Abstract
OBJECTIVES
Concomitant tricuspid valve surgery with mitral valve surgery is recommended for patients with severe functional tricuspid regurgitation (TR). However, the treatment for 2+ TR (mild TR) remains controversial. Here, we evaluated the long-term results of untreated 2+ TR in patients undergoing mitral valve surgery.
METHODS
We retrospectively reviewed the records of 96 patients with untreated 2+ TR among 885 patients who underwent mitral valve surgery from 2003 to 2010. Exclusion criteria were tricuspid valve surgery (TVS), emergency surgery, primary TR and pacemaker lead through the tricuspid valve. We assessed survival and freedom from heart failure. The freedom from 3+ (moderate) or 4+ (severe) TR was investigated by echocardiographic data at pre- and postoperative week 1, then at 1, 3, 5, 7 and 10 postoperative years, which were compared with those in patients who had 2+ TR preoperatively and underwent concomitant TVS in the same period (n = 47).
RESULTS
The mean follow-up was 7.1 ± 2.7 years. There was no 30-day mortality. The survival rate was 97.5% at 5 years and 87.5% at 10 years. The independent risk factors for mortality were age (OR 1.2, P = 0.03) and left ventricular ejection fraction (OR 0.9, P = 0.03). Untreated 2+ TR improved transiently within the first postoperative year (P < 0.001), but progressed again in the mid- to long term. Freedom from ≥3+ TR was 64.2% at 5 years and 46.7% at 10 years, which was significantly lower than that from ≥3+ TR in patients who underwent concomitant TVS (P = 0.006). The independent risk factors for TR progression (≥3 + TR) were age (OR 1.1, P = 0.005), atrial fibrillation (OR 2.2, P = 0.04) and tricuspid annular diameter (TAD) index (mm/m2; OR 1.1, P = 0.02). Receiver operating characteristic curves showed that the optimal TAD index cut-off value was 21.0 for long-term survival [area under the curve (AUC) = 0.72] and 21.2 for TR progression (AUC = 0.64).
CONCLUSIONS
Although untreated, 2+ TR significantly improved after mitral valve surgery, it then progressed again in the mid- to long term. Therefore, concomitant TVS should be considered in patients with 2+ TR who have dilated tricuspid annulus or atrial fibrillation, if feasible.
Keywords: Tricuspid regurgitation, Mitral valve surgery, Long term, Tricuspid annular diameter, Atrial fibrillation
INTRODUCTION
Functional tricuspid regurgitation (TR) is frequently present in patients with degenerative mitral valve disease [1]. TR is mainly caused by dilatation of the tricuspid annulus and tethering of the tricuspid valve leaflets secondary to right ventricular dysfunction due to chronic pressure and volume overload [2, 3]. Historically, TR secondary to mitral valve disease was thought to improve after mitral valve surgery [4, 5], which has led to a conservative non-surgical approach to TR. However, recent studies have not supported this concept [6–11]. Indeed, right ventricular dilatation causes deformity of the tricuspid annulus shape and displacement of the papillary muscles, which contributes to reduction of the tricuspid valve leaflet coaptation and resulting residual regurgitation [3, 12]. Therefore, mitral valve surgery alone in a subset of patients cannot be expected to result in effective TR control.
Survival is worse for patients with moderate and severe TR than for those without TR [13]. The 2014 American Heart Association/American College of Cardiology (AHA/ACC) guidelines for valvular heart disease recommend tricuspid valve surgery (TVS) for patients with severe TR undergoing left-sided valve surgery (Class 1), and suggest that TVS can be beneficial for patients with mild, moderate or greater TR with either tricuspid annular dilatation or prior evidence of right heart failure (Class 2a) [14]. However, there are a few studies showing the long-term outcomes and prognosis of TR in patients with mild TR (2+ TR). General agreement regarding appropriate patient selection and optimal index of surgical treatment is clinically important. Thus, the aim of the present study was to evaluate the long-term results of untreated 2+ TR patients undergoing mitral valve surgery.
MATERIALS AND METHODS
Patients
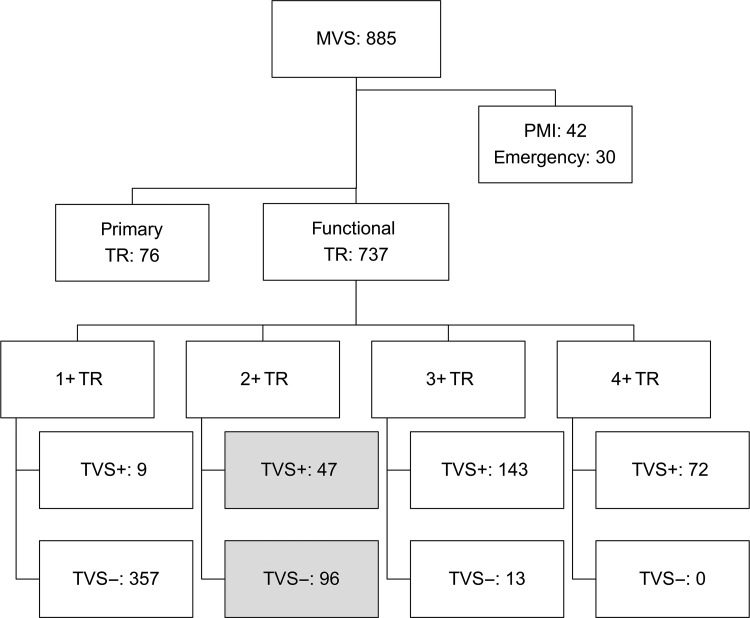
This retrospective study was approved by the Institutional Review Board of the National Cerebral and Cardiovascular Centre of Osaka, Japan, and individual patient consent was waived. From 2003 to 2010, 885 patients underwent mitral valve surgery for mitral stenosis or regurgitation. Of these, 76 patients had primary TR (infectious endocarditis 58, Marfan syndrome 4, tumour 1, congenital heart disease 8 and rheumatic disease 5), 30 had emergency surgery and 42 had pacemaker leads through the tricuspid annulus pre- or postoperatively. Of the remaining 737 patients, 96 2+ TR patients without concomitant TVS were included for analysis. As a reference, 47 patients with 2+ TR with TVS were also included for analysis (Fig. 1).
Figure 1:
Study algorithm. MVS: mitral valve surgery; TR: tricuspid regurgitation; TVS+: with concomitant tricuspid valve surgery; TVS−: without concomitant tricuspid valve surgery; PMI: pre- or postoperative implantation of pacemaker through the tricuspid annulus.
Table 1 summarizes the perioperative characteristics of included 96 2+ TR patients without TVS (TVS− group) and 47 2+ TR patients with concomitant TVS (TVS+ group). The mean age was 64.4 ± 10.2 years in the TVS− group and 64.3 ± 7.7 in the TVS+ group, respectively, and 51 and 36% were male, respectively. There were significant differences between the two groups in body surface area (BSA; 1.55 ± 0.1 vs 1.49 ± 0.2 m2, P = 0.03), mean disease duration, which indicates the period from diagnosis to surgery (24 vs 162 months, P < 0.001), preoperative New York Heart Association (NYHA) class (P = 0.001), preoperative atrial fibrillation (55 vs 83%, P = 0.001) and rheumatic mitral aetiology (28 vs 62%, P < 0.001).
Table 1:
Perioperative characteristics
| Variables [mean(±SD) or n (%)] | Group |
P-value | |
|---|---|---|---|
| TVS− (N = 96) | TVS+ (N = 47) | ||
| Preoperative | |||
| Male sex | 49 (51) | 17 (36) | 0.1 |
| Age (years) | 64.4 (10.2) | 64.3 (7.7) | 1.0 |
| BSA (m2) | 1.55 (0.1) | 1.49 (0.2) | 0.03 |
| Disease duration (m) | 24 (30) | 162 (151) | <0.001 |
| BNP (pg/ml) | 406 (743) | 217 (213) | 0.1 |
| Reoperation | 17 (18) | 10 (21) | 0.7 |
| NYHA class | |||
| 2 | 56 (58) | 17 (36) | 0.001 |
| 3 | 30 (31) | 29 (62) | |
| 4 | 10 (11) | 1 (2) | |
| CAD | 15 (16) | 3 (6) | 0.2 |
| AF | 53 (55) | 39 (83) | 0.001 |
| Rheumatic aetiology | 27 (28) | 29 (62) | <0.001 |
| MR | 68 (71) | 18 (38) | <0.001 |
| MS | 28 (29) | 29 (62) | <0.001 |
| AR | 13 (14) | 3 (6) | 0.3 |
| AS | 9 (9) | 3 (6) | 0.8 |
| LVEDD (mm) | 57 (8) | 53 (8) | 0.01 |
| LAD (mm) | 54 (11) | 58 (11) | 0.05 |
| LVEDVI (ml/m2) | 111 (43) | 99 (29) | 0.1 |
| LVEF (%) | 52 (10) | 53 (11) | 0.6 |
| C.I. (l/min/m2) | 2.6 (0.7) | 2.4 (0.8) | 0.3 |
| Systolic PAP (mmHg) | 36 (12) | 34 (10) | 0.3 |
| TAD (mm) | 31 (4) | 32 (4) | 0.5 |
| TAD index (mm/m2) | 20 (3) | 21 (3) | 0.06 |
| Operative | |||
| MV replacement | 45 (47) | 31 (66) | 0.03 |
| MV repair | 51 (53) | 16 (34) | 0.03 |
| Concomitant procedure | |||
| CABG | 14 (15) | 3 (6) | 0.2 |
| AVR | 17 (18) | 5 (11) | 0.3 |
| Maze | 43 (45) | 20 (43) | 0.9 |
| CPB (min) | 162 (59) | 148 (38) | 0.1 |
| AXC (min) | 114 (40) | 109 (34) | 0.5 |
TVS−: without concomitant tricuspid valve surgery; TVS+: with concomitant tricuspid valve surgery; SD: standard deviation; BSA: body surface area; BNP: brain natriuretic peptide; NYHA: New York Heart Association; CAD: coronary artery disease; AF: atrial fibrillation; MR: mitral regurgitation; MS: mitral stenosis; AR: aortic regurgitation; AS: aortic stenosis; LVEDD: left ventricular end-diastolic diameter; LAD: left atrial diameter; LVEDVI: left ventricular end-diastolic volume index; LVEF: left ventricular ejection fraction; C.I.: cardiac index; PAP: pulmonary artery pressure; TAD: tricuspid annulus diameter; TAD index: TAD/BSA; MV: mitral valve; CABG: coronary artery bypass grafting; AVR: aortic valve replacement; Maze: modified Cox maze procedure; CPB: cardiopulmonary bypass time; AXC: aortic cross-clamping time.
In mitral valve disease aetiology, mitral regurgitation was 71% in the TVS− group and 38% in the TVS+ group, and mitral stenosis 29% in the TVS− group and 62% in the TVS+ group (P < 0.001).
Echocardiography/catheter examination
All patients underwent M-mode two-dimensional and Doppler transthoracic echocardiography for the measurement of preoperative cardiac dimensions, transvalvular pressure gradients and valve regurgitation severity [15, 16]. The tricuspid annular diameter (TAD) was measured as the point of attachment of the septal and posterior leaflets to the atrioventricular junction in the transthoracic apical four-chamber view in late diastole at the time of maximal tricuspid opening [17, 18]. We measured the TAD index, which is the TAD indexed to the individual's BSA (mm/m2). TR severity was quantified as none (0+), trivial (1+), mild (2+), moderate (3+) and severe (4+) [15, 16]. Clinical follow-up data were collected through routine perioperative surveys, including transthoracic echocardiography performed within 1 week before and after the operation and at postoperative 1-, 3-, 5-, 7- and 10-year follow-up. Cardiac catheter evaluation was also performed preoperatively. All other data were collected from medical records, and all patients completed the course of follow-up examinations.
On preoperative echocardiographic examination, the mean left ventricular end-diastolic diameter was 57 ± 8 mm in the TVS− group and 53 ± 8 in the TVS+ group (P = 0.01), and the left atrial diameter was 54 ± 11 and 58 ± 11 mm, respectively. There was no difference in the mean TAD and TAD index (P = 0.5; Table 1).
Surgical procedures
A full median sternotomy was performed in all patients. Cardiopulmonary bypass was established between the ascending aorta and both venae cavae. Temperature was normally decreased to 32–34°C. Tepid blood cardioplegia was administered in an antegrade and/or retrograde fashion every 20–30 min. In patients with atrial fibrillation, a modified Cox maze procedure was performed using a cryoablator prior to mitral valve surgery, if indicated [19]. Mitral valve surgery was performed through a right-sided left atriotomy under cardioplegic arrest. Mitral valve replacement or mitral valve repair was performed according to the aetiology and dysfunction.
Mitral valve replacement was performed in 47% of TVS− patients and 66% in TVS+ (P = 0.03), whereas mitral valve repair was performed in 53 and 34%, respectively (P = 0.03). Concomitant procedures included coronary artery bypass grafting in 15% of TVS− and 6% of TVS+ patients (P = 0.2), aortic valve replacement in 18 and 11% (P = 0.3) and a modified Cox maze procedure in 45 and 43% (P = 0.9), respectively. The mean cardiopulmonary bypass time was 162 ± 59 min in TVS− and 148 ± 38 in TVS+ (P = 0.1), and the mean aorta cross-clamp time was 114 ± 40 min, 109 ± 34 (P = 0.5), respectively (Table 1).
TVS was performed according to the surgeons' decision. But it was performed in patients who had had severer TR in the past or would likely develop severe TR, with respect to the duration of disease or rheumatic aetiology. TVS was performed by annuloplasty with the De Vega method or ring annuloplasty using prosthesis.
End-points
The primary end-point was 5-year and 10-year survival. The secondary end-points were freedom from heart failure (defined as NYHA class 3 or 4) and freedom from 3+ or 4+ TR.
Statistical methods
Statistical analysis was performed, using the non-parametric Mann–Whitney U-test for continuous variables and ordinal variables (independent samples), the Wilcoxon signed-rank test for ordinal variables (paired samples) and Fisher's exact test for categorical variables. Results are described as mean ± standard deviation for continuous variables, and as counts and percentages for categorical variables. A multivariate analysis with Cox proportional hazards modelling, using univariate predictors with a P-value of ≤0.1 as the entry criterion was performed to determine the risks for late mortality, heart failure and postoperative TR progression (≥3+). Time-to-event data (survival, freedom from heart failure and 3+ or 4+ TR) were analysed using the Kaplan–Meier method. The curve was discontinued, when the patients at risks were less than 10. The receiver operating characteristic (ROC) curve was presented to determine an appropriate cut-off value of the TAD index as a predictor of late mortality and postoperative TR progression. We determined the optimal cut-off value point by Youden index, which is equivalent to maximizing the sum of sensitivity and specificity. P-values of <0.05 were considered statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, version 3.2.2) [20].
RESULTS
Survival and freedom from heart failure
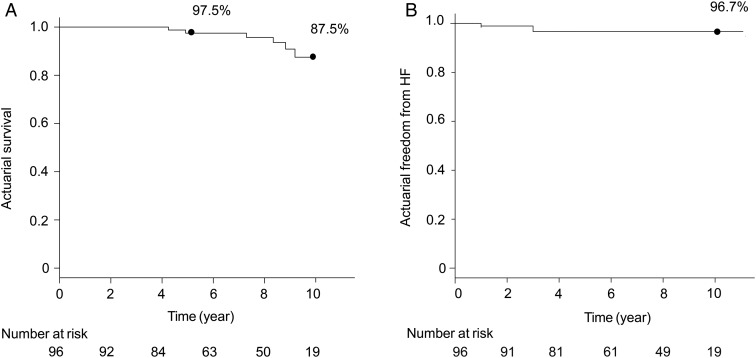
Follow-up was complete for all included patients, and the mean follow-up period was 7.1 ± 2.7 years. There were no deaths within postoperative 30 days. The survival rate was 97.5% at 5 years and 87.5% at 10 years (Fig. 2A). Univariate analysis revealed that long-term survival was associated with age (P = 0.002), preoperative NYHA class (P = 0.02), left ventricular ejection fraction (LVEF; P = 0.09) and TAD index (P = 0.1). Multivariate analysis revealed that the independent risk factors for long-term survival were age [OR 1.2, 95% confidence interval (CI) 1.0–1.4, P = 0.03] and LVEF (OR 0.9, 95% CI 0.9–1.0, P = 0.03; Table 2).
Figure 2:
(A) Actuarial survival rate, analysed using the Kaplan–Meier method. (B) Actuarial freedom from heart failure (HF; NYHA class ≥3).
Table 2:
Risk factors for long-term survival and heart failure
| Variables | Univariate | Multivariate | 95% CI | P-value |
|---|---|---|---|---|
| P-value | Odds ratio | |||
| Risk factors for long-term survival | ||||
| Age (years) | 0.002 | 1.2 | 1.0–1.4 | 0.03 |
| NYHA class | 0.02 | 2.4 | 0.6–8.3 | 0.2 |
| Male sex | 0.4 | |||
| Mitral stenosis | 0.4 | |||
| Atrial fibrillation | 0.7 | |||
| Disease duration (m) | 0.6 | |||
| LVEDD (ml) | 0.4 | |||
| LAD (mm) | 0.2 | |||
| LVEF (%) | 0.09 | 0.9 | 0.9–1.0 | 0.03 |
| TAD index (mm/m2) | 0.1 | 1.0 | 0.8–1.3 | 0.9 |
| Risk factors for heart failure | ||||
| Age (years) | 0.1 | 1.1 | 0.9–1.3 | 0.3 |
| NYHA class | 0.07 | 3.5 | 0.6–19.4 | 0.2 |
| Male sex | 0.6 | |||
| Atrial fibrillation | 0.5 | |||
| Disease duration (m) | 0.6 | |||
| LVEDD (ml) | 0.4 | |||
| LAD (mm) | 0.6 | |||
| LVEF (%) | 0.03 | 0.9 | 0.8–0.9 | 0.04 |
CI: confidence interval; TR: tricuspid regurgitation; NYHA: New York Heart Association; LVEDD: left ventricular end-diastolic diameter; LAD: left atrial diameter; LVEF: left ventricular ejection fraction; TAD: tricuspid annulus diameter; TAD index: TAD/body surface area.
The rate of freedom from heart failure (NYHA class ≥3) was 96.7% at 10 years (Fig. 2B). Univariate analysis revealed that freedom from heart failure was associated with age (P = 0.1), preoperative NYHA class (P = 0.07) and LVEF (P = 0.03). Multivariate analysis revealed that LVEF was an independent risk factor for heart failure (OR 0.9, 95% CI 0.8–0.9, P = 0.04, Table 2).
Postoperative tricuspid regurgitation progression
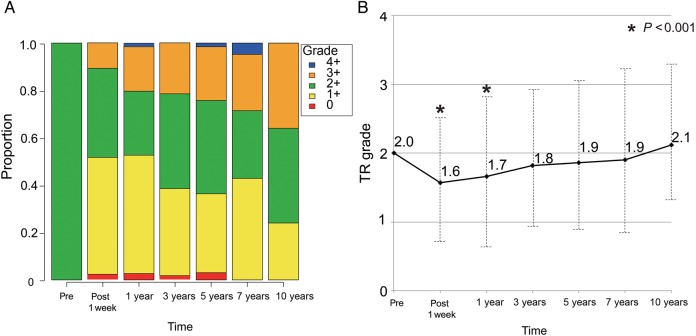
Figure 3A shows the proportion of TR grade at different points relative to operation. Untreated 2+ TR significantly improved temporarily postoperatively. The mean postoperative TR grade at 1 week and 1 year postoperative TR grade were 1.6 ± 0.7 and 1.7 ± 0.9, respectively (compared with preoperative grade, P < 0.001 for both). TR grade then progressed again in the mid- to long term (Fig. 3B). Freedom from 3+ or 4+ TR was 64.2% at 5 years and 46.7% at 10 years (Fig. 4) This was significantly lower than that from ≥3+ TR in patients who underwent concomitant TVS (100% at 5 years, P = 0.006). Univariate analysis revealed that postoperative TR progression (≥3+) was significantly associated with age, preoperative atrial fibrillation and preoperative TAD index (all P < 0.05). Multivariate analysis revealed that the independent risk factors for postoperative TR progression (≥3+) were age (OR 1.1, 95% CI 1.0–1.1, P = 0.005), atrial fibrillation (OR 2.2, 95% CI 1.0–4.5, P = 0.04) and preoperative TAD index (OR 1.1, 95% CI 1.0–1.2, P = 0.02, Table 3).
Figure 3:
(A) The proportion of tricuspid regurgitation (TR) grade at different points relative to operation. (B) Mean tricuspid regurgitation (TR) grade at different points relative to operation. Follow-up echocardiography examinations were performed at 1 week, and 1, 3, 5, 7 and 10 years postoperative. Pre: preoperative. *P < 0.001 compared with the preoperative grade.
Figure 4:
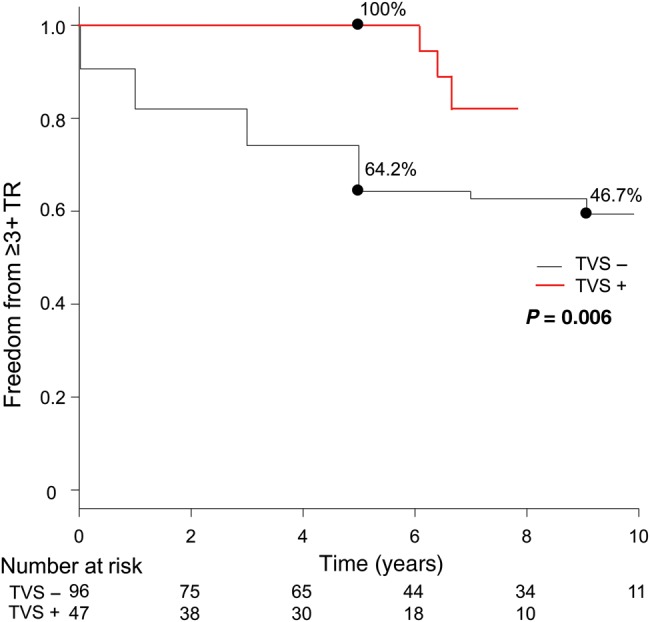
Comparison of actuarial rate of freedom from postoperative tricuspid regurgitation (TR) progression (≥3) between the two groups. TVS− group: without concomitant tricuspid valve surgery, solid line; TVS+ group: with concomitant tricuspid valve surgery, dashed line.
Table 3:
Risk factors for TR progression (≥3+)
| Variables | Univariate | Multivariate | 95% CI | P-value |
|---|---|---|---|---|
| P-value | Odds ratio | |||
| Age (years) | 0.002 | 1.1 | 1.0–1.1 | 0.005 |
| NYHA class | 0.9 | |||
| Atrial fibrillation | 0.02 | 2.2 | 1.0–4.5 | 0.04 |
| Rheumatic aetiology | 0.2 | |||
| Disease duration (m) | 0.5 | |||
| TAD index (mm/m2) | 0.004 | 1.1 | 1.0–1.2 | 0.02 |
| LVEDD (ml) | 0.09 | 1.0 | 0.9–1.0 | 0.2 |
| LAD (mm) | 0.8 |
CI: confidence interval; TR: tricuspid regurgitation; NYHA: New York Heart Association; LVEDD: left ventricular end-diastolic diameter; LAD: left atrial diameter; TAD: tricuspid annulus diameter; TAD index: TAD/body surface area.
Cut-off value of tricuspid annular diameter index
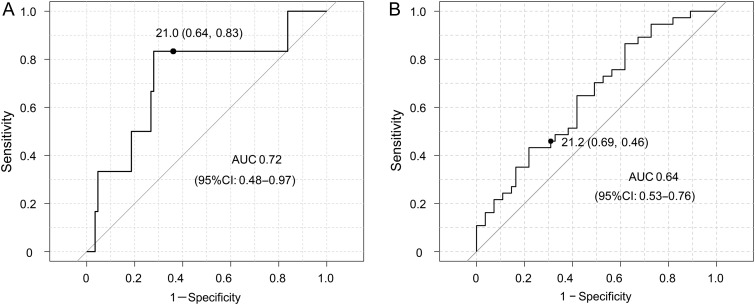
TAD index was an independent risk factor for postoperative TR progression. Hence, the ROC curve was expressed to determine an appropriate cut-off value of the TAD index as a predictor of late mortality and postoperative TR progression. The ROC curves showed an optimal TAD index cut-off value of 21.0 for late mortality and 21.2 for TR progression. The sensitivities for late mortality and TR progression were 83 and 46%, respectively, and the specificities were 64 and 69%, respectively. The areas under the ROC curves were 0.72 (95% CI 0.48–0.97) for late mortality and 0.64 (95% CI 0.53–0.76) for TR progression (Fig. 5A and B).
Figure 5:
Receiver operating characteristic (ROC) curves for late mortality (A) and postoperative tricuspid regurgitation (TR) progression (≥3) (B). The optimal tricuspid annular diameter (TAD) index cut-off values were 21.0 and 21.2 mm/m2, respectively. The sensitivities were 83 and 46%, respectively, and the specificities were 64 and 69%, respectively. The areas under the ROC curves (AUC) were 0.72 and 0.64, respectively.
DISCUSSION
This study showed that untreated 2+ TR temporarily improved after mitral valve surgery, but then progressed again in the mid- to long term. While the freedom from 3+ or 4+ TR was 64.2% at 5 years, it was only 46.7% at the 10-year follow-up. Our early recovery findings support previous reports that TR secondary to mitral valve disease may improve after mitral valve surgery [4, 5, 21]. However, our data suggest that this effect is reversible in the mid- to long-term period [7, 22]. On the other hand, there were no patients who developed ≥3+ TR postoperatively, when concomitant TVS was performed. The freedom from ≥3+ TR in the TVS+ group was also significantly better even among the patients who had longer disease duration and more rheumatic aetiology. Therefore, concomitant TVS is recommended, if applicable, because the durability of tricuspid valve surgery seems warranted.
Although the decrease in pulmonary artery pressure after mitral valve surgery reduces the afterload for the tricuspid valve and improves untreated TR just after mitral valve surgery, remodelling of the right ventricle and tricuspid valve is likely to cause TR progression [10]. As right ventricular remodelling leads to tricuspid annulus dilatation and the tethering of the tricuspid valve leaflets, these structural changes can cause TR progression in the mid- to long term after mitral valve surgery [3]. Previous studies found that right ventricular dilatation and dysfunction, tricuspid annular dilatation (>40 mm or >21 mm/m2), preoperative moderate or greater TR and atrial fibrillation [6, 8–11, 18] increased the likelihood of TR progression. We presented that the three factors (age, atrial fibrillation and TAD index) were associated with TR progression. These phenomena are associated with right ventricular remodelling. Remodelling of the right ventricle and tricuspid valve due to chronic pressure and volume overload may be a key factor in postoperative TR progression.
As our data showed that age is an independent risk factor for TR progression, tricuspid valve repair for older patients should be considered, especially if they are receiving concomitant cardiac surgery. Tricuspid valve repair concomitant with mitral valve surgery may provide the prognostic benefits and extend the patients' life expectancy. Risk scores such as the EuroSCORE or STS score and frailty may be useful for deciding whether to indicate tricuspid valve repair.
Although there were a few studies regarding 2+ TR prognosis, we presented that more than half of untreated 2+ TR can progress to 3+ or 4+ TR in the long term. Therefore, even in patients with 2+ TR, tricuspid valve repair should be considered when patients have dilated tricuspid annulus or atrial fibrillation, or are elderly, if feasible.
The 2014 AHA/ACC guidelines suggest that risk factors for progression of TR include tricuspid annulus dilatation (>21 mm/m2 diameter indexed to BSA), and that tricuspid valve repair is recommended with dilatation of this size [14]. Although the statistical power was not strong, ROC data also suggested that the optimal TAD index cut-off value for late mortality was 21.0, whereas the optimal TAD index cut-off value for TR progression (≥3) was 21.2.
While there are no established indices in right ventricular remodelling or dysfunction, our data suggest that the TAD index may be a simple and effective indicator, in addition to the mode of tricuspid leaflet coaptation and tricuspid leaflet tethering [23]. Thus, the TAD index can be used to consider surgical indication for the tricuspid valve.
Tricuspid valve repair using a prosthesis results in better outcomes than suture repair, and a three-dimensional prosthetic ring has provided excellent survival and freedom from recurrence [10, 18, 24, 25]. Our previous study showed that tricuspid valve repair using the MC3 annuloplasty ring (Edwards Lifesciences, Irvine, CA, USA) for moderate to severe TR provided 75% freedom from moderate or greater TR 5 years postoperative, with low surgical mortality [24]. Combined with these results, aggressive tricuspid valve repair using a prosthetic ring is reasonable although the surgical approach for right ventricular remodelling besides annuloplasty has not been established.
In conclusion, untreated 2+ TR improved temporarily after mitral valve surgery, but then progressed in the mid- to long term. Therefore, concomitant TVS should be considered in patients with 2+ TR if they have dilated tricuspid annulus or atrial fibrillation, or are elderly, if feasible.
Limitations
This study was a retrospective analysis, and therefore unknown confounders may have influenced outcomes beyond those controlled in the multivariate analysis. No information was provided regarding preoperative right atrial dimensions or degree of leaflet tethering. An important limitation is the relatively small sample size. Future randomized controlled studies are required to determine whether tricuspid valve repair is useful to prevent postoperative TR progression due to right ventricular remodelling. However, our data showed a relatively long-term outcome for the patients who did not undergo tricuspid valve repair for 2+ TR. Although the ESC/EACTS 2012 guidelines recommend tricuspid annuloplasty in patients with only 1+ TR in the presence of tricuspid annulus dilatation, we did not include patients with 0+ or 1+ TR, regardless of the size of the tricuspid annulus. Further studies including 0+ or 1+ TR with dilated tricuspid annulus are required.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr M. De Bonis (Milan, Italy): The first consideration I would like to do is a suggestion. I would like to suggest that you limit your analysis, in terms of Kaplan-Meier curves for survival and freedom from events to five years rather than ten years, because the number of patients at risk you do have at ten years is very small. So really, you cannot draw any conclusion at such long-term follow-up.
Having said that, I would say that a major limitation of the study is the fact that most of the patients, preoperatively had 2+ tricuspid regurgitation and very few of them, seventeen patients indeed, had 3+ tricuspid regurgitation. Now, the problem with this small sample size is, that when you do the comparative analysis between the two groups, actually you have many doubts in terms of its statistical significance because of the very small number of patients in the tricuspid regurgitation subgroup. Moreover, those seventeen patients were very different from the 3+ tricuspid regurgitation, because they had a longer duration of the disease, they had more advanced pathology. So my suggestion would be possibly to remove those seventeen patients and just focus all the analysis on the 2+ moderate tricuspid regurgitation and I would like to have your comment on that.
Then the other point is, that half of your patients did have rheumatic mitral valve disease. This was not really addressed in your analysis. Of course, you might have had a significant progression of tricuspid regurgitation mainly because of the aetiology of tricuspid regurgitation, which was rheumatic in most of them. So why you did not include the aetiology among the predictors of progression of tricuspid regurgitation, and if you did that afterwards, did you find any correlation?
Dr Kusajima: We indicated tricuspid regurgitation due to rheumatic disease as primary tricuspid regurgitation, therefore, those patients were excluded from this study. However, rheumatic heart disease is sometimes associated with advanced tricuspid regurgitation; in fact, 27% of our cohort was rheumatic disease. As you implied, it is sometimes difficult to distinguish between primary tricuspid regurgitation and secondary tricuspid regurgitation with rheumatic mitral valve disease. Maybe by eliminating primary rheumatic tricuspid regurgitation there was no significant difference between the two groups.
Dr M. Salehi (Tehran, Iran): Is there any difference between the method of repair according to the severity of tricuspid regurgitation?
Dr Kusajima: Pardon?
Dr Salehi: Is there any difference between the method of the repair according to the severity of tricuspid regurgitation? Is there any difference between the method that you do for severe tricuspid regurgitation or mild tricuspid regurgitation?
Dr O. Alfieri (Milan, Italy): It is not related directly to this paper, but he asked whether you do a different type of repair according to the severity of tricuspid regurgitation.
Dr T. Fujita (Osaka, Japan): This is a good point. This cohort of patients didn't have tricuspid repair. In our institute, though, we historically did a De Vega repair and then changed to a ring repair for tricuspid regurgitation despite the severity of tricuspid regurgitation. Also to answer the first question for Dr De Bonis, why did we include moderate tricuspid regurgitation patients? Of course, the patients who had moderate tricuspid regurgitation were the sicker patients due to bad left ventricle function and the long disease duration. Therefore the survival curve was different in the two groups.
But the progression of the tricuspid regurgitation is quite similar in the two groups, even with mild tricuspid regurgitation patients deteriorated in the long term, meaning that this is important in comparing these two groups.
Dr Alfieri: Would you change the recommendation of the guidelines on the basis of your study suggesting a repair even if with mild tricuspid regurgitation and dilated annulus?
Dr Fujita: You are quite right, because this is long-term follow-up, so starting 2003. In this time the guidelines changed. So we started aggressively to treat moderate tricuspid regurgitation during that period. Regarding the concept of dilated annulus, more than 21 mm/m2 and these data were corrected from relatively large body surface area patients. In our data in which cohort the average body surface area was only 15.5, the cut-off point of TAD index for better long-term survival was 20.8 mm/m2. Even with a small patient, the TAD index works for leading to this result, which means this study was important, I think.
Dr R. Sharony (Tel Aviv, Israel): In our centre, we are very liberal intervening on tricuspid incompetence while performing left heart valve surgery. According to the current guidelines it is recommended to perform annuloplasty, if the annulus diameter is more than 40 mm. However, in our centre, even in the presence of mild to moderate functional tricuspid regurgitation, while the annulus size is measured between 35 and 40 mm by echocardiography, we generally use to perform annuloplasty. This ‘aggressive’ approach is mainly applied for patients with rheumatic aetiology of mitral valve disease.
REFERENCES
- 1.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol 2009;53:401–18. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda S, Gillinov AM, Song JM, Daimon M, Kongsaerepong V, Thomas JD et al. . Echocardiographic insights into atrial and ventricular mechanisms of functional tricuspid regurgitation. Am Heart J 2006;152:1208–14. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda S, Gillinov AM, McCarthy PM, Stewart WJ, Song JM, Kihara T et al. . Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation 2006;114:I-582–7. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald NS, Ross J, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation 1967;35:I-63–9. [DOI] [PubMed] [Google Scholar]
- 5.Duran CM, Pomar JL, Colman T, Figueroa A, Revuelta JM, Ubago JL. Is tricuspid valve repair necessary? J Thorac Cardiovasc Surg 1980;80:849–60. [PubMed] [Google Scholar]
- 6.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127–32. [DOI] [PubMed] [Google Scholar]
- 7.Calafiore AM, Gallina S, Iaco AL, Contini M, Bivona A, Gagliardi M et al. . Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? A propensity score analysis. Ann Thorac Surg 2009;87:698–703. [DOI] [PubMed] [Google Scholar]
- 8.Chan V, Burwash IG, Lam BK, Auyeung T, Tran A, Mesana TG et al. . Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg 2009;88:1209–15. [DOI] [PubMed] [Google Scholar]
- 9.Di Mauro M, Bivona A, Iaco AL, Contini M, Gagliardi M, Varone E et al. . Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg 2009;35:635–40. [DOI] [PubMed] [Google Scholar]
- 10.Benedetto U, Melina G, Angeloni E, Refice S, Roscitano A, Comito C et al. . Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2012;143:632–8. [DOI] [PubMed] [Google Scholar]
- 11.Navia JL, Brozzi NA, Klein AL, Ling LF, Kittayarak C, Nowicki ER et al. . Moderate tricuspid regurgitation with left-sided degenerative heart valve disease: to repair or not to repair? Ann Thorac Surg 2012;93:59–69. [DOI] [PubMed] [Google Scholar]
- 12.Ton-Nu TT, Levine RA, Handschumacher MD, Dorer DJ, Yosefy C, Fan D et al. . Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation 2006;114:143–9. [DOI] [PubMed] [Google Scholar]
- 13.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA et al. . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 15.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C et al. . European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–32. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. [DOI] [PubMed] [Google Scholar]
- 17.Foale R, Nihoyannopoulos P, McKenna W, Kleinebenne A, Nadazdin A, Rowland E et al. . Echocardiographic measurement of the normal adult right ventricle. Br Heart J 1986;56:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van de Veire NR, Braun J, Delgado V, Versteegh MI, Dion RA, Klautz RJ et al. . Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 2011;141:1431–9. [DOI] [PubMed] [Google Scholar]
- 19.Fujita T, Kobayashi J, Toda K, Nakajima H, Iba Y, Shimahara Y et al. . Long-term outcome of combined valve repair and maze procedure for nonrheumatic mitral regurgitation. J Thorac Cardiovasc Surg 2010;140:1332–7. [DOI] [PubMed] [Google Scholar]
- 20.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianchi G, Solinas M, Bevilacqua S, Glauber M. Which patient undergoing mitral valve surgery should also have the tricuspid repair? Interact CardioVasc Thorac Surg 2009;9:1009–20. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz O, Suri RM, Dearani JA, Sundt TM, Daly RC, Burkhart HM et al. . Functional tricuspid regurgitation at the time of mitral valve repair for degenerative leaflet prolapse: the case for a selective approach. J Thorac Cardiovasc Surg 2011;142:608–13. [DOI] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al. . Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 24.Hata H, Fujita T, Shimahara Y, Sato S, Kobayashi J. Early and mid-term outcomes of aggressive tricuspid annuloplasty with the MC3 ring. J Heart Valve Dis 2014;23:601–8. [PubMed] [Google Scholar]
- 25.Parolari A, Barili F, Pilozzi A, Pacini D. Ring or suture annuloplasty for tricuspid regurgitation? A meta-analysis review. Ann Thorac Surg 2014;98:2255–63. [DOI] [PubMed] [Google Scholar]