Abstract
OBJECTIVES
Annuloplasty bands and rings are widely used to treat functional tricuspid regurgitation (TR). However, the question as to which is the ideal annuloplasty device remains open. Early and late outcomes of tricuspid valve annuloplasty with flexible band (B-TVA) or rigid ring (R-TVA) are compared in the present study.
METHODS
Between 1999 and 2014, 462 consecutive patients (mean age, 69.2 ± 9.5 years) with grade ≥1+ functional TR (graded from 0 to 3+) underwent either B-TVA (n = 345; mean EuroSCORE II 9.2 ± 10.8%) or R-TVA (n = 117; mean EuroSCORE II 12 ± 13.4%) in addition to other cardiac procedures at the authors' institution.
RESULTS
One-to-one propensity score-matched analysis resulted in 98 pairs with similar baseline characteristics and operative risk. Hospital mortality was 7.5% after B-TVA and 12% after R-TVA (P = 0.14). R-TVA was associated with higher rates of low cardiac output (10.1 vs 17.9%, P = 0.025) and transient complete atrioventricular block (10.3 vs 17.2%, P = 0.046). Among the matched pairs, there were no significant differences in hospital mortality (5.1 vs 9.2%, P = 0.27) and perioperative complications. Both in overall series and matched pairs, between B-TVA and R-TVA patients, there were no significant differences in freedom from all-cause death (P = 0.29 and 0.91), cardiac and cerebrovascular deaths (P = 0.63 and 0.87) and grade ≥2+ TR (P = 0.68 and 0.77). Right atrial and tricuspid valve reverse remodelling combined with right ventricular reverse remodelling occurred after R-TVA but not after B-TVA.
CONCLUSIONS
B-TVA and R-TVA are equally effective in the treatment of functional TR. However, R-TVA causes over time a more complete right heart reverse remodelling.
Keywords: Echocardiography, Prosthesis, Reverse remodelling, Surgery, Valve regurgitation
INTRODUCTION
Functional tricuspid regurgitation (TR) results from dilatation/dysfunction of the right-sided cardiac chambers, with enlargement of the tricuspid annulus and tethering of the tricuspid valve leaflets, which are apparently normal [1, 2]. Although TR can usually be effectively treated with valve annuloplasty, a significant recurrent regurgitation negatively impacts functional class and late survival of the patients [2–6], to whom a new surgical chance is rarely given because reoperations for recurrent TR carry high mortality rates [7]. In this context, it seems ever more urgent the need for establishing which is the optimal surgical treatment (provided there is) for functional TR.
Tricuspid valve annuloplasty (TVA), using an annuloplasty device, band or ring, is widely adopted to treat functional TR [2–7]. Whereas in almost all patients of almost all studies suture TVA seems to offer poor long-term outcomes when compared with device TVA [8, 9], to date there is no clear evidence of the superiority of one annuloplasty device over the other in the sense of long-term control of regurgitation [10].
In the present study, the authors have reviewed retrospectively their 16-year experience in tricuspid valve repair for functional TR using device annuloplasty. Early and late outcomes of TVA with flexible band (B-TVA) or rigid ring (R-TVA) were compared. The focus was placed on both the freedom from significant TR after repair and the right heart reverse remodelling.
PATIENTS AND METHODS
Study patients
From January 1999 to 2014, 462 consecutive patients (mean age, 69.2 ± 9.5 years) with grade ≥1+ functional TR (graded from 0 to 3+) underwent primary TVA either with band (B-TVA group; n = 345, 74.7%) or with ring (R-TVA group; n = 117, 25.3%) in addition to other cardiac procedures at the authors' institution. TR was secondary to mitral valve disease (n = 415, 89.8%), aortic valve disease (n = 148, 32%), ischaemic heart disease (n = 135, 29.2%) or atrial septal defect (n = 5, 1.1%). The baseline characteristics and risk profiles of the patients are listed in Table 1. Unless otherwise stated, definitions were those employed for the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II), which was used to evaluate the operative risk [11].
Table 1:
Baseline characteristics and risk profiles of patients (N = 462)a,b
| Characteristics | Overall series |
PS-matched pairs |
||||
|---|---|---|---|---|---|---|
| B-TVA (N = 345) |
R-TVA (N = 117) |
P-value | B-TVA (N = 98) |
R-TVA (N = 98) |
P-value | |
| Age, years | 69.2 ± 9.3 | 69.3 ± 10.2 | 0.95 | 69.3 ± 9.7 | 68.3 ± 10.7 | 0.51 |
| >75 | 109 (31.6) | 39 (33.3) | 0.73 | 30 (30.6) | 31 (31.6) | 0.89 |
| Female gender | 180 (52.2) | 61 (52.1) | 1 | 60 (61.2) | 52 (53.1) | 0.25 |
| BMI, kg/m2 | 25.4 ± 4.3 | 24.7 ± 4.4 | 0.14 | 24.8 ± 4.3 | 24.6 ± 4.6 | 0.82 |
| >30 | 50 (14.5) | 13 (11.1) | 0.36 | 16 (16.3) | 11 (11.2) | 0.3 |
| Diabetes on insulin | 19 (5.5) | 5 (4.3) | 0.6 | 2 (2) | 3 (3.1) | 1 |
| Serum haemoglobin, g/l | 13 ± 1.8 | 12.5 ± 1.8 | 0.0032 | 12.7 ± 1.8 | 12.7 ± 1.8 | 0.99 |
| <11 | 43 (12.5) | 25 (21.4) | 0.019 | 18 (18.4) | 16 (16.3) | 0.71 |
| Chronic lung diseasec | 37 (10.7) | 11 (9.4) | 0.69 | 10 (10.2) | 9 (9.2) | 0.81 |
| GFR, ml/mind | 61.9 ± 25.1 | 58.4 ± 25.1 | <0.0001 | 59.6 ± 24.5 | 58.7 ± 25 | 0.79 |
| 50–85c | 185 (53.6) | 64 (54.7) | 0.84 | 47 (48) | 54 (55.1) | 0.32 |
| <50c | 112 (32.5) | 38 (32.5) | 1 | 34 (34.7) | 32 (32.7) | 0.76 |
| Chronic dialysis | 8 (2.3) | 4 (3.4) | 0.74 | 1 (1) | 2 (2) | 1 |
| Extracardiac arteriopathyc | 38 (11) | 23 (19.7) | 0.017 | 13 (13.3) | 15 (15.3) | 0.68 |
| Poor mobilityc | 0 | 1 (0.9) | 0.25 | 0 | 0 | – |
| Atrial fibrillation | 110 (31.9) | 30 (25.6) | 0.2 | 30 (30.6) | 28 (28.6) | 0.75 |
| Permanent pacemaker | 4 (1.2) | 1 (0.9) | 0.63 | 2 (2) | 1 (1) | 1 |
| Congestive heart failure | 206 (59.7) | 90 (76.9) | 0.0008 | 76 (77.6) | 73 (74.5) | 0.62 |
| Signs of right heart failure | ||||||
| Jugular venous distension | 100 (29) | 22 (18.8) | 0.031 | 26 (26.5) | 21 (21.4) | 0.4 |
| Palpable hepatomegaly/pulsatile liver | 77 (22.3) | 20 (17.1) | 0.23 | 22 (22.4) | 17 (17.3) | 0.37 |
| Ascites/important peripheral oedema | 9 (2.6) | 6 (5.1) | 0.22 | 4 (4.1) | 4 (4.1) | 1 |
| Unstable angina | 34 (9.9) | 16 (13.7) | 0.25 | 14 (14.3) | 10 (10.2) | 0.38 |
| Recent myocardial infarctionb | 15 (4.3) | 4 (3.4) | 0.79 | 3 (3.1) | 4 (4.1) | 1 |
| Coronary artery disease | 100 (29) | 38 (32.5) | 0.48 | 24 (24.5) | 29 (29.6) | 0.42 |
| LV ejection fraction, % | 55.2 ± 12.3 | 56.4 ± 12.4 | <0.0001 | 55.2 ± 12.7 | 56.1 ± 13 | 0.62 |
| 30–50c | 91 (26.4) | 24 (20.5) | 0.2 | 21 (21.4) | 20 (20.4) | 0.86 |
| ≤30c | 16 (4.6) | 5 (4.3) | 0.86 | 6 (6.1) | 5 (5.1) | 0.75 |
| RV fractional area change, % | 38.3 ± 9.9 | 37.7 ± 9.9 | 0.57 | 38.2 ± 12.1 | 37.6 ± 9.5 | 0.7 |
| <35 | 35 (10.1) | 16 (12.8) | 0.31 | 13 (13.3) | 13 (13.3) | 1 |
| Pulmonary hypertensione | 77 (22.3) | 29 (24.8) | 0.58 | 34 (34.7) | 25 (25.5) | 0.16 |
| TR severity | ||||||
| 1+, mild | 109 (31.6) | 26 (22.2) | 0.15 | 34 (34.7) | 33 (33.7) | 0.99 |
| 2+, moderate | 153 (44.3) | 60 (51.3) | 43 (43.9) | 44 (44.9) | ||
| 3+, severe | 83 (24.1) | 31 (26.5) | 21 (21.4) | 21 (21.4) | ||
| Previous cardiac surgery | 40 (11.6) | 18 (15.4) | 0.29 | 14 (14.3) | 14 (14.3) | 1 |
| Active endocarditisb | 0 | 4 (3.4) | 0.004 | 0 | 0 | – |
| Critical statec | 15 (4.3) | 10 (8.5) | 0.083 | 6 (6.1) | 5 (5.1) | 0.75 |
| Aortic disease | 13 (3.7) | 6 (5.1) | 0.59 | 5 (5.1) | 5 (5.1) | 1 |
| Surgical procedures, 3 or morec | 169 (49) | 63 (53.8) | 0.36 | 45 (45.9) | 48 (49) | 0.67 |
| Surgical priorityc | ||||||
| Elective | 253 (73.3) | 71 (60.7) | 0.027 | 60 (61.2) | 65 (66.3) | 0.75 |
| Urgent | 89 (25.8) | 43 (36.8) | 37 (37.8) | 32 (32.7) | ||
| Emergency | 3 (0.9) | 2 (1.7) | 1 (1) | 1 (1) | ||
| Salvage | 0 | 1 (0.9) | 0 | 0 | ||
| Expected operative risk (by EuroSCORE II), %f | 9.2 ± 10.8 | 12 ± 13.4 | 0.015 | 10.5 ± 11.5 | 9.9 ± 10.5 | 0.7 |
B-TVA: band tricuspid valve annuloplasty; BMI: body mass index; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GFR: glomerular filtration rate; LV: left ventricular; PS: propensity score; R-TVA: ring tricuspid valve annuloplasty; RV: right ventricular; SD: standard deviation; TR: tricuspid regurgitation.
aValues are the number of patients with percentages in brackets or mean ± SD.
bPatients' clinical characteristics and echocardiographic parameters were compared using the χ2, Fisher's exact or McNemar's test for dichotomous variables, and the Student's t-test, Mann–Whitney U or Wilcoxon rank-sum test for continuous variables. The matched and unmatched patients were compared with paired or unpaired tests, respectively.
cDefinitions were those employed for EuroSCORE II (ref. [11]).
dThe creatinine clearance rate, calculated according to the Cockcroft–Gault formula, was used for approximating the GFR.
eSystolic pulmonary artery pressure ≥60 mmHg.
fRef. [11].
Echocardiographic assessment
All patients underwent two-dimensional and Doppler echocardiographic assessment preoperatively, intraoperatively, at discharge and during the follow-up period. Evaluations were performed according to the international echocardiographic recommendations [12]. The mechanisms involved in TR were confirmed by both an intraoperative echocardiographic examination and a direct evaluation of the pathological tricuspid valves. The severity of TR was graded as follows: grade 0 = null or trivial; grade 1 = mild (regurgitant jet area <5 cm2; vena contracta width <3 mm); grade 2 = moderate (regurgitant jet area ≥5 cm2 but <10 cm2; vena contracta width ≥3 mm but <7 mm) and grade 3 = severe (regurgitant jet area >10 cm2; vena contracta width ≥7 mm; effective regurgitant orifice area ≥20 mm2) [13]. The tricuspid annular diameter was measured at end-diastole in the four-chamber view; tethering area of the tricuspid valve (defined as the area enclosed by the annular plane and the three leaflets) and the tenting height of the tricuspid valve leaflets (defined as the minimal distance between the leaflet coaptation and the tricuspid annular plane) were measured at mid-systole in the four-chamber view. The systolic pulmonary artery pressure was estimated by measuring the peak velocity of the tricuspid regurgitant jet at continuous-wave Doppler analysis, and using the modified Bernoulli's equation. The transtricuspid velocity, measured by a continuous-wave Doppler technique, was used to calculate the transtricuspid mean pressure gradient after TVA. The right ventricular (RV) fractional area change was retrospectively measured by reviewing recorded echocardiographic examinations, and adopted for RV systolic functional assessment. The left ventricular (LV) end-diastolic and end-systolic volumes were estimated using the biplane method of discs (modified Simpson's rule), and the LV ejection fraction was calculated. Regional LV function was analysed using a 16-segment model on multiple short-axis views, with each segment being analysed and scored on the basis of its motion and systolic thickening. The LV wall motion score index was derived as a sum of individual segment scores divided by the number of segments visualized.
Surgery
Surgery was carried out via a median sternotomy with bicaval venous cannulation and cardiopulmonary bypass. Myocardial protection was achieved with either multidose cold blood cardioplegia or (since July 2009) single-dose crystalloid solution [Custodiol–histidine–tryptophan–ketoglutarate (Custodiol-HTK®) solution; Essential Pharma, Newtown, PA, USA], both delivered in an antegrade and retrograde mode.
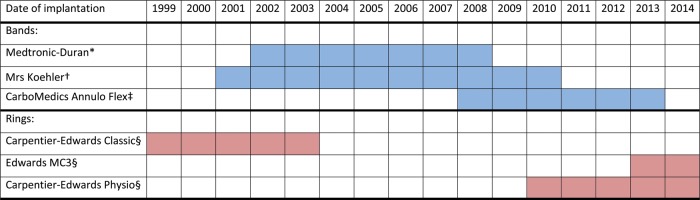
Tricuspid valve repair was usually performed during cross-clamping of the ascending aorta (the valve repair technique within a beating heart was occasionally adopted) either with band or with ring annuloplasty. Six different models of tricuspid annuloplasty devices have been used: three flexible bands [14, 15], one classic rigid ring [16] and two three-dimensional rigid rings [17] (Table 2 and Fig. 1). Both tricuspid valve sizing and annuloplasty device implantation were performed with specific obturators and holders. The tricuspid valve was sized using the straight margin of the obturators to measure the base of the septal leaflet. The selected annuloplasty band was then implanted onto the tricuspid annulus by 2-0 braided sutures placed circumferentially along the hinge of the anterior and posterior leaflets; the selected annuloplasty ring was implanted along the hinge of the anterior and posterior leaflets and part of the septal leaflet. Annular plication was obtained by passing the suture ends closer to each other onto the band (or the ring) compared with the corresponding bites in the annulus, with most of the plication made along the posterior leaflet [18].
Table 2:
Operative data (N = 462)a,b
| Procedure | Overall series |
PS-matched pairs |
||||
|---|---|---|---|---|---|---|
| B-TVA (N = 345) |
R-TVA (N = 117) |
P-value | B-TVA (N = 98) |
R-TVA (N = 98) |
P-value | |
| Tricuspid valve annuloplasty | ||||||
| Band | ||||||
| Medtronic-Duran®c | 66 (19.1) | – | 16 (16.3) | – | ||
| Koehler mrs®d | 98 (28.4) | – | 26 (26.5) | – | ||
| CarboMedics Annulo Flex®e | 181 (52.5) | – | 56 (57.1) | – | ||
| Ring | ||||||
| Carpentier–Edwards Classic®f | – | 25 (21.4) | – | 23 (23.5) | ||
| Edwards MC3®f | – | 24 (20.5) | – | 22 (22.4) | ||
| Carpentier–Edwards Physio®f | – | 68 (58.1) | – | 53 (54.1) | ||
| Annuloplasty device size, mm | 32 ± 2.1 | 31.8 ± 2.4 | 0.39 | 31.7 ± 2 | 31.9 ± 2.3 | 0.52 |
| Concomitant procedure | ||||||
| Mitral valve surgery | 310 (89.9) | 105 (89.7) | 1 | 84 (85.7) | 90 (91.8) | 0.17 |
| Aortic valve surgery | 109 (31.6) | 39 (33.3) | 0.73 | 39 (39.8) | 28 (28.6) | 0.098 |
| Thoracic aortic surgery | 14 (4.1) | 6 (5.1) | 0.62 | 6 (6.1) | 5 (5.1) | 0.75 |
| Coronary bypass surgery | 96 (27.8) | 35 (29.9) | 0.66 | 23 (23.5) | 29 (29.6) | 0.33 |
| LV reconstruction | 4 (1.2) | 0 | 0.31 | 0 | 0 | – |
| Cryosurgical ablation for atrial fibrillation | 67/110 (60.9) | 14/30 (46.7) | 0.16 | 17/30 (56.7) | 14/28 (50) | 0.61 |
| Pacemaker implantation and lead placing | 6 (1.7) | 5 (4.3) | 0.16 | 2 (2) | 3 (3.1) | 1 |
| Atrial septal defect closure | 3 (0.9) | 2 (1.7) | 0.6 | 1 (1) | 2 (2) | 1 |
| Otherg | 14 (4.1) | 3 (2.6) | 0.58 | 4 (4.1) | 2 (2) | 0.68 |
| Aortic cross-clamping time, min | 125 ± 38 | 125 ± 37 | 0.98 | 125 ± 42 | 124 ± 36 | 0.85 |
| Use of Custodiol-HTK® solutionh | 143 (41.4) | 67 (57.3) | 0.003 | 42 (42.9) | 52 (53.1) | 0.15 |
| Cardiopulmonary bypass time, min | 164 ± 51 | 173 ± 54 | 0.095 | 161 ± 55 | 172 ± 53 | 0.17 |
| Duration of surgery, min | 308 ± 90 | 327 ± 96 | 0.051 | 299 ± 91 | 323 ± 94 | 0.068 |
B-TVA: band tricuspid valve annuloplasty; HTK: histidine–tryptophan–ketoglutarate; PS: propensity score; R-TVA: ring tricuspid valve annuloplasty; SD: standard deviation; LV: left ventricular.
aValues are the number of patients with percentages in brackets or mean ± SD.
bData were compared using the χ2 or Fisher's exact test for dichotomous variables, and the Student's t-test for continuous variables. The matched and unmatched patients were compared with paired or unpaired test, respectively.
cMedtronic, Minneapolis, MN, USA.
dKoehler, Bellshill, Scotland.
eSorin-CarboMedics, Austin, TX, USA.
fEdwards Lifesciences, Irvine, CA, USA.
gPatent foramen ovale closure = 3, septal myectomy = 2, pericardiectomy = 3, pulmonic valve replacement = 1, coronary fistula ligature = 1, femoral–femoral bypass = 2, carotid endarterectomy = 2, mastectomy = 1, splenectomy = 1 and right hemicolectomy = 1.
hEssential Pharma, Newtown, PA, USA.
Figure 1:
The six different models of tricuspid annuloplasty devices that have been implanted between 1999 and 2014 at the authors' institution and the corresponding period of implantation. Asterisk denotes Medtronic, Minneapolis, MN, USA. Dagger denotes Koehler, Bellshill, Scotland. Double dagger denotes Sorin-CarboMedics, Austin, TX, USA. Sections denote Edwards Lifesciences, Irvine, CA, USA.
The concomitant surgical procedures are listed in Table 2.
Follow-up
Deaths and complications were defined according to the guidelines proposed by Akins et al. [19]. Perioperative data were recorded prospectively for all patients in a computed data registry (FileMaker® Pro 12.0; FileMaker, Inc., Santa Clara, CA, USA). The clinical and echocardiographic follow-up was conducted yearly at the present authors' cardiac units. For each discharged patient, the echocardiographic records before operation, at hospital discharge and during the follow-up period were reviewed, separately, by two cardiologists with experience in echocardiographic examination. For every hospital discharged patient, the present authors have obtained and recorded an echocardiographic study that has been performed within 1 year from the date of conclusion of follow-up, or from the date of death. All comparative analyses of echocardiographic measurements were performed between preoperative imaging recorded early before surgery (baseline) and postoperative imaging at the latest follow-up. For the purposes of this study, the follow-up was closed on 1 March 2015.
Approval to conduct the study was acquired from the Institutional Ethics Committee, based on retrospective data retrieval, having waived the need for patients to provide their individual written consent.
Statistical methods
Data were expressed as the number of patients with the percentage in brackets, or mean ± standard deviation. A risk factor analysis for hospital death was performed. All significant variables from the univariable analysis were entered into a multivariable analysis using the binary logistic regression model and the odds ratio, with 95% confidence interval (CI), was calculated for each significant variable. The Cox proportional-hazards model was used to determine the influence of patients' characteristics and echocardiographic measurements on late survival. The hazard ratio with 95% CI was calculated for each variable. The Grambsch–Therneau test was used to verify that the proportionality assumption holds true. Since the study groups significantly differed in a number of baseline variables, a multivariate analysis was performed using logistic regression and a propensity score was calculated to estimate the probability of being assigned either to the B-TVA or to the R-TVA group. The area under the receiver operating characteristic curve was used to represent the regression probabilities. This propensity score was calculated in a non-parsimonious way including preoperative variables and operative data. Data were matched using the probability scale. One-to-one propensity matching was performed. The caliper width chosen was 0.2 times the standard deviation of the propensity score. Non-parametric estimates and curves of freedom from all-cause death, cardiac and cerebrovascular deaths and grade ≥2+ TRs were generated with the Kaplan–Meier method. Comparisons between survival curves were made by the log-rank test. A value of P < 0.05 was considered to be statistically significant. Analyses were performed with IBM SPSS Statistics (IBM Software Group).
RESULTS
The overall series
The B-TVA and R-TVA groups were markedly different in a number of baseline characteristics and comorbidities, and the mean expected operative risk in R-TVA patients was greater than that in B-TVA patients (P = 0.015; Table 1). There were no intergroup differences in operative data, except for the use of Custodiol-HTK solution (P = 0.003; Table 2). Hospital mortality was 7.5% after B-TVA and 12% after R-TVA (P = 0.14; Table 3). Glomerular filtration rate <50 ml/min (P = 0.0002) and previous cardiac surgery (P = 0.0046) were risk factors for hospital death according to multivariable analysis (Supplementary Table 1). Prolonged invasive ventilation, low cardiac output, multiple blood transfusion and mediastinal re-exploration for bleeding and tamponade were the most frequent major perioperative complications. Atrial fibrillation (new-onset) was more frequent in B-TVA patients (P = 0.0049). R-TVA was associated with higher rates of transient complete atrioventricular block (P = 0.046) and low cardiac output (P = 0.025; Table 3).
Table 3:
Hospital mortality, perioperative complications and hospital course of patients (N = 462)a,b
| Event | Overall series |
PS-matched pairs |
||||
|---|---|---|---|---|---|---|
| B-TVA (N = 345) |
R-TVA (N = 117) |
P-value | B-TVA (N = 98) |
R-TVA (N = 98) |
P-value | |
| In-hospital death | 26 (7.5) | 14 (12) | 0.14 | 5 (5.1) | 9 (9.2) | 0.27 |
| Stroke | 4 (1.2) | 0 | 0.36 | 3 (3.1) | 0 | 0.25 |
| Prolonged (>48 h) invasive ventilation | 64 (18.6) | 30 (25.6) | 0.1 | 18 (18.4) | 21 (21.4) | 0.59 |
| Atrial fibrillation, new-onset | 96/231c (41.6) | 21/86c (24.4) | 0.0049 | 25/66c (37.9) | 17/69c (24.6) | 0.097 |
| Transient complete atrioventricular block | 35/341d (10.3) | 20/116d (17.2) | 0.046 | 10/96d (10.4) | 14/97d (14.4) | 0.67 |
| Myocardial infarction | 1 (0.3) | 0 | 0.75 | 0 | 0 | – |
| Low cardiac outpute | 35 (10.1) | 21 (17.9) | 0.025 | 9 (9.2) | 14 (14.3) | 0.27 |
| Use of adrenergic agonists | 256 (74.2) | 91 (77.8) | 0.44 | 78 (79.6) | 73 (74.5) | 0.4 |
| Intra- and postoperative use of IABP | 7 (2) | 3 (2.6) | 0.49 | 0 | 1 (1) | 0.5 |
| Use of ECMO | 2 (0.6) | 0 | 0.56 | 0 | 0 | – |
| Acute kidney injuryf | 19 (5.5) | 12 (10.3) | 0.076 | 3 (3.1) | 7 (7.1) | 0.19 |
| Renal replacement therapy | 13 (3.8) | 8 (6.8) | 0.17 | 2 (2) | 5 (5.1) | 0.44 |
| Bleeding peptic ulcer | 1 (0.3) | 1 (0.9) | 0.44 | 0 | 0 | – |
| Mesenteric ischaemia | 3 (0.9) | 1 (0.9) | 0.73 | 1 (1) | 1 (1) | 1 |
| Acute pancreatitis | 0 | 1 (0.9) | 0.25 | 0 | 0 | – |
| Multiorgan failure | 22 (6.4) | 11 (9.4) | 0.27 | 4 (4.1) | 5 (5.1) | 1 |
| Sepsis | 8 (2.3) | 2 (1.7) | 0.51 | 1 (1) | 1 (1) | 1 |
| 48-h chest tube output, ml | 1371 ± 1510 | 1836 ± 2336 | 0.084 | 1412 ± 1552 | 1763 ± 2306 | 0.27 |
| Multiple blood transfusion (>2 RBCs) | 120 (34.8) | 33 (28.2) | 0.19 | 37 (37.8) | 26 (26.5) | 0.093 |
| Mediastinal re-explorationg | 69 (20) | 24 (20.5) | 0.92 | 20 (20.4) | 19 (19.4) | 0.86 |
| Hospital stay, days | 17.6 ± 22.6 | 18.7 ± 19.8 | 0.74 | 17.7 ± 14.6 | 17.3 ± 17.4 | 0.18 |
B-TVA: band tricuspid valve annuloplasty; ECMO: extracorporeal membrane oxygenator; HTK: histidine–tryptophan–ketoglutarate; IABP: intra-aortic balloon pumping; PS: propensity score; RBCs: packed red blood cells; R-TVA: ring tricuspid valve annuloplasty; SD: standard deviation.
aValues are the number of patients with percentages in brackets or mean ± SD.
bPerioperative data were compared using the χ2 or Fisher's exact test for dichotomous variables, and the Mann–Whitney U-test or Wilcoxon rank-sum test for continuous variables. The matched and unmatched patients were compared with the paired or unpaired test, respectively.
cPatients with preoperative stable sinus rhythm or paroxysmal atrial fibrillation.
dPatients without preoperative pacemaker.
eLow cardiac output was defined as three consecutive cardiac index measurements <2.0 l/min/m2 despite adequate preload, afterload and inotropic support or intra-aortic balloon pumping.
fPostoperative acute kidney injury was defined as postoperative serum creatinine >2.0 mg/l in the patients without preoperative renal impairment, and postoperative increase in serum creatinine of at least 1.0 mg/l above baseline in the patients with preoperative renal impairment.
gThrough resternotomy or subxyphoid window.
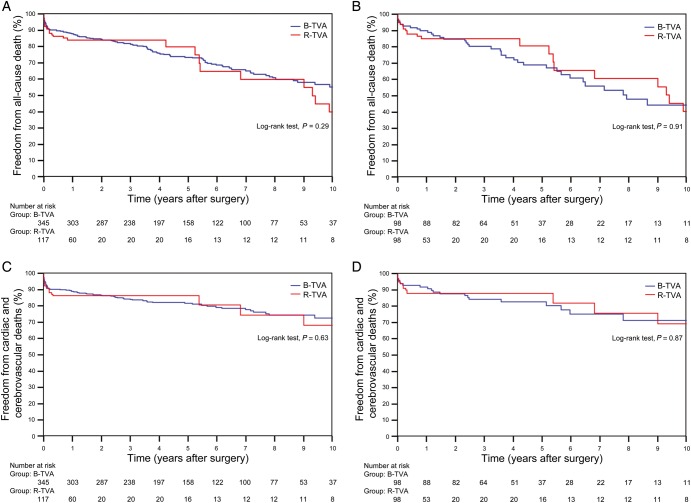
The follow-up was 100% complete for the 422 remaining patients, and a total of 1998.1 cumulative patient-years were reviewed. In the B-TVA group, the mean follow-up was of 5.7 ± 3.1 years; there were 112 deaths, 72 cardiac or cerebrovascular deaths and 40 non-cardiac non-cerebrovascular deaths. In the R-TVA group, the mean follow-up was of 3.2 ± 4.2 years; there were 28 deaths, 19 cardiac or cerebrovascular deaths and 9 non-cardiac non-cerebrovascular deaths. Between the two groups, there were no significant differences in the non-parametric estimates of freedom from all-cause death (P = 0.29; Fig. 2A) and cardiac and cerebrovascular deaths (P = 0.63; Fig. 2C). Old age (P < 0.0001), extracardiac arteriopathy (P < 0.0001), congestive heart failure (P = 0.042), LV ejection fraction of <50% (P = 0.0003) and RV fractional area change of <35% (P = 0.0049) were predictors of late death (Supplementary Table 2). The New York Heart Association class, the Canadian Cardiovascular Society class and the signs of right heart failure were improved (P < 0.01) for both B-TVA and R-TVA patients. Postoperative echocardiographic assessment showed a significant reduction in both the central venous pressure and the systolic pulmonary artery pressure; the RV fractional area change was improved. In B-TVA patients, although the LV ejection fraction was decreased (P = 0.045), the LV wall motion score index remained unchanged (P = 0.55; Table 4).
Figure 2:
Non-parametric curves (Kaplan–Meier model) of freedom from (A and B) all-cause death (including hospital mortality) and (C and D) cardiac and cerebrovascular deaths (including hospital mortality). (A and C) The overall series: in B-TVA patients, the 1-, 5- and 10-year non-parametric estimates of freedom from all-cause death were 87.8 (95% CI: 86–89.6), 73.4 (95% CI: 70.9–75.9) and 55.2% (95% CI: 51.3–59.1), respectively, and the 1-, 5- and 10-year non-parametric estimates of freedom from cardiac and cerebrovascular deaths were 89 (95% CI: 87.3–90.7), 81.5 (95% CI: 79.3–83.2) and 72.6% (95% CI: 69.2–76), respectively; in R-TVA patients, the 1-, 5- and 10-year non-parametric estimates of freedom from all-cause death were 84 (95% CI: 80.5–87.5), 79.8 (95% CI: 74.5–85.1) and 39.9% (95% CI: 29.6–50.2), respectively, and the 1-, 5- and 10-year non-parametric estimates of freedom from cardiac and cerebrovascular deaths were 86.3 (95% CI: 83.1–89.5), 80.5 (95% CI: 74.2–86.8) and 68.1% (95% CI: 58.4–77.8), respectively. (B and D) The PS-matched pairs. The number of patients remaining at risk is reported. B-TVA: tricuspid valve annuloplasty with flexible band; PS: propensity score; R-TVA: tricuspid valve annuloplasty with rigid ring.
Table 4:
Postoperative echocardiographic changes (N = 422)a,b,c
| Parameter | Overall series |
PS-matched pairs |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-TVA (N = 319) |
R-TVA (N = 103) |
B-TVA (N = 93) |
R-TVA (N = 89) |
|||||||||
| Baseline | Follow-up | P-value | Baseline | Follow-up | P-value | Baseline | Follow-up | P-value | Baseline | Follow-up | P-value | |
| TR severity | ||||||||||||
| 0, null or trivial | – | 143 | <0.0001 | – | 61 | <0.0001 | – | 45 | <0.0001 | – | 55 | <0.0001 |
| 1+, mild | 103 | 151 | 22 | 37 | 33 | 41 | 32 | 30 | ||||
| 2+, moderate | 143 | 21 | 55 | 3 | 41 | 7 | 40 | 2 | ||||
| 3+, severe | 73 | 4 | 26 | 2 | 19 | 0 | 17 | 2 | ||||
| Mean transtricuspid pressure gradient, mmHg | – | 3 ± 2.2 | – | – | 2.5 ± 1.4 | – | – | 2.6 ± 1.6 | – | – | 2.3 ± 1.3 | – |
| Central venous pressure, mmHg | 8.3 ± 2.1 | 7.7 ± 1.7 | 0.0012 | 9.3 ± 2.6 | 7.8 ± 1.3 | <0.001 | 8.6 ± 2.2 | 8 ± 2 | 0.11 | 9 ± 2.5 | 7.8 ± 1.4 | 0.01 |
| Systolic pulmonary artery pressure, mmHg | 47.6 ± 15.4 | 39 ± 13.6 | <0.0001 | 54.6 ± 15.7 | 39.5 ± 12.9 | <0.0001 | 52.7 ± 17 | 41 ± 16.1 | 0.0011 | 52.4 ± 15.3 | 38.7 ± 13.1 | 0.0011 |
| RV fractional area change, % | 38.5 ± 10 | 40.8 ± 7.7 | 0.0097 | 37.7 ± 9.9 | 43 ± 9.8 | 0.006 | 38.2 ± 12.1 | 41.7 ± 9 | 0.1 | 37.6 ± 9.5 | 42.9 ± 10.3 | 0.017 |
| LV ejection fraction, % | 56.4 ± 12.3 | 54.2 ± 11.9 | 0.045 | 56.8 ± 12 | 53.2 ± 13.3 | 0.13 | 56.8 ± 11.6 | 56.6 ± 12.7 | 0.93 | 57 ± 12.7 | 53.1 ± 13.4 | 0.15 |
| LV wall motion score index | 1.21 ± 0.42 | 1.24 ± 0.4 | 0.55 | 1.24 ± 0.4 | 1.3 ± 0.4 | 0.45 | 1.12 ± 0.26 | 1.15 ± 0.31 | 0.63 | 1.24 ± 0.43 | 1.29 ± 0.43 | 0.61 |
B-TVA: band tricuspid valve annuloplasty; LV: left ventricular; PS: propensity score; R-TVA: ring tricuspid valve annuloplasty; RV: right ventricular; SD: standard deviation; TR: tricuspid regurgitation.
aThe hospital discharged patients.
bValues are the number of patients with percentages in brackets or mean ± SD.
cEchocardiographic parameters were compared using the McNemar's test for dichotomous variables, and the Student's t-test, Mann–Whitney U-test or Wilcoxon rank-sum test for continuous variables. The matched and unmatched patients were compared with paired or unpaired tests, respectively.
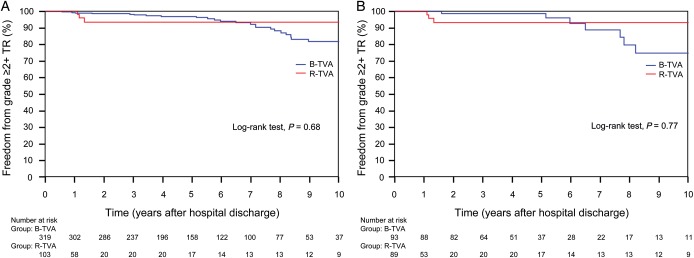
Residual TR was null or trivial for all discharged patients. During the follow-up, the grade of TR was improved (P < 0.0001) for both B-TVA and R-TVA patients (Table 4). Out of the 30 (7.1%) patients who had moderate (n = 24) or severe (n = 6) recurrent TR, no patient underwent reoperation due to TR. The actual recurrence rate of TR in B-TVA and R-TVA patients was 7.8 and 4.9%, respectively (P = 0.3), and there was no significant difference between the two groups as the non-parametric estimate of freedom from grade ≥2+ TR (P = 0.68). In B-TVA patients, there was a mean increase of recurrent TR of 2 ± 2% by year. In R-TVA patients, otherwise, recurrent TR remained at a constant rate starting from over 1 year from hospital discharge (Fig. 3A). In both groups of patients, there were no cases of significant tricuspid stenosis (Table 4).
Figure 3:
Non-parametric curves (Kaplan–Meier model) of freedom from grade ≥2+ TR. (A) The overall series: in B-TVA patients, the 1-, 5- and 10-year non-parametric estimates of freedom from grade ≥2+ TR were 99.3 (95% CI: 98.8–99.8), 96.9 (95% CI: 95.8–98) and 81.8% (95% CI: 78–85.6), respectively; in R-TVA patients, the 1-, 5- and 10-year non-parametric estimates of freedom from grade ≥2+ TR were 98.1 (95% CI: 96.2–100), 93.6 (95% CI: 90–97.2) and 93.6% (95% CI: 90–97.2), respectively. (B) The PS-matched pairs. The number of patients remaining at risk is reported. B-TVA: tricuspid valve annuloplasty with flexible band; PS: propensity score; R-TVA: tricuspid valve annuloplasty with rigid ring; TR: tricuspid regurgitation.
The propensity score-matched pairs
A propensity score was estimated by logistic regression. The area under the receiver operating characteristic curve was 0.76 (95% CI: 0.72–0.81). One-to-one propensity score-matched analysis resulted in 98 pairs with similar baseline characteristics and operative risk (Table 1). Among the matched pairs, there were no significant differences in operative data (Table 2), hospital mortality (P = 0.27), perioperative complications (Table 3), freedom from all-cause death (P = 0.91; Fig. 2B), cardiac and cerebrovascular deaths (P = 0.87; Fig. 2D) and grade ≥2+ TR (P = 0.77; Fig. 3B) between B-TVA and R-TVA patients.
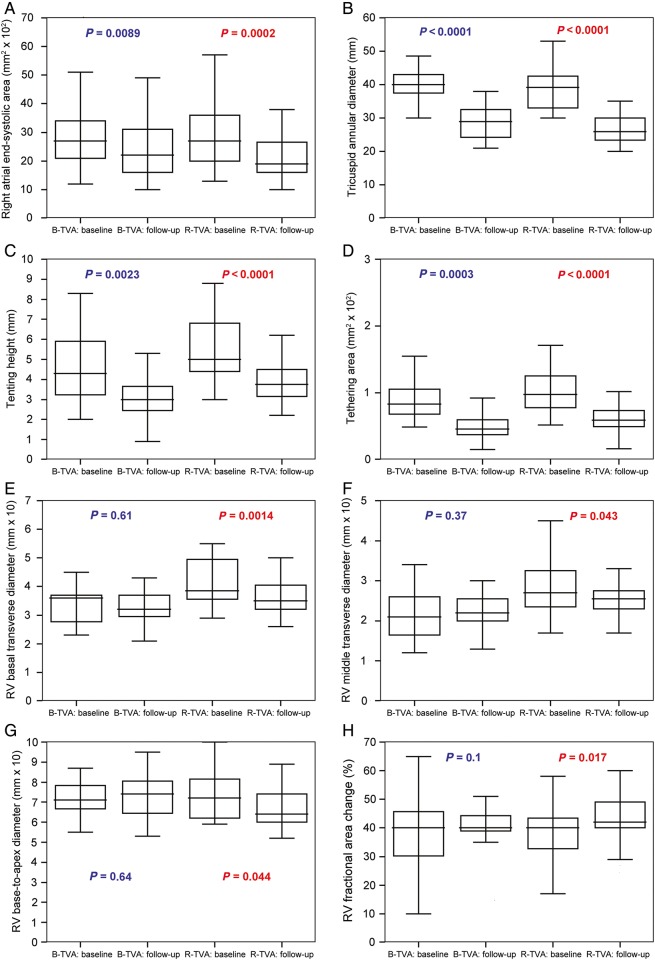
The central venous pressure (P = 0.01), the systolic pulmonary artery pressure (P = 0.0011) and the RV fractional area change (P = 0.017) were all improved in R-TVA patients; in B-TVA patients, only the systolic pulmonary artery pressure was improved (P = 0.0011; Table 4 and Fig. 4). The measured dimensions of the right atrium (P = 0.0002), the tricuspid annulus (P < 0.0001) and the right ventricle (P < 0.045) were all decreased after R-TVA; after B-TVA, only the dimensions of the right atrium (P = 0.0089) and the tricuspid annulus (P < 0.0024) were decreased (Fig. 4).
Figure 4:
The right heart reverse remodelling in the PS-matched pairs: (A) the right atrium, (B–D) the tricuspid valve and (E–H) the right ventricle. Box-and-whisker plot: the central box represents the values from the lower to upper quartile; the middle line represents the median; a line extends from the minimum to the maximum value. B-TVA: tricuspid valve annuloplasty with flexible band; PS: propensity score; R-TVA: tricuspid valve annuloplasty with rigid ring; RV: right ventricular.
DISCUSSION
Valve annuloplasty is the preferred surgical treatment for functional TR today, with a recurrence rate, defined as postoperative moderate or severe TR, ranging from 2.5 to 5.5% at 1-year follow-up [2]. Significant degrees of annular reduction requiring durability are best accomplished with bands or rings [2–6], suture annuloplasty also being employed but for mild-to-moderate annular dilatation [8, 9]. Throughout the years, various annuloplasty devices have been devised and implanted onto the tricuspid annulus of patients with functional TR in order to enable annular reverse remodelling after surgery [10, 14–18, 20]. To simplify, three device employment trends across time have been demonstrated: (i) the use of standard rigid rings was predominant in the last-1990s; (ii) flexible bands were increasingly employed from the early-2000s and (iii) three-dimensional rigid rings were mostly used in recent years [21]. These employment trends have been the same in the present authors' experience as well (Fig. 1). However, despite an extended use of annuloplasty devices to treat functional TR, there are few studies that compare specifically band versus ring annuloplasty. Bands and rings seem to be equally effective in the control of regurgitation even though rings have sometimes lower rates of recurrent TR [10, 22]. Actually, at least on a speculative basis, annuloplasty bands could offer specific benefits (over rings) due to the inherent flexibility and the simpler design and technique of implantation. There is a lower risk of device dehiscence or fracture and tricuspid stenosis even after undersized annuloplasty. There is virtually no risk of injuring the conduction tissue and the right coronary artery, or the aortic box during implantation within a beating heart. Finally, flexible bands could best preserve RV function and help the RV functional recovery after surgery [10, 14, 15, 18]. Nevertheless, despite all these benefits, there is no evidence of the superiority of B-TVA over R-TVA. If anything, the opposite is true [10]. To date, however, no investigator has explored and compared the pattern of reverse remodelling of the right heart with either after B-TVA or R-TVA.
Among the 462 consecutive patients with functional TR who underwent primary device annuloplasty at the authors' institution from 1999 throughout 2014, B-TVA and R-TVA were performed in 345 and 117 patients, respectively. The immediate and long-term outcomes were reviewed retrospectively and compared in the present study. Almost all patients underwent surgery due to a concomitant left-sided heart valve disease. Rheumatic valve disease, myxomatous degeneration, mitral annular calcification and ischaemic heart disease were the most frequent causes of mitral valve disease. Degenerative calcification and bicuspid aortic stenosis were the most common causes of isolated aortic valve disease. Mitral insufficiency or steno-insufficiency and aortic stenosis were the most frequent mechanisms of valve failure. In every patient, surgery was carried out via a median sternotomy. Tricuspid valve repair was usually performed during cross-clamping of the ascending aorta and six various models of tricuspid annuloplasty device—three flexible bands [14, 15], one classic rigid ring [16] and two three-dimensional rigid rings [17]—have been used, separately, during six different periods of the study (which were all longer than 1 year).
In both the B-TVA and R-TVA groups, the average risk profile of the patients was high, mainly due to the high prevalence of severe renal impairment among the recorded comorbidities and of severe signs and symptoms of heart failure and advanced coronary artery disease. Furthermore, previous cardiac surgery, active endocarditis, critical state and salvage surgical priority were not exclusion criteria for the patients of the study. In particular, the mean expected operative risk (by EuroSCORE II) in R-TVA patients was higher than that in B-TVA patients due to more frequent extracardiac arteriopathy, congestive heart failure and active endocarditis and the higher surgical priority. Hospital mortality in R-TVA group was consistently higher than that in the B-TVA group even though the difference was not significant. Severe renal impairment and previous cardiac surgery were predictors of hospital death, and low cardiac output and multiorgan failure were the most frequent causes. Prolonged invasive ventilation, low cardiac output, acute kidney injury, multiple blood transfusion and mediastinal re-exploration for bleeding and tamponade were frequent major perioperative complications. In the literature, poor immediate outcomes are usually reported after surgery for TR secondary to left-sided heart valve disease [6, 8–10, 14–18, 20–23]. This is due to the need for prolonged surgical times to treat multiple valve disease in the presence of LV and/or RV dysfunction and pulmonary hypertension. In the present series of patients, 832 surgical procedures were performed in addition to TVA, with a procedure-to-patient ratio of 2.8 in both the B-TVA and R-TVA groups. Indeed, combined mitral and aortic valve surgery, coronary artery bypass grafting and cryosurgical ablation for atrial fibrillation were common and some patients underwent concomitant aortic surgery or LV reconstruction. Since 2009, myocardial protection using the Custodiol-HTK solution has been adopted precisely in order to reduce the duration of operation and has been used more frequently in R-TVA patients. In these patients, the duration of surgery was consistently longer (albeit almost significantly) even though there were apparently no other intergroup differences in operative data. Among the perioperative complications, atrial fibrillation was more frequent in the B-TVA group and R-TVA patients experienced higher rates of low cardiac output and transient complete atrioventricular block. However, these differences were not confirmed among the propensity score-matched pairs.
During the follow-up, dyspnoea, angina and the signs of right heart failure were improved. Freedom from all-cause death and cardiac and cerebrovascular deaths were comparable, or compared favourably, with those cited in previous reports dealing with outcomes of patients with functional TR undergoing surgery [6, 8–10, 14–18, 20–23]. The treatment of every left-sided, valvular and non-valvular, lesion, the use of cryosurgical ablation in about half patients with atrial fibrillation, myocardial revascularization of every critically stenotic vessel ≥1 mm of diameter and the extensive use of one (93.1%) or both internal thoracic arteries (24.4%) in ischaemic heart disease may give reason of these good late results. Old age, extracardiac arteriopathy, congestive heart failure and the LV/RV dysfunction were predictors of decreased late survival. Among the matched pairs, there were no significant differences between B-TVA and R-TVA patients in freedom from all-cause death and cardiac and cerebrovascular deaths.
TR was null or trivial early after repair and was controlled within grade 1+ in ∼93% of patients after hospital discharge. Freedom from recurrent grade ≥2+ TR was very good in both B-TVA and R-TVA patients and compared favourably with previous data of the literature [6, 8–10, 14–18, 20–23]. The use of an annuloplasty device even for patients with mild TR (but only on condition that there were severe tricuspid annular dilatation, RV dysfunction or pulmonary hypertension) [24, 25] in addition to the effective and lasting treatment of left-sided heart valve diseases (also confirmed by pulmonary artery pressure reduction after surgery) may give reason of these encouraging results. The analysis performed on the echocardiographic, dimensional and functional changes in the right-sided cardiac chambers of the propensity score-matched pairs either after B-TVA or after R-TVA has identified two different patterns of right heart reverse remodelling: after R-TVA, the right atrial and the tricuspid valve reverse remodelling combined with the RV reverse remodelling (and functional improvement); after B-TVA, there were significant changes in neither the RV dimensions nor function. To synthesize, TR was controlled within grade 1+ in both B-TVA and R-TVA patients, but only for R-TVA patients there was a complete right heart reverse remodelling that includes the RV. Speculations can be made to explain this interesting result. Among the R-TVA patients with recurrent grade 1+ or less of TR, there could be a greater rate of patients with null or trivial regurgitation (against B-TVA patients). A lower coaptation reserve of the tricuspid valve leaflets in B-TVA patients could help higher grades of regurgitation during exercise or other stress conditions. These hypotheses should be investigated by means of stress or three-dimensional echocardiographic assessment or magnetic resonance imaging. Anyhow, these results could account for the constant rate of recurrent TR after R-TVA (but not after B-TVA) and justify the increasingly extended use in recent years of three-dimensional annuloplasty rings [17, 20–22] even for less than severe functional TR [24, 25].
Study limitations
The primary limitations of the present study were the retrospective nature of the analysis, and the fact that the patients were evaluated at different times after surgery. Geometric changes in the tricuspid valve apparatus and cardiac chambers were assessed using two-dimensional echocardiography and no three-dimensional evaluation was carried out. As patients with concomitant coronary artery bypass grafting were also included, all postoperative changes in RV volumes and function and TR degree could not be attributed exclusively to TVA and correction of the left-sided cardiac lesions. An additional point was that no comparison was made between annuloplasty bands (or rings) of different design. The mechanisms of late failure of TVA were not explored. Finally, the length of follow-up was different between B-TVA and R-TVA patients. Consequently, the results obtained can in no way be considered conclusive and should be confirmed by future prospective studies including three-dimensional echocardiographic assessment or magnetic resonance imaging.
CONCLUSION
The early surgical treatment of functional TR using an annuloplasty device also for less than severe regurgitation provides a significant reduction in the tricuspid annulus that effectively reduces leaflet tenting and increases leaflet coaptation. This tricuspid valve reverse remodelling improves tricuspid repair durability and helps to create an effective reverse remodelling of the right heart. Although flexible band and rigid ring annuloplasty seem to be equally effective in the long-term treatment of functional TR, there are two different patterns of right heart reverse remodelling, which is more complete when a ring has been used.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
REFERENCES
- 1.Goldstone AB, Howard JL, Cohen JE, MacArthur JW Jr, Atluri P, Kirkpatrick JN et al. Natural history of coexistent tricuspid regurgitation in patients with degenerative mitral valve disease: implications for future guidelines. J Thorac Cardiovasc Surg 2014;148:2802–9. [DOI] [PubMed] [Google Scholar]
- 2.Di Mauro M, Bezante GP, Di Baldassarre A, Clemente D, Cardinali A, Acitelli A et al. Functional tricuspid regurgitation: an underestimated issue. Int J Cardiol 2013;168:707–15. [DOI] [PubMed] [Google Scholar]
- 3.Filsoufi F, Chikwe J, Carpentier A. Rationale for remodelling annuloplasty to address functional tricuspid regurgitation during left-sided valve surgery. Eur J Cardiothorac Surg 2015;47:1–3. [DOI] [PubMed] [Google Scholar]
- 4.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol 2009;53:401–8. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda S, Gillinov AM, McCarthy PM, Stewart WJ, Song JM, Kihara T et al. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation 2006;114:I582–7. [DOI] [PubMed] [Google Scholar]
- 6.De Meester P, De Cock D, Van De Bruaene A, Gabriels C, Buys R, Helsen F et al. Additional tricuspid annuloplasty in mitral valve surgery results in better clinical outcome. Heart 2015;101:720–6. [DOI] [PubMed] [Google Scholar]
- 7.Jeganathan R, Armstrong S, Al-Alao B, David T. The risk and outcomes of reoperative tricuspid valve surgery. Ann Thorac Surg 2013;95:119–24. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Gu C, Men X, Zhang J, You B, Zhang H et al. Repair of functional tricuspid regurgitation: comparison between suture annuloplasty and rings annuloplasty. Ann Thorac Surg 2014;97:1286–92. [DOI] [PubMed] [Google Scholar]
- 9.Parolari A, Barili F, Pilozzi A, Pacini D. Ring or suture annuloplasty for tricuspid regurgitation? A meta-analysis review. Ann Thorac Surg 2014;98:2255–63. [DOI] [PubMed] [Google Scholar]
- 10.Zhu TY, Wang JG, Meng X. Is a rigid tricuspid annuloplasty ring superior to a flexible band when correcting secondary tricuspid regurgitation? Interact CardioVasc Thorac Surg 2013;17:1009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–44. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 13.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–32. [DOI] [PubMed] [Google Scholar]
- 14.Jung SH, Je HG, Song JM, Choo SJ, Chung CH, Yun SC et al. Outcomes following use of a modified Duran ring tricuspid valve reconstruction procedure for secondary tricuspid regurgitation. Circ J 2010;74:925–30. [DOI] [PubMed] [Google Scholar]
- 15.Gatti G, Marcianò F, Antonini-Canterin F, Pinamonti B, Benussi B, Pappalardo A et al. Tricuspid valve annuloplasty with a flexible prosthetic band. Interact CardioVasc Thorac Surg 2007;6:731–5. [DOI] [PubMed] [Google Scholar]
- 16.Onoda K, Yasuda F, Takao M, Shimono T, Tanaka K, Shimpo H et al. Long-term follow-up after Carpentier-Edwards ring annuloplasty for tricuspid regurgitation. Ann Thorac Surg 2000;70:796–9. [DOI] [PubMed] [Google Scholar]
- 17.Filsoufi F, Salzberg SP, Coutu M, Adams DH. A three-dimensional ring annuloplasty for the treatment of tricuspid regurgitation. Ann Thorac Surg 2006;81:2273–7. [DOI] [PubMed] [Google Scholar]
- 18.Gatti G, Maffei G, Lusa AM, Pugliese P. Tricuspid valve repair with the Cosgrove-Edwards annuloplasty system: early clinical and echocardiographic results. Ann Thorac Surg 2001;72:764–7. [DOI] [PubMed] [Google Scholar]
- 19.Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg 2008;135:732–8. [DOI] [PubMed] [Google Scholar]
- 20.Ratschiller T, Guenther T, Guenzinger R, Noebauer C, Kehl V, Gertler R et al. Early experiences with a new three-dimensional annuloplasty ring for the treatment of functional tricuspid regurgitation. Ann Thorac Surg 2014;98:2039–44. [DOI] [PubMed] [Google Scholar]
- 21.Navia JL, Nowicki ER, Blackstone EH, Brozzi NA, Nento DE, Atik FA et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg 2010;139:1473–82. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy PM, Bhudia SK, Rajeswaran J, Hoercher KJ, Lytle BW, Cosgrove DM et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127:674–85. [DOI] [PubMed] [Google Scholar]
- 23.Díez-Villanueva P, Gutiérrez-Ibañes E, Cuerpo-Caballero GP, Sanz-Ruiz R, Abeytua M, Soriano J et al. Direct injury to right coronary artery in patients undergoing tricuspid annuloplasty. Ann Thorac Surg 2014;97:1300–5. [DOI] [PubMed] [Google Scholar]
- 24.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1–44. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.