Abstract
OBJECTIVES
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a rare but unique subtype of non-small-cell lung cancer (NSCLC). Our study aimed to evaluate clinicopathological characteristics and the value of surgical treatment for LELC and explore the relevant prognostic factors in a relatively large cohort.
METHODS
We retrospectively reviewed the medical records of 39 lung LELC patients who underwent pulmonary resection with curative intent between January 2009 and December 2013. The clinical and pathological characteristics, survival data and relevant prognostic factors were analysed.
RESULTS
The median age of lung LELC patients was 47 years (36–81), and 32 of 39 patients were non-smokers (82.1%). Positive expression of P63 and CK5/6 was shown in all the tested LELC specimens. In situ hybridization of Epstein–Bar virus-encoded RNA (EBER) was performed in 36 patients and all of them were positive. However, epidermal growth factor receptor (EGFR) mutational analysis was done in 19 patients and all of them were wild-type. The median follow-up time was 26.0 months in our cohort, and 6-, 12-, 24- and 36-month recurrence-free survival (RFS) rates were 92, 82, 73 and 73%, respectively. Patients with positive lymph nodes experienced significantly worse postoperative RFS than those with negative ones (P = 0.002). Multivariate survival analysis confirmed that only lymph node involvement [RR 0.051; 95% confidence interval, 0.003–0.991, P = 0.049] was an independent prognostic factor.
CONCLUSIONS
Primary lung LELC is closely associated with Epstein–Bar virus infection but not involved in EGFR mutation pathway. Radical surgery could achieve a good outcome for resectable pulmonary LELC, and regional lymph node status is a vital prognostic factor.
Keywords: Lymphoepithelioma-like carcinoma, Lung cancer, Surgery, Prognosis
INTRODUCTION
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a very rare subtype of non-small-cell lung cancer (NSCLC) which was first reported in 1987 [1]. LELC has similar morphology with undifferentiated nasopharyngeal carcinoma (NPC) and is reported as an Epstein–Barr virus (EBV)-associated epithelial neoplasm [1, 2]. According to World Health Organization classification, LELC belongs to the group of large cell carcinoma. However, LELC is regarded as a unique form of neoplasm in terms of epidemiology, aetiology and histopathology [3]. So far, there are only about 200 cases of LELC documented in the literature, and most of these cases were reported in Southeast Asia including Southern China [2], Hong Kong [4] and Taiwan [5]. LELC has been rarely reported in the Caucasian population with no >20 cases [6]. Interestingly, there is no gender predilection regarding its occurrence, and the mean age of LELC patients was reported to be 10 years younger than other lung cancer patients. Furthermore, LELC seems to be not relevant to cigarette smoking, which indicates that other aetiological factors play more important roles [3]. All kinds of modalities have been reported to treat LELC in the literature [2]. However, because of insufficient cases, it remains unknown about the optimal treatment for primary pulmonary LELC. In this retrospective study, we aimed to evaluate clinicopathological characteristics and the value of surgical treatment for LELC, and explore the relevant prognostic factors in a relatively large cohort.
MATERIALS AND METHODS
Patients
The study was approved by the Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. We retrospectively evaluated 52 consecutive patients with lung LELC who underwent pulmonary resection with curative intent between January 2009 and December 2013. We verified and updated the survival data in the patient records through June 2015 using the database. A total of 11 cases were excluded from the study because of an incomplete medical record or being lost to follow-up. Another 2 cases were excluded because of occult pleural drop metastases identified intraoperatively. Therefore, 39 patients were finally enrolled for analysis in this retrospective study. Informed consent for the collection of medical information was obtained from all patients at their first visit.
The preoperative staging workup included chest radiography, computed tomography (CT) of chest and upper abdomen, CT or magnetic resonance imaging (MRI) of the brain, bronchoscopy with or without biopsy, and respiratory function test. Particularly, all 39 patients in our analysis had nasopharyngoscopy to rule out primary NPC. For the 7 patients who were already diagnosed with LELC by bronchoscopic biopsies preoperatively, the subsequent nasopharyngoscopy was performed before surgery. The remaining 32 cases who were confirmed to be LELC by postoperative histology, however, would have their nasopharyngoscopy after surgery. Eleven patients had positron emission tomography–CT (PET/CT), whereas 15 had endobronchial ultrasound (EBUS) in their preoperative workup to rule out regional lymph node and/or distant metastasis. For other patients, the mediastinal staging information was evaluated by CT scan, which defined the short axis of lymph nodes <1 cm as no nodal involvement. All the pulmonary lesions were considered as resectable with clinical N0/N1 and M0 diseases according to the preoperative assessment and were offered pulmonary resection with curative intent. All cases were staged according to the TNM staging system of the American Joint Committee on Cancer (AJCC Staging Manual, 7th edition) [7]. Adjuvant therapies were applied in line with the recommendation of National Comprehensive Cancer Network (NCCN) Guidelines. Hence, adjuvant therapy was given to all the Stage II and IIIa patients but not to the ones with Stage IA diseases. For Stage IB patients, chemotherapy was only applied for the ones with high-risk factors, which included poorly differentiated tumours, vascular invasion, wedge resection, tumours >4 cm, visceral pleural involvement and incomplete lymph node sampling. Follow-up information was obtained from patients' medical records or by telephone interview.
Immunohistochemistry
Immunoperoxidase stain was done on 4-μm-thick paraffin sections. The slides were deparaffinized in xylene and then hydrated prior to antigen retrieval by microwaving in sodium citrate buffer (pH 6.0). The slides were then incubated with a peroxidase block and then the primary antibody. After a PBS wash, the slides were incubated first with the secondary antibody and then with 3,3′-diaminobenzidine, then counterstained with haematoxylin (Hematoxylin 7211; Richard-Allen Scientific, Kalamazoo, MI, USA). The peroxidase block, secondary antibody and 3,3′-diaminobenzidine were all obtained from the DakoCytomation EnVision System (Glostrup, Denmark). The primary antibodies used in the study are listed in Table 1.
Table 1:
Immunohistochemistry results for lung lymphoepithelioma-like carcinoma
| Antibody | Source | Clone | Positive expression (%) |
|---|---|---|---|
| P63 | DAKO (Carpinteria, California) | VS38c | 34/34 (100%) |
| CK 5/6 | DAKO (Carpinteria, California) | D5/16 B4 | 23/23 (100%) |
| CK 7 | DAKO (Carpinteria, California) | OV-TL 12/30 | 2/30 (6.67%) |
| CgA | DAKO (Carpinteria, California) | DAK-A3 | 3/31 (9.68%) |
| Syn | Santa Cruz Biotechnology (Santa Cruz, California) | LB 509 | 1/30 (3.33%) |
| TTF-1 | DAKO (Carpinteria, California) | 8G7G3/1 | 6/36 (16.67%) |
| Ki-67 | DAKO (Carpinteria, California) | MIB-1 | 9/9 (100%) |
In situ hybridization of Epstein–Bar virus-encoded RNA
In situ hybridization was performed on formalin-fixed, paraffin-embedded tissue sections using fluorescein-conjugated EBV (EBER) RNA probe (Dako; Code Y 5200), which is complementary to the two nuclear EBER RNAs encoded by the EBV. According to the manufacturer's instructions, briefly, 4-μm sections were deparaffinized and digested with proteinase K. After the probe was added and incubated at 55°C for 1.5 h, the sections were washed with a stringent solution. A chromogen, BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt and nitroblue tetrazolium chloride), was then added and counterstained with haematoxylin.
Mutational analysis of epidermal growth factor receptor
Epidermal growth factor receptor (EGFR) tyrosine kinase exons 18, 19, 20 and 21 were amplified by nested PCR, using specific primers. The primers and amplification conditions have been described previously [8]. The resulting PCR amplicons were purified with a Gel/PCR DNA Fragments Extraction kit and sequenced using the BigDye Terminator kit (Applied Biosystems, Foster City, CA, USA) and ABI Prism 3700 DNA Analyzer (Applied Biosystems) according to the manufacturer's instructions. All sequencing reactions were carried out in both forward and reverse directions, using tracings from at least two independent PCRs.
End-points and statistical analysis
Since there were only 2 patients who died after surgery during the follow-up (1 died from cancer recurrence, the other from other cause), the primary end-points of this retrospective study were recurrence-free survival (RFS). Local recurrence was defined as any recurrence within the same lung or ipsilateral lymph nodes. Distant recurrence was defined as any recurrence other than local recurrence. Survival curves were calculated by the Kaplan–Meier method and were compared by the log-rank test. Time to event (recurrence) was calculated from the date of surgery to the date of event. In event-free subjects, the time variable was censored at the date of last follow-up. Univariate and multivariate analysis of prognostic factors was performed using Cox's regression model. A significant difference was defined as a two-tailed P-value of less than 0.05. All of the statistical analyses were performed using the SPSS 16.0 for Windows software system (SPSS, Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
The demographic and clinicopathological parameters of the 39 patients are listed in Table 2. Patients in our cohort were young with a median age of 47 years. More female patients (61.5%) and non-smokers (82.1%) were observed in the study. Cough was the most common symptom (35.9%), which was followed by haemoptysis (15.4%), and 16 patients were asymptomatic when the lung lesions were incidentally found. Of the total, 36 patients received radical lobectomy and 2 had pneumonectomy. The remaining 1 patient had therapeutic wedge resection because of borderline lung function. There was no surgery-related mortality. All patients recovered well and were discharged uneventfully. A total of 13 patients received surgery as the sole treatment. The remaining 26 patients received adjuvant therapies, of which 24 patients received sole adjuvant chemotherapy including 5 Stage IB patients with visceral pleural involvement; 1 patient had sole adjuvant radiation and 1 patient received adjuvant chemoradiation. The radiation dosage for both patients was 45 Gy. The regimen of adjuvant chemotherapy included docetaxel plus cisplatin/carboplatin (8 cases, 32.0%), pemetrexed plus cisplatin/carboplatin (7 cases, 28.0%), gemcitabine plus cisplatin/carboplatin (8 cases, 32.0%) and paclitaxel plus cisplatin/carboplatin (2 cases, 8.0%).
Table 2:
Clinicopathological characteristics of all patients
| Characteristic | Patients with LELC (n = 39) |
|---|---|
| Sex | |
| Male | 15 (38.5%) |
| Female | 24 (61.5%) |
| Age (years) | |
| Median | 47 |
| Range | 36–81 |
| Main complaint | |
| Cough | 14 (35.9%) |
| Haemoptysis | 6 (15.4%) |
| Chest pain | 3 (7.7%) |
| Asymptomatic | 16 (41.0%) |
| Smoking history | |
| Former or current smoker | 7 (17.9%) |
| Non-smoker | 32 (82.1%) |
| Locations | |
| Right upper lobe | 5 (12.8%) |
| Right middle lobe | 12 (30.8%) |
| Right lower lobe | 3 (7.7%) |
| Right middle and lower lobes | 1 (2.6%) |
| Left upper lobe | 7 (17.9%) |
| Left lower lobe | 11 (28.2%) |
| Tumour diameter (cm) | 4.27±0.29 |
| Operation | |
| Lung wedge resection | 1 (2.6%) |
| Lobectomy | 33 (84.6%) |
| Bilobectomy | 3 (7.7%) |
| Pneumonectomy | 2 (5.1%) |
| T stage | |
| T1 | 9 (23.1%) |
| T2 | 26 (66.7%) |
| T3 | 4 (10.3%) |
| N stage | |
| N0 | 23 (59.0%) |
| N1 | 7 (17.9%) |
| N2 | 9 (23.1%) |
| TNM stage | |
| I | 18 (46.2%) |
| II | 10 (25.6%) |
| IIIa | 11 (28.2%) |
| Adjuvant therapy | |
| Sole chemotherapy | 24 (61.5%) |
| Sole radiation | 1 (2.6%) |
| Chemoradiation | 1 (2.6%) |
| No adjuvant therapy | 13 (33.3%) |
Imaging characteristics
CT findings of the 39 patients with primary pulmonary LELC are described in Table 3. Slightly more lung lesions were detected peripherally in our cohort (56.4%). Lobulated and spiculated signs were very common features on the CT scan; coexistence of both signs was identified in 4 cases (10.3%) in our study. Upon contrast study, attenuation patterns varied among all the lesions. Of the total, 26 cases (66.7%) showed homogeneous enhancement, while 13 (33.3%) demonstrated heterogeneous enhancement. Vascular and/or bronchial encasement occurred quite frequently (28.2%), but only one tumour caused bronchial obstruction and subsequent pneumonia. In our group, 11 patients had preoperative PET/CT exams and all of them showed that the pulmonary lesions were moderately to intensively FDG avid, which were consistent with lung malignancies.
Table 3:
CT characteristics of all patients with primary pulmonary LELC
| CT characteristic | Patients with LELC (n = 39) |
|---|---|
| Tumour site | |
| Peripheral | 22 (56.4%) |
| Central | 17 (43.6%) |
| Tumour contour | |
| Lobular | 28 (71.8%) |
| Spiculated | 15 (38.4%) |
| Tumour definition | |
| Well-defined | 25 (64.1%) |
| Poorly defined | 14 (35.9%) |
| Enhancement pattern | |
| Homogeneous | 26 (66.7%) |
| Heterogeneous | 13 (33.3%) |
| With cavitation | 1 (2.6%) |
| Relationship with pleura | |
| Invasion or potential invasion | 24 (61.5%) |
| No invasion | 15 (38.5%) |
| Vascular and/or bronchial encasement | 11 (28.2%) |
| Obstructive pneumonia | 1 (2.6%) |
| Pleural effusion | 0 |
| Pericardial effusion | 0 |
Immunohistochemistry, Epstein–Bar virus-encoded RNA and epidermal growth factor receptor status
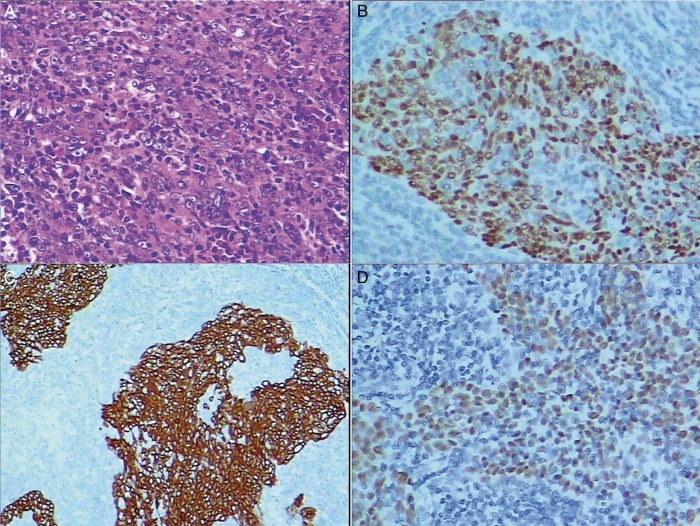
Immunohistochemistry tests were performed in combination with histology for most of the cases. The common biomarkers are listed in Table 1. Positive findings were noted in the expression of P63 (34/34, 100%, Fig. 1B) and CK5/6 (23/23, 100%, Fig. 1C), while negative expression was demonstrated in CK7 (28/30, 93.33%), CgA (28/31, 90.32%), Syn (29/30, 96.67%) and TTF1 (30/36, 83.33%). Ki-67 was tested on nine specimens. All the results were positive, but the staining percentages were different. More than 75% of positive staining was found in 3 cases followed by 20% of positive staining identified in 2 cases. Furthermore, 50, 40, 15 and 10% positive expressions were present in the other four specimens, respectively. In situ hybridization for EBER was performed in 36 patients and positive signals in the nuclei of neoplastic cells were identified in all the tested samples (100%, Fig. 1D). EGFR mutational analysis was carried out in 19 patients of our cohort, and all of them were wild-type.
Figure 1:
(A) Lymphoepithelioma-like carcinoma is an undifferentiated carcinoma with predominant lymphocytic infiltration (haematoxylin and eosin-stained, original magnification ×200). (B) Positive P63 expression (original magnification ×200). (C) Positive CK5/6 expression (original magnification ×100). (D) Positive signals in the nuclei of cancer cells (in situ hybridization for EBER, original magnification ×200). EBER: Epstein–Bar virus-encoded RNA.
Recurrence-free survival and relevant prognostic factors
Survival data for 39 patients were updated by 1 June 2015. There were only 2 deaths in our cohort. One patient died from cancer recurrence; the other from exacerbation of COPD and pneumonia. However, local recurrence or distant metastasis happened in 9 patients during the follow-up, among whom mediastinal lymph node recurrence was found in 4 cases, bone metastases occurred in 3 patients, malignant pleural or peritoneal effusion was diagnosed in 2 and 1 cases, respectively, and 1 patient had liver metastases. The median and mean follow-up time were 26.0 and 26.7 months (3–61 months); and the 6-, 12-, 24- and 36-month RFS rates were 92, 82, 73 and 73%, respectively (Fig. 2).
Figure 2:
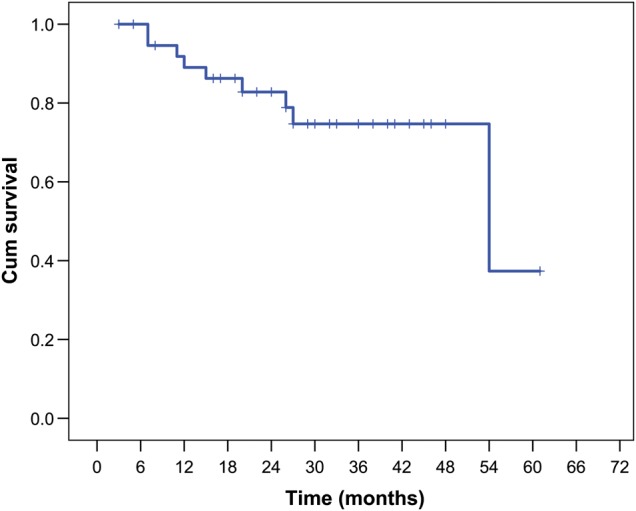
Recurrence-free survival for the 39 patients with primary pulmonary lymphoepithelioma-like carcinoma.
We performed survival analysis using the Cox proportional hazards model to identify factors involved in RFS (Table 4). Age, gender, smoking history, tumour size, regional lymph node involvement, pathological stage, adjuvant radiation and chemotherapy had been taken into account. Both lymph node status and pathological stage were found to be significant prognostic factors for RFS (P = 0.017 and P = 0.027, respectively). However, further multivariate analysis confirmed that only lymph node involvement (RR 0.051; 95% confidence interval, 0.003–0.991, P = 0.049) was an independent prognostic factor.
Table 4:
Univariate and multivariate recurrence-free survival analysis by the Cox proportional hazards model
| Variable | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% Confidence interval | P-value | Hazard ratio | 95% Confidence interval | P-value | |
| Age (years) | ||||||
| ≤50 vs >50 | 4.801 | 0.588–39.186 | 0.143 | 1.013 | 0.927–1.108 | 0.773 |
| Gender | ||||||
| Male vs female | 0.493 | 0.100–2.446 | 0.387 | 0.576 | 0.087–3.806 | 0.567 |
| Smoking | ||||||
| Smoker vs non-smoker | 0.642 | 0.079–5.231 | 0.679 | 1.228 | 0.121–12.505 | 0.862 |
| Tumour diameter (cm) | ||||||
| ≤3.0 vs >3.0 | 0.026 | 0.000–10.391 | 0.232 | 0.930 | 0.614–1.408 | 0.732 |
| Lymph node involvement | ||||||
| Negative vs positive | 0.078 | 0.009–0.636 | 0.017* | 0.051 | 0.003–0.991 | 0.049* |
| TNM stage | ||||||
| I–II vs IIIa | 0.199 | 0.047–0.833 | 0.027* | 1.092 | 0.169–7.071 | 0.927 |
| Adjuvant therapy | ||||||
| Yes vs no | 3.245 | 0.399–26.400 | 0.271 | 0.800 | 0.046–13.972 | 0.879 |
*Statistically significant.
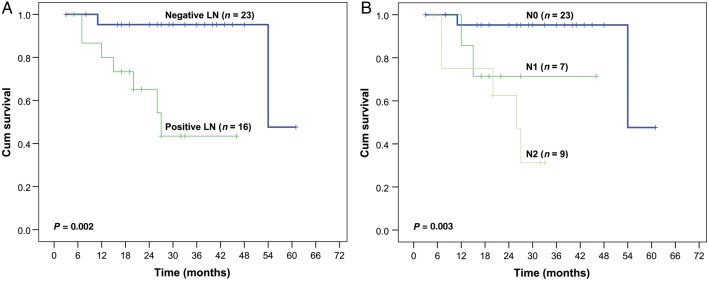
Then, we further studied the impact of lymph node status on RFS of LELC patients in stratified analysis. The Kaplan–Meier survival curve (Fig. 3A) showed that patients with positive lymph nodes experienced significantly worse postoperative survival than those with negative ones (P = 0.002). According to the TNM staging system of NSCLC, N stage could be further categorized into N0 (no regional lymph node metastasis), N1 (metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes) and N2 (metastasis in ipsilateral mediastinal and/or subcarinal lymph nodes) diseases. In the stratified analysis split by different N status (Fig. 3B), a trend was again observed that more advanced lymph node involvement was correlated to poorer prognosis (P = 0.003).
Figure 3:
(A) A comparison of recurrence-free survival for the patients with and without regional lymph node involvement. (B) A comparison of recurrence-free survival for the patients with stage N0, N1 and N2 diseases.
DISCUSSION
Lymphoepithelioma refers to an undifferentiated carcinoma with predominant lymphocytic infiltration. The primary lung LELC was first described by Begin et al. [1] in 1987, which was histologically identical with the undifferentiated NPC. LELC is a rare and clinicopathologically distinctive lung cancer, representing only 0.9% of all primary lung cancers. And almost all the reported pulmonary LELC patients were East Asians, with a much smaller number of cases in the Western population [3]. Interestingly, the mean age of LELC patients was reported to be 10 years younger than that of other patients of lung cancer. Moreover, in our study, LELC did affect a relatively young population with a median age of 47 years. This finding was consistent with previous studies [2, 5]. More female patients were present in our series (24 vs 15; 1.6:1), which was in line with the report of Chang et al. [5] but in contrast to others [2, 9]. Despite these conflicting results, it is certain that LELC has no male predilection like other histological subtypes of lung cancer. Another interesting finding was 82.1% of patients in our cohort never smoked. The less association with cigarette smoking indicated that it might not be the main aetiological factor for lung LELC. Therefore, further exploration of the true carcinogenic factors for LELC is warranted.
Although immunohistochemistry was not performed deliberately to identify the correlation between the biomarkers and LELC in this retrospective study, it did provide some clues regarding the biological characteristics of lung LELC. All the patients in our series had positive staining in CK5/6 and P63 expression, which suggested lung LELC originated from epithelial tissue and should belong to squamous cell carcinoma. Because of the similarity to NPC, a suspicion of an aetiological role of EBV has been raised since 1987 [1]. The subsequent researches have further confirmed the presence of EBV in LELC by polymerase chain reaction (PCR) for EBV DNA, in situ hybridization for EBV DNA and RNA, and immunohistochemistry for EBV-associated proteins [3]. There was no exception in our cohort that all the tested samples had shown positive EBER signals in the nuclei of cancer cells. However, whether EBV is a requisite factor in the pathogenesis of LELC remains controversial. For instance, one research from non-Asian countries demonstrated that all 6 patients were negative for EBV. This might suggest there is no association between EBV and lung LELC in the Western population [10]. Therefore, an ethnic predisposition, apart from EBV infection, might also contribute to the tumorigenesis of primary lung LELC.
It is known that ∼10% of patients with NSCLC in the USA and 35% in East Asia have tumour-associated EGFR mutations [8, 11]. So efforts were also made to explore the relation between EGFR mutations and lung LELC. In a recent study from Taiwan, EGFR mutations were detected in 8 of 46 lung LELC cases (17.4%) with a majority of exon 21 mutations but without L858R [5]. However, neither the series of Liang et al. (11 cases) [5] nor our cohort (19 cases) displayed the same molecular profile. All tested samples in both studies were of wild-type EGFR, which suggested that patients with lung LELC might not be able to benefit from EGFR tyrosine kinase inhibitors.
Regarding the prognosis of lung LELC, Han et al. compared 32 lung LELC with 84 non-LELC NSCLC cases. All LELC patients received surgical resection and the results showed that LELC patients experienced a significantly better 5-year survival than non-LELC patients with Stage II (P < 0.025) and Stage III/IV diseases (P < 0.05) [9]. A similar picture was also obtained from the study of Liang et al. [2], which demonstrated that the 2- and 5-year overall survival rates were 88 and 62%, respectively. With a median follow-up time of 26.0 months in our study, there were only 2 patients who died of cancer recurrence or a non-cancer cause, while 9 patients had local recurrence or distant metastasis; and the 3-year RFS rate was 73%. In the further attempt to discern the prognosticators for lung LELC, only regional lymph node status was identified as an independent prognostic factor for RFS. In addition, more advanced lymph node involvement seemed to be related to worse prognosis. These results were again consistent with those in the research of Liang et al. [2], although lymph node status was only included in univariate analysis but not in multivariate analysis. Surprisingly, the prognosis of lung LELC was not proved to be related to tumour size, histological stage and adjuvant therapy in our study, which indicated that the biological behaviour of LELC might be fairly different from other types of NSCLCs. Therefore, further research focusing on the mechanism of dedifferentiation, hyperproliferation, invasion and the metastatic potential of LELC at the molecular level is necessary to understand this disease more comprehensively.
In conclusion, primary lung LELC is a rare but distinct subtype of NSCLC. It tends to affect non-smokers in a younger population without male gender predilection. Molecular testing demonstrates that lung LELC is closely associated with EBV infection but not involved in the EGFR mutation pathway. Radical surgery could achieve a good outcome for resectable pulmonary LELC, and regional lymph node status is regarded as a vital prognostic factor. However, because of its low incidence, further research studies are warranted in order to determine its biological profile and optimal treatment protocol.
Conflict of interest: none declared.
REFERENCES
- 1.Begin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987;36:280–3. [DOI] [PubMed] [Google Scholar]
- 2.Liang Y, Wang L, Zhu Y, Lin Y, Liu H, Rao H et al. . Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748–58. [DOI] [PubMed] [Google Scholar]
- 3.Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology 2006;11:539–45. [DOI] [PubMed] [Google Scholar]
- 4.Ho JC, Lam WK, Wong MP, Wong MK, Ooi GC, Ip MS et al. . Lymphoepithelioma-like carcinoma of the lung: experience with ten cases. Int J Tuberc Lung Dis 2004;8:890–5. [PubMed] [Google Scholar]
- 5.Chang YL, Wu CT, Shih JY, Lee YC. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci 2011;102:282–7. [DOI] [PubMed] [Google Scholar]
- 6.Morbini P, Riboni R, Tomaselli S, Rossi A, Magrini U. Eber- and LMP-1-expressing pulmonary lymphoepithelioma-like carcinoma in a Caucasian patient. Hum Pathol 2003;34:623–5. [DOI] [PubMed] [Google Scholar]
- 7.Goldstraw P. The 7th Edition of TNM in Lung Cancer: what now? J Thorac Oncol 2009;4:671–3. [DOI] [PubMed] [Google Scholar]
- 8.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al. . EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. [DOI] [PubMed] [Google Scholar]
- 9.Han AJ, Xiong M, Gu YY, Lin SX, Xiong M. Lymphoepithelioma-like carcinoma of the lung with a better prognosis. A clinicopathologic study of 32 cases. Am J Clin Pathol 2001;115:841–50. [DOI] [PubMed] [Google Scholar]
- 10.Castro CY, Ostrowski ML, Barrios R, Green LK, Popper HH, Powell S et al. . Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol 2001;32:863–72. [DOI] [PubMed] [Google Scholar]
- 11.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW et al. . Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39. [DOI] [PubMed] [Google Scholar]