Highlight
Comparing plant responses with changes in the availability of either mineral nutrients or water reveals some differences but also similarities, mostly involving hormonal long-distance signalling.
Key words: Mineral nutrients, water deficit, intracellular and long-distance signalling, abscisic acid, auxin, cytokinins, sugars, growth, root architecture.
Abstract
Changes in resource (mineral nutrients and water) availability, due to their heterogeneous distribution in space and time, affect plant development. Plants need to sense these changes to optimize growth and biomass allocation by integrating root and shoot growth. Since a limited supply of water or nutrients can elicit similar physiological responses (the relative activation of root growth at the expense of shoot growth), similar underlying mechanisms may affect perception and acquisition of either nutrients or water. This review compares root and shoot responses to availability of different macronutrients and water. Attention is given to the roles of root-to-shoot signalling and shoot-to-root signalling, with regard to coordinating changes in root and shoot growth and development. Involvement of plant hormones in regulating physiological responses such as stomatal and hydraulic conductance is revealed by measuring the effects of resource availability on phytohormone concentrations in roots and shoots, and their flow between roots and shoots in xylem and phloem saps. More specific evidence can be obtained by measuring the physiological responses of genotypes with altered hormone responses or concentrations. We discuss the similarity and diversity of changes in shoot growth, allocation to root growth, and root architecture under changes in water, nitrate, and phosphorus availability, and the possible involvement of abscisic acid, indole-acetic acid, and cytokinin in their regulation. A better understanding of these mechanisms may contribute to better crop management for efficient use of these resources and to selecting crops for improved performance under suboptimal soil conditions.
Introduction
Plant growth and productivity depend greatly on water and mineral nutrient availability in the soil and their capture by the roots. Arable farmers try to minimize shortfalls in availability through skilled and well-informed management of the soil. Less appreciated is that a better understanding of how plants cope with suboptimal water and mineral nutrient supply can also improve crop management and help select more resilient crop genotypes for the future.
This review integrates hitherto dispersed information on whole-plant responses to changes in water and nutrient supply. It compares root and shoot responses to these changes (first perceived by the root system) and evaluates root-to-shoot signalling mechanisms, which help ensure that shoot behaviour harmonizes with root supply of water and mineral nutrients, especially nitrogen (N) and phosphorus (P). Since similar mechanisms appear to underpin the perception of nutrient or water availability and their acquisition, some unifying principles emerge. We shall successively address the perception of external changes in nitrate, phosphorus, or water by the roots themselves (e.g. gene regulation, root branching and elongation, nutrient and water uptake, and root hydraulic conductivity) and then follow the effects of long-distance root-to-shoot signalling on shoot responses such as those regulating stomatal and growth responses. A much less widely recognized influence of reverse signalling from shoots to roots on root/shoot relationships will also be considered. While it is not always easy to separate local responses from those regulated at the whole-plant level, this succession provides a convenient conceptual framework for analysis.
Intracellular signalling in roots under changing resource availability
This section assesses recent progress in identifying the molecular basis of the initial sensing of changes in nutrient and water supply. Since these early intracellular signalling responses are resource specific (Morcuende et al., 2007), nutrient and water availability are considered separately.
Nitrate
Many studies have addressed responses to nitrate re-supply in previously N-starved plants (see below). Transcriptomic analysis revealed rapid changes (within minutes) in root gene expression after transfer of N-starved plants to nitrate solution (Krouk et al., 2010b ). Primary nitrate responses involve changes in gene expression of enzymes catalysing nitrate reduction and amino acid assimilation, as well as nitrate transporters, such as NRT2 (high-affinity transporter) (Forde, 2002). These primary nitrate responses were detected in nitrate reductase mutants, suggesting direct induction by nitrate itself (Wang et al., 2004). Nitrate receptor function is presently attributed to nitrate transporters (called transceptors due to combined functions of receptor and transporter), with the most convincing evidence of transceptor function being established for the low-affinity nitrate transporter CHL1/NRT1 (Gojon et al., 2011).
Initial upregulation of expression of some genes involved in the primary nitrate response is subsequently followed by their downregulation. Thus, the transcript level of NTR2.1 increased within 30min of exposure to 25mM KNO3, peaked by 3h, and then declined to a steady level (Ho et al., 2009). Applying inhibitors of N assimilation indicated that downregulation of gene expression was mediated by ammonium, glutamine, and other amino acids (Zhuo et al., 1999). Consequently, the effect may be classified as feedback regulation resulting from accumulation of the products of nitrate metabolism. Recent reports have implicated transcription factors LBD37/38/39 (lateral organ boundary domain) in negatively regulating the primary nitrate response (Rubin et al., 2009). Conversely, the transcription factor NLP7 (characterized as a ‘master regulator’ of nitrate-induced responses) and protein kinase CIPK8 interacting with calcineurin B-like protein (CBL) can positively regulate the primary nitrate response (Krapp et al., 2014). How these apparently opposing mechanisms enable adequate uptake of nitrate becomes clear from comparison with its operation under N starvation.
Effects of nitrate starvation have been less studied than its re-supply (De Jong et al., 2014). Expression of Nrt2;1 was persistently upregulated by NO3 starvation in Arabidopsis plants probably due to its release from feedback repression by N metabolites (Lejay et al., 1999). Interestingly, expression of genes for transcription factors LBD37/38/39 that negatively regulate nitrate uptake displayed very low expression under N-limited conditions (Rubin et al., 2009). Furthermore, nlp7 mutants showed a phenotype typical of nitrogen-starved plants, irrespective of N supply, suggesting that NLP7 is required to suppress N-starvation responses (Castaings et al., 2011). Thus, interaction of the factors involved in the N response (Fig. 1) enable adequate nitrate acquisition and metabolism depending on it its level in the root environment.
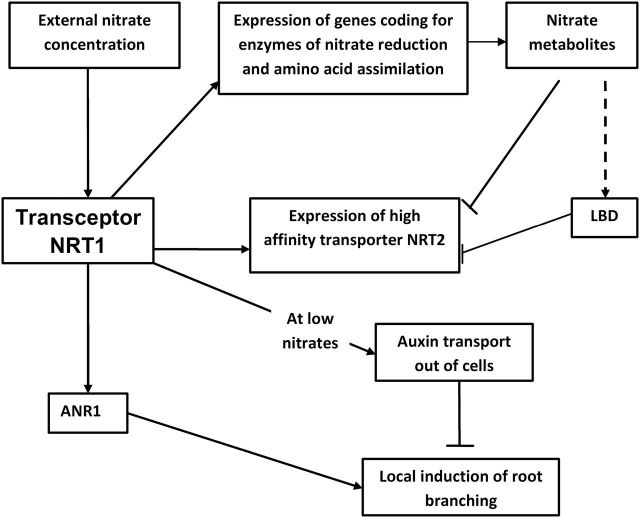
Fig. 1.
Simplified scheme of nitrate signalling. Changes in nitrate concentration are perceived by transceptors (e.g. low-affinity nitrate transporter NRT1), enabling changes in expression of enzymes catalysing nitrate reduction and amino acid assimilation, as well as nitrate transporters, such as NRT2 (high-affinity transporter). Initial upregulation of NRT2 expression following N re-supply to previously N-starved plants is subsequently followed by feedback downregulation resulting from the accumulation of the products of nitrate metabolism. This is probably mediated by transcription factors LBD37/38/39 (lateral organ boundary domain, negative regulator of the primary nitrate response). Gene expression for transcription factors LBD37/38/39 is very low under N-limited conditions. NRT1 acts upstream of ANR1 (a nitrate-regulated member of the MADS-box family of transcription factors), regulating increased root branching under a localized supply of nitrate. At low nitrate concentrations, NRT1 functions as an auxin transporter out of the root tip, thus inhibiting lateral root growth. Lines ending in arrows and bars indicate positive and negative effects, respectively. Dotted lines indicate uncertainty in the response.
The NLA (nitrogen limitation adaptation) gene encoding a ubiquitin ligase is involved in controlling responses to N starvation (Peng et al., 2007). Senescence-mediated nitrogen remobilization does not occur in nla mutants, implicating NLA in nitrogen recycling. Evidence of the control of nitrate uptake by NLA is missing, although recent data suggest its participation in regulating the activity of phosphate transporters (Park et al., 2014; see below).
Phosphorus (P)
Phosphate ([inorganic P (Pi)] starvation responses (PSRs) in plants include regulatory components, in which transcription is either activated or repressed by low Pi, and many downstream target genes (Lei et al., 2011). Reversion of PSRs by Pi re-supply can help identify their primary characteristic (Yang and Finnegan, 2010). Based on homology studies, orthologues of IPS (induced by phosphate starvation non-coding RNA involved in P homeostasis, e.g. regulation of the Pi transporters) were discovered in Arabidopsis and cereal species (Huang et al., 2011). Changes in expression of target genes enable adaptive responses facilitating external Pi acquisition, mobilizing internal and external organic Pi, limiting Pi consumption, and adjusting Pi recycling internally (Lin et al., 2014).
As with nitrate signalling, numerous regulatory components exhibit either negative (e.g. ubiquitin conjugase PHO2) or positive effects (e.g. transcription factor WRKY75 and SPX1 proteins) (Yang and Finnegan, 2010) on PSR. PHO2 was confirmed to be a target gene for the microRNA miR399 having miR399 target sites in the 5-untranslated region of its transcripts (Yang and Finnegan, 2010). The MYB transcription factor PHR1 was the first molecular determinant shown to be required for Pi starvation-dependent responses. Changes in the expression of most of the regulatory components of Pi-starvation signalling, and their target genes, are dependent on PHR1. SUMO E3 ligase SIZ1 acts upstream of PHR1 and enables a control mechanism that acts both negatively and positively on different PSRs (Miura et al., 2005). More recent data show cross-talk between Pi and N response pathways. NLA together with PHO2 destabilize the phosphate transporter PHT1;4 via ubiquitin-mediated proteolysis and maintain the protein at a low level, thus limiting Pi uptake and preventing imbalance between P and N under N starvation (Park et al., 2014). This complicated regulatory network probably co-ordinates numerous processes involved in adaptation of plants to changes in Pi availability. A simplified scheme of PSR is presented in Fig. 2.
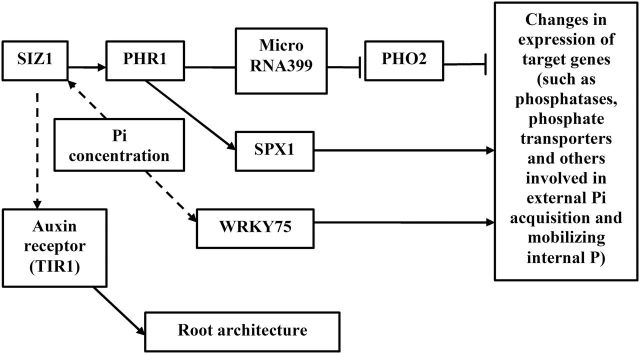
Fig. 2.
Simplified scheme of PSR. Although the presence of P sensors was suggested, their exact position remains unclear. P responses include regulatory components controlling expression of target genes. These regulatory components exhibit either negative (e.g. ubiquitin conjugase PHO2, target gene for miRNA399) or positive (e.g. transcription factor WRKY75 and SPX1 proteins) effects. The MYB transcription factor PHR1 is required for Pi starvation-dependent responses, with SUMO E3 ligase SIZ1 acting upstream of PHR1. SIZ1 may also control the auxin receptor TIR1, thereby modifying the root system architecture. Lines and arrows are as in Fig. 1 legend.
Water
The search for mechanisms sensing water shortage has led to the discovery of several putative osmosensors. Most are analogous to the yeast osmosensor SLN1, activating the mitogen-activated protein kinase (MAPK) cascade (Kumar et al., 2013). The Arabidopsis SLN1 homologue, AtHK1, complemented the salt-sensitive growth defect of yeast sln1/sho1 mutants (Urao et al., 1999). However, direct evidence for a role of AtHK1 as an osmosensor in plants is still missing. Another histidine kinase, CRE1, which was identified as a cytokinin receptor, is also able to complement the yeast sln1 mutant in the presence of cytokinin (Inoue et al., 2001). Although the functional importance of osmotic signalling components was related mostly to stomatal closure (Baxter et al., 2014), an MAPK cascade may be implicated in root osmotic adjustment under water deficit.
While nutrient transporters are the main targets of N and P cellular signalling, the membrane water-channel aquaporins (AQPs) are involved in dehydration responses. Decreased root hydraulic conductance due to reduced AQP activity was detected during the initial phases of water deficit caused by drought (Aroca et al., 2012). Adding the neutral osmolyte polyethylene glycol to the maize root zone inhibits AQPs, thereby decreasing diffusional water transfer after only 10min (Ionenko et al., 2012). Rapid changes in AQP activity may be due to post-transcriptional modifications of water channels (Maurel et al., 2008), thereby altering membrane permeability for water. A correlation between apoplastic water potential and phosphorylation of ZmPIPs and SoPIP2;1 was also demonstrated (Hachez et al., 2012).
In maize, the plasma-membrane AQP genes PIP1;1, PIP1;5 and PIP2;4 were induced after root osmotic adjustment (Zhu et al., 2005). Under these conditions, the decline in AQP activity, which initially prevented water losses by root cells (Steudle, 2000), was no longer necessary, and increased AQP expression could maintain water flow. Thus, rapid and delayed cellular responses in roots can enable adaptation of water relationships to water deficit. A scheme of root responses to osmotic-induced dehydration is presented in Fig. 3.
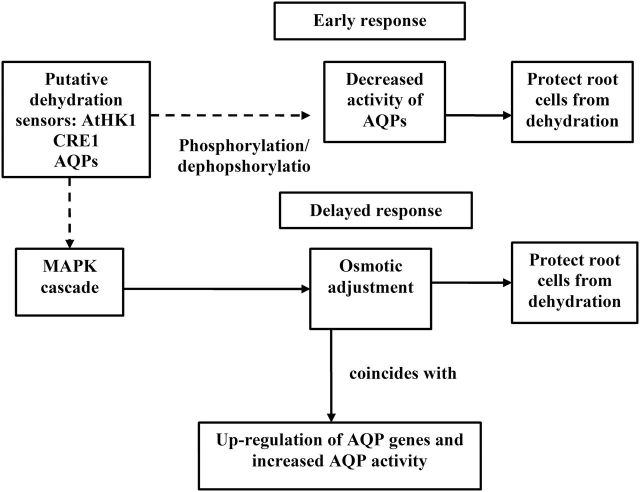
Fig. 3.
Scheme of some root responses to osmotic-induced dehydration. Early and delayed responses are deduced from experiments applying osmotic stress and comprise events occurring within minutes (early) to several days (delayed) (data from Aroca et al., 2012). Dehydration is perceived by putative osmosensors [AtHKT1, CRE, AQPs (a sensor role of AQPs; Hill et al., 2004)]. AtHKT1 and CRE are analogous to the yeast osmosensor SLN1 activating the MAPK cascade. Decreased root hydraulic conductance due to reduced AQP activity (phosphorylation/dephosphorylation events) is an early response to protect cells from dehydration. The MAPK cascade may be implicated in root osmotic adjustment under water deficit, with plasma membrane AQPs genes being induced after root osmotic adjustment (delayed response). Lines and arrows are as in Fig. 1 legend.
Interestingly, P and nitrate supply also change expression of AQP genes and hydraulic conductance (Liu et al., 2008; Calderon-Vazquez et al., 2008).
Thus, although distinct signalling cascades are involved in the response to availability of either water or the main nutrients, a common feature is that they enable fast changes in activity of specific transporters necessary for the capture of resources.
Root architecture: environmental regulation and importance
Many of the mechanisms discussed above were first identified in single-celled microbes (e.g. inhibition of N assimilation by their end products). However, plants possess higher-order mechanisms, due to their more complex organization, that allow the flexible and responsive architecture of organs such as root systems, facilitating acquisition of water and nutrients.
Enhanced root growth (either in absolute terms or relative to shoot growth) is a common response to reduced availability of water, nitrates, and phosphates, although each of these factors influence root elongation and branching in specific ways. Although exact root growth responses are sometimes species specific and also depend on factors other than resource availability (e.g. soil structure), there is agreement concerning optimal root architecture for efficient acquisition of water, nitrate, and phosphate. Typically, root traits enabling ‘topsoil foraging’ enhance Pi acquisition when this element is limiting (Lynch, 2013). This may be related to localization of Pi mainly in the upper soil layers due to low solubility and leaching. This topsoil-foraging ideotype was modelled in hydroponically and agar-grown Arabidopsis plants where roots of low-P-supplied plants were commonly shallower due to inhibition of primary root elongation and stimulation of lateral root formation (Giehl et al., 2014).
In contrast, a ‘steep, cheap, and deep’ ideotype was proposed for optimal acquisition of water and N in deep soil strata (Lynch, 2013). This pattern of root architecture is important for both N and water acquisition, since nitrate is highly soluble and moves deeper in the soil during the growing season, and water availability is typically greater at depth. Furthermore, a low nitrate (0.05mM) supply stimulated primary root elongation (Linkohr et al., 2002; Tian et al., 2005), while primary root growth was maintained under water deficit (Voothuluru and Sharp, 2013). However, the effects of low N on lateral root growth are contradictory, probably dependent on sugar concentration, with high sugar levels inhibiting lateral root growth in N-starved plants (Little et al., 2005).
Inhibition of lateral root development by drought stress is considered an important adaptive response (Xiong et al., 2006). However, moderate soil drying (25% decline in soil water content compared with well-watered plants) accelerated elongation of some lateral roots of maize and wheat plants (Ito et al., 2006). Although longer roots penetrating a deeper soil layer enable water uptake when upper soil layers are drying, a shallow yet extensive root system may allow exploitation of light rainfalls that fail to infiltrate the soil to a great depth (Hodge, 2010). Similarly, a dimorphic root system, having both shallow and deep roots to enable acquisition of mineralized N in the topsoil as well as leached N at depth, is considered advantageous (Richardson et al., 2009). Such a dimorphic root system was detected in plants under local application of fertilizers (placement in bands 15cm apart at 8–10cm depth). Consequently, wheat roots were shorter with more laterals at the site of high nutrient content, while they were longer outside the nutrient-rich patch enabling deeper penetration of the soil, with this pattern of root architecture increasing the drought resistance of plants grown with localized placement of fertilizers under field conditions (Trapeznikov et al., 2003).
Some components of intercellular nutrient signalling are implicated in root growth responses. Thus, ANR1 (a nitrate-regulated member of the MADS-box family of transcription factors) regulated increased root branching under localized supply of nitrate (Zhang and Forde, 1998). Downregulating ANR1 expression prevented lateral root branching in response to localized nitrate supply.
It is clear that changes in root architecture are important for the efficient acquisition of water and nutrients. Since hormones control root growth, discussion of the mechanisms by which they regulate root growth is necessary.
Nutrient and water availability affects root phytohormone status and local hormonal functions
Changes in availability of water and nutrients influence the expression of genes controlling hormone metabolism, intercellular transport and signalling. However, measuring gene expression alone does not always predict the effects on plant hormone concentrations, since the activity of enzymes catalysing both hormone synthesis and decay are frequently elevated simultaneously (e.g. both IPT genes coding for isopentenyltransferase catalysing cytokinin synthesis and those for cytokinin oxidase were upregulated in plants re-supplied with nitrate; Sakakibara et al., 2006). The effects of water and nutrient availability on hormone concentrations (cytokinins, auxin, and abscisic acid) and the involvement of hormones in adaptation to the level of water and nutrients are discussed below in succession.
Cytokinins
Although the importance of cytokinins in responses to N and P starvation have frequently been discussed, many authors do not measure root cytokinin concentrations directly, instead relying on indirect evidence of cytokinin involvement in nutrient deficit responses. Thus, increased cytokinin oxidase expression occurs under Pi starvation (Uhde-Stone et al., 2003), water deficit (Brugiere et al., 2003), and nutrient deficiency (Vysotskaya et al., 2009), implying cytokinin degradation. Furthermore, treatment with exogenous cytokinins can repress expression of AtIPS1 (non-coding RNA, specifically responsive to Pi) and other Pi-starvation-induced genes (Martin et al., 2000), while ahk3 mutant plants deficient in cytokinin perception show reduced repression of several Pi starvation-responsive genes by cytokinins (Franco-Zorrilla et al., 2005). Since exogenous cytokinins inhibit expression of nitrate, Pi, and sulfate transporters, a nutrient-induced increase in cytokinin concentrations has been suggested as a negative-feedback regulation of nutrient uptake (Kiba et al., 2011). Thus, cytokinins seem to be intimately associated with responses to water and nutrient availability and subsequent physiological and morphological adaptations.
An increased cytokinin concentration inhibited root elongation under high N (20 mМ nitrate) (Tian et al., 2005), and exogenous cytokinin (0.5 μM kinetin) inhibited Arabidopsis root elongation (Laplaze et al., 2007). Furthermore, roots in tobacco plants overexpressing a cytokinin oxidase gene were longer (compared with WT plants), thereby increased drought resistance (Werner et al., 2010). Thus, cytokinins are clearly implicated in regulating root growth under changing N and water availability. However, inhibition of root elongation under P starvation (1 μM phosphate) is unlikely to be cytokinin mediated, as low P is presumed to decrease cytokinin concentrations (Franco-Zorrilla et al., 2005). Although indirect evidence suggests that a decrease in cytokinins contributes to the PSR (Martin et al., 2000), further measurements of root tip cytokinin concentrations of Pi-starved plants are required.
Abscisic acid (ABA)
Although numerous studies attribute plant drought responses to increased production of ABA by roots in drying soil (Wilkinson et al., 2012; Puértolas et al., 2013), root ABA concentrations of nutrient-deficient plants are less frequently measured. Although root ABA concentrations increased 2- to 3-fold in Arabidopsis following N deficiency (Balazadeh et al., 2014), only transient increases were detected in barley plants (Brewitz et al., 1995). Dilution of nutrients led to root -tip ABA accumulation in wheat, while root bulk ABA concentrations were similar to those in well-fed plants (Vysotskaya et al., 2008). This diversity of root ABA accumulation responses suggests that other potential regulatory variables (e.g. root water potential, root nutrient status) need investigating. ABA can also influence root hydraulic conductance, due possibly to its effect on AQP activity (Zhu et al., 2005; Maurel et al., 2008). Conversely, the role of nutrient deprivation-induced changes in root hydraulic conductance (discussed above) in mediating root ABA concentrations deserves further attention.
The role of ABA in mediating growth responses to nutrient deficits is less clear, since it does not always accumulate following nutrient starvation (see above). However, increased ABA concentration in the primary root tip maintained its elongation in nutrient-starved wheat (Vysotskaya et al., 2008). Conversely, lateral root growth may be inhibited by ABA, as ABA-deficient Arabidopsis mutants had more lateral roots under osmotic stress (Xiong et al., 2006) and excessively high nitrate (De Smet et al., 2006). Since some reports suggest osmotic stress may mediate the effects of high nitrate concentrations (Roycewicz and Malamy, 2012), future studies should address the possibility that inhibition of lateral root emergence by excessively high nitrates may arise from stimulating ABA accumulation.
Auxin
Auxin is well known for regulating root initiation and lateral root development, and changes in water or nutrient availability can modify auxin concentration, distribution, and signalling. The axr4 mutant failed to respond to a localized nitrate treatment, suggesting an overlap between the auxin- and N-response pathways in regulating lateral root growth (Zhang et al., 1999). Hypothetically, the low-affinity nitrate transporter NRT1 is implicated in the auxin-controlled response of lateral roots to nitrate availability (Krouk et al., 2010a ), with its putative capacity for auxin transport out of the root tip under low nitrate inhibiting lateral root growth. In contrast, the auxin transport activity of NRT1 is inhibited at high nitrate concentration, causing auxin accumulation within lateral root primordia and lateral root growth.
Adding auxin to P-sufficient roots mimicked the effects of P starvation (inhibited primary root growth and increased branching), suggesting involvement in root growth responses to P availability (Ribot et al., 2008). Furthermore, P starvation changed auxin concentrations measured in root apices, zones of initiation of lateral primordia, and elongating lateral roots (Nacry et al., 2005). However, root auxin concentration may not always alter following P starvation, suggesting that P-induced changes in sensitivity to auxin are important, possibly dependent on auxin receptor (TIR1) and the transcription auxin responsive factor (ARF19) (Pérez-Torres et al., 2008).
Data concerning involvement of auxins in responses to water deficit are scarce. In transgenic rice overexpressing the OsGH3-2 gene coding for an enzyme catalysing conjugation of the auxin indole-acetic acid (IAA) to amino acids, IAA content was lower than in wild-type plants, which was accompanied by a reduced number of lateral roots and drought hypersensitivity (Du et al., 2012), contrary to expectation that reduced growth of lateral roots increases drought resistance (Xiong et al., 2006).
Although plant hormone concentrations respond to changes in water and nutrient availability and probably regulate adaptive root reactions, they are also involved in long-distance signalling between roots and shoots (see below).
Long-distance signalling pathways regulate plant responses to the availability of mineral nutrients and water
Shoot-to-root signalling can be important in regulating root responses. For example, nitrate re-supply to N-starved roots initially upregulates expression of the NRT2.1 gene, which is normally downregulated afterwards (Little et al., 2005). However, in split-root experiments, NRT2.1 expression remains elevated in roots supplied with 1mM nitrate, when the remaining roots are N starved (Gansel et al., 2001). Thus, split-root experiments indicate that NRT2.1 expression may be controlled by shoot-to-root signals of N demand or some signals originating from starved roots. A recent report suggested that the small C-terminal-encoded peptide CEP1 serves as a signal emitted from the starved roots (Tabata et al., 2014). In split-root experiments, induction of SEP genes in the root directly experiencing N starvation or its treatment with 1 μM CEP1 was accompanied by upregulation of NRT2.1 in the untreated distant roots.
Another example demonstrating the dependence of the root response on whole-plant nutrient status comes from experiments with Pi-starved plants. Upregulation of the IPS1 gene and genes for Pi transporters and Pi uptake activity in P-starved roots was repressed by Pi supply to other roots (Franco-Zorrilla et al., 2005). It was suggested that part of the root system takes up sufficient nutrients for the whole plant and that corresponding nutrient-starvation response are systemically downregulated in the remaining roots (Martin et al., 2000). Thus, root responses are seemingly regulated by both nutrient supply and demand.
Similarly, the dependence of local root responses on long-distance signalling from other plant parts can regulate root branching. Although supplying 12mM nitrate to parts of the root system stimulated lateral root growth, branching was inhibited when the same concentration was supplied to the whole root system (Scheible et al., 1997). It was suggested that nitrate regulates root branching both locally and systemically: its external presence stimulates lateral root initiation or/and elongation of those roots in direct contact with nitrate, while endogenous plant nitrate concentrations (above a certain threshold) inhibit lateral root elongation (Forde, 2002).
To understand systemic whole-plant responses to nutrient and water availability, it is important to identify signals originating from roots that can be transferred to shoots and then back to the roots.
Nutrients and hydraulic long-distance signalling
Nutrients themselves are the most obvious candidates for the role of root-to-shoot signals of their own availability. Shoot gene expression (about 300 genes) changed 20min after nitrate re-supply to the roots (Castaings et al., 2011), suggesting direct effects of nitrate signalling prior to nitrate assimilation in the roots.
Although it was considered that nitrate ions are not phloem mobile, and hence cannot inform roots of shoot N demand (Imsande and Touraine, 1994), more recent evidence indicates phloem nitrate transport to ensure nitrate remobilization to sink tissues (Krapp et al., 2014). Consequently, either nitrates or their metabolites may serve as long-distance systemic signals indicating shoot N status to the roots and regulating root responses such as inhibiting nitrate uptake. However, in hni mutants affected in systemic feedback repression of root nitrate uptake by shoot N status, phloem amino acid concentrations were inversely correlated with repression of the nitrate transporter NRT2.1 (Girin et al., 2010), suggesting that amino acids are not systemic signals.
Pi is both xylem and phloem mobile, and decreased basipetal Pi flow may act as a long-distance signal communicating information on shoot P status to the roots (Lin et al., 2014). Under P starvation, leaf growth inhibition was attributed to low root P export and shoot accumulation (Amtmann et al., 2006). However plants underexpressing PHO1 (a gene participating in Pi transport from roots to shoots) maintained their shoot growth comparable to a Pi-sufficient wild-type plant, despite their low Pi content, suggesting that leaf Pi content is not the only growth-regulating factor (Rouached et al., 2011).
In split-root experiments, when some roots were P starved, phosphate from Pi-replete roots was proposed to act as a signal suppressing P starvation induced genes in the P-starved roots. However downregulation of the Mt4 gene (belonging to the Mt4/TPSI1 family, widely used as molecular indicators of Pi starvation) occurred before increased P levels sourced from P-replete roots, suggesting that phosphate is not the systemic signal (Dinant and Suárez-López, 2012).
Stomatal closure in response to soil water deficit is a well-studied example of long-distance root-to-shoot signalling seemingly related to hydraulic signalling. Nevertheless, although stomata can close in response to hydraulic signals (Kudoyarova et al., 2013, and references therein), stomatal closure of plants exposed to partial root zone drying, when leaf water potential was maintained, suggests the action of chemical signals exported from roots in drying soil rather than hydraulic signalling (Blackman and Davies, 1983) (see below).
Hydraulic signals are important not only for adaptation to water availability but also for nutrient uptake, since transpiration-driven ‘mass flow’ of soil water can increase nutrient flow to the root surface (Cramer et al., 2009). Root hydraulic conductance increased rapidly in the presence of locally increased nitrate availability contributing to water uptake by roots in nitrate-rich soil, and it was suggested that nitrate concentration was translated into a hydraulic signal transmitted rapidly throughout the plant (Gorska et al., 2008). Furthermore, decreased root hydraulic conductance in response to P (Radin and Eidenbock 1984) and N (Dodd et al., 2002) starvation can decrease leaf turgor, thereby inhibiting leaf growth and causing stomatal closure. Nevertheless, experiments that maintained leaf turgor following nutrient deprivation (by applying a pneumatic pressure to the roots) failed to sustain leaf growth, suggesting that leaf water relationships are not the primary limiting factor (Dodd et al., 2002), and that other signals (other than water and nutrients) are important regulators of shoot responses.
ABA and cytokinins as long-distance signals
Nitrate re-supply to N-starved plants rapidly stimulates root cytokinin biosynthesis and xylem export to the shoots. Furthermore, the ability of cytokinins to regulate expression of at least some nitrate-inducible genes suggests that these hormones are important in nitrate signalling (Sakakibara et al., 2006). In contrast, diluting the nutrient solution (including a 10-fold decrease in nitrate concentration) had no effect on root cytokinin export (Vysotskaya et al., 2009). Nevertheless, nutrient shortage decreased shoot cytokinin concentrations, which was attributed to cytokinin oxidase activation. Applying fluridone (an ABA biosynthesis inhibitor) to nutrient-starved plants alleviates both the increase in shoot cytokinin oxidase enzyme activity and decreased shoot cytokinin content (Vysotskaya et al., 2009), suggesting ABA mediation of shoot cytokinin status, which may be common to both nutrient deprivation and soil drying (Davies et al., 2005; Kudoyarova et al., 2007).
Enhanced xylem ABA export from roots when the soil is allowed to dry can precede any decrease in leaf water status (Zhang and Davies, 1989; Puértolas et al., 2013). The importance of ABA export from roots in mediating drought long-distance signalling was demonstrated by experiments showing increased foliar ABA concentrations when a greater root biomass was exposed to partially dry soil, independent of any changes in leaf water relationships (Martin-Vertedor and Dodd, 2011).
ABA accumulation in response to nutrient starvation depends on the nutrient dynamics. Abrupt withdrawal of the nitrate supply increased the xylem sap ABA concentration (Dodd et al., 2003), whereas gradual nitrate depletion did not affect it (Palmer et al., 1996). Alternatively, gradual P depletion increased the root xylem sap ABA concentration and foliar ABA concentration by 6- and 2-fold, respectively (Jeschke et al., 1997). Resolving the impacts of specific nutrient deficiencies, and the rate of stress imposition, on root ABA and cytokinin status and subsequent xylem export deserves further attention if plant responses to multiple abiotic stresses are to be predicted.
Changes in hormone export from roots and their shoot concentration are involved in regulating shoot responses to availability of soil resources. While stomatal closure in response to soil drying has mainly been associated with increased xylem ABA concentrations (Davies et al., 2005), decreased shoot cytokinin concentration and export from the root system may also be important (Kudoyarova et al., 2007). Cytokinins can directly influence stomatal opening and counteract ABA-induced stomatal closure (Davies et al., 2005; Dodd 2005). A new insight is that cytokinin-induced upregulation of nitrate transporters in leaves (Kiba et al., 2011) may be related to stomatal opening, as the nitrate transporter NRT1 regulates nitrate accumulation in guard cells (Castaings et al., 2011). Whether guard-cell nitrate status modulates local cytokinin accumulation (and possible guard-cell autonomous regulation in response to shoot nutrient status) should be assessed.
Alternatively, stomatal closure in response to nutrient starvation was correlated with increased stomatal sensitivity to ABA (Jeschke and Hartung, 2000), which may occur in stressed plants due to apoplastic alkalization (Wilkinson and Davies, 2008). Under increased pH, weak acids such as ABA dissociate and cannot pass through the plasmalemma, causing apoplastic accumulation and stomatal closure after binding to external-facing receptors in the plasmalemma (Hartung 1983). Stomatal closure in response to apoplastic ABA may be ascribed to the guanine nucleotide-binding protein-coupled receptor GCR1 (one of multiple ABA-perception sites located in the plasma membrane and positively regulating ABA signalling; Wang and Zhang, 2008). Nitrate uptake by anion/proton symport systems (Santi et al., 2003) explains apoplastic alkalization at high nitrate concentrations (Wilkinson and Davies, 2008). Xylem sap alkalization in response to nitrate deprivation may also cause stomatal closure independent of any increase in xylem ABA concentration, although the mechanisms of pH changes in this case are unclear (Dodd et al., 2003).
Thus, changes in stomatal conductance related to root cytokinin and ABA export and shoot accumulation, as well as stomatal sensitivity to these hormones, present a good example of the common response to availability of water and nutrients regulating water balance and nutrient flow into the plants.
Hormonal signalling is also important for the control of shoot growth in response to changes in availability of resources. Independent of the source of nitrate-induced foliar cytokinin accumulation (upregulation of foliar IPT gene expression or translocation of root-synthesized cytokinins to shoots), their elevated concentration is likely to mediate both cell division and elongation (since both processes are inhibited in shoots of cytokinin deficient plants; Werner et al., 2003). Although diluting the nutrient medium of hydroponically grown plants decreased both leaf cytokinin concentration and leaf growth (Vysotskaya et al., 2009), exogenous cytokinin supply prevented shoot growth inhibition (Kuiper et al., 1989).
Short-term experiments (1–2 weeks) with partial root zone drying have also emphasized the importance of root-to-shoot chemical signals in regulating leaf growth. Decreased foliar cytokinin concentrations following partial root zone drying (Kudoyarova et al., 2007) probably contributed to decreased leaf growth by decreasing cell division and expansion. Thus, as with transpiration, similar increases of ABA and decreases of cytokinins in response to water deficit and nutrient starvation may limit shoot growth.
Recirculation of cytokinins and ABA in plants is important for plant adaptation to availability of resources. Basipetal phloem cytokinin transport can inhibit nutrient-starvation-induced upregulation of transporter and other gene responses in roots (Martin et al., 2000; Franco-Zorrilla et al., 2005; Kiba et al., 2011). However, attempts to inhibit P-starvation responses by supplying cytokinins to part of the roots resulted only in local, and not systemic, effects (starvation responses still occurred in roots that were not treated with cytokinins; Franco-Zorrilla et al., 2005). Nevertheless, manipulating cytokinin concentrations in distant plant organs may be difficult. Split-root experiments demonstrated that cytokinins accumulated only in treated (local cytokinin application) roots, and their content did not change in untreated roots (Kudoyarova et al., 2014a ). In roots treated with exogenous zeatin, cytokinin transport to the shoots was prevented due to their active uptake by root cells (Kudoyarova et al., 2014b ). This may explain the absence of a systemic response of Pi-starved plants to exogenous cytokinins but does not imply that changes in endogenous cytokinins do not serve as long-distance signals.
The importance of ABA transported from shoots for root adaptation to foliar water deficit (induced by high atmospheric evaporative demand) was shown by inhibiting phloem transport by cooling the shoot base, which restricted root ABA accumulation and thus root hydraulic conductance (Kudoyarova et al., 2011). Although it was suggested that an ABA-dependent signalling pathway operates within the developing lateral root primordium (Walch-Liu et al., 2006), long-distance ABA signalling cannot be excluded, since this hormone is phloem mobile (Jeschke and Hartung, 2000), and changes in the availability of mineral nutrients influence phloem transport of ABA (Vysotskaya et al., 2008).
Auxins
Although auxin concentration and sensitivity may be modified in roots themselves participating in local root growth responses (see above), auxin transport from the shoot into the root controls lateral root development (Reed et al., 1998). Auxin concentration in phloem sap and roots was lower in maize supplied with high nitrate, and was correlated with reduced root growth (Tian et al., 2008). Furthermore, the release from systemic inhibition of lateral root emergence detected after transfer of Arabidopsis plants from high to low nitrate medium was accompanied by increased root auxin concentrations (Walch-Liu et al., 2006). Consequently, auxin transport from shoots to roots may well be important for adaptive systemic root growth responses to availability of soil resources.
Thus, alongside ABA and cytokinins discussed above, auxins transported from shoots to roots through the phloem are probable systemic signals integrating whole-plant responses to mineral and water deficiency, although further study is necessary to integrate what is known about the individual hormones. One example of such integration is the capacity of cytokinins to influence root responses (such as lateral root emergence) indirectly by affecting phloem transport of auxins from shoots to roots (Hachiya et al., 2014).
Sugars
Under water or nutrient deficits, sugars accumulate in leaves and roots (De Jong et al., 2014; Lin et al., 2014), since photosynthesis is usually less affected than shoot growth (Pinheiro and Chaves, 2011). This linkage of shoot growth retardation and accumulation of sugars is clearly outlined in the case of water deficit (Pinheiro and Chaves, 2011) but the same also should be true for nutrient starvation. Decreased use of sugars in cell-wall synthesis of growing cells is likely to make an important contribution to sugar accumulation in nutrient- and water-starved plants, as may changes in the enzyme activities involved in sugar- and starch-related pathways (De Jong et al., 2014; Lin et al., 2014).
Higher apoplastic sugar accumulation may be related to decreased stomatal conductance under low N. It was suggested that accumulated sucrose is carried towards the stomata by the transpiration stream and stimulates stomatal closure via hexokinase (Granot et al., 2014).
Accumulated sugars are available for transport to roots to support their growth. Transport is favoured by the increased sugar concentration at the source end of the phloem, elevating the pressure gradient driving carbohydrate transport in sieve tubes, especially under drought (Sevanto, 2014), but this also should apply to P- and N-starvation responses accompanied by sugar accumulation.
Apart from being a substrate for root growth, sugar transported from the shoots also acts as a signal controlling the activity of nutrient transporters (Lejay et al., 2008) and root branching (Jain et al., 2007). Sucrose was suggested to effect lateral root growth via regulation of auxin transport from shoot to root (Jain et al., 2007). NRT2 gene expression is regulated diurnally, and decreased expression during the dark period is reversed by supplying the roots with sucrose (Lejay et al., 1999). Expression of many transporter genes is hexokinase dependent (Lejay et al., 2008). Alongside hexokinase-mediated stimulation of NRT2 expression, some glucose metabolites activate nitrate transport through putative post-transcriptional modification of transporters independently of hexokinase signalling (De Jong et al., 2014).
Impaired phloem loading of sucrose in pho3 mutants leads to attenuated P-deficiency responses (compared with wild-type plants) (Zakhleniuk et al., 2001). Thus, sucrose translocation in the phloem meets the criteria for a causal intermediary signal linking P availability to adaptive root responses (Hammond and White, 2008).
Collectively these results suggest that sucrose transported from the shoot via the phloem contributes to adaptive responses in roots to availability of nutrients and water.
Conclusions
Comparing plant responses with changes in the availability of either mineral nutrients or water reveals some differences (mainly the specific molecular mechanisms involved in root perception of different stresses; Figs 1–3) but also similarities, mostly involving hormonal long-distance signalling (Fig. 4).
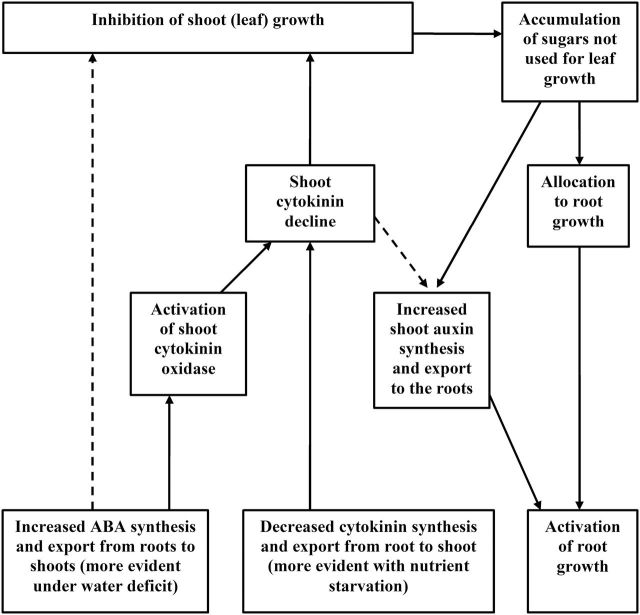
Fig. 4.
Scheme showing similar whole-plant effects of water and nutrient deficits. Decreased resource availability generally increases root-to-shoot ABA export and/or decreases cytokinin export. Furthermore, shoot ABA accumulation can decrease shoot cytokinin concentration by activating cytokinin oxidase. These changes in shoot hormone content inhibit leaf growth (more rapidly and to a greater extent than photosynthesis), causing foliar sugar accumulation and its transport to roots (probably controlled by cytokinin decline) due to decreased demand of sink leaves. Sugars act as both substrates for root growth and a signal controlling auxin transport from shoots to roots and its distribution in the roots.
Decreased resource availability generally increases root-to-shoot ABA export and/or decreases cytokinin export. Furthermore, shoot ABA accumulation can decrease shoot cytokinin concentration by activating cytokinin oxidase. These changes in shoot hormone content inhibit leaf growth (more rapidly and to a greater extent than photosynthesis), causing foliar sugar accumulation and its transport to roots due to the decreased demand of sink leaves. Sugars act both as substrate for root growth and a signal controlling auxin transport from shoots to roots and its distribution in the roots (Fig. 4). However, future experiments need to discriminate the effects of exogenous (supplied in vitro) and endogenous sugar concentrations. The importance of cross-talk between sugar and hormonal signalling for adaptation to resource availability has not been addressed sufficiently and should be studied in the future.
Although allocation to root growth is a typical response to the shortage of nitrate, phosphate, and water, assimilates delivered to the roots are differentially distributed enabling their efficient uptake. Auxins are believed to be involved in root architectural changes, but more information is needed to understand how subtle changes in auxin distribution between root cells are achieved under different resource limitations. Unfortunately, the DR5 reporter construct, frequently used to study auxin cellular signalling, does not distinguish between changes in cellular auxin concentration and their sensitivity to auxin (e.g. Pérez-Torres et al., 2008; Krouk et al., 2010a ). The use of antibodies allows the distribution and concentrations of auxins, ABA, and cytokinins to be measured in the same root sections via immunolocalization (Vysotskaya et al., 2007; Kudoyarova et al., 2014a ). This may contribute to a better understanding of the control of root architecture and may allow the design of better root systems to minimize resource limitations when fertilizers or water are in scarce supply.
An improved understanding of the processes affecting resource acquisition should contribute to increasing crop yield and food security. Further studies of the hormonal relationships of transgenic lines knocked out in their primary sensing mechanisms of nutrient availability (e.g. lbd; Rubin et al., 2009) may indicate interactions between intra- and intercellular signalling. Transcription factors involved in nutrient signalling (such as LBD37/38/39, NLP7, WRKY75, and PHR1 mentioned above) may affect phytohormone levels or their transport (which was suggested for LBD by Rubin et al., 2009), but confirmation has not been forthcoming. Future progress in crop improvement in suboptimal environments may depend on attempts to couple more tightly intercellular and long-distance signalling of resource availability.
Acknowledgements
The work was funded by the Ministry of Education and Science (N 01201456413) and Russian Foundation for Basic Research (N 12-04-01111, N 15-04-04750 and N 14-04-97077).
Glossary
Abbreviations:
- ABA
abscisic acid
- AQP
aquaporin
- IAA
indole-acetic acid
- MAPK
the mitogen-activated protein kinase
- N
nitrogen
- P
phosphorus
- Pi
inorganic phosphate
- PSR
phosphate starvation responses.
References
- Amtmann A, Hammond JP, Armengaud P, White PJ. 2006. Nutrient sensing and signalling in plants: potassium and phosphorus. Advances in Botanical Research 43, 209–257. [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM. 2012. Regulation of root water uptake under abiotic stress conditions. Journal of Experimental Botany 63, 43–57. [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Schildhauer J, Araujo WL, Munne-Bosch S, Fernie AR, Proost S, Humbeck K, Mueller-Roeber B. 2014. Reversal of senescence by N resupply to N-starved Arabidopsis thaliana: transcriptomic and metabolomic consequences. Journal of Experimental Botany 65, 3975–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signalling. Journal of Experimental Botany 65, 1229–1240. [DOI] [PubMed] [Google Scholar]
- Blackman PG, Davies WJ. 1983. The effects of cytokinins and ABA on stomatal behaviour of maize and Commelina . Journal of Experimental Botany 34, 1619–1626. [Google Scholar]
- Brewitz E, Larsson C-M, Larsson M. 1995. Influence of nitrate supply on concentrations and translocation of abscisic acid in barley (Hordeum vulgare). Physiologia Plantarum 95, 499–506. [Google Scholar]
- Brugiere N, Jiao S, Hantke S, Zinselmeier C, Roessler JA, Niu X, Jones RJ, Habben JE. 2003. Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiology 132, 1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Vazquez C, Ibarra-Laclette E, Caballero-Perez J, Herrera-Estrella L. 2008. Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant and species specific levels. Journal of Experimental Botany 59, 2479–2497. [DOI] [PubMed] [Google Scholar]
- Castaings L, Marchive C, Meyer C, Krapp A. 2011. Nitrogen signaling in Arabidopsis: how to obtain insights into a complex signalling network. Journal of Experimental Botany 62, 1391–1397. [DOI] [PubMed] [Google Scholar]
- Cramer MD, Hawkins H-J, Verboom GA. 2009. The importance of nutritional regulation of plant water flux. Oecologia 161, 15–24. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Kudoyarova G, Hartung W. 2005. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. Journal of Plant Growth Regulation 24, 285–295. [Google Scholar]
- De Jong F, Thodey K, Lejay LV, Bevan MW. 2014. Glucose elevates nitrate transporter 2.1 protein levels and nitrate transport activity independently of its hexokinase1-mediated stimulation of nitrate transporter 2.1 expression. Plant Physiology 164, 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Zhang H, Inze D, Beeckman T. 2006. A novel role for abscisic acid emerges from underground. Trends in Plant Sciences 11, 434–439. [DOI] [PubMed] [Google Scholar]
- Dinant S, Suárez-López P. 2012. Multitude of long-distance signal molecules acting via phloem. In: Witzany G, Baluska F, eds. Biocommunication of plants. New York: Springer, 89–121. [Google Scholar]
- Dodd IC. 2005. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant and Soil 274, 251–270. [Google Scholar]
- Dodd I, Munns R, Passioura J. 2002. Dose shoot water status limit leaf expansion of nitrogen deprived barley. Journal of Experimental Botany 53, 1765–1770. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Tan LP, He J. 2003. Do increases in xylem sap pH and/or ABA concentration mediate stomatal closure following nitrate deprivation? Journal of Experimental Botany 54, 1281–1288. [DOI] [PubMed] [Google Scholar]
- Du H, Wu N, Fu J, Wang S, Li X, Xiao J, Xiong L. 2012. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. Journal of Experimental Botany 63, 6467–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG. 2002. Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology 53, 203–224. [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Martin AC, Leyva A, Paz-Ares J. 2005. Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiology 138, 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansel X, Munos S, Tillard P, Gojon A. 2001. Differential regulation of the NO3 and NH4 + transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. The Plant Journal 26, 143–155 [DOI] [PubMed] [Google Scholar]
- Giehl RFH, Giehl RFH, Wiren N. 2014. It’s time to make changes: modulation of root system architecture by nutrient signals. Journal of Experimental Botany 65, 769–778. [DOI] [PubMed] [Google Scholar]
- Girin T, El-Kafafi S, Widiez T, Erban A, Hubberten HM, Kopka J, Hoefgen R, Gojon A, Lepetit M. 2010. Identification of Arabidopsis mutants impaired in the systemic regulation of root nitrate uptake by the nitrogen status of the plant. Plant Physiology 153, 1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojon A, Krouk G, Perrine-Walker F, Laugier E. 2011. Nitrate transceptor(s) in plants. Journal of Experimental Botany 62, 2299–2308. [DOI] [PubMed] [Google Scholar]
- Gorska A, Ye Q, Holbrook NM, Zwieniecki MA. 2008. Nitrate control of root hydraulic properties in plants: translating local information to whole plant response. Plant Physiology 148, 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot D, Kelly G, Stein O, David-Schwartz R. 2014. Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. Journal of Experimental Botany 65, 809–819. [DOI] [PubMed] [Google Scholar]
- Hachez C, Veselov D, Ye Q, Reinhardt H, Knipfer T, Fricke W, Chamout F. 2012. Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant, Cell & Environment 35, 185–198. [DOI] [PubMed] [Google Scholar]
- Hachiya T, Sugiura D, Kojima M, Sato S, Yanagisawa S, Sakakibara H, Terashima I, Noguchi K. High. 2014. CO2 triggers preferential root growth of Arabidopsis thaliana via two distinct systems under low pH and low N stresses. Plant Cell Physiology 55, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JP, White PJ. 2008. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany 59, 93–109. [DOI] [PubMed] [Google Scholar]
- Hartung W. 1983. The site of action of abscisic acid at the guard cell plasmalemma of Valerianella locusta . Plant, Cell & Environment 6, 427–428. [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. 2009. CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194. [DOI] [PubMed] [Google Scholar]
- Hodge A. 2010. Roots: the acquisition of water and nutrients from the heterogeneous soil environment. Progress in Botany 71, 307–337. [Google Scholar]
- Huang CY, Shirley N, Genc Y, Shi BJ, Langridge P. 2011. Phosphate utilization efficiency correlates with expression of low affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiology 156, 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J, Touraine B. 1994. Demand and the regulation of nitrate uptake. Plant Physiology 105, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. 2001. Identification of CRE1 as a cytokinin receptor from Arabidopsis . Nature 409, 1060–1063. [DOI] [PubMed] [Google Scholar]
- Ionenko IF, Dautova NR, Anisimov AV. 2012. Early changes of water diffusional transfer in maize roots under the influence of water stress. Environmental and Experimental Botany 76, 16–23. [Google Scholar]
- Ito K, Tanakamaru K, Morita S, Abe J, Inanaga S. 2006. Lateral root development, including responses to soil drying, of maize (Zea mays) and wheat (Triticum aestivum) seminal roots. Physiologia Plantarum 127, 260–267. [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. 2007. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis . Plant Physiology 144, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD, Hartung W. 2000. Root–shoot interactions in mineral nutrition. Plant and Soil 226, 57–69. [Google Scholar]
- Jeschke WD, Kirkby EA, Peuke AD, Pate JS, Hartung W. 1997. Effects of P deficiency on assimilation and transport of nitrate and phosphate in intact plants of castor bean (Ricinus communis L.). Journal of Experimental Botany 48, 75–91. [Google Scholar]
- Kiba T, Kudo T, Kojima M, Sakakibara H. 2011. Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. Journal of Experimental Botany 62, 1399–1409. [DOI] [PubMed] [Google Scholar]
- Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince AS, Chaillou S, Ferrario-Mйry S, Meyer C, Daniel-Vedele F. 2014. Nitrate transport and signalling in Arabidopsis . Journal of Experimental Botany 65, 789–798. [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. 2010. a . Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18, 927–937. [DOI] [PubMed] [Google Scholar]
- Krouk G, Mirowski P, LeCun Y, Shasha DE, Coruzzi GM. 2010. b . Predictive network modeling of the high-resolution dynamic plant transcriptome in response to nitrate. Genome Biology 11, R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoyarova G, Veselova S, Hartung W, Farhutdinov R, Veselov D, Sharipova G. 2011. Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 233, 87–94 [DOI] [PubMed] [Google Scholar]
- Kudoyarova GR, Kholodova VP, Veselov DS. 2013. Current state of the problem of water relations in plants under water deficit. Russian Journal of Plant Physiology 60, 165–175. [Google Scholar]
- Kudoyarova GR, Korobova AV, Akhiyarova GR, Arkhipova TN, Zaytsev DY, Prinsen E, Egutkin NL, Medvedev SS, Veselov SY. 2014. b . Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). Journal of Experimental Botany 65, 2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoyarova GR, Melentiev AI, Martynenko EV, Arkhipova TN, Shendel GV, Kuz’mina LY, Dodd IC, Veselov SY. 2014. a . Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiology and Biochemistry 83, 285–291. [DOI] [PubMed] [Google Scholar]
- Kudoyarova GR, Vysotskaya LB, Cherkozyanova A, Dodd IC. 2007. Effect of partial rootzone drying on the concentration of zeatintype cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. Journal of Experimental Botany 58, 161–168. [DOI] [PubMed] [Google Scholar]
- Kuiper D, Kuiper P J C, Lambers H, Schuit J, Staal M. 1989. Cytokinin concentration in relation to mineral nutrition and benzyladenine treatment in Plantago major ssp. pleiosperma Physiologia Plantarum 75, 511–517.
- Kumar MN, Jane WN, Verslues PE. 2013. Role of the putative osmosensor arabidopsis Histidine Kinase1 in dehydration avoidance and low-water-potential response. Plant Physiology 161, 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, et al. 2007. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19, 3889–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Zhu C, Liu Y, Karthikeyan AS, Bressan RA, Raghothama KG, Liu D. 2011. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis . New Phytologist 189, 1084–1095. [DOI] [PubMed] [Google Scholar]
- Lejay L, Tillard P, Lepetit M, Olive F, Filleur S, Daniel-Vedele F, Gojon A. (1999). Molecular and functional regulation of two NO3-uptake systems by N- and C-status of Arabidopsis plants. The Plant Journal 18, 509–519 [DOI] [PubMed] [Google Scholar]
- Lejay L, Wirth J, Pervent M, Cross JMF, Tillard P, Gojon A. 2008. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiology 146, 2036–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Huang TK, Leong SJ, Chiou TJ. 2014. Long-distance call from phosphate: systemic regulation of phosphate starvation responses. Journal of Experimental Botany 65, 1817–1827. [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. 2002. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis . The Plant Journal 29, 751–760. [DOI] [PubMed] [Google Scholar]
- Little YD, Rao H, Oliva S, Daniel-Vedel F, Krapp A, Malamy JE. 2005. The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proceedings of the National Academy Sciences, USA 102, 13693–13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Han L, Chen F, Bao J, Zhang F. 2008. Microarray analysis reveals early responsive genes possibly involved in localized nitrate stimulation of lateral root development in maize (Zea mays L.). Plant Science 175, 272–282. [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, del Pozo JC, Iglesias J, Rubio V, Solano R, de la Pena A, Leyva A, Paz-Ares J. 2000. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis . The Plant Journal , 24 559–568. [DOI] [PubMed] [Google Scholar]
- Martin-Vertedor AI, Dodd IC. 2011. Root-to-shoot signalling when soil moisture is heterogeneous: increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf ABA concentration. Plant, Cell & Environment 34, 1164–1175. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology 59, 595–624. [DOI] [PubMed] [Google Scholar]
- Miura K, Rus A, Sharkhuu A, et al. 2005. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proceedings of the National Academy of Sciences, USA 102, 7760–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, et al. , 2007. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant, Cell & Environment 30, 85–112. [DOI] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Onckelen HV, Rossignol M, Doumas P. 2005. A role for auxin redistribution in the response of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiology 138, 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SJ, Berridge DM, McDonald AJS, Davies WJ. 1996. Control of leaf expansion in sunflower (Helianthus annuus L.) by nitrogen nutrition. Journal of Experimental Botany 47, 359–368. [Google Scholar]
- Park BS, Seo JS, Chua N-H. 2014. Nitrogen limitation adaptation recruits phosphate to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 26, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Hannam C, Gu H, Bi Y, Rothstein SJ. 2007. A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. The Plant Journal 50, 320–337. [DOI] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. 2008. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20, 3258–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. 2011. Photosynthesis and drought: can we make metabolic connections from available data? Journal of Experimental Botany 62, 869–882. [DOI] [PubMed] [Google Scholar]
- Puértolas J, Alcobendas R, Alarcón J, Dodd IC. 2013. Long-distance abscisic acid signalling under different vertical soil moisture gradients depends on bulk root water potential and average soil water content in the root zone. Plant, Cell & Environment 36, 1465–1475. [DOI] [PubMed] [Google Scholar]
- Radin JW, Eidenbock MP. 1984. Hydraulic conductance as a factor limiting leaf expansion of phosphorus-deficient cotton. Plant Physiology 75, 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. 1998. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis . Plant Physiology 118, 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot C, Wang Y, Poirier Y. 2008. Expression analyses of three members of the AtPHO1 family reveal differential interactions between signaling pathways involved in phosphate deficiency and the responses to auxin, cytokinin, and abscisic acid. Planta 227, 1025–1036. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C. 2009. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil 321, 305–339. [Google Scholar]
- Rouached H, Stefanovic A, Secco D, Bulak Arpat A, Gout E, Bligny R, Poirier Y. 2011. Uncoupling phosphate deficiency from its major effects on growth and transcriptome via PHO1 expression in Arabidopsis . The Plant Journal 65, 557–570. [DOI] [PubMed] [Google Scholar]
- Roycewicz P, Malamy JE. 2012. Dissecting the effects of nitrate, sucrose and osmotic potential on Arabidopsis root and shoot system growth in laboratory assays. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR. 2009. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis . Plant Cell 21, 3567–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H, Takei K, Hirose N. 2006. Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends in Plant Science 11, 440–448. [DOI] [PubMed] [Google Scholar]
- Santi S, Locci G, Monte R, Pinton R, Varanini Z. 2003. Induction of nitrate uptake in maize roots: expression of a putative high-affinity nitrate transporter and plasma membrane H+-ATPase isoforms. Journal of Experimental Botany 54, 1851–1864. [DOI] [PubMed] [Google Scholar]
- Scheible WR, Lauerer M, Schulze ED, Caboche M, Stitt M. 1997. Accumulation of nitrate in the shoot acts as a signal to regulate shoot–root allocation in tobacco. The Plant Journal 11, 671–691. [Google Scholar]
- Sevanto S. 2014. Phloem transport and drought. Journal of Experimental Botany 65, 1751–1759. [DOI] [PubMed] [Google Scholar]
- Steudle E. 2000. Water uptake by roots: effects of water deficits. Journal of Experimental Botany 51, 1531–1542. [DOI] [PubMed] [Google Scholar]
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y. 2014. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346, 343–346. [DOI] [PubMed] [Google Scholar]
- Tian Q, Chen F, Liu J, Zhang F, Mi G. 2008. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. Journal of Plant Physiology 165, 942–951. [DOI] [PubMed] [Google Scholar]
- Tian Q, Chen F, Zhang F, Mi G. 2005. Possible involvement of cytokinin in nitrate-mediated root growth in maize. Plant Soil 277, 185–196. [Google Scholar]
- Trapeznikov VK, Ivanov II, Kudoyarova GR. 2003. Effect of heterogeneous distribution of nutrients on root growth, ABA content and drought resistance of wheat plants. Plant and Soil 252, 207–214. [Google Scholar]
- Uhde-Stone C, Zinn KE, Ramirez-Yanez M, Li A, Vance CP, Allan DL. 2003. Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiology 131, 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, Shinozaki K. 1999. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell 11, 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voothuluru P, Sharp RE. 2013. Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress. I. Increased levels are specific to the apical region of growth maintenance. Journal of Experimental Botany 64, 1223–1233. [DOI] [PubMed] [Google Scholar]
- Vysotskaya LB, Korobova AV, Kudoyarova GR. 2008. Abscisic acid accumulation in the roots of nutrient-limited plants: its impact on the differential growth of roots and shoots. Journal of Plant Physiology 165, 1274–1279. [DOI] [PubMed] [Google Scholar]
- Vysotskaya LB, Korobova AV, Veselov SY, Dodd IC, Kudoyarova GR. 2009. ABA mediation of shoot cytokinin oxidase activity: assessing its impacts on cytokinin status and biomass allocation of nutrient deprived durum wheat. Functional Plant Biology 36, 66–72. [DOI] [PubMed] [Google Scholar]
- Vysotskaya LB, Veselov SY, Veselov DS, Filippenko VN, Ivanov EA, Ivanov II, Kudoyarova GR. 2007. Immunohistological localization and quantification of IAA in studies of root growth regulation. Russian Journal of Plant Physiology 54, 827–832. [Google Scholar]
- Walch-Liu P, Ivanov II, Filleur S, Gan Y, Remans T, Forde BG. 2006. Nitrogen regulation of root branching. Annals of Botany 97, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Tischner R, Gutierrez RA, Hoffman M, Xing X, Chen M, Coruzzi G, Crawford NM. 2004. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiology 136, 2512–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Zhang DP. 2008. Abscisic acid receptors: multiple signal perception sites. Annals of Botany 101, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15, 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmulling T. 2010. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22, 3905–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. 2008. Manipulation of the apoplastic pH of intact plants mimics stomatal and growth responses to water availability and microclimatic variation. Journal of Experimental Botany 59, 619–631. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Kudoyarova GR, Veselov DS, Arkhipova TN, Davies WJ. 2012. Plant hormone interactions: innovative targets for crop breeding and management. Journal of Experimental Botany 63, 3499–3509. [DOI] [PubMed] [Google Scholar]
- Xiong L, Wang RG, Mao G, Koczan JM. 2006. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiology 142, 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Finnegan PM. 2010. Regulation of phosphate starvation responses in higher plants. Annals of Botany 105, 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhleniuk OV, Raines CA, Lloyd JC. 2001. Pho3: a phosphorus-deficient mutant of Arabidopsis thaliana (L.) Heynh. Planta 212, 529–534. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Forde BG. 1998. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409 [DOI] [PubMed] [Google Scholar]
- Zhang HM, Jennings A, Barlow PW, Forde BG. 1999. Dual pathways for regulation of root branching by nitrate. Proceedings of the National Academy of Sciences, USA 96, 6529–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davies WJ. 1989. Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant, Cell & Environment 12, 73–81. [Google Scholar]
- Zhu C, Schraut D, Hartung W, Schaffner AR. 2005. Differential responses of maize MIP genes to salt stress and ABA. Journal of Experimental Botany 56, 2971–2981. [DOI] [PubMed] [Google Scholar]
- Zhuo D, Okamoto M, Vidmar JJ, Glass ADM. 1999. Regulation of a putative high-affinity nitrate transporter (NRT2; 1At) in roots of Arabidopsis thaliana . The Plant Journal. 17, 563–568. [DOI] [PubMed] [Google Scholar]