This review highlights the function of hormone and cell cycle regulation in the growth-immunity cross-talk and discusses the contribution of root cell types therein?
Key words: Cell cycle, cell identity, cell type specificity, growth under stress, hormone signalling, immunity, root apical meristem, root development, stress adaptation.
Abstract
Biotic stress and diseases caused by pathogen attack pose threats in crop production and significantly reduce crop yields. Enhancing immunity against pathogens is therefore of outstanding importance in crop breeding. However, this must be balanced, as immune activation inhibits plant growth. This immunity-coupled growth trade-off does not support resistance but is postulated to reflect the reallocation of resources to drive immunity. There is, however, increasing evidence that growth–immunity trade-offs are based on the reconfiguration of hormone pathways, shared by growth and immunity signalling. Studies in roots revealed the role of hormones in orchestrating growth across different cell types, with some hormones showing a defined cell type-specific activity. This is apparently highly relevant for the regulation of the cell cycle machinery and might be part of the growth–immunity cross-talk. Since plants are constantly exposed to Immuno-activating microbes under agricultural conditions, the transition from a growth to an immunity operating mode can significantly reduce crop yield and can conflict our efforts to generate next-generation crops with improved yield under climate change conditions. By focusing on roots, we outline the current knowledge of hormone signalling on the cell cycle machinery to explain growth trade-offs induced by immunity. By referring to abiotic stress studies, we further introduce how root cell type-specific hormone activities might contribute to growth under immunity and discuss the feasibility of uncoupling the growth–immunity cross-talk.
Introduction
As sessile organisms, plants are repeatedly challenged as their environment changes during their lifetime. The ability of plants to perceive and respond to these changes in an adaptive way facilitates survival and reproduction. Environmental stresses are highly variable in their temporal occurrence and physical nature (e.g. abiotic or biotic) while their intensity (e.g. amplitude and complexity) defines the stress severity on plants. Among the regular and thus predictable re-occurring changes is the day–night cycle and the different seasons in temperate climates. Plants have evolved adaptive systems such as the circadian clock (e.g. to integrate the day–night cycle), which are part of developmental programmes (e.g. senescence or vernalization) to adjust growth and reproduction accordingly (Huijser and Schmid, 2011; Hsu and Harmer, 2014). Plants have also evolved morphogenetic traits to overcome or avoid stress. For instance, roots sense nutrient and water content in the soil and actively reorganize root architecture to access areas with superior resource availabilities (Gifford et al., 2013; Bao et al., 2014). Biotic stress induced by pathogen or herbivore attack, in contrast, is unpredictable, and plants must immediately activate immune pathways to ward off the invader. It is a significant challenge for plants to sense biotic stress severity adequately and respond appropriately. Any failure would allow invaders to feed on plants, resulting in plant disease and plant death. On the other hand, activation of immunity is also associated with trade-offs that can significantly affect growth and yield.
Plant immunity defines plant responses to stop pathogen invasion and is based on highly sensitive receptor-based perception systems activating antimicrobial signalling cascades. Pathogens such as bacteria and fungi are generally recognized by plasma membrane-localized or intracellular receptors that initiate signalling cascades to activate transcription factors in order to induce stress-adaptive genes (Boller and Felix, 2009; Stergiopoulos and de Wit, 2009; Bernoux et al., 2011; Heidrich et al., 2012). More precisely, plant immunity is turned on upon the recognition of conserved microbial molecules known as microbe-associated molecular patterns (MAMPs), such as bacterial flg22 and elf18 (active epitopes of bacterial flagellin and elongation factor-Tu, respectively) or fungal chitin. Plasma membrane-localized pattern recognition receptors (PRRs) specifically recognize these MAMPs to trigger immune responses (Gómez-Gómez et al., 1999; Kunze et al., 2004; Wan et al., 2008; Petutschnig et al., 2010). This so-called pattern-triggered immunity (PTI) includes different immune pathways such as the rapid apoplastic production of reactive oxygen species (termed the ROS burst) and the phosphorylation of mitogen-activated protein kinases (MAPKs) and Ca2+-mediated activation of Ca2+-dependent protein kinases (CDPKs) that activate transcription factors to induce defence genes (Felix et al., 1999; Asai et al., 2002; Boudsocq et al., 2010; Macho et al., 2012). The modular composition of immune signalling basically consisting of receptors, signalling cascades, and gene expression is highly effective as it rapidly integrates different signalling streams. Based on this signalling concept, plant immunity provides protection against the majority of pathogens (Jones and Dangl, 2006; Boller and Felix, 2009).
PTI signalling is based on the concerted action of synergistically and antagonistically interacting pathways that add to the robustness and effectivity of immunity (Tsuda et al., 2009; Pieterse et al., 2012). The MAPK and CDPK pathways, for instance, independently and synergistically activate defence genes (Boudsocq et al., 2011). Hormones significantly contribute to plant immunity and can synergistically or antagonistically activate defence genes. For instance, immunity against necrotrophic pathogens crucially depends on the synergistic interaction of jasmonate (JA) and ethylene (ET) or JA and abscisic acid (ABA) pathways, respectively. In turn, salicylic acid (SA), as part of the immunity to stop biotrophic pathogens, involves the suppression of JA signalling (Pieterse et al., 2009, 2012).
Though immunity is highly effective in protecting plants, it antagonizes plant growth (Bowling et al., 1994; Gómez-Gómez et al., 1999; Jirage et al., 2001; Kunze et al., 2004). Biotic stress induces a redistribution of resources such as energy currents and signalling processes to activate immunity (Navarro et al., 2004; Bolton, 2009; Tsuda et al., 2009; Pieterse et al., 2012) that might account for the inhibition of growth. In this review, we will introduce our current understanding of the growth–immunity antagonism and propose current models to explain this conflict. In addition to the energy reallocation hypothesis which considers growth suppression by immunity as a resource trade-off (Smedegaard-Petersen and Tolstrup, 1985), we will discuss the observed growth inhibition as a function of conflictive cross-talk of hormone signalling pathways participating in immunity and growth-associated cell cycle regulation. By focusing on roots, we will finally consider how immunity affects the spatio-temporal function of hormones in root growth and development. Since plants are constantly attacked by microbes under both agricultural and natural conditions, this switch from a ‘growth’ to a ‘stress’ operating mode can prevent crops from harnessing their full genetic yield potential (Brown, 2002). As well as the economic impacts on crop production, this growth to stress switch also bears conflictive potential in view of our efforts to generate next-generation crops with improved stress resistance and yield under climate change conditions.
What is plant growth and how is it regulated?
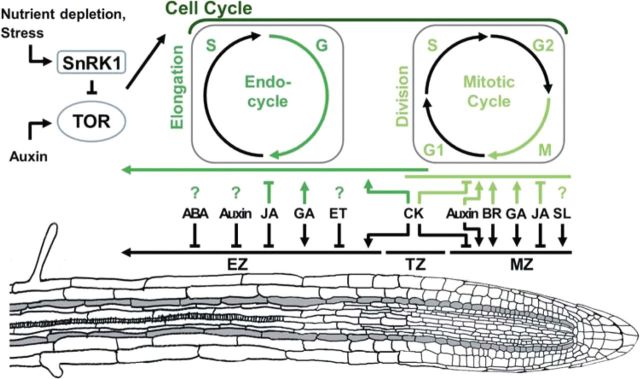
Plant growth is the result of the well-co-ordinated interaction of cell cycle and cell growth in shoots and roots (Sablowski and Carnier Dornelas, 2014). In the root apical meristematic zone (MZ), the cell cycle continuously delivers new cells through mitotic activity to maintain root growth (Fig. 1). Above the MZ, in the elongation zone (EZ), cells stop dividing and instead undergo rapid cell elongation via the endocycle, a cell cycle variant under which cells replicate their DNA without entering mitosis. This enhances cell ploidy and associates the endocycle with cell growth, which is characterized by cell expansion and dependent on vacuole-mediated cell turgor and of cytoplasmic growth driven by anabolic metabolism and protein synthesis in the EZ (Breuer et al., 2014; Edgar et al., 2014; Sablowski and Carnier Dornelas, 2014). The MZ and EZ are separated by a short transition zone (TZ). Here, cells show slight elongation, while some mitotic activity can still be found (Fig. 1). Thus, root growth is a succession of processes (e.g. cell division, endocycle) taking place at different root zones. Though hormones play an essential role in regulating growth via the cell cycle, its molecular basis is only partly understood (Fig. 1).
Fig. 1.
Hormone function in root growth. Indicated are the supportive or inhibitory effects of the different hormones on growth in the meristematic zone (MZ), transition zone (TZ), and elongation zone (EZ). The effect of these hormones on cell division in the MZ and endoreplication via the endocycle is unknown for most hormones (?). ABA, abscisic acid; AUX, auxin; BR, brassinosteroids; CK, cytokinins; ET, ethylene; GA, gibberellins; JA, jasmonates; SA, salicylic acid; SL, strigolactone; TOR, target of rapamycin; SNRK1, Snf1-related AMP-activated kinase.
The cell cycle regulates cell proliferation via four defined phases to organize DNA synthesis and mitosis. Cell cycle progression is mainly mediated by two classes of cyclin-dependent kinases (CDKs) that function and gain specificity in complex with various cyclins (Cycs) (De Veylder et al., 2007; Van Leene et al., 2010; Nowack et al., 2012). The formation of CDK–Cyc complexes is supported by the induction of different Cyc and CDK genes by auxin (CYCA2;3, CYCB1;1, and CDKB2;1), cytokinin (CK; CYCD), and brassinosteroid (BR; CYCB1) (Riou-Khamlichi et al., 1999; González-García et al., 2011; Wang and Ruan, 2013). DNA synthesis is initiated after CDKA in complex with CycD and CYCA3 phosphorylates and thus inactivates retinoblastoma-related (RBR) protein (De Veylder et al., 2007). RBR inactivation allows E2Fa-DPa and E2Fb-DPb transcription factor dimer formation to regulate genes involved in DNA replication and chromatin remodelling (De Veylder et al., 2002; Ramirez-Parra et al., 2003; Vandepoele et al., 2005; Magyar et al., 2012). Auxin has been identified to support this step further by stabilizing E2Fb (Magyar et al., 2005). At the time that cells enter mitosis, CDKA–CycB1/CycD3 and CDKB–CycB/CycA2 complexes have activated Myb3R transcription factors to regulate genes involved in vesicular trafficking and other processes mediating mitosis and cytokinesis (Ito et al., 2001; Araki et al., 2004).
KIP-related proteins (KRPs) that are conserved in all eukaryotes function in concert with plant-specific Siamese (SIM) and Siamese-related (SMR) proteins as CDK inhibitors. Auxin and gibberellic acid (GA) suppress KRP2 and SIM, thereby supporting cell division, while ABA enhances KRP1 expression (Wang et al., 1998). In addition, the Cullin-ring finger E3 ligase anaphase-promoting complex/cyclosome (APC/C) blocks the entry into mitosis to maintain the endocycle. To achieve this, cell division cycle 20 (CDC20) and cell cycle switch 52 (CCS52) interact and thus activate APC/C to degrade Cycs such as CycA2;3. In complex with CDKB1, these interactions negatively regulate the endocycle (Cebolla et al., 1999; Boudolf et al., 2009). CKs assist the endocycle by inducing CCS52a1 through ARR2 in the TZ (Takahashi et al., 2013). The endocycle E2F transcription factors are suppressors of the endocycle. DP-E2F-like 1 (DEL1)/E2Fe, for instance, interferes with endocycle entry by misregulating CCS52a1 expression (Lammens et al., 2008), and E2Fa interacts with RBR to form a transcriptional repressor complex that binds to the promoter of CCS52a1 and CCS52a2 to prevent endocycle entry (Magyar et al., 2012).
In addition to hormonal control, the cell cycle is a function of the energy status of the plant. Plant organs possess a developmental and growth plasticity to cope with fluctuations in internal resources (e.g. nutrient availability) and external (e.g. environmental stress) conditions. This plasticity is partly anchored in the cell cycle as plant organs compensate disturbances in cell proliferation by the adaptation of cell sizes, to maintain overall organ size (Sablowski and Carnier Dornelas, 2014). However, conditions that ultimately trigger cell growth cessation also impair cell proliferation and elongation (Henriques et al., 2014). In contrast to the low energy input process of turgor-driven cell elongation, cytoplasmic growth (and thus cell growth) depends on available cellular energy and nutrient resources. Plants therefore require a sensing and integration system that translates cellular energy/nutrient status into appropriate growth and cell cycle outputs, also taking into account cellular and environmental changes. As in other eukaryotes, target of rapamycin (TOR) kinase and Snf1-related AMP-activated kinase (SNRK1) signalling pathways in plants control cell growth by integrating energy availability and environmental stimuli (Baena-González et al., 2007; Robaglia et al., 2012). SNRK1 monitors the nutrient and stress status of cells and reduces anabolic processes to enable energy homeostasis and sustain growth under unfavourable conditions (Baena-González and Sheen, 2008). TOR, in turn, is directly involved in the regulation of translational processes as well as in growth-promoting transcription (Xiong et al., 2013; Henriques et al., 2014). This indicates an opposite effect of SNRK1 and TOR signalling pathways on growth. Moreover, SNRK1 negatively regulates TOR under stress or nutrient depletion (Fig. 1). It is currently unclear how TOR and SNRK1 perceive cellular energy/nutrient status and environmental stress and how this is communicated to the cell cycle programme. A recent study, however, revealed that, driven by photosynthesis-derived glucose, TOR induces primary metabolism genes including protein and cell wall anabolism in roots and also phosphorylates E2Fa to activate cell cycle S phase in the root apical meristem (RAM). It indicates a direct connection of TOR and plant growth by an alternative activation of cell proliferation and anabolic pathways (Xiong et al., 2013). Intriguingly, auxin is able to activate TOR (Schepetilnikov et al., 2013) (Fig. 1). How this interaction is associated with other known auxin activities on cell cycle regulation is currently unknown.
Biotic stress and immunity inhibit plant growth
If not lethal, biotic stress and plant disease as a result of pathogen colonization inhibit growth of affected plants. The reason for these symptoms is thought to reflect energy and nutrient undersupply. In addition to altering plant primary metabolism to recruit nutrients to foster their own reproduction, plant pathogens disturb root system architecture during infection, which can affect root function and hence the capacities for water and nutrient acquisition. This suggests that disease symptoms such as stunted growth are a direct consequence of nutrient and energy depletion.
Immune elicitors (MAMPs) of pathogens affect shoot and root growth to a degree that can be very similar to disease symptoms. Studies with the MAMPs flg22 and elf18 or with plants constitutively expressing resistance genes indicated that an activated immune system interferes with plant growth and development (Gómez-Gómez et al., 1999; Jirage et al., 2001; Kunze et al., 2004). This indicates that a highly sensitive or hyperactive immune system can activate traits that are highly disadvantageous for crop growth and thus yield. In fact, field studies with barley indicated that even a regular PTI activation by microbes of the phyllosphere and rhizosphere reduces crop yield by ~10% (Smedegaard-Petersen and Tolstrup, 1985). The potential of such an immunity trade-off is obvious in Arabidopsis mutants with constitutively activated immunity, which show up to 90% yield reduction (Jirage et al., 2001; Bartels et al., 2009). Considering the impact of immunity on growth and yield raises the question of the molecular origin of this trade-off.
Why does immunity inhibit plant growth?
The effectiveness of immunity depends on different strategies in which the reallocation of resources for the de novo synthesis of stress-adaptive proteins and secondary metabolites as well as the reconfiguration of cell signalling by hormones are of critical importance (Bolton, 2009; Robert-Seilaniantz, 2011; Pieterse et al., 2012; Neilson et al., 2013; Henriques et al., 2014) (Fig. 2). Biotic stress integration obviously requires a co-ordinated redirection of cell processes in which stress is prioritized over growth signalling. As a consequence, energy and nutrient resources are allocated to a diverse set of stress-adaptive responses. The observed growth inhibition under stress is therefore believed to reflect a competition for energy and nutrient resources, as both growth and stress adaption demands cannot be covered at the same time (Smedegaard-Petersen and Tolstrup, 1985; Purrington, 2000; Heil and Baldwin, 2002).
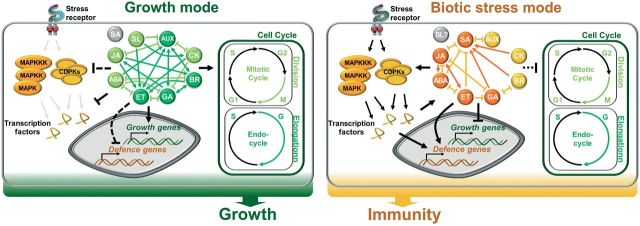
Fig. 2.
Schematic overview of hormone signalling in growth and immunity. The cell cycle is central to plant growth and is affected by hormone signalling. Upon biotic stress, stress receptors activate immune signalling and cells operate in a stress mode. Immune signalling rewires hormone signalling in order to substantiate immunity. Under the stress mode, immuno-associated hormone signalling is postulated to suppress growth at the transcriptional and post-translational level. Under growth conditions, AUX, BR, ET, and GA show the highest interconnections, in contrast to ABA, ET, GA, JA, and SA under biotic stress. Dashed lines, predicted link. MAPK, mitogen-activated protein kinase; CDPK, Ca2+-dependent protein kinase; ABA, abscisic acid; AUX, auxin; BR, brassinosteroids; CK, cytokinins; ET, ethylene; GA, gibberellins; JA, jasmonates; SA, salicylic acid; SL, strigolactone.
This hypothesis of competition for limited resources is, however, challenged by other studies. Hormones are essential for the regulation of growth and immunity. Obviously, hormone signalling networks substantially differ depending on whether cells operate under a growth or immunity mode (Pieterse et al., 2009; Wolters and Jürgens, 2009; Bennett and Scheres, 2010; Vanstraelen and Benková, 2012; Jung and McCouch, 2013; Huot et al., 2014) (Fig. 2). Although data presented in Fig. 2 display hormone growth networks in whole roots in comparison with immunity networks in whole plants, they suggest a close link between the alteration of hormone interactions and the growth–immunity cross-talk. Consistent with this hypothesis, growth-inhibiting MAMP treatments suppress signalling of the growth hormone auxin (Wang et al., 2007; Navarro et al., 2008) and the mutually inhibitory cross-talk of JA and GA in different plants (e.g. rice) (Hou et al., 2010; Wild et al., 2012; Yang et al., 2012; Heinrich et al., 2013). However, the growth–immunity cross-talk is not unidirectional. Recent studies indicated that growth suppresses PTI responses by interfering with BR signalling (Albrecht et al., 2012; Belkhadir and Jaillais, 2012; Lozano-Duran et al., 2013; Shi et al., 2013; Fan et al., 2014; Malinovsky et al., 2014). Importantly, immunity-triggered growth inhibition might be explained by JA- and SA-mediated inhibition of the cell cycle. Chen et al. (2011) demonstrated that JA suppressed CDKA;1 and CYCB1;1. Moreover, the transcription factor MYC2, a positive regulator of JA signalling, was found to bind to the promoter of Plethora 1 (PLT1) and PLT2 (Chen et al. 2011). Together with auxin, PLTs are essential for stem cell maintenance, root meristem activity, and root zone patterning (Mähönen et al., 2014). Recent studies further suggested the stabilization of DELLAs by JA signalling (Yang et al., 2012). DELLAs are inhibitors of GA signalling that also induce the CDKA and CDKB inhibitors KRP2, SIM, and SMR (Achard et al., 2009). The growth inhibitory activity of SA, in turn, might be based on a cross-talk with auxin signalling as SA treatment stabilized auxin-inhibiting AUX/IAA proteins (Wang et al., 2007). However, an antagonistic effect of SA on auxin-mediated cell cycle regulation is currently unknown.
Remarkably, despite this highly complex interdependence of intertwined or conflictive hormone signalling networks (Denance et al., 2013; Huot et al., 2014), the growth–immunity cross-talk appears to be detachable. Studies of the mutualistic fungus Piriformospora indica showed that the growth–immunity cross-talk is separated in JA mutants (Jacobs et al., 2011). While the fungus suppressed flg22-triggered growth inhibition, flg22-triggered immune signalling (e.g. ROS burst) was unimpaired. This therefore negates an immuno-relevant function of signalling processes underlying growth inhibition. Moreover, chitin is equally as potent as flg22 or elf18 as an activator of immunity in plants (Wan et al., 2008; Petutschnig et al., 2010). Although these three MAMPs share signalling pathways and trigger highly similar immune responses (e.g. oxidative burst, callose deposition, immunity gene induction) chitin does not inhibit growth. In addition, Luna et al. (2014) recently identified the molecular nature of growth suppression by the chemical immuno-activating agent α-aminobutyric acid (BABA). BABA induces immunity by binding to the aspartyl-tRNA synthetase IMPAIRED IN BABA-INDUCED IMMUNITY 1 (IBI1). This interaction blocks aspartyl-tRNA synthesis by IBI1 and results in the accumulation of uncharged tRNAAsp. It was shown that the protein kinase GCN2 recognizes these uncharged tRNAs and phosphorylates the translation initiation factor elF2α to stop protein synthesis, resulting in plant growth inhibition. Interestingly, BABA was still able to induce immunity but did not inhibit growth in the gcn2 mutant (Luna et al., 2014). Considering the importance of GCN2 for cell growth, this study further suggests that the observed hormone-induced growth trade-offs under stress might be based on an impairment of protein synthesis.
Taken together, energy and nutrient distribution must be rearranged upon stress in order to achieve stress resistance (Bolton, 2009). However, the observed growth arrest upon stress is apparently not a consequence of the reallocation of nutrient and energy resources. Rather, the hormone signalling network is redirected. Since hormones appear to have defined spatio-temporal functions in root growth, root cell type-specific studies can help us to elucidate the redirection of hormone signalling during the growth–immunity cross-talk.
Cell type specificities of hormonal growth pathways
Root growth needs to be highly regulated in order to ensure proper establishment and maintenance of the different developmental zones as well as co-ordinated cell expansion along the longitudinal and radial axis (Fig. 3A, B). If we hypothesize that immunity–growth cross-talk can be uncoupled in roots, it will be crucial to understand the underlying regulatory mechanisms of growth under immunity at the developmental and cell type level. Hormones are prime candidates when looking for a starting point to manipulate such trade-offs. They are known to act in interdependent networks, linking growth and development to immunity (Wolters and Jürgens, 2009; Robert-Seilaniantz, 2011; Depuydt and Hardtke, 2011; Pieterse et al., 2012). As discussed above, cell cycle progression is also directly influenced by hormones (Gutierrez, 2009; Takatsuka and Umeda, 2014).
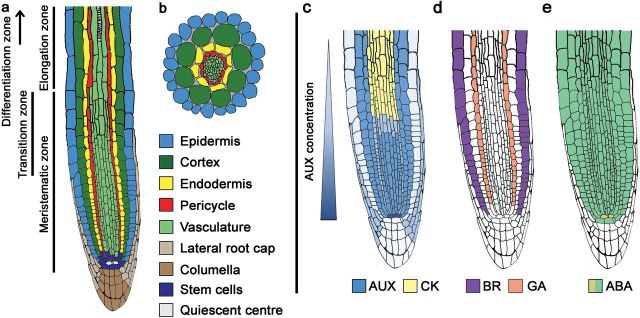
Fig. 3.
Root organization and cell type specificities of hormone signalling. (a) Developmental zones and cell types of Arabidopsis roots. The pericycle and the cell types of the vasculature form the root stele. The quiescent centre (QC) plus surrounding stem cells build the stem cell niche (SCN). (b) Cross-section of a mature root showing the concentric arrangement of root cell types. (c–e) Different modes of cell context-specific hormone signalling. (c) AUX distribution (blue) is a main regulator of root patterning and meristem function (Blilou et al., 2005; Kieffer et al., 2010). AUX shows a general basipetal gradient across the root tip (Petersson et al., 2009; Brunoud et al., 2012). CK (yellow) acts in the TZ vasculature to promote cell differentiation (Dello Ioio et al., 2007). (d) Epidermal BR signalling (grey) regulates cell division and cell size (Hacham et al., 2011; Fridman et al., 2014). GA (red) exerts control over meristem development and cell elongation through the endodermis (Ubeda-Tomás et al., 2008, 2009). (e) ABA inhibits cell division in the RAM (Zhang et al., 2010). While QC maintenance positively affects root growth (beige), it is negatively influenced by inhibition of cell division in the remaining meristem (green).
Over the last years, hormone signalling was found to be greatly influenced by cellular context (Dello Ioio et al., 2007, 2008; Dinneny et al., 2008; Gifford et al., 2008; Ubeda-Tomás et al., 2008, 2009; Hacham et al., 2011; Iyer-Pascuzzi et al., 2011; Bargmann et al., 2013; Duan et al., 2013; Geng et al., 2013; Fridman et al., 2014). This offers the fascinating possibility to manipulate subsets of hormone functions by specifically targeting signalling components in distinct cell types. So far, most information about this topic comes from studies on root tips, where hormone signalling was either activated or disrupted in distinct cell types. Although these studies focused on growth and development, the generated mutant lines will prove extremely useful for investigating cell context-dependent hormone responses during immune activation. Furthermore, this research provides valuable insights into the emerging concepts of cell type-specific hormone signalling, that need to be understood to decipher growth–immunity trade-offs.
Auxin and cytokinin: concentration gradient and ac tivity in a defined root zone
The auxin–CK circuit constitutes the main hormonal regulator of RAM size, which is modified by the activities of other hormones. While auxin was shown to promote stem cell maintenance and cell division, CK supports cell differentiation (Blilou et al., 2005; Dello Ioio et al., 2007, 2008; Ruzicka et al., 2009; Petricka et al., 2012; Vanstraelen and Benková, 2012; Moubayidin et al., 2013).
The distribution of auxin, both longitudinally and radially, strongly influences root patterning (Kieffer et al., 2010). Recent research has refined our understanding of cellular specificities of auxin distribution and signalling in the root tip (Petersson et al., 2009; Brunoud et al., 2012; Bargmann et al., 2013) (Fig. 3C). Quantification of auxin levels in different cell types showed a gradient, with a maximum in the quiescent centre (QC), high levels in the stele, endodermis and cortex, and low levels in the epidermis (Petersson et al., 2009). This map of auxin distribution was largely confirmed by use of the highly sensitive auxin sensor DII-VENUS (Brunoud et al., 2012). In addition, Bargmann et al. (2013) found transcription of most auxin-regulated genes to be cell context dependent. This means that the identity of a given cell, which is determined by cell type, developmental status, and the combination of both, can intersect with auxin signalling and modify the auxin response. A cell response to auxin is therefore determined by its internal composition and the concentration of the hormone, which is actively regulated by the plant.
In contrast to auxin, CK was shown to exert its control over meristem size specifically through signalling in the TZ stele (Dello Ioio et al., 2007) (Fig. 3C). The regulation of cell differentiation in the whole TZ requires vascular CK signalling through the ARABIDOPSIS HISTIDINE KINASE 3 (AHK3) receptor and the response regulators ARR1 and ARR12 (Dello Ioio et al., 2007, 2008). ARR1 indirectly influences cell cycle progression through SHORT HYPOCOTYL 2 (SHY2), an AUX/IAA protein expressed in the vasculature of the TZ (Weijers et al., 2005; Dello Ioio et al., 2008). AUX/IAA proteins are negative regulators of auxin signalling and are degraded in the presence of auxin (Mockaitis and Estelle, 2008). Increased SHY2 protein levels in the vasculature cause redistribution of auxin (Dello Ioio et al., 2008). As a result, the auxin–CK balance is shifted towards CK, therefore favouring cell differentiation instead of proliferation in all tissues of the TZ (Dello Ioio et al., 2008).
CK also provides a good example for another level of complexity in hormone signalling. Bishopp et al. (2011) investigated the basipetal transport of shoot CK in the root phloem. Specific depletion of this CK element had no effect on root meristem size, but altered root vasculature patterning. Therefore, hormones produced in different source tissues appear to determine distinct developmental functions. This raises the question of whether it will be possible to manipulate subsets of plant growth and stress responses by interfering with hormone biosynthesis in specific tissues.
Brassinosteroids and gibberellic acid: distinct cell type-specific activities
In contrast to the broader activity of auxin and CK, BR and GA promote root growth through distinct cell types (Ubeda-Tomás et al., 2008, 2009; Hacham et al., 2011; Fridman et al., 2014) (Fig. 3D). BR regulates cell proliferation and elongation in the root via the epidermis (González-García et al., 2011; Hacham et al., 2011; Fridman et al., 2014) (Fig. 3D). In mutant studies, balanced BR signalling was found to be necessary for optimal meristem development, as both reduced and increased BR signalling led to a reduction in root meristem size (González-García et al., 2011; Hacham et al., 2011). Expression of the BR receptor BRI1 in non-hair epidermal cells of the bri1 mutant was sufficient to restore meristem size (Hacham et al., 2011). Thus, BRI1 signalling in the epidermis is sufficient to control meristematic cell expansion and activity. Moreover, BR signalling was found to have opposing effects on cell elongation, depending on the relative abundance of BRI1 in non-hair compared with hair epidermal cells (Fridman et al., 2014).
GA signalling occurs via degradation of growth-repressing DELLA proteins (Peng et al., 1997; Silverstone et al., 1998, 2001; Olszewski et al., 2002). In an elegant study, Ubeda-Tomas and colleagues (2008) identified the endodermis as the primary target site for GA-induced root cell elongation. Expression of the non-degradable DELLA protein variant ga insensitive (gai) (Peng et al., 1997) exclusively in this tissue layer led to disrupted cell elongation, while gai expression in other cell layers had no effect on root growth (Ubeda-Tomás et al., 2008). In addition to its effect on cell elongation, GA controls cell proliferation in the meristem (Achard et al., 2009; Ubeda-Tomás et al., 2009). Therefore, GA signalling reduces the expression of cell cycle inhibitors KRP2 and SIM, providing another direct link between hormone signalling and cell cycle control (Achard et al., 2009). Regulation of meristem size further requires GA perception in the endodermis (Ubeda-Tomás et al., 2009). In 2013, Shani and colleagues observed the accumulation of fluorescently labelled GA in the root endodermis. Importantly, this also indicated the existence of an active GA transport mechanism in roots that is dependent on endodermal cell identity (Shani et al., 2013).
Abscisic acid: versatile integrator in the meristem
ABA plays a central role in plant adaptation to various biotic and abiotic stresses, but has important developmental functions under homeostatic conditions as well (Raghavendra et al., 2010; Finkelstein, 2013; Nakashima and Yamaguchi-Shinozaki, 2013). In the RAM and the lateral root meristem (LRM), ABA was shown to inhibit division of QC cells (Zhang et al., 2010). Through this, ABA promotes maintenance of the stem cell niche and thus positively influences root growth. Inhibition of cell proliferation by ABA in the other parts of the root meristem, however, suppresses root growth. Thus, ABA is perceived throughout the RAM but has opposite effects on overall root growth by exerting the same regulatory function on different cell types within the meristem (Zhang et al., 2010) (Fig. 3E). Recently, highly sensitive ABA sensors have been developed that will open up new possibilities in studying the roles of ABA at cell type and longitudinal resolution (Jones et al., 2014; Waadt et al., 2014).
Cell context-dependent abiotic stress signalling: what can we learn?
Is there a direct link between hormones, stress, cellular context, and root growth and development? Considering the importance of cell type-specific activities of hormones in root growth, it seems likely that the observed growth–immunity cross-talk will depend on a cell type-specific redirection of hormone signalling. Unfortunately, the question has not been addressed concerning biotic stress so far. This can, however, be postulated from various cell type-specific transcriptome studies of abiotic stress and nitrogen depletion (Gifford et al., 2008; Dinneny et al., 2008; Iyer-Pascuzzi et al., 2011; Geng et al., 2013). These studies revealed cell context-dependent hormone signalling to be crucial during adaptation to abiotic stress and changing environments.
ABA, for example, is known for its involvement in plant responses to various adverse environments (Raghavendra et al., 2010; Finkelstein, 2013; Nakashima and Yamaguchi-Shinozaki, 2013). For several abiotic stresses, ABA signalling was shown to be directly linked to proteins controlling cell identity (Iyer-Pascuzzi et al., 2011). Cell identity regulators have been defined as genes with a known function in the determination or maintenance of a cell type (Dinneny et al., 2008; Iyer-Pascuzzi et al., 2011; Bargmann et al., 2013). Indeed, many of these genes showed differential expression during abiotic stress responses. One of the proteins that probably links stress responses and cell identity is SCARECROW (SCR) (Iyer-Pascuzzi et al., 2011). SCR is a transcription factor expressed in the endodermis where it regulates the expression of cell cycle components to control ground tissue patterning (Scheres et al., 1995; Di Laurenzio et al., 1996; Sozzani et al., 2010). Several ABA-responsive genes have been identified as direct targets of SCR (Iyer-Pascuzzi et al., 2011; Cui et al., 2012). In addition, in phosphate-limiting conditions, SCR levels depend on PDR2, a protein involved in inorganic phosphate sensing (Ticconi et al., 2004, 2009). These studies exemplify the intricate interconnection between cell identity regulators and stress adaptation. Given their involvement in hormone signalling, development, and stress sensing, it can be expected that cell identity regulators also influence the immunity–growth cross-talk. Studies focusing on cell type-specific transcriptomics in Arabidopsis roots have uncovered core gene sets defining certain cell types, thus greatly widening the list of possible cell type regulators (Birnbaum et al., 2003; Brady et al., 2007; Gifford et al., 2008; Dinneny et al., 2008; Mustroph et al., 2009; Iyer-Pascuzzi et al., 2011; Bruex et al., 2012; Bargmann et al., 2013; Geng et al., 2013; Lan et al., 2013; Simon et al., 2013). It is yet to be determined which of them are linked to immune signalling, but these findings open up an exciting opportunity to identify new links between growth and defence responses.
Concerning stress, hormonal signalling at cellular resolution has been best studied under high salinity conditions and revealed a redirection of hormone activities and salt stress-induced growth inhibition (Dinneny et al., 2008; Duan et al., 2013; Geng et al., 2013). The response of primary root growth to high salt concentrations can be divided into several distinct phases (Geng et al., 2013). Initial growth reduction leads to a quiescent period without growth. This is followed by a recovery phase in which growth is reinitiated and subsequently maintained at a lower rate in the homeostasis phase. Tissue-specific hormone signalling is crucial in governing these growth phases. For instance, profiling at cell type resolution allowed the identification of both broad (several cell types show the same response) and cell type-specific ABA responses. It also revealed the endodermis and pericycle as the main sites for ABA-dependent primary root growth recovery (Geng et al., 2013). In lateral roots, the endodermis was found to be the primary site of ABA-mediated regulation of growth in response to salt treatment (Duan et al., 2013).
Intriguingly, growth of the different root meristems (RAM and LRM) is affected quite differently by salt stress (Duan et al., 2013; Geng et al., 2013). In the primary root, the salt-induced quiescent phase lasts several hours and is associated with ABA-induced growth suppression (Geng et al., 2013). The same effect has been observed for lateral roots, but here the meristem was found to be considerably more sensitive to ABA, resulting in a quiescent phase that lasted several days (Duan et al., 2013). The findings raised the question of whether lateral roots are generally hypersusceptible to growth-inhibiting hormones. However, application of an ET precursor caused stronger growth reduction in the primary than in the lateral roots. Thus both root types seem to be equipped with a unique set of signalling components to interpret stress and hormone signalling, with a major impact on root system architecture (Duan et al., 2013). These results should be taken into account when studying immunity–growth trade-offs in roots, since a sole focus on primary root length or fresh weight might overlook more subtle effects that occur in individual meristems or cell types.
The classic defence hormone JA was identified to play a role in salt stress adaptation as well (Geng et al., 2013). JA signalling was activated during salt stress and found to be involved in growth suppression (Geng et al., 2013). While JA is a key regulator of many defence responses, it has recently been shown to inhibit root growth by regulating the expression of several cell cycle-related genes (Chen et al., 2011; Pieterse et al., 2012). The inhibitory effect of JA signalling on growth during salt stress occurred via the inner root tissues (Geng et al., 2013). In contrast, induction of the JA signalling inhibitor JAI3 was observed specifically in the epidermis, indicating cell type specificity of JA-related stress signalling. Intriguingly, genes associated with defence responses were enriched among the JA-induced genes (Geng et al., 2013). This raises the question of whether these defence proteins play a role during salt adaptation. Alternatively, roots might activate JA signalling solely to adjust root growth patterns, and activation of JA-related defences might occur as an unspecific side effect. It will be most interesting to see if JA-associated growth suppression during salt stress can be decoupled from the observed activation of defence responses. This example also demonstrates how research on immunity–growth trade-offs can profit from studies addressing abiotic stress responses.
Conclusions
Immunity inhibits plant growth with potentially very negative impacts on crop yield. The effect of immunity cannot only be attributed to the reallocation of limited resources, but, rather, stress perception redirects cell signalling from a growth to a stress mode. Roots are a quintessential example of an organ whose diverse functions are orchestrated by the interaction of metabolically and functionally very different cell types. This orchestration across all cell types is mediated by hormones, with some hormones characterized by their strict cell type-specific activity. In addition to a synergistic or antagonistic interaction in the regulation of signalling (e.g. co-activation/suppression of transcription factors) within and across cell boundaries, hormones are anchored in cell cycle regulation. The cell type-specific localization of cell cycle genes and the known regulation of cell cycle modules or pathways by hormones might be part of the growth–immunity cross-talk. In fact, recent studies indicated a direct link of immunity proteins (e.g. CPR5) in the regulation of central cell cycle regulators as well as of the cell cycle on SA signalling (Bao and Hua, 2014; Chandran et al., 2014; Wang et al., 2014). This further underlines the importance of cell identity and developmental status in the hormonal regulation of root growth during immunity. Though recent studies have broadened our knowledge of root immunity (Millet et al., 2010; Jacobs et al., 2011; Beck et al., 2014), high-resolution transcriptomic data would be needed to unveil underlying regulatory principles. Identifying the signalling pathways involved in regulating the growth–immunity cross-talk in combination with localizing their exact site of action would open up opportunities to adjust the growth–immunity trade-off and could enable researchers to uncouple immunity from growth or at least drastically mitigate its negative effects.
Acknowledgements
This work was supported by a Biotechnology and Biological Sciences Research Council New Investigator BB/H109502/1 grant and a BBSRC BB/H019502/1 grant to MLG, as well as funding by the Deutsche Forschungsgemeinschaft (SCHA1444/3-3) and the University of Warwick to PS.
References
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GTS, Genschik P. 2009. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current Biology 19, 1188–1193. [DOI] [PubMed] [Google Scholar]
- Albrecht C, Boutrot F, Segonzac C, Schwessinger B, Gimenez-Ibanez S, Chinchilla D, Rathjen JP, de Vries SC, Zipfel C. 2012. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proceedings of the National Academy of Sciences, USA 109, 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Ito M, Soyano T. 2004. Mitotic cyclins stimulate the activity of c-Myb-like factors for transactivation of G2/M phase-specific genes in tobacco. Journal of Biological Chemistry 279, 32979–32988. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. 2002. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983. [DOI] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942. [DOI] [PubMed] [Google Scholar]
- Baena-González E, Sheen J. 2008. Convergent energy and stress signaling. Trends in Plant Science 13, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Aggarwal P, Robbins NE, et al. 2014. Plant roots use a patterning mechanism to position lateral root branches toward available water. Proceedings of the National Academy of Sciences, USA 111, 9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z, Hua J. 2014. Interaction of CPR5 with cell cycle regulators UVI4 and OSD1 in Arabidopsis. PLoS One 9, e100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann BOR, Vanneste S, Krouk G, et al. 2013. A map of cell type-specific auxin responses. Molecular Systems Biology 9, 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels S, Anderson JC, González Besteiro MA, Carreri A, Hirt H, Buchala A, Métraux J-P, Peck SC, Ulm R. 2009. MAP kinase phosphatase1 and protein tyrosine phosphatase1 are repressors of salicylic acid synthesis and SNC1-mediated responses in Arabidopsis. The Plant Cell 21, 2884–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Wyrsch I, Strutt J, Wimalasekera R, Webb A, Boller T, Robatzek S. 2014. Expression patterns of flagellin sensing 2 map to bacterial entry sites in plant shoots and roots. Journal of Experimental Botany 65, 6487–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Jaillais Y. 2012. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proceedings of the National Academy of Sciences, USA 109, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Scheres B. 2010. Root development—two meristems for the price of one? Current Topics in Developmental Biology 91, 67–102. [DOI] [PubMed] [Google Scholar]
- Bernoux M, Ellis JG, Dodds PN. 2011. New insights in plant immunity signaling activation. Current Opinion in Plant Biology 14, 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN. 2003. A gene expression map of the Arabidopsis root. Science 302, 1956–1960. [DOI] [PubMed] [Google Scholar]
- Bishopp A, Lehesranta S, Vatén A, Help H, El-Showk S, Scheres B, Helariutta K, Mähönen AP, Sakakibara H, Helariutta Y. 2011. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Current Biology 21, 927–932. [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44. [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Bolton MD. 2009. Primary metabolism and plant defense—fuel for the fire. Molecular Plant-Microbe Interactions 22, 487–497. [DOI] [PubMed] [Google Scholar]
- Boudolf V, Lammens T, Boruc J, et al. 2009. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiology 150, 1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng S-H, Sheen J. 2010. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 464, 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X. 1994. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. The Plant Cell 6, 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Orlando D a, Lee J-Y, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. 2007. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318, 801–806. [DOI] [PubMed] [Google Scholar]
- Breuer C, Braidwood L, Sugimoto K. 2014. Endocycling in the path of plant development. Current Opinion in Plant Biology 17, 78–85. [DOI] [PubMed] [Google Scholar]
- Brown JKM. 2002. Yield penalties of disease resistance in crops. Current Opinion in Plant Biology 5, 339–344. [DOI] [PubMed] [Google Scholar]
- Bruex A, Kainkaryam RM, Wieckowski Y, et al. 2012. A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genetics 8, e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoud G, Wells DM, Oliva M, et al. 2012. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482, 103–106. [DOI] [PubMed] [Google Scholar]
- Cebolla A, Vinardell JM, Kiss E, Oláh B, Roudier F, Kondorosi A, Kondorosi E. 1999. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO Journal 18, 4476–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran D, Rickert J, Huang Y, Steinwand MA, Marr SKK, Wildermuth MCC. 2014. Atypical E2F transcriptional repressor DEL1 acts at the intersection of plant growth and immunity by controlling the hormone salicylic acid. Cell Host and Microbe 15, 506–513. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sun J, Zhai Q, et al. 2011. The basic helix–loop–helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. The Plant Cell 23, 3335–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Hao Y, Kong D. 2012. SCARECROW has a SHORT-ROOT-independent role in modulating the sugar response. Plant Physiology 158, 1769–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. 2007. Cytokinins determine arabidopsis root-meristem size by controlling cell differentiation. Current Biology 17, 678–682. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322, 1380–1384. [DOI] [PubMed] [Google Scholar]
- Denance N, Sanchez-Vallet A, Goffner D, Molina A. 2013. Disease resistance or growth: the role of plant hormones in balancing immune responses and fitness costs. Frontiers in Plant Science 4, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Hardtke CS. 2011. Hormone signalling crosstalk in plant growth regulation. Current Biology 21, R365–R373. [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, et al. 2002. Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO Journal 21, 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Inzé D. 2007. The ins and outs of the plant cell cycle. Nature Reviews Molecular Cell Biology 8, 655–665. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. 1996. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86, 423–433. [DOI] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. 2008. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320, 942–945. [DOI] [PubMed] [Google Scholar]
- Duan L, Dietrich D, Ng CH, Chan PMY, Bhalerao R, Bennett MJ, Dinneny JR. 2013. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. The Plant Cell 25, 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B, Zielke N, Gutierrez C. 2014. Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nature Reviews Molecular Cell Biology 15, 197–210. [DOI] [PubMed] [Google Scholar]
- Fan M, Bai M-Y, Kim J-G, et al. 2014. The bHLH transcription factor HBI1 mediates the trade-off between growth and pathogen-associated molecular pattern-triggered immunity in Arabidopsis. The Plant Cell 26, 828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran J, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal 18, 265–276. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. 2013. Abscisic acid synthesis and response. The Arabidopsis Book 11, e0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman Y, Elkouby L, Holland N, Vragović K, Elbaum R, Savaldi-Goldstein S. 2014. Root growth is modulated by differential hormonal sensitivity in neighboring cells. Genes and Development 28, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Wu R, Wee CW, Xie F, Wei X, Chan PMY, Tham C, Duan L, Dinneny JR. 2013. A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. The Plant Cell 25, 2132–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford ML, Banta JA, Katari MS, Hulsmans J, Chen L, Ristova D, Tranchina D, Purugganan MD, Coruzzi GM, Birnbaum KD. 2013. Plasticity regulators modulate specific root traits in discrete nitrogen environments. PLoS Genetics 9, e1003760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. 2008. Cell-specific nitrogen responses mediate developmental plasticity. Proceedings of the National Academy of Sciences, USA 105, 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Felix G, Boller T. 1999. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. The Plant Journal 18, 277–284. [DOI] [PubMed] [Google Scholar]
- González-García M-P, Vilarrasa-Blasi J, Zhiponova M, Divol F, Mora-García S, Russinova E, Caño-Delgado AI. 2011. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 138, 849–859. [DOI] [PubMed] [Google Scholar]
- Gutierrez C. 2009. The Arabidopsis cell division cycle. The Arabidopsis Book 7, e0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacham Y, Holland N, Butterfield C, Ubeda-Tomas S, Bennett MJ, Chory J, Savaldi-Goldstein S. 2011. Brassinosteroid perception in the epidermis controls root meristem size. Development 138, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich K, Blanvillain-Baufumé S, Parker JE. 2012. Molecular and spatial constraints on NB-LRR receptor signaling. Current Opinion in Plant Biology 15, 385–391. [DOI] [PubMed] [Google Scholar]
- Heil M, Baldwin IT. 2002. Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends in Plant Science 7, 61–67. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Hettenhausen C, Lange T, Wünsche H, Fang J, Baldwin IT, Wu J. 2013. High levels of jasmonic acid antagonize the biosynthesis of gibberellins and inhibit the growth of Nicotiana attenuata stems. The Plant Journal 73, 591–606. [DOI] [PubMed] [Google Scholar]
- Henriques R, Bögre L, Horváth B, Magyar Z. 2014. Balancing act: matching growth with environment by the TOR signalling pathway. Journal of Experimental Botany 65, 2691–2701. [DOI] [PubMed] [Google Scholar]
- Hou X, Lee LYC, Xia K, Yan Y, Yu H. 2010. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Developmental Cell 19, 884–894. [DOI] [PubMed] [Google Scholar]
- Hsu PY, Harmer SL. 2014. Wheels within wheels: the plant circadian system. Trends in Plant Science 19, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser P, Schmid M. 2011. The control of developmental phase transitions in plants. Development 138, 4117–4129. [DOI] [PubMed] [Google Scholar]
- Huot B, Yao J, Montgomery BL, He SY. 2014. Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Molecular Plant 7, 1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Araki S, Matsunaga S, Itoh T, Nishihama R, Machida Y, Doonan JH, Watanabe A. 2001. G2/M-phase-specific transcription during the plant cell cycle is mediated by c-Myb-like transcription factors. The Plant Cell 13, 1891–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer-Pascuzzi AS, Jackson T, Cui H, Petricka JJ, Busch W, Tsukagoshi H, Benfey PN. 2011. Cell identity regulators link development and stress responses in the Arabidopsis root. Developmental Cell 21, 770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Zechmann B, Molitor A, Trujillo M, Petutschnig E, Lipka V, Likpa V, Kogel K-H, Schäfer P. 2011. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiology 156, 726–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Zhou N, Cooper B, Clarke JD, Dong X, Glazebrook J. 2001. Constitutive salicylic acid-dependent signaling in cpr1 and cpr6 mutants requires PAD4. The Plant Journal 26, 395–407. [DOI] [PubMed] [Google Scholar]
- Jones AM, Danielson JÅH, Manojkumar SN, Lanquar V, Grossmann G, Frommer WB. 2014. Abscisic acid dynamics in roots detected with genetically encoded FRET sensors. eLife 3, e01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Jung JKH, McCouch S. 2013. Getting to the roots of it: genetic and hormonal control of root architecture. Frontiers in Plant Science 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer M, Neve J, Kepinski S. 2010. Defining auxin response contexts in plant development. Current Opinion in Plant Biology 13, 12–20. [DOI] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. 2004. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. The Plant Cell 16, 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens T, Boudolf V, Kheibarshekan L, et al. 2008. Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset. Proceedings of the National Academy of Sciences, USA 105, 14721–14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Li W, Lin W-D, Santi S, Schmidt W. 2013. Mapping gene activity of Arabidopsis root hairs. Genome Biology 14, R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Durán R, Macho A, Boutrot F. 2013. The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. eLife 2, e00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, van Hulten M, Zhang Y, et al. 2014. Plant perception of β-aminobutyric acid is mediated by an aspartyl-tRNA synthetase. Nature Chemical Biology 10, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho AP, Boutrot F, Rathjen JP, Zipfel C. 2012. Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiology 159, 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, De Veylder L, Atanassova A, Bakó L, Inzé D, Bögre L. 2005. The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. The Plant Cell 17, 2527–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Horváth B, Khan S, Mohammed B, Henriques R, De Veylder L, Bakó L, Scheres B, Bögre L. 2012. Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. EMBO Journal 31, 1480–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mähönen AP, ten Tusscher K, Siligato R, et al. 2014. PLETHORA gradient formation mechanism separates auxin responses. Nature 515, 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinovsky FG, Batoux M, Schwessinger B, Youn JH, Stransfeld L, Win J, Kim S-K, Zipfel C. 2014. Antagonistic regulation of growth and immunity by the Arabidopsis basic helix–loop–helix transcription factor homolog of brassinosteroid enhanced expression2 interacting with increased leaf inclination1 binding bHLH1. Plant Physiology 164, 1443–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel FM. 2010. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. The Plant Cell 22, 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M. 2008. Auxin receptors and plant development: a new signaling paradigm. Annual Review of Cell and Developmental Biology 24, 55–80. [DOI] [PubMed] [Google Scholar]
- Moubayidin L, Di Mambro R, Sozzani R, et al. 2013. Spatial coordination between stem cell activity and cell differentiation in the root meristem. Developmental Cell 26, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Zanetti ME, Jang CJH, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J. 2009. Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K. 2013. ABA signaling in stress-response and seed development. Plant Cell Reports 32, 959–970. [DOI] [PubMed] [Google Scholar]
- Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, Jones JDG. 2008. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Current Biology 18, 650–655. [DOI] [PubMed] [Google Scholar]
- Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JDG. 2004. The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiology 135, 1113–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson EH, Goodger JQD, Woodrow IE, Møller BL. 2013. Plant chemical defense: at what cost? Trends in Plant Science 18, 250–258. [DOI] [PubMed] [Google Scholar]
- Nowack MK, Harashima H, Dissmeyer N, Zhao X, Bouyer D, Weimer AK, De Winter F, Yang F, Schnittger A. 2012. Genetic framework of cyclin-dependent kinase function in Arabidopsis. Developmental Cell 22, 1030–1040. [DOI] [PubMed] [Google Scholar]
- Olszewski N, Sun T-P, Gubler F. 2002. Gibberellin signaling: biosynthesis, catabolism, and response pathways. The Plant Cell 14 Suppl, S61–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. 1997. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes and Development 11, 3194–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson S, V, Johansson AI, Kowalczyk M, Makoveychuk A, Wang JY, Moritz T, Grebe M, Benfey PN, Sandberg G, Ljung K. 2009. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. The Plant Cell 21, 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Winter CM, Benfey PN. 2012. Control of Arabidopsis root development. Annual Review of Plant Biology 63, 563–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petutschnig EK, Jones AME, Serazetdinova L, Lipka U, Lipka V. 2010. The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. Journal of Biological Chemistry 285, 28902–28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. 2009. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. 2012. Hormonal modulation of plant immunity. Annual Review of Cell and Developmental Biology 28, 489–521. [DOI] [PubMed] [Google Scholar]
- Purrington CB. 2000. Costs of resistance. Current Opinion in Plant Biology 3, 305–308. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. 2010. ABA perception and signalling. Trends in Plant Science 15, 395–401. [DOI] [PubMed] [Google Scholar]
- Ramirez-Parra E, Fründt C, Gutierrez C. 2003. A genome-wide identification of E2F-regulated genes in Arabidopsis. The Plant Journal 33, 801–811. [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. 1999. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Robaglia C, Thomas M, Meyer C. 2012. Sensing nutrient and energy status by SnRK1 and TOR kinases. Current Opinion in Plant Biology 15, 301–307. [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A. 2011. Hormone crosstalk in plant disease and defense: more than just jasmonate–salicylate antagonism. Annual Review of Phytopathology 49, 317–343. [DOI] [PubMed] [Google Scholar]
- Ruzicka K, Simásková M, Duclercq J, Petrásek J, Zazímalová E, Simon S, Friml J, Van Montagu MCE, Benková E. 2009. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proceedings of the National Academy of Sciences, USA 106, 4284–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R, Carnier Dornelas M. 2014. Interplay between cell growth and cell cycle in plants. Journal of Experimental Botany 65, 2703–2714. [DOI] [PubMed] [Google Scholar]
- Schepetilnikov M, Dimitrova M, Mancera-Martínez E, Geldreich A, Keller M, Ryabova LA. 2013. TOR and S6K1 promote translation reinitiation of uORF-containing mRNAs via phosphorylation of eIF3h. EMBO Journal 32, 1087–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, Laurenzio L Di, Willemsen V, Hauser M, Janmaat K, Weisbeek P, Benfey PN. 1995. Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development 62, 53–62. [Google Scholar]
- Shani E, Weinstain R, Zhang Y, Castillejo C, Kaiserli E, Chory J, Tsien RY, Estelle M. 2013. Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proceedings of the National Academy of Sciences, USA 110, 4834–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Shen Q, Qi Y, Yan H, Nie H, Chen Y, Zhao T, Katagiri F, Tang D. 2013. BR-SIGNALING KINASE1 physically associates with FLAGELLIN SENSING2 and regulates plant innate immunity in Arabidopsis. The Plant Cell 25, 1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Ciampaglio CN, Sun T. 1998. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. The Plant Cell 10, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone AL, Jung HS, Dill A, Kawaide H, Kamiya Y, Sun TP. 2001. Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. The Plant Cell 13, 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Bruex A, Kainkaryam RM, Zheng X, Huang L, Woolf PJ, Schiefelbein J. 2013. Tissue-specific profiling reveals transcriptome alterations in Arabidopsis mutants lacking morphological phenotypes. The Plant Cell 25, 3175–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedegaard-Petersen V, Tolstrup K. 1985. The limiting effect of disease resistance on yield. Annual Review of Phytopathology 23, 475–490. [Google Scholar]
- Sozzani R, Cui H, Moreno-Risueno M a, Busch W, Van Norman JM, Vernoux T, Brady SM, Dewitte W, Murray JAH, Benfey PN. 2010. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature 466, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos I, de Wit PJGM. 2009. Fungal effector proteins. Annual Review of Phytopathology 47, 233–263. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kajihara T, Okamura C, Kim Y, Katagiri Y, Okushima Y, Matsunaga S, Hwang I, Umeda M. 2013. Cytokinins control endocycle onset by promoting the expression of an APC/C activator in arabidopsis roots. Current Biology 23, 1812–1817. [DOI] [PubMed] [Google Scholar]
- Takatsuka H, Umeda M. 2014. Hormonal control of cell division and elongation along differentiation trajectories in roots. Journal of Experimental Botany 65, 2633–2643. [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. 2004. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. The Plant Journal 37, 801–814. [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Lucero RD, Sakhonwasee S, Adamson AW, Creff A, Nussaume L, Desnos T, Abel S. 2009. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proceedings of the National Academy of Sciences, USA 106, 14174–14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. 2009. Network properties of robust immunity in plants. PLoS Genetics 5, e1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Federici F, Casimiro I, Beemster GTS, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ. 2009. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Current Biology 19, 1194–1199. [DOI] [PubMed] [Google Scholar]
- Ubeda-Tomás S, Swarup R, Coates J, Swarup K, Laplaze L, Beemster GTS, Hedden P, Bhalerao R, Bennett MJ. 2008. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nature Cell Biology 10, 625–628. [DOI] [PubMed] [Google Scholar]
- Vandepoele K, Vlieghe K, Florquin K, Hennig L, Beemster GTS, Gruissem W, Van de Peer Y, Inzé D, De Veylder L. 2005. Genome-wide identification of potential plant E2F target genes. Plant Physiology 139, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leene J, Hollunder J, Eeckhout D, et al. 2010. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Molecular Systems Biology 6, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstraelen M, Benková E. 2012. Hormonal interactions in the regulation of plant development. Annual Review of Cell and Developmental Biology 28, 463–487. [DOI] [PubMed] [Google Scholar]
- Waadt R, Hitomi K, Nishimura N, Hitomi C. 2014. FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 3, e01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhang X-C, Neece D, Ramonell KM, Clough S, Kim S-Y, Stacey MG, Stacey G. 2008. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. The Plant Cell 20, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X. 2007. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Current Biology 17, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC. 1998. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. The Plant Journal 15, 501–510. [DOI] [PubMed] [Google Scholar]
- Wang L, Ruan Y-L. 2013. Regulation of cell division and expansion by sugar and auxin signaling. Frontiers in Plant Science 4, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gu Y, Zebell SG, Anderson LK, Wang W, Mohan R, Dong X. 2014. A noncanonical role for the CKI–RB–E2F cell-cycle signaling pathway in plant effector-triggered immunity. Cell Host and Microbe 16, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G. 2005. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO Journal 24, 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild M, Daviere J-M, Cheminant S, Regnault T, Baumberger N, Heintz D, Baltz R, Genschik P, Achard P. 2012. The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. The Plant Cell 24, 3307–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H, Jürgens G. 2009. Survival of the flexible: hormonal growth control and adaptation in plant development. Nature Reviews Genetics 10, 305–317. [DOI] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J. 2013. Glucose–TOR signalling reprograms the transcriptome and activates meristems. Nature 496, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D-L, Yao J, Mei C-S, et al. 2012. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proceedings of the National Academy of Sciences, USA 109, E1192–E1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A, Rong H, Jürgens G, Paul Knox J, Wang MH. 2010. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. The Plant Journal 64, 764–774. [DOI] [PubMed] [Google Scholar]