39, 1067–1073. [Google Scholar]
- Barceló J, Vázquez MD, Poshenrieder C. 1988. Cd induced structural and ultrastructural changes in the vascular system of bush bean stems. Botanica Acta 101, 254–261. [Google Scholar]
- Baryla A, Carrier P, Franck F, Coulomb C, Sahut C, Havaux M. 2001. Leaf chlorosis in oil seed rape plants (Brassica napus) grown on cadmium-polluted soil: causes and consequences for photosynthesis and growth. Planta 212, 696–709. [DOI] [PubMed] [Google Scholar]
- Beaudette PC, Chlup M, Yee J, Emery RJN. 2007. Relationships of root conductivity and aquaporin gene expression in Pisum sativum: diurnal patterns and the response to HgCl2 and ABA. Journal of Experimental Botany 58, 1291–1300. [DOI] [PubMed] [Google Scholar]
- Becerril JM, Gonzalez-Murua C, Munoz-Rueda A, de Felipe MR. 1989. Changes induced by cadmium and lead in gas exchange and water relations of clover and lucerne. Plant Physiology and Biochemistry 27, 913–918. [Google Scholar]
- Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ. 2009. Rhizosphere bacteria containing ACC deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytologist 181, 413–423. [DOI] [PubMed] [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R. 1999. The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant Journal 18, 565–570. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Zhang J. 1991. Root signals and the regulation of growth and development of plants in drying soil. Annual Reviews in Plant Physiology and Plant Molecular Biology 42, 55–76. [Google Scholar]
- De Silva ND, Cholewa E, Ryser P. 2012. Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.). Journal of Experimental Botany 63, 5957–5966. [DOI] [PubMed] [Google Scholar]
- Disante KB, Cortina J, Vilagrosa A, Fuentes D, Hernández EI, Ljung K. 2014. Alleviation of Zn toxicity by low water availability. Physiologia Plantarum 150, 412–424. [DOI] [PubMed] [Google Scholar]
- Dodd IC. 2005. Root-to-shoot signalling: assessing the roles of “up” in the up and down world of long-distance signalling in planta . Plant and Soil 274, 257–275. [Google Scholar]
- Dodd IC, Ferguson BJ, Beveridge CA. 2008. Apical wilting and petiole xylem vessel diameter of the rms2 branching mutant of pea are shoot controlled and independent of a long-distance signal regulating branching. Plant and Cell Physiology 49, 791–800. [DOI] [PubMed] [Google Scholar]
- Frick A, Järvå M, Ekvall M, Uzdavinys P, Nyblom M, Törnroth-Horsefield S. 2013. Mercury increases water permeability of a plant aquaporin through a non-cysteine-related mechanism. Biochemistry Journal 454, 491–499. [DOI] [PubMed] [Google Scholar]
- Hollenbach B, Schreiber L, Hartung W, Dietz KJ. 1997. Cadmium leads to stimulated expression of the lipid transfer protein genes in barley: implications for the involvement of lipid transfer proteins in wax assembly. Planta 203, 9–19. [DOI] [PubMed] [Google Scholar]
- Huang B, Hatch E, Goldsbrough PB. 1987. Selection and characterization of cadmium tolerant cells in tomato. Plant Science 52, 211–221. [Google Scholar]
- Kasim WA. 2007. Physiological consequences of structural and ultra-structural changes induced by Zn stress in Phaseolus vulgaris. I. Growth and photosynthetic apparatus. International Journal of Botany 3, 15–22. [Google Scholar]
- Lefevre I, Marchal G, Meerts P, Correal E, Lutts S. 2009. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environmental and Experimental Botany 65, 142–152. [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ. 2011. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany 62, 21–37. [DOI] [PubMed] [Google Scholar]
- Maggio A, Joly RJ. 1995. Effects of mercuric chloride on the hydraulic conductivity of tomato root systems. Plant Physiology 109, 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic I, Kastori R, Kristic L, Lukovic J. 2007. Steady presence of cadmium and nickel affects root anatomy, accumulation and distribution of essential ions in maize seedlings. Biologia Plantarum 51, 589–592. [Google Scholar]
- Malkov NV, Belimov AA, Safronova VI. 2007. Heavy metal tolerance of pea mutant SGECdt: new possibility for mechanistic study of metal toxicity for plant–microbe interaction. In: Provorov NA, ed. Abstracts of Postgraduate Course “Applied and fundamental aspects of responses, signaling and developmental process in the root-microbe systems” and Meeting of the Research Consortium on Evolution of Plant-Microbe Interactions . St.-Petersburg: ARRIAM Press, 69. [Google Scholar]
- Maurel C, Verdoucq L, Luu D-T, Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annual Reviews of Plant Biology 59, 595–624. [DOI] [PubMed] [Google Scholar]
- Nedjimi B, Daoud Y. 2009. Cadmium accumulation in Atriplex halimus subsp. Schweinfurthii and its influence on growth, proline, root hydraulic conductivity and nutrient uptake. Flora - Morphology, Distribution, Functional Ecology of Plants 204, 316–324. [Google Scholar]
- Ortega-Villasante C, Rellan-Alvarez R, Del Campo FF, Carpena-Ruiz RO, Hernandez LE. 2005. Cellular damage induced by cadmium and mercury in Medicago sativa . Journal of Experimental Botany 56, 2239–2251. [DOI] [PubMed] [Google Scholar]
- Pascual I, Antolín MC, García C, Polo A, Sánchez-Díaz M. 2004. Plant availability of heavy metals in a soil amended with a high dose of sewage sludge under drought conditions. Biology and Fertility of Soils 40, 291–299. [Google Scholar]
- Perfus-Barbeoch L, Leonhardt L, Vavasseur A, Forestier C. 2002. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant Journal 32, 539–548. [DOI] [PubMed] [Google Scholar]
- Poschenrieder C, Barceló J. 1999. Water relations in heavy metal stressed plants. In: Prasad MNV, Hagemeyer J, eds. Heavy Metal Stress in Plants: From Molecules to Ecosystems . Berlin Heidelberg: Springer-Verlag, 207–229. [Google Scholar]
- Poschenrieder C, Gunse B, Barceló J. 1989. Influence of cadmium on water relations, stomatal resistance and abscisic acid content in expanding bean leaves. Plant Physiology 90, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postaire J, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, Schaffner AR, Maurel C. 2010. PIP1 Aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis . Plant Physiology 152, 1418–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedpelska E, Muszynska A, Polatajko A, Wierzbicka M. 2008. Influence of cadmium stress on aquaporins activity in Arabidopsis halleri (L.) O’Kane & Al-Shehbaz spp halleri cells. In: Metal Ions in Biology and Medicine . Montrouge: John Libbey Eurotext, 804–810. [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. 1988. A monoclonal antibody to (S)-abscisic acid: its characterisation and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta 173, 330–339. [DOI] [PubMed] [Google Scholar]
- Rellan-Alvarez R, Ortega-Villasante C, Alvarez-Fernandez A, del Campo FF, Hernandez LE. 2006. Stress responses of Zea mays to cadmium and mercury. Plant and Soil 279, 41–50. [Google Scholar]
- Savage DF, Stroud RM. 2007. Structural basis of aquaporin inhibition by mercury. Journal of Molecular Biology 368, 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas L, Mistrik I, Huttova J, Haluskova L, Valentovicova K, Zelinova V. 2010. Role of reactive oxygen species-generating enzymes and hydrogen peroxide during cadmium, mercury and osmotic stresses in barley root tip. Planta 231, 221–231. [DOI] [PubMed] [Google Scholar]
- Tazawa M, Asai K, Iwasaki N. 1996. Characteristics of Hg- and Zn-sensitive water channels in the plasma membrane of Chara cells. Botanical Acta 5, 388–396. [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, et al. 2007. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiology 143, 1905–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsyganov VE, Belimov AA, Borisov AY, Safronova VI, Georgi M, Dietz K-J, Tikhonovich IA. 2007. A chemically induced new pea (Pisum sativum L.) mutant SGECdt with increased tolerance to and accumulation of cadmium. Annals of Botany 99, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsyganov VE, Belimov AA, Safronova VI, Naumkina TS, Borisov AY, Dietz K-J, Tikhonovich IA. 2004. A new pea cadmium tolerant mutant is a unique tool for studying molecular plant-microbe interactions under cadmium stress. In: Tikhonovich I, Lugtenberg B, Provorov N, eds. Biology of Plant–Microbe Interactions . St. Paul: IS MPMI, 506–509. [Google Scholar]
- Verdoucq L, Grondin A, Maurel C. 2008. Structure–function analysis of plant aquaporin AtPIP2;1 gating by divalent cations and protons. Biochemical Journal 415, 409–416. [DOI] [PubMed] [Google Scholar]
- Zhu R, Macfie SM, Ding Z. 2005. Cadmium-induced plant stress investigated by scanning electrochemical microscopy. Journal of Experimental Botany 56, 2831–2838. [DOI] [PubMed] [Google Scholar]