Abstract
OBJECTIVES
In Sweden, two centres perform lung transplantation for a population of about 9 million and the entire population is covered for lung transplantation by government health insurance. Lund University Hospital is one of these centres. This retrospective report reviews the 25-year experience of the Skåne University Hospital Lung Transplant Program with particular emphasis on short-term outcome and long-term survival but also between different subgroups of patients and types of transplant [single-lung transplantation (SLTx) versus double-lung transplantation (DLTx)] procedure performed.
METHODS
Between January 1990 and June 2014, 278 patients underwent lung transplantation at the Skåne University Hospital Sweden. DLTx was performed in 172 patients, SLTx was performed in 97 patients and heart–lung transplantation was performed in 9 patients. In addition, 15 patients required retransplantation (7 DLTx and 8 SLTx).
RESULTS
Overall 1-, 5-, 10-, 15- and 20-year survival rates were 88, 65, 49, 37 and 19% for the whole cohort. DLTx recipients showed 1-, 5-, 10- and 20-year survival rates of 90, 71, 60 and 30%, compared with SLTx recipients with 1-, 5-, 10- and 20-year survival rates of 83, 57, 34 and 6% (P < 0.05), respectively. Comparing the use of intraoperative extracorporeal membrane oxygenation, extracorporeal circulation (ECC) and no circulatory support in the aspect of survival, a significant difference in favour of intraoperative ECC was seen.
CONCLUSIONS
Superior long-term survival rates were seen in recipients diagnosed with cystic fibrosis, α1-antitrypsin deficiency and pulmonary hypertension. DLTx showed better results compared with SLTx especially at 10 years post-transplant. In the present study, we present cumulative incidence rates of bronchiolitis obliterans syndrome of 15% at 5 years, 26% at 10 years and 32% at 20 years post-transplant; these figures are in line with the lowest rates presented internationally.
Keywords: Lung, Transplantation, Survival, Long-term follow-up, Cystic fibrosis
INTRODUCTION
Lung transplantation (LTx) and heart–lung transplantation (HLTx) are established medical interventions for treating irreversible, end-stage pulmonary disease in patients in whom standard medical treatment has been proven to be insufficient [1].
Postoperative survival of LTx depends on various factors such as general and organ-specific recipient status, donor organ condition and operative technique. The prolonged survival rates obtained during the 1980s and 1990s most probably reflect improvements in organ preservation, operative technique, immunosuppression, recipient and donor organ selection, and prophylactic as well as direct treatment of infections in the patient [2].
These advancements in preventing early mortality and morbidity have allowed a broader list of indications for LTx with a gradual liberalization of the lung donor selection criteria, yielding an overall increase in LTxs even if this number is still restricted by organ availability.
Sweden has a population of 9 million and all citizens receive government health insurance. There are two active LTx centres in the country; Lund University Hospital is one of these centres. This retrospective report reviews the 25-year experience of the Skåne University Hospital Lung Transplant Program with particular emphasis on short-term outcome and long-term survival but also between different subgroups of patients and type of transplant procedure performed.
PATIENTS AND METHODS
Between January 1990 and June 2014, 278 patients underwent LTx at the Skåne University Hospital, Sweden. Double-lung transplantation (DLTx) was performed in 172 patients, single-lung transplantation (SLTx) was performed in 97 patients and HLTx in 9 patients. Of these, 129 were male and 149 were female. Retransplantation (ReTx) was performed in 15 patients. Among the ReTx recipients, of whom 5 were female and 10 were male, 7 recipients had a DLTx and 8 had an SLTx.
In the present study, the median age was 51 years with a range of 12–71 years. The major indications were defined as chronic obstructive pulmonary disease (COPD) (n = 67), cystic fibrosis (CF) (n = 54), α1-antitrypsin deficiency (AAT1) (n = 55), pulmonary fibrosis (PF) (n = 38), pulmonary hypertension (PH) (n = 39) and a group deemed as ‘other’ (n = 25) including bronchiectasis, sarcoidosis, bronchioalveolar cancer, silicosis, acute respiratory distress syndrome (ARDS) and graft-vs-host disease (GVHD).
Transplant procedure
HLTx was performed via median sternotomy in 7 patients and via a ‘clamshell’ (bilateral anterolateral thoracotomy with a transverse sternotomy) incision in the 4th intercostal space in 2 patients. SLTx and DLTx were performed in standard fashion. SLTx was performed through a posterolateral thoracotomy in 86 patients, via clamshell in 7 patients and via median sternotomy in 4 patients. DLTx was performed through a ‘clamshell incision’ in 146 patients, via median sternotomy in 17 patients and via anterolateral thoracotomy in 9 patients.
Preoperative respiratory support was used in 13 operations (CF 4, PF 5, ReTx 3, PH 1). Preoperative extracorporeal membrane oxygenation (ECMO) support was used in 12 operations (CF 6, PF 3, ARDS 1, PH 1, ReTx 1).
Intraoperative circulatory support in the form of extracorporeal circulation (ECC) was used in 105 cases, and intraoperative ECMO was used in 73 cases. Intraoperative circulatory support was not used in 115 cases.
Recipient selection
Recipients were selected according to the guidelines by the consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation [3]. Inclusion criteria were patients diagnosed with chronic pulmonary disease who were unresponsive to additional medical and/or surgical treatment. Lung transplant candidates typically had a life expectancy of <18 months and were reliant on supplemental oxygen with severe exercise intolerance. Prospective candidates were generally less than 65 years of age, and if older, candidates were investigated for additional comorbidities. Before entering the transplantation programme, all candidates underwent a clinical evaluation, a comprehensive history and a physical examination. The following investigations were conducted in all patients:
evaluation of the renal glomerular filtration rate (GFR) by iohexol clearance;
human leukocyte antigen antibody and typing;
immunological screening;
microbiological culturing;
haematological and biochemical laboratory measurements;
virology screening;
dental examination (general status including an oral panoramic radiograph);
densitometry (evaluating osteoporosis and vertebral morphometry);
stress test with arterial blood gas measured during work and during rest, lung scintigraphy (ventilation/perfusion scan) and spirometry (evaluating pulmonary function and diffusion capacity by carbon monoxide);
Doppler of the carotid arteries (>50 years) detecting potential arteriosclerosis;
CT scan of the chest (possible malignancies, emphysema/fibrosis), and in most cases abdominal CT scan;
echocardiogram (assessing ventricular function and potential PH);
coronary angiography of coronary blood vessels; and
24 h pH evaluation, sometimes followed by gastroscopy.
Patients were then reviewed by a multidisciplinary team before they were accepted for transplantation. Recipient and donor matching was based on AB0 blood type and organ size.
Donor organ selection and preservation
Criteria for potential donor lungs included the following: donor age generally less than 65 years, no signs of significant pulmonary infection, chest X-ray clear of suspicious malignancies, normal arterial blood gas (PaO2 at 40 kPa or more with FiO2 of 1.0 and positive end-expiratory pressure of 5 cmH2O) and no known history of significant lung disease. Toxoplasma serology, microscopy, culture of urine and tracheal secretions and cytomegalovirus (CMV) screening were routinely implemented. Pretransplantation, explanted organs were macroscopically inspected for defects, and samples were sent for fungal and bacterial culturing.
The high success rate and the scarcity of donor organs have led to less restrictive donor selection criteria at our centre. Marginal donors have been increasingly accepted such as donors older than 65 years but who have no additional comorbidities, donors with a smoking history, mild hypertension and diabetes mellitus, and donors with an infection not considered an absolute contraindication to lung donation.
From the programme's inception, procured lungs were perfused in an antegrade fashion with EuroCollins solution at a low perfusion pressure (<20 mmHg). In 1993, the preservation solution was switched from EuroCollins to low-potassium dextran (Perfadex, Vitrolife, Göteborg, Sweden). The procured lungs were perfused antegradely with 80 ml/kg of Perfadex® solution containing 1.0 ml isotonic trometamol (Addex-THAM 3.3 mmol/ml, Fresenius Kabi AB, Uppsala, Sweden), 2 ml calcium chloride (0.45 mmol/ml) and 3 ml nitroglycerine (5 mg/ml, BMM Pharma AB, Stockholm, Sweden) at a low perfusion pressure (<20 mmHg). The Perfadex mixture is still used today. The lungs were semi-inflated before harvesting. Explanted organs were maintained at approximately 4–8°C.
Immunosuppression
Maintenance of immunoregulation has remained more or less constant throughout the programme centred on a protocol of cyclosporine, corticosteroids and azathioprine or mykofenolatmofetil as a lifelong treatment. Blood cyclosporine concentration was sustained between 145 and 245 µg/l. Most patients were also given induction therapy with antithymocyte immunoglobulin.
Antimicrobial and infection prophylaxis
Prophylactic administration of broad-spectrum antibiotics (currently Carbapenem, or Meronem) were given until cultures were available and we could target treatment for specific infection. Antiviral therapy was directed against CMV for patients with positive CMV serology. Preoperative screening for CMV was performed routinely in both recipients and donors to predict the level of needed antiviral therapy. Antifungal prophylaxis included low-dose flukonazol against Candida and sulfametoxazol/trimethoprim for pneumocystis carinii. Broad-spectrum antibiotics including colistin were used for targeting Pseudomonas infection if present.
Patients remained in critical care until adequate pain control was achieved and they were mobilized. In addition, patients were required to be infection-free and have stable immunosuppression treatment with no liver and renal insufficiency.
Follow-up
Patients followed a planned clinical regime and were reviewed the first year at 3, 6 and 12 months and annually thereafter. During the first year, densitometry, echocardiogram and an appointment with a dietician were scheduled. The follow-up programme consisted of the following tests:
renal GFR by iohexol clearance;
haematological and biochemical lab analysis and serology/virology lab measurements;
6-min walk test, performed at each follow-up. Assessing the patients expected work percentage determined on expected walking distance (m), average velocity (km/h) and, if oxygen support was made available, saturated O2 levels (%);
general examination by physician;
high-resolution computed tomography of the chest (may include contrast dye on suspicion of any malignancies);
spirometry, lung scintigraphy and work test;
oral glucose tolerance test for CF patients if not already diabetic; and
bronchoscopy with trans-bronchial biopsy (TBB) and bronchoalveolar lavage (BAL) at 3 and 6 months and on suspicion of rejection.
Rejection
Rejection was classified as either acute rejection characterized by perivascular and interstitial mononuclear cell infiltrates or chronic rejection in the form of obliterative bronchiolitis characterized by dense scarring and eosinophilic infiltrates [4]. If rapid deterioration of pulmonary function was detected as a sign of rejection, bronchiolitis obliterans syndrome (BOS) [5] being an example, bronchoscopies with TBB were conducted for diagnosis and antirejection treatment was initiated with pulsed methylprednisolone (Solu-Medrol, Pfizer AB, Sollentuna, Sweden) often along with tacrolimus (Prograf, Astellas Pharma AB, Malmö, Sweden) or everolimus (Certican, Novartis AB, Täby, Sweden) as a replacement for cyclosporine.
Statistical methods
Primary stratification of the material was made into two sets of cohorts. The first cohort was based on the main indication for LTx, with the following indicator cohorts: COPD, AAT1, CF, PH and PF. The second set divided the material based on type of LTx: DLTx, SLTx or HLTx. Overall survival was measured at 1, 3, 5, 10, 15 and 20 years after primary LTx by the Kaplan–Meier method and compared between the two sets of cohorts using the log-rank test, sample sizes allowing. Patients were censored if they reached the end of the study period, June 2014, or were lost to follow-up. All causes of death were incorporated into the survival analyses. Continuous variables are expressed as median (range) and categorical variables as number (%). Statistical comparisons of continuous variables were made by Mood's median test. Statistical comparisons of categorical variables were made by χ2 analysis or Fisher's exact test. Kaplan–Meier survival estimates were expressed as % -Survival [95% confidence interval (CI), point estimate]. For all statistical analyses, a P-value of less than 0.05 was considered significant. All calculations above were performed using SPSS Version 19.0 (IBM Corp., Armonk, NY, USA). A subanalysis of this study was the occurrence of BOS after primary LTx. In this analysis, death acted as a competing risk event to BOS. In a competing-risks model, we analysed the incidence of BOS and death as two separate outcomes. Specifically, we estimated and compared the cumulative incidence functions for BOS and death using Gray's test; see Gray (1988). All calculations regarding competing risks were performed using R with the CMPRSK package (available at http://www.r-project.org).
RESULTS
Type of transplant and indications
Number and type of transplant are illustrated in Fig. 1. The indications for LTx by type of transplant are presented in Table 1, and the transplantation indications by era are presented in Table 2. The group PH contains patients with primary pulmonary hypertension (PPH) (n = 28), secondary pulmonary hypertension (SPH) (n = 4) and patients with Eisenmenger's syndrome (EIS) as a major indication (n = 7).
Figure 1:
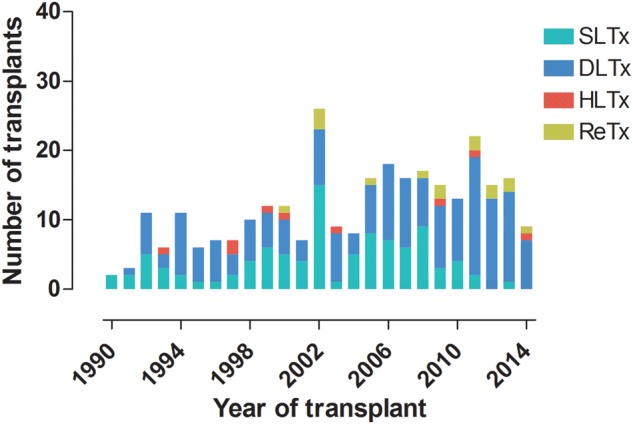
Number and type of transplant performed by year at Skåne University Hospital. HLTx: heart–lung transplant; DLTx: double-lung transplant; SLTx: single-lung transplant; ReTx: re-transplant.
Table 1:
Indications for lung transplantations by type of transplant
| Indications | Double lung (n = 179) | Single lung (n = 105) | Heart–lung (n = 9) |
|---|---|---|---|
| Pulmonary hypertension | 15% (n = 26) | 5% (n = 5) | 89% (n = 8) |
| Cystic fibrosis | 29% (n = 52) | 1% (n = 1) | 11% (n = 1) |
| Pulmonary fibrosis | 11% (n = 20) | 17% (n = 18) | 0% (n = 0) |
| COPD | 15% (n = 28) | 37% (n = 39) | 0% (n = 0) |
| Retransplantation | 4% (n = 7) | 8% (n = 8) | 0% (n = 0) |
| AAT1 | 15% (n = 26) | 27% (n = 29) | 0% (n = 0) |
| Othera | 11% (n = 20) | 5% (n = 5) | 0% (n = 0) |
aIncludes bronchiectasis, sarcoidosis, bronchioalveolar cancer, silicosis, ARDS and GVHD.
GVHD: graft-vs-host disease; COPD: chronic obstructive pulmonary disease; AAT1: α1-antitrypsin deficiency.
Table 2:
Indications for transplantation by era
| Indication | 1990–1993 (n = 22) | 1994–1997 (n = 30) | 1998–2001 (n = 41) | 2002–2005 (n = 59) | 2006–2009 (n = 66) | 2010–2014 (n = 75) |
|---|---|---|---|---|---|---|
| Pulmonary fibrosis | 18% (n = 4) | 0% (n = 0) | 17% (n = 7) | 5% (n = 3) | 20% (n = 13) | 15% (n = 11) |
| Cystic fibrosis | 9% (n = 2) | 6% (n = 2) | 20% (n = 8) | 17% (n = 10) | 21% (n = 14) | 24% (n = 18) |
| Pulmonary hypertension | 36% (n = 8) | 27% (n = 8) | 12% (n = 5) | 12% (n = 7) | 6% (n = 4) | 9% (n = 7) |
| ReTx | 0% (n = 0) | 0% (n = 0) | 2% (n = 1) | 7% (n = 4) | 5% (n = 3) | 9% (n = 7) |
| COPD | 14% (n = 3) | 20% (n = 6) | 17% (n = 7) | 30% (n = 18) | 29% (n = 19) | 18% (n = 14) |
| AAT1 | 23% (n = 5) | 30% (n = 9) | 20% (n = 8) | 22% (n = 13) | 13% (n = 9) | 15% (n = 11) |
| Othera | 0% (n = 0) | 17% (n = 5) | 12% (n = 5) | 7% (n = 4) | 6% (n = 4) | 10% (n = 7) |
aIncludes bronchiectasis, sarcoidosis, bronchioalveolar cancer, silicosis, ARDS and GVHD.
ReTx: retransplantation; COPD: chronic obstructive pulmonary disease; AAT1: α1-antitrypsin deficiency; GVHD: graft-vs-host disease.
Survival
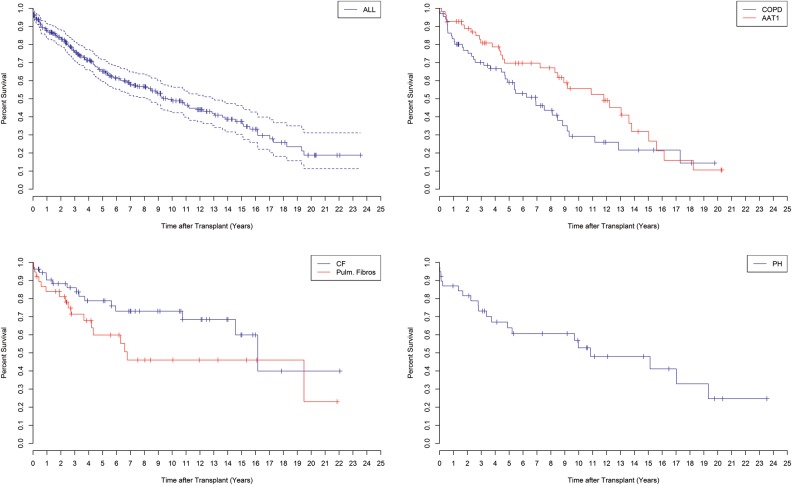
The overall 1-, 3-, 5-, 10-, 15- and 20-year survival rates for the entire cohort of patients in terms of percentage of survival with an upper/lower 95% CI were 88% (CI 84–92), 76% (CI 71–82), 65% (CI 59–72), 49% (CI 42–56), 37% (CI 30–46) and 19% (CI 11–31), respectively (Fig. 2).
Figure 2:
Overall survival after lung transplantation at Skåne University Hospital from January 1990 to June 2014, with a total of 278 patients (top left). Survival for recipients with COPD and AAT1 (P > 0.05) (top right). Survival for recipients with CF and PF (P > 0.05) (bottom, left). Survival for recipients with PH (bottom right). COPD: chronic obstructive pulmonary disease; AAT1: α1-antitrypsine deficiency; CF: cystic fibrosis; PH: pulmonary hypertension; PF: pulmonary fibrosis.
Patients with COPD showed 1-, 3-, 5-, 10- and 15-year survival rates of 83% (CI 75–93), 70% (CI 60–82), 59% (CI 48–73), 29% (CI 18–47) and 22% (CI 12–41), respectively, compared to patients with AAT1 at 1-, 3-, 5-, 10-, 15- and 20-year survival rates of 93% (CI 86–99), 81% (CI 71–92), 70% (CI 58–84), 56% (CI 42–73), 32% (CI 19–55) and 11% (CI 3–37), respectively (P > 0.05).
Patients with CF showed overall survival rates of 90% at 1 year (CI 83–99), 86% at 3 years (CI 77–96), 79% at 5 years (CI 68–92), 73% at 10 years (CI 61–88), 60% at 15 years (CI 43–85) and 40% at 20 years (CI 17–96) compared with survival rates in PF patients at equivalent time intervals of 84% (CI 73–97), 71% (CI 58–88), 60% (CI 45–80), 46% (CI 30–70), 46% (CI 30–70) and 23% (CI 5–98), respectively (P > 0.05) .
Recipients diagnosed with PH had 1-, 3-, 5-, 10-, 15- and 20-year survival rates of 87% (CI 77–98), 73% (CI 60–90), 64% (CI 50–82), 53% (CI 38–74), 48% (CI 33–70) and 25% (CI 10–59), respectively.
Survival by type of transplant
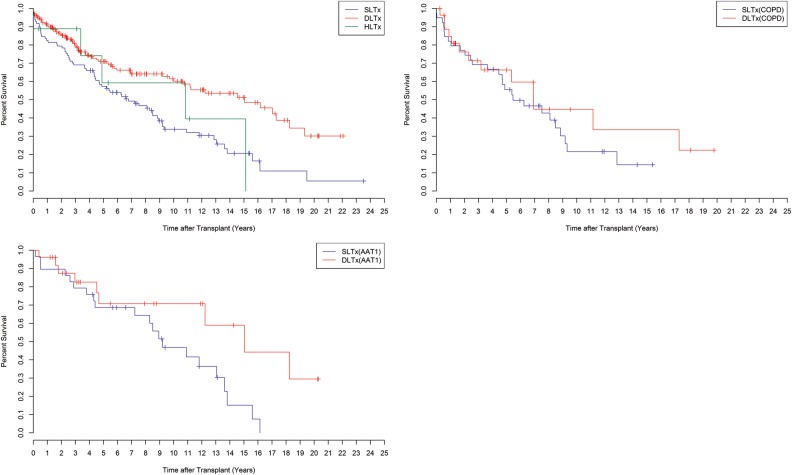
Pulmonary transplant survival by type of transplant (DLTx, SLTx and HLTx) is shown in Fig. 3. Survival rates for DLTx patients were 90% at 1 year (CI 86–95), 80% at 3 years (CI 74–87), 71% at 5 years (CI 64–79), 60% at 10 years (CI 52–70), 51% at 15 years (CI 42–63) and 30% at 20 years (CI 18–49) compared with SLTx recipients with 1-, 3-, 5-, 10-, 15- and 20-year survival rates of 83% (CI 75–90), 69% (CI 61–79), 57% (CI 48–68), 34% (CI 25–46), 21% (CI 12–34) and 6% (CI 1–31), respectively. HLTx recipients showed 1-, 3-, 5-, 10- and 15-year survival rates of 89% (CI 71–100), 89% (CI 71–100), 59% (CI 32–100), 59% (CI 32–100) and 40% (CI 14–100), respectively. A significant difference in survival was shown between the three groups: DLTx, SLTx and HLTx (P < 0.05). In the comparison of SLTx and DLTx, a P < 0.05 is obtained. No significant difference was shown between HLTx and DLTx (P > 0.05) and between HLTx and SLTx (P > 0.05).
Figure 3:
Survival by type of transplant after lung transplantation at Skåne University Hospital from January 1990 to June 2014. HLTx (n = 9), DLTx (n = 172) and SLTx (n = 97) (P < 0.05) (top left). Survival in COPD patients by type of transplants, SLTx versus DLTx (P > 0.05) (top right). Survival in AAT1 patients by type of transplant, SLTx versus DLTx (P < 0.05) (bottom left). COPD: chronic obstructive pulmonary disease; HLTx: heart–lung transplantation; DLTx: double-lung transplantation; SLTx: single-lung transplantation.
In addition to survival by type of transplant, an observation of survival is illustrated in two groups: COPD and AAT1 in terms of DLTx vs SLTx (Fig. 3). In COPD patients, 1-, 3- 5-, 10- and 15-year survival rates for DLTx recipients were 85% (CI 72–100), 71% (CI 55–92), 66% (CI 49–89), 45% (CI 26–77) and 34% (CI 15–73) compared with those of SLTx recipients at the same time intervals of 82% (CI 71–95), 69% (CI 56–85), 56% (CI 42–74), 22% (CI 10–45) and 14% (CI 5–42), respectively (P > 0.05). In AAT1 patients, 1-,3- 5-, 10-, 15- and 20-year survival rates for DLTx recipients were 96% (CI 89–100), 83% (CI 68–100), 71% (CI 53–94), 71% (CI 53–94), 59% (CI 37–93) and 30% (CI 10–87) in comparison with those of SLTx recipients at the same time intervals of 90% (CI 79–100), 79% (CI 66–96), 69% (CI 54–88), 47% (CI 31–71) and 15% (CI 5–49), respectively (P < 0.05) (Fig. 3).
Survival by type of intraoperative circulatory support (ECC, ECMO or no circulatory support)
Survival rates for recipients with intraoperative circulatory support were 94% at 1 year (CI 87–97), 84% at 3 years (CI 75–90), 71% at 5 years (CI 60–79), 57% at 10 years (CI 44–68), 45% at 15 years (CI 32–57) and 26% at 20 years (CI 13–40) compared with recipients with intraoperative ECMO with a 1-, 3-, 5-, 10- and 15-year survival rates of 95% (CI 88–98), 85% (CI 75–91), 72% (CI 60–81), 49% (CI 32–64) and 49% (CI 32–64), respectively . Recipients with no intraoperative support showed 1-, 3-, 5-, 10-, 15- and 20-year survival rates of 88% (CI 81–94), 76% (CI 64–82), 68% (CI 59–75), 47% (CI 37–56), 30% (CI 19–42) and 15% (CI 5–30), respectively. No significant difference in survival was seen between the three groups: intraoperative circulatory support, intraoperative ECMO and no support (P = 0.100). In comparison between the subgroups a significant difference was seen between the use of intraoperative ECC and intraoperative ECMO, in favour of intraoperative ECC (P = 0.049). No significant difference was shown between intraoperative ECMO and no intraoperative support (P = 0.295). Between intraoperative ECC and no intraoperative support, no significant difference was shown (P = 0.149).
Ventilator support, intensive care and postoperative stay
The overall median time the recipients needed ventilator support postoperatively was 1.99 days (range, 0.04–95.00 days). The median duration of the intensive care unit (ICU) stay was 6.60 days (range, 0.49–105.00 days), and the total median postoperative hospital stay was 42.65 days (range, 11.68–175.66 days) for the same cohort (Table 3).
Table 3:
Time of ventilator support, time in the ICU and total hospital time with regard to type of transplant and diagnosis
| Indications | Ventilator time (days) | P-value | ICU time (days) | P-value | Hospital time (days) | P-value |
|---|---|---|---|---|---|---|
| LTx type | 0.001 | <0.001 | 0.386 | |||
| SLTx | 1.0 (0.04–62) | 4 (0.5–81) | 42 (12–134) | |||
| DLTx | 2.0 (0.06–95) | 7.6 (1–105) | 42 (12–176) | |||
| HLTx | 7.0 (1.00–22) | 57.3 (24–91) | 67 (33–98) | |||
| Diagnosis | 0.007 | 0.001 | 0.645 | |||
| COPD | 1.0 (0.04–95) | 5.2 (0.5–95) | 44 (12–164) | |||
| AAT1 | 1.1 (0.22–62) | 4 (1–66) | 43 (12–176) | |||
| CF | 1.9 (0.06–30) | 7.7 (1–75) | 41 (12–122) | |||
| PF | 2.6 (0.44–62) | 8 (2–81) | 46 (20–127) | |||
| SPH, EIS | 10.5 (1.00–65) | 36 (4–69) | 47 (33–87) | |||
| PPH | 4.5 (0.69–80) | 12.5 (1–105) | 47 (23–147) | |||
| Sarcoidosis | 5.8 (0.40–37) | 19.4 (2–73) | 58 (32–120) | |||
| GVHD | 6.3 (1.00–15) | 11.6 (4–22) | 38 (30–79) | |||
| Bronchiectasis | 2.0 (1.31–9) | 5.8 (3–35) | 41 (31–64) | |||
| Total | 2.0 (0.04–9) | 6.6 (0.5–105) | 43 (12–176) |
ICU: intensive care unit; COPD: chronic obstructive pulmonary disease; AAT1: α1-antitrypsin deficiency; CF: cystic fibrosis; PF: pulmonary fibrosis; SPH: secondary pulmonary hypertension; EIS: Eisenmenger’s syndrome; PPH: primary pulmonary hypertension; GVHD: graft-vs-host disease.
In terms of type of transplant, the median respiratory time was 1.00 day (range, 0.04–62.00 days) for SLTx recipients, 2.01 days (range, 0.06–95.00 days) for DLTx recipients and 7.00 days (range, 1.00–22.00 days) for HLTx recipients (P < 0.05). The median ICU stay was 4.00 days (range, 0.49–81.00 days) for SLTx recipients, 7.60 days (range, 1.00–105.00 days) for DLTx recipients and 57.33 days (range, 24.00–91.00 days) for HLTx recipients (P < 0.05). The median total hospital stay was 42 days (range, 11.69–133.64 days) for SLTx recipients, 42.3 days (11.81–175.66 days) for DLTx recipients and 67.37 days (range, 33.00–98.00 days) for HLTx recipients (P > 0.05) (Table 3).
In terms of diagnosis, the median and the range for the duration of ventilator support, ICU stay and total hospital stay are given in Table 3.
Overall cumulative incidence of bronchiolitis obliterans syndrome and death
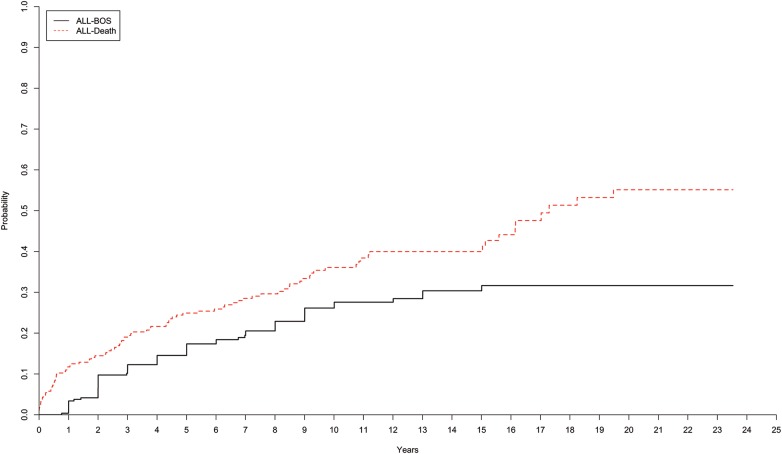
The overall 5-, 10-, 15- and 20-year incidence rates of BOS are presented (percentage of probability ± standard error) for the entire cohort of patients in Fig. 4. The incidence rates of BOS among the recipients at 5 years was 15 ± 2%, at 10 years 26 ± 3%, at 15 years 30 ± 3% and at 20 years 32 ± 4%. The overall incidence rates of death for the entire cohort of recipients were 25 ± 3% (5 years), 36 ± 3% (10 years), 40 ± 4% (15 years) and 55 ± 5% (20 years).
Figure 4:
Cumulative incidence of BOS and death, after lung transplantation at Skåne University Hospital from January 1990 to June 2014, for all recipients. BOS: bronchiolitis obliterans syndrome; SLTx: single-lung transplantation; HLTx: heart–lung transplantation; DLTx: double-lung transplantation.
Mortality
Mortality is shown at two time windows: >12 and <12 months postoperatively (Table 4). The group called ‘other causes’ is defined as mortality caused by myocardial and cerebral ischaemia, and multiple organ failure in addition to other causes related to the patient's age and health status.
Table 4:
Causes of death according to recipient diagnosis and time after transplant
| Diagnosis; cause of death | <12 months | >12 months | P-value |
|---|---|---|---|
| Total: 278 | |||
| Pulmonary fibrosis (n = 38) | |||
| Total number of deaths | 6 | 11 | 0.903 |
| Death from organ rejection | 1 (17%) | 2 (18%) | |
| Death from infection | 1 (17%) | 4 (36%) | |
| Death from malignancy | 2 (33%) | 3 (27%) | |
| Death from other causes | 2 (33%) | 2 (18%) | |
| COPD (n = 67) | |||
| Total number of deaths | 11 | 29 | 0.458 |
| Death from organ rejection | 4 (36%) | 7 (24%) | |
| Death from infection | 2 (18%) | 8 (28%) | |
| Death from malignancy | – | 5 (17%) | |
| Death from other causes | 5 (46%) | 9 (31%) | |
| AAT1 (n = 55) | |||
| Total number of deaths | 4 | 26 | 0.718 |
| Death from organ rejection | – | 6 (23%) | |
| Death from infection | 2 (50%) | 7 (27%) | |
| Death from malignancy | – | 4 (15%) | |
| Death from other causes | 2 (50%) | 9 (35%) | |
| Pulmonary hypertension (n = 39) | |||
| Total number of deaths | 5 | 15 | 0.208 |
| Death from organ rejection | – | 5 (33%) | |
| Death from infection | – | 2 (13%) | |
| Death from malignancy | – | – | |
| Death from other causes | 5 (100%) | 8 (53%) | |
| Cystic fibrosis (n = 54) | 0.712 | ||
| Total number of deaths | 5 | 10 | |
| Death from organ rejection | 1 (20%) | 6 (60%) | |
| Death from infection | 2 (40%) | 1 (10%) | |
| Death from malignancy | – | 2 (20%) | |
| Death from other causes | 2 (40%) | 1 (10%) | |
| Other (n = 25) | 0.190 | ||
| Total number of deaths | 3 | 11 | |
| Death from organ rejection | – | 5 (46%) | |
| Death from infection | 1 (33%) | – | |
| Death from malignancy | – | 2 (18%) | |
| Death from other causes | 2 (67%) | 4 (36%) | |
The group called ‘other causes’ is defined as patients with mortality caused by myocardial and cerebral ischaemia, and multiple organ failure such as renal and liver in addition to other causes related to the patient's old age and individual health status.
COPD: chronic obstructive pulmonary disease; AAT1: α1-antitrypsin deficiency; CF: cystic fibrosis; PF: pulmonary fibrosis.
DISCUSSION
The number of transplants at our centre has increased since 1990. The frequency of SLTx reached its peak in 2002 and significantly declined thereafter, in favour of DLTx (Fig. 1). This change can be attributed to the results of published studies that showed significantly better long-term survival rates in patients undergoing DLTx rather than SLTx [6–8].
CF patients were infrequent at the beginning of the programme, with a modest 2 patients in 1990–1993 with an increase to 18 patients in 2010–2014. The most common diagnosis for LTx at our clinic was COPD, peaking at about 30% in 2002–2005 and in 2006–2009, but declining to 18% in 2010–2014. The variance probably reflects the number of patients with COPD on the waiting list.
The overall 1-, 5-, 10-, 20- and 25-year survival rates of the entire cohort were 88, 65, 49, 37 and 19%, respectively, and the overall median survival for the whole cohort was 9.8 years. The results of the present study are in line with the highest survival rates presented internationally, where follow-up is often limited to 10 years [9–11]. One of the largest published series from a single centre was a 20-year experience of 521 lung transplants in 501 recipients by de Perrot et al. [12] with documented 5-, 10- and 15-year survival rates of 55, 35 and 27%, respectively.
In our study, the best long-term survival was achieved by DLTx recipients with 1-, 10- and 20-year survival rates of 90, 60 and 30%, compared with SLTx recipients with 1-, 10- and 20-year survival rates of 83, 34 and 5%, respectively (P < 0.05). These results further support a clinical programme favouring DLTx instead of SLTx. It has been suggested that better long-term survival after DLTx is due to better pulmonary functional recovery and to reduced graft-related mortality when compared with SLTx [6, 7]. HLTx recipients at equivalent time intervals showed survival rates of 89% (1 year) and 59% (10 years), with no recipient evaluable at 20 years. However, the HLTx group consisted of a limited patient group (n = 9) and should therefore be analysed with caution.
CF patients and AAT1 patients had the best survival rates at our clinic, showing a median survival of 16.2 and 11.8 years, respectively. We speculate that these encouraging figures regarding CF are a result of our lung clinical department specializing in CF. The groups with the least chance of survival were observed among patients who underwent LTx due to COPD and PF, who only had a median survival of 6.9 and 6.8 years post-transplant, respectively. COPD recipients represent an older patient group often with comorbidities such as heart and vessel disease that might explain the discouraging figures. However, our survival rates regarding these recipients are in line with survival rates presented internationally [12].
In the present study, PH recipients had 1-, 5-, 10- and 20-year survival rates of 87, 64, 53 and 25% and a median survival of 10.8 years. These results are better than those found in other international studies that report a 1-year survival rate of 60% for such recipients [13, 14]. We speculate that this high rate of survival may be an outcome of our selection of PH patients as recipients of DLTx or HLTx [15].
Patients were supported by a ventilator a median of 2 days post-transplant (Table 3). Divided into types of transplant, the SLTx recipients showed a significantly lower ventilator time of only 1 day, followed by DLTx recipients of 2 days and finally HLTx recipients of 7 days (P < 0.05). We speculate that SLTx patients have their remaining native lung to depend on in the early postoperative phase and might therefore better handle a reperfusion injury in the transplanted lung. An HLTx represents the most challenging transplantation procedure both peri- and postoperatively compared with both SLTx and DLTx. Recipients who received HLTx mainly consisted of two complex patient groups of PH and EIS, which we believe explains the significantly longer time on ventilator support. Regarding ICU stay, the same pattern emerges: SLTx (4 days), DLTx (8 days) and HLTx (57 days) (P < 0.05).
Interestingly, when divided by diagnoses, the shortest median ventilator support time and shortest median ICU time were seen among COPD (1 day, 5 days), AAT1 (1 day, 4 days) and CF patients (2 days, 8 days). SPH and EIS patients showed the longest median duration of ventilator support (11 days) as well as ICU stay (36 days). We speculate that the younger age of the CF and AAT1 patient groups might explain the shorter recovery time. The vast number of SLTx procedures in COPD patients may also explain that group's shorter recovery time. Groups with the longest time of recovery (sarcoidosis, PH) exhibited systemic illnesses often with additional comorbidities. Poor preoperative health status seems to predispose these patients to a longer recovery time, reflected by prolonged ICU stay and respiratory time.
As for the parameter of total hospital time, no significant correlation was found regarding type of transplant or diagnosis. This is to be expected considering potential episodes of CMV pneumonitis and possible acute rejections that might affect the patient outcome of total hospital stay [16, 17].
BOS reflects small airway obliteration that results in chronic rejection, and is a major risk factor for survival after 90 days [16]. However, it is less probable for the recipient to develop BOS within the first year, as reports from international studies have shown that the cumulative incidence quickly increases only within the first 5 years [17–19]. In the present study, we present cumulative incidence rates of BOS of 15% at 5, 26% at 10 and 32% at 20 years post-transplant; these figures are in line with the lowest rates presented internationally. For comparison, Burton et al. [20] presented with a cohort of 346 patients cumulative incidence rates of BOS of 57% (5 years) and 77% (10 years).
Limitations
A limitation of this study is the rather broad period of time, since donor selection has evolved over the past 25 years. There have also been significant changes in the care of transplant patients that affect outcome variables such as survival depending on the year of transplantation. Surgical and anaesthesia techniques have also been refined and improved, as have the management and treatment of these patients perioperatively and in the intensive care unit. Also the introduction of new pharmaceuticals as well as provision of more focus on specific prophylactic treatments for these patients might affect results. Recipient inclusion criteria have broadened over the years and now preoperative ECMO support or ventilator support are no longer contraindications for LTx, representing a complex recipient clientele.
CONCLUSIONS
Sweden has a population of 9 million and all citizens receive government health insurance. There are two active LTx centres in the country; Lund University Hospital is one of these centres. In the present study, we present the 25-year experience of LTx at Skåne University Hospital in Lund, Sweden. Our study shows excellent survival rates with overall 1-, 5-, 10-, 20- and 25-year survival rates of 88, 65, 49, 37 and 19%, respectively, giving our recipients one of the highest survival rates internationally. The best long-term survival was seen in recipients diagnosed with CF, AAT1 and PH. DLTx showed better results than SLTx especially after 10 years post-transplant. In the present study, we present a cumulative incidence rate of BOS of 15% at 5, 26% at 10 and 32% at 20 years post-transplant; these figures are in line with the lowest rates presented internationally. Challenges remain in terms of mortality such as in reducing the incidence of infection, but most of all in preventing chronic graft dysfunction as the major obstacle to survival with LTx.
Funding
The study was supported by the Skane University Hospital Foundation.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors thank Lena Mared, Leif Eriksson, Jan-Otto Solem and Stig Steen, all co-workers at the Department of Cardiothoracic Surgery, Pulmonary Medicine and Thoracic Intensive Care and Anesthesia, Skåne University Hospital, Lund University, Sweden.
REFERENCES
- 1.Van Trigt P, Davis RD, Shaeffer GS, Gaynor JW, Landolfo KP, Higginbotham MB et al. Survival benefits of heart and lung transplantation. Ann Surg 1996;223:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosenpud JD, Bennett LE, Keck BM, Fiol B, Boucek MM, Novick RJ. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official report-1999. J Heart Lung Transplant 1999;18:611–26. [DOI] [PubMed] [Google Scholar]
- 3.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ et al. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–55. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229–42. [DOI] [PubMed] [Google Scholar]
- 5.Burton CM, Iversen M, Mortensen J, Carlsen J, Andersen CB, Milman N et al. Post-transplant baseline FEV1 and the development of bronchiolitis obliterans syndrome: an important confounder? J Heart Lung Transplant 2007;26:1127–34. [DOI] [PubMed] [Google Scholar]
- 6.Cassivi SD, Meyers BF, Battafarano RJ, Guthrie TJ, Trulock EP, Lynch JP et al. Thirteen-year experience in lung transplantation for emphysema. Ann Thorac Surg 2002;74:1663–9; discussion 9–70. [DOI] [PubMed] [Google Scholar]
- 7.Hertz MI, Taylor DO, Trulock EP, Boucek MM, Mohacsi PJ, Edwards LB et al. The registry of the international society for heart and lung transplantation: nineteenth official report-2002. J Heart Lung Transplant 2002;21:950–70. [DOI] [PubMed] [Google Scholar]
- 8.Neurohr C, Huppmann P, Thum D, Leuschner W, von Wulffen W, Meis T et al. Potential functional and survival benefit of double over single lung transplantation for selected patients with idiopathic pulmonary fibrosis. Transpl Int 2010;23:887–96. [DOI] [PubMed] [Google Scholar]
- 9.Speich R, Nicod LP, Aubert JD, Spiliopoulos A, Wellinger J, Robert JH et al. Ten years of lung transplantation in Switzerland: results of the Swiss Lung Transplant Registry. Swiss Med Wkly 2004;134:18–23. [DOI] [PubMed] [Google Scholar]
- 10.Burton CM, Milman N, Carlsen J, Arendrup H, Eliasen K, Andersen CB et al. The Copenhagen National Lung Transplant Group: survival after single lung, double lung, and heart-lung transplantation. J Heart Lung Transplant 2005;24:1834–43. [DOI] [PubMed] [Google Scholar]
- 11.Sigurdardottir V, Bjortuft O, Eiskjaer H, Ekmehag B, Gude E, Gustafsson F et al. Long-term follow-up of lung and heart transplant recipients with pre-transplant malignancies. J Heart Lung Transplant 2012;31:1276–80. [DOI] [PubMed] [Google Scholar]
- 12.de Perrot M, Chaparro C, McRae K, Waddell TK, Hadjiliadis D, Singer LG et al. Twenty-year experience of lung transplantation at a single center: influence of recipient diagnosis on long-term survival. J Thorac Cardiovasc Surg 2004;127:1493–501. [DOI] [PubMed] [Google Scholar]
- 13.Egan TM, Detterbeck FC, Mill MR, Bleiweis MS, Aris R, Paradowski L et al. Long term results of lung transplantation for cystic fibrosis. Eur J Cardiothorac Surg 2002;22:602–9. [DOI] [PubMed] [Google Scholar]
- 14.Harringer W, Wiebe K, Struber M, Franke U, Niedermeyer J, Fabel H et al. Lung transplantation—10-year experience. Eur J Cardiothorac Surg 1999;16:546–54. [DOI] [PubMed] [Google Scholar]
- 15.Fadel E, Mercier O, Mussot S, Leroy-Ladurie F, Cerrina J, Chapelier A et al. Long-term outcome of double-lung and heart-lung transplantation for pulmonary hypertension: a comparative retrospective study of 219 patients. Eur J Cardiothorac Surg 2010;38:277–84. [DOI] [PubMed] [Google Scholar]
- 16.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med 2010;182:784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant 1998;17:1255–63. [PubMed] [Google Scholar]
- 18.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report—2010. J Heart Lung Transplant 2010;29:1104–18. [DOI] [PubMed] [Google Scholar]
- 19.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant 2007;26:681–6. [DOI] [PubMed] [Google Scholar]
- 20.Burton CM, Iversen M, Carlsen J, Mortensen J, Andersen CB, Steinbruchel D et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant 2009;28:888–93. [DOI] [PubMed] [Google Scholar]