Abstract
OBJECTIVES
Little is known regarding the transit-time flow measurement (TTFM) variables in grafts anastomosed to chronically totally occluded vessels (CTOs). We aimed to establish the TTFM cut-off values for detecting graft failure in bypass grafts anastomosed to chronically totally occluded arteries and clarify the relationship between early graft failure and the grade of collateral circulation/regional wall motion of the CTO territory.
METHODS
Among 491 patients who underwent isolated coronary artery bypass grafting (CABG) from 2009 to 2015, 196 cases with CTOs underwent postoperative coronary angiography within 1 month after CABG. Two hundred and forty-one CTOs in all patients were examined. Thirty-two CTOs (13%) were not bypassed and 214 conduits were anastomosed to CTOs and underwent intraoperative TTFM. Arterial conduits and saphenous vein grafts (SVGs) were used in 102 and 112 cases, respectively. Among the arterial conduit procedures that were performed, 78 involved the left internal thoracic artery (LITA), 10 involved the right internal thoracic artery (RITA) and 14 involved the right gastroepiploic artery (rGEA). Any graft showing Fitzgibbon type B or O lesions on angiography was considered to be a failing graft.
RESULTS
The insufficiency rates for LITA, RITA, rGEA and SVG procedures were 5.1, 10, 14.3 and 7.1%, respectively. The TTFM variables recorded in failing grafts had a significantly lower mean flow (Qmean) and higher pulsatility index (PI) compared with patent grafts. Furthermore, akinetic or dyskinetic wall motion in the territory of bypassed CTOs was observed at a significantly higher rate in failing grafts. A multivariable regression analysis and receiver operating characteristic analysis revealed good predictors of early graft failure as follows: a Qmean value of < 11.5 ml/min for arterial conduits, a PI value of >5.85 and akinetic/dyskinetic wall motion in the CTO territory for SVGs. The Rentrop collateral grade was not associated with early graft failure.
CONCLUSIONS
The Qmean value and PI value by the TTFM are useful to detect early graft failure in conduits anastomosed to CTOs. The collateral grade is not associated with graft failure; however, bypass grafting to CTOs with akinetic/dyskinetic wall motion should be carefully considered.
Keywords: Chronic total occlusion, Coronary artery bypass grafting, Transit-time flow measurement, Rentrop collateral grade, Graft failure
INTRODUCTION
With a prevalence rate of ∼18% in patients with significant coronary artery disease who undergo non-emergency coronary angiography (CAG), coronary chronic total occlusions (CTOs) are a frequent finding in today's catheterization laboratories [1]. The presence of a CTO is associated with higher rates of referral for coronary artery bypass grafting (CABG) due to the complex nature of percutaneous coronary intervention [2, 3]. Most CTOs are successfully bypassed; however, revascularization failures are sometimes observed in CTOs in areas other than the left anterior descending artery (LAD) branch [4, 5]. The CTOs targeted for bypass is often an atherosclerotic narrowing coronary artery with diffuse disease or heavy calcification, as is frequently observed in CTOs in non-LAD areas. High vascular resistance in CTOs results in poor blood runoff from the bypass graft, and contributes to graft failure.
At present, transit-time flow measurement (TTFM) is the most common intraoperative method for assessing graft function. Several retrospective studies have indicated that TTFM is useful for detecting grafts with impaired flow [6], and that TTFM values show a satisfactory level of correlation with early postoperative CAG flow data [7–9]. The reported cut-off TTFM values to distinguish failing grafts have been heterogeneous. According to different groups, the mean flow (Qmean) is set at 10 or 15 ml/min and the pulsatility index (PI) is set at 3 or 5 [10]. However, we occasionally encounter normal grafts showing a Qmean below or a PI above the cut-off values, e.g. when grafts are anastomosed to CTOs. To date, very little is known about the cut-off TTFM values in grafts anastomosed to CTOs.
Coronary collaterals in CTOs are typically observed and vary in extent from patient to patient. Although rich collateral circulation to the CTO can potentially compete with the graft flow, its influence on graft patency is not well known. Furthermore, the relationship between graft failure and regional wall motion of the territory supplied by a CTO is unknown.
Thus, in the present study, we focused on early graft failure in the grafts anastomosed to CTOs and examined the following issues: (i) the cut-off TTFM values for detecting graft failure in bypass grafts anastomosed to CTOs and (ii) the relationship between early graft failure and the grade of collateral circulation/regional wall motion of the CTO territory.
MATERIALS AND METHODS
From January 2009 to October 2015, 491 patients underwent isolated CABG in our institution. Of these patients, 196 patients with CTOs who underwent both intraoperative TTFM and postoperative CAG within 1 month after surgery were selected. One hundred and sixty-six patients were male and 30 patients were female, and the mean age was 66 ± 10 years. The preoperative coronary angiograms were reviewed by two cardiologists who were blinded to the subsequent clinical outcomes, and the extent of perfusion distal to the CTO lesion through the collateral feeding arteries was scored according to the criteria of Rentrop et al. [11], as follows: Grade 0 = no filling; Grade 1 = filling of side branches only, without visualization of the epicardial segment; Grade 2 = partial epicardial vessel filling by collaterals and Grade 3 = complete epicardial vessel filling by collaterals.
Operative procedures
CABG surgery was performed in the standard manner, either with a cardiopulmonary bypass (n = 112, 57%, including 19 beating on-pump cases) or without (n = 84, 43%).
No sequential anastomoses were included in this series. All arterial grafts were used as in situ grafts, while all saphenous vein grafts (SVGs) were used in an aortocoronary bypass fashion.
All of the patients underwent preoperative echocardiography and their left ventricular function was evaluated. The regional wall motion of the territory supplied by a CTO was assessed according to American Society of Echocardiography guidelines [12, 13], and a regional wall motion score was applied to each CTO territory according to the following classifications: 1 = normal contraction, 2 = hypokinetic (reduced contraction), 3 = akinetic (no contraction), 4 = dyskinetic (paradoxical motion during systole) and 5 = aneurysmal (diastolic deformation).
This retrospective study was approved by our institutional ethics committee and institutional review board (570-2).
Intraoperative flow measurement
Graft flow tracing was recorded intraoperatively just before sternal closure with a transit-time flowmeter (VQ1001; Medi-Stim AS, Oslo, Norway). The haemodynamic condition remained stable with a mean blood pressure between 70 and 90 mmHg during the flow measurement. TTFM analysis can provide the following parameters: mean flow calculated across five cardiac cycles (Qmean) and PI as the ratio of the difference between the maximum flow (Qmax) and the minimum flow (Qmin) ((Qmax − Qmin)/Qmean).
Postoperative angiography
All of the patients underwent postoperative angiography within 1 month after CABG. The angiograms were independently reviewed by two or more cardiologists. Each graft was assessed according to the Fitzgibbon classification [14]. Grafts that showed type B or O lesions were considered to be failing grafts.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation. Normally distributed continuous variables were analysed with an unpaired Student's t-test, and data that were not normally distributed were analysed with the Mann–Whitney U-test; the χ2 or Fisher's exact tests were used for categorical variables. A univariable logistic regression analysis was initially performed for each of the potential predictor variables of early graft failure. The selection of the independent variables was performed using a multivariable forward stepwise selection method. The optimal Qmean and PI cut-off values for predicting early graft failure were determined by a receiver operating characteristic (ROC) curve analysis. The area under the curve, sensitivity, 95% confidence interval and P-value were also reported. The optimal cut-off value was defined as the value that provided the maximum level of sensitivity and specificity. The relationship between the TTFM variables (Qmean and PI) and the Rentrop collateral grade was examined by one-way analysis of variance (one-way ANOVA) or the Kruskal–Wallis test. Within the three groups of the Rentrop grade, post hoc multiple comparisons were made by using the Tukey test. P-values of <0.05 were considered to be statistically significant. The SPSS 20.0 (IBM Corp., Armonk, NY, USA) software program was used for all of the statistical analyses.
RESULTS
Comparison between bypassed and non-bypassed chronically totally occluded arteries
One hundred and forty-seven patients (75%), 48 patients (24.5%) and 1 patient (5.1%) had 1, 2 and 3 three CTOs, respectively. A total of 246 CTOs were analysed. The distribution of the 246 CTO lesions were as follows: 114 (46%) in the right coronary artery (RCA), 83 (34%) in the LAD, 44 (18%) in the left circumflex artery (LCx) and 5 (2%) in the diagonal branch (Diag) (Table 1).
Table 1:
A comparison of bypassed and non-bypassed chronically totally occluded arteries
| All (n = 246) | Bypassed (n = 214) | Non-bypassed (n = 32) | P-value | |
|---|---|---|---|---|
| Chronically totally occluded artery | ||||
| LAD | 83 | 82 | 1 | |
| Diag | 5 | 4 | 1 | |
| LCx | 44 | 28 | 16 | |
| RCA | 114 | 100 | 14 | |
| Rentrop collateral grade | 2.4 ± 0.7 | 2.5 ± 0.6 | 1.9 ± 0.8 | <0.001 |
| Regional wall score | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.9 ± 0.7 | 0.88 |
| Akinesis or dyskinesis in CTO artery territory | 14 (6%) | 9 (4%) | 5 (16%) | 0.023 |
The distribution of CTO lesions was compared using the χ2 test, and the ratio of akinesis/dyskinesis was compared by Fisher's exact test.
CTO: chronic total occlusion; LAD: left anterior descending artery; Diag: diagonal artery; LCx: left circumflex artery; RCA: right coronary artery.
Of the 246 CTOs in 196 patients, 32 CTOs (13%) were not bypassed due to heavy calcification and/or small vessel diameter of the coronary arteries with diffuse atherosclerosis or vessels that supplied little or no viable myocardium. Three of 32 non-bypassed CTOs had diffuse heavy calcification. The mean diameter of non-bypassed CTOs was 0.47 ± 0.21 mm. Seven of 32 non-bypassed CTOs circulated through non-viable myocardium that was definitively assessed using preoperative SPECT. One of the 83 LAD–CTOs, 1 of the 5 Diag–CTOs, 14 of the 114 RCA–CTOs and 16 of the 44 LCx–CTOs were not bypassed. In total, 196 grafts were included in the subsequent analysis. The grafts used for distal anastomoses were the left internal thoracic artery (LITA) (n = 78), the right internal thoracic artery (RITA) (n = 10), the right gastroepiploic artery (rGEA) (n = 14) and an SVG (n = 112). The distribution of CTO lesions differed between the bypassed group and non-bypassed group (P < 0.001 by the χ2 test). The rate of LCx–CTOs was higher in the non-bypassed group compared with the bypassed group (50 vs 13%). The Rentrop collateral grade was significantly higher in the bypassed CTOs than in the non-bypassed arteries (2.5 ± 0.6 vs 1.9 ± 0.8, P < 0.001). The regional wall motion score did not differ significantly between the territories that were supplied by a bypassed CTO and those that were not bypassed (1.7 ± 0.6 vs 1.9 ± 0.7, P = 0.88); however, the rate of akinesis or dyskinesis in the territory of the CTOs was lower in the bypassed CTOs than in the non-bypassed arteries (4 vs 16%, P = 0.023).
The angiographic results
Angiography revealed that 199 grafts were normal and fully patent and 15 grafts were failing, with the overall insufficiency rate being 7.0%. The target territories and the different types of conduits that were used for CABG surgery are presented in Table 2. There were no statistically significant differences in the incidence rates of graft failure with the different types of conduits (P = 0.64), or among the target territories (P = 0.79).
Table 2:
Coronary artery bypass grafts and their target arteries
| LAD | Diag | LCx | RCA | Total | Insufficiency rate (%) | |
|---|---|---|---|---|---|---|
| LITA | 75 (4) | 0 | 3 (0) | 0 | 78 (4) | 5.1 |
| RITA | 5 (1) | 0 | 3 (0) | 2 (0) | 10 (1) | 10.0 |
| rGEA | 0 | 0 | 0 | 14 (2) | 14 (2) | 14.3 |
| SVG | 2 (1) | 4 (0) | 22 (3) | 84 (4) | 112 (8) | 7.1 |
| Total | 82 (6) | 4 (0) | 28 (3) | 100 (6) | 214 (15) | 7.0 |
| Insufficiency rate (%) | 7.3 | 0 | 10.7 | 6.0 | 7.0 |
The number in parentheses indicates the number of abnormal grafts according to the early postoperative angiography.
LITA: left internal thoracic artery; RITA: right internal thoracic artery; rGEA: right gastroepiploic artery; SVG: saphenous vein graft; LAD: left anterior descending artery; Diag: diagonal artery; LCx: left circumflex artery; RCA: right coronary artery.
Normal versus abnormal grafts
The TTFM variables measured in the patent grafts were compared with the 15 grafts that were angiographically demonstrated to be abnormal (Table 3). The Qmean of the patent grafts was 40.9 ± 27.1 ml/min, while that of the abnormal grafts was 21.3 ± 16.2 ml/min. The PI of the patent grafts was 2.8 ± 1.8, while that of the abnormal grafts was 5.5 ± 4.7. The abnormal grafts showed significantly lower Qmean and higher PI values than the patent grafts. There was no significant difference between the patent and abnormal grafts with regard to the preoperative Rentrop collateral grade of the target CTOs or the regional wall motion score of the territory supplied by the bypassed CTOs. However, the rate of akinesis or dyskinesis observed in the territory of the bypassed CTOs was significantly different between patent and abnormal grafts (P = 0.018). Six (3%) of 199 patent grafts were anastomosed to CTOs with akinetic or dyskinetic wall motion. On the other hand, 3 (20%) of 15 abnormal grafts were anastomosed to CTOs with akinetic or dyskinetic wall motion.
Table 3:
A comparison of the TTFM variables, Rentrop collateral grade and regional wall motion score in patent and abnormal grafts
| Patent graft (n = 199) | Failing graft (n = 15) | P-value | |
|---|---|---|---|
| Overall | |||
| Qmean (ml/min) | 40.9 ± 27.1 | 21.3 ± 16.2 | 0.006 |
| PI | 2.8 ± 1.8 | 5.5 ± 4.7 | 0.002 |
| Rentrop grade | 2.5 ± 0.6 | 2.4 ± 0.6 | 0.563 |
| Regional wall score | 1.7 ± 0.6 | 1.9 ± 0.9 | 0.568 |
| Akinesis, dyskinesis | 6 (3%) | 3 (20%) | 0.018 |
| Arterial | |||
| Qmean (ml/min) | 40.1 ± 25.0 | 16.4 ± 13.8 | 0.016 |
| PI | 2.8 ± 1.7 | 4.9 ± 5.1 | 0.063 |
| Rentrop grade | 2.4 ± 0.6 | 2.3 ± 0.8 | 0.517 |
| Regional wall score | 1.7 ± 0.6 | 2.1 ± 1.1 | 0.307 |
| Akinesis, dyskinesis | 5 (5%) | 2 (29%) | 0.072 |
| SVG | |||
| Qmean (ml/min) | 41.7 ± 29.0 | 25.6 ± 17.9 | 0.126 |
| PI | 2.7 ± 1.8 | 6.0 ± 4.5 | 0.009 |
| Rentrop grade | 2.6 ± 0.6 | 2.5 ± 0.5 | 0.832 |
| Regional wall score | 1.6 ± 0.5 | 1.6 ± 0.7 | 0.885 |
| Akinesis, dyskinesis | 1 (1%) | 1 (13%) | 0.138 |
The ratio of akinesis/dyskinesis in grafted CTO territories was compared using Fisher's exact test.
Qmean: mean flow; PI: pulsatility index; TTFM: transit-time flow measurement; SVG: saphenous vein graft; CTO: chronic total occlusion.
When the analysis was subsequently stratified according to arterial or venous conduits, patent arterial grafts demonstrated higher Qmean values (P = 0.016), while patent vein grafts showed lower PI values (P = 0.009) compared with abnormal grafts. A univariable regression analysis revealed that both the Qmean [odds ratio (OR), 0.95; P = 0.006) and PI (OR, 1.35; P < 0.001) values were significant predictors of early graft failure in all grafts. Furthermore, grafting to CTOs with akinetic or dyskinetic wall motion was a significant predictor (OR, 8.04; P = 0.007). A multivariable regression model revealed that the PI value was the only significant independent predictor of early graft failure. In a multivariable analysis in which the arterial and vein conduits were separated, we found that the Qmean value was a significant predictor of arterial graft failure (OR, 0.91; P = 0.031), while the PI value (OR, 1.51; P = 0.003) and grafting to akinetic/dyskinetic CTO lesions (OR, 26.0; P = 0.037) were predictors of venous graft failure. In a regression analysis, neither the Rentrop collateral grade nor the regional wall motion score was found to be associated with early graft failure (Table 4).
Table 4:
A logistic regression analysis for the prediction of early graft failure
| Graft | Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | ||
| Overall | |||||||
| Rentrop collateral grade | 0.78 | 0.33–1.82 | 0.562 | ||||
| Regional wall score | 1.73 | 0.74–4.05 | 0.204 | ||||
| Akinesis, dyskinesis | 8.04 | 1.79–36.2 | 0.007 | ||||
| TTFM Qmean | 0.95 | 0.91–0.98 | 0.006 | ||||
| TTFM PI | 1.35 | 1.15–1.60 | <0.001 | 1.23 | 1.02–1.49 | 0.028 | |
| Arterial graft | |||||||
| Rentrop collateral grade | 0.643 | 0.17–2.42 | 0.514 | ||||
| Regional wall score | 2.40 | 0.83–7.0 | 0.108 | ||||
| Akinesis, dyskinesis | 7.2 | 1.11–46.8 | 0.039 | ||||
| TTFM Qmean | 0.90 | 0.83–0.98 | 0.012 | 0.91 | 0.83–0.99 | 0.031 | |
| TTFM PI | 1.26 | 1.01–1.57 | 0.041 | ||||
| SVG | |||||||
| Rentrop collateral grade | 0.88 | 0.28–2.77 | 0.83 | ||||
| Regional wall score | 1.07 | 0.27–4.2 | 0.92 | ||||
| Akinesis, dyskinesis | 14.7 | 0.83–261 | 0.067 | 26.0 | 1.2–553 | 0.037 | |
| TTFM Qmean | 0.97 | 0.93–1.01 | 0.129 | ||||
| TTFM PI | 1.47 | 1.14–1.90 | 0.003 | 1.51 | 1.16–2.0 | 0.003 | |
CI: confidence interval; TTFM: transit-time flow measurement; Qmean: mean flow; PI: pulsatility index; SVG: saphenous vein graft.
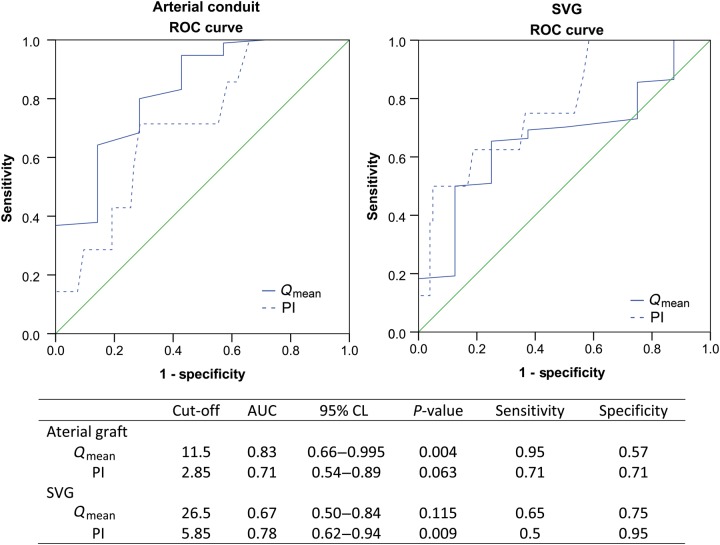
The results of an ROC analysis according to the type of graft are presented in Fig. 1. The analysis was split according to arterial or vein conduits. The cut-off value for detecting abnormal grafts in arterial conduits to CTOs was a Qmean value <11.5 ml/min (P = 0.004)—however, a PI value of >2.85 was not found to be a statistically significant indicator of graft failure (P = 0.063). On the other hand, the cut-off value for the prediction of graft failure in venous conduits was a PI value > 5.85 (P = 0.009), while a Qmean value of <26.5 ml/min was not found to be a good indicator of graft failure (P = 0.115).
Figure 1:
The ROC analysis representing the cut-off TTFM values for predicting early graft failure. Qmean: mean flow; PI: pulsality index; AUC: area under curve; CL: confidence limit; ROC: receiver operating characteristic; TTFM: transit-time flow measurement; SVG: saphenous vein graft.
Transit-time flow measurement variables and the Rentrop collateral grade in normal grafts
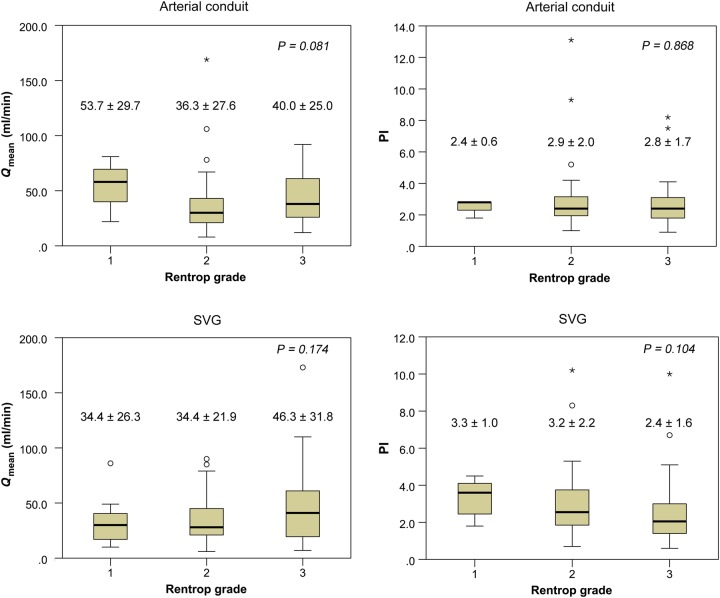
There was no significant correlation between the TTFM variables and the Rentrop collateral grade in the grafts anastomosed to CTOs (Fig. 2) according to one-way ANOVA or the Kruskal–Wallis test. In arterial conduits, however, a higher Qmean and lower PI were likely to be observed in Rentrop grade 1 compared with Rentrop grades 2 and 3. On the other hand, in SVGs, a higher Qmean and lower PI tended to be observed in Rentrop grade 3 compared with Rentrop grades 1 and 2.
Figure 2:
The relationship between the TTFM variables and the Rentrop collateral grade in normal grafts by one-way ANOVA or the Kruskal–Wallis test. There was no statistically significant correlation between the TTFM variables and the Rentrop collateral grade in the grafts anastomosed to CTOs. TTFM: transit-time flow measurement; CTO: chronic total occlusion; PI: pulsality index; SVG: saphenous vein graft; Qmean: mean flow.
DISCUSSION
A few studies have evaluated the rate of patency and TTFM variables in bypass grafts that are anastomosed to CTOs [15]. However, little is known regarding the cut-off values of TTFM variables for the prediction of graft failure when the graft is anastomosed to CTOs or the relationship between graft failure and the collateral grade. The association between regional wall motion and graft failure also remains unclear. The main findings of the present study were as follows. (i) TTFM was useful for detecting graft failure in CABG surgery involving CTOs, however the cut-off values of Qmean and PI for graft failure differed from the previous reports. (ii) Neither the preoperative collateral grade nor the regional wall motion score was associated with an incidence of graft failure; however, bypass grafting to CTO territories with akinetic or dyskinetic wall motion was a potential risk factor of graft failure.
Graft patency and flow versus collateral grade or regional wall motion
No association was observed between graft failure and the Rentrop collateral grade. A regression test demonstrated that the Rentrop collateral grade was not a predictor of graft failure. This indicates that the native collateral flow does not cause graft occlusion, although it is well developed.
Then, one question arising from clinical observation is whether a well-developed collateral flow competes with the graft flow. Several reports have attempted to elucidate the relationship between the fate of bypass grafts and the preoperative collateral grade of the bypassed target vessels. Kaku et al. [16] reported that the mean graft flow of the LITA grafted to an LAD–CTO decreased, while its PI increased with increasing Rentrop collateral grades. They considered that there is potential competition between the rich collateral flow and the LITA graft flow when collateral perfusion distal to a CTO is well-developed (high Rentrop grade), although they did not demonstrate any influence of well-developed collateral grade on early graft failure. Our study did not demonstrate this type of significant interaction between the collateral grade and the graft flow in both arterial and venous conduits. In arterial conduits, however, a higher Qmean and lower PI were likely to be observed in Rentrop grade 1 compared with Rentrop grades 2 and 3. This is similar to the results reported by Kaku et al. [16]. In our study, the number of cases with Rentrop collateral Grades 0 and 1 might be too small to demonstrate a significant interaction between the TTFM variables and the collateral grade.
On the other hand, in SVGs, a higher Qmean and lower PI tended to be observed in Rentrop grade 3 compared with Rentrop grades 1 and 2. This observation is consistent with the results of Verhoye et al. [17]. They studied the collateral blood flow between left CABGs and chronically occluded right coronary circulation in patients with triple vessel disease. The authors anastomosed a LITA to the LAD, an RITA to the LCx and an SVG to the RCA–CTO. They then measured the TTFM Qmean value through the LITA and RITA before and after unclamping of the SVG anastomosed to an RCA–CTO. A significant decrease was observed in blood flow across an RITA grafted to the LCx after unclamping the SVG anastomosed to an RCA–CTO. They additionally demonstrated a significant relationship between the difference in the RITA flow before versus after unclamping the SVGs to RCA–CTOs and the preoperative Rentrop collateral grade of RCA–CTOs. These findings suggest that the coronary blood flow to the RCA–CTO was provided through a collateral pathway from the LCx. Moreover, the findings indicate that some reverse flow from the RCA to the LCx through a collateral channel was obtained after the RCA–CTO was bypassed using an SVG and that this flow to the LCx resulted in the flow competition against the RITA anastomosed to the LCx. This phenomenon might explain our results that the Qmean tended to increase and the PI tended to decrease in SVGs anastomosed to CTOs with Rentrop grade 3 compared with Rentrop grades 1 and 2. The blood flow to those CTOs through the collateral pathway from the native LAD or LCx did not compete with the flow of the SVGs. Conversely, SVGs anastomosed to CTOs with well-developed collateral circulation could obtain good runoff with an identically high Qmean value or a low PI value. A potential reason for this might be the existence of reverse flow from a bypassed SVG–CTO to the other territories through the well-developed collateral channel, which results in higher graft flow to more vascular beds in addition to the CTO territory. In our study, however, there was no significant interaction between the TTFM variables and Rentrop grade in SVGs as well. Although we analysed the correlation in 84 SVGs anastomosed to RCA–CTOs and found similar trends, we could not demonstrate a significant correlation between the TTFM variables and the Rentrop collateral grade (Qmean P = 0.273; PI, P = 0.171). A greater accumulation of cases is necessary to clarify whether this type of interaction exists in SVGs anastomosed to CTOs.
Whether the regional wall motion affects graft function should also be considered. In the present study, the regional wall motion score of the CTO territory was not found to be associated with early graft failure. However, akinetic or dyskinetic wall motion in the CTO territory was significantly observed in failing grafts compared with patent grafts. A univariable regression test showed this to be a significant predictor for early graft failure, and a multivariable regression test revealed it to be a risk factor of SVG graft failure. Indeed, a higher Qmean and a lower PI were observed in CTOs with a normal or hypokinetic area compared with those with an akinetic or dyskinetic area; however, these differences were not significant (Qmean 40.2 ± 27.2 vs 25.8 ± 14.0 ml/min, P = 0.118; PI 2.92 ± 2.0 vs 4.8 ± 4.9, P = 0.256). This is reasonable because the blood flow demands are less in the akinetic or dyskinetic area compared with the normal or hypokinetic area. However, Glineur et al. reported that no association was observed between graft patency or function and the regional wall motion of the RCA territory when the RCA was grafted with the SVG, RGEA or RITA. We should therefore consider a more precise analysis of myocardial viability in CTOs. In some cases, akinetic wall motion does not indicate non-viability, but rather hibernating myocardium due to ischaemia. For such cases, regional functional recovery is obtained in the akinetic area after coronary bypass surgery. Murashita et al. reported that preoperative SPECT is useful to predict this type of recovery [18]. In the present study, only 29% of 246 patients underwent preoperative SPECT and only 60 of bypassed CTOs were assessed. Therefore, we could not precisely demonstrate that akinetic or dyskinetic wall motion in the CTO territory was a significant risk factor; however, surgeons should carefully consider whether to perform bypass surgery for CTOs with an akinetic or dyskinetic area, and SPECT might be needed for the decision.
Transit-time flow measurement cut-off values for the prediction of graft failure
We analysed the Qmean and PI cut-off values for detecting graft failure in grafts anastomosed to CTOs. A Qmean of <11.5 ml/min was found to be a good predictor of arterial graft failure, while a PI of >5.85 was a predictor of venous graft failure. These values differed from the TTFM cut-off values previously reported in the literature [7–10]. According to different groups, the optimal Qmean cut-off value is 10 or 15 ml/min, while the PI cut-off value is 3 or 5 [10, 19]. Previous studies analysed data sets that included both coronary arteries with stenosis (not CTOs) and CTO arteries as bypassed target vessels. According to the study of Kieser et al. [19], a PI value between 3 and 5 may indicate diffuse disease of the grafted artery. Their study demonstrated that the graft was actually patent even when the PI value was >3. CTOs often demonstrate poor runoff due to high vascular resistance caused by diffuse arteriosclerotic disease. Therefore, an optimal PI cut-off value of 3 or 5 is not improbable in a patent SVG anastomosed to a CTO with poor runoff.
As stated above, the arterial graft flow to a CTO might be affected by the collateral network from other territories after revascularization. Therefore, even an arterial conduit with a Qmean value of <15 ml/min can be a patent graft. It is therefore little wonder that the cut-off value of the Qmean obtained by the results of the present study for predicting the failure of bypass grafts to CTOs is below that of previous reports.
Study limitations
The present study is associated with several limitations. First, it was a retrospective investigation with a relatively small number of patients. Its main limitation is undoubtedly the unavoidable selection bias. Whether the CTOs should be bypassed was determined according to the anatomical and pathological findings in CTO vessels, and the clinical data of an electrocardiogram, echocardiography and CAG. It is difficult to randomize patients because of ethical reasons. Second, we did not evaluate the myocardial viability in all patients using SPECT, which would have been useful to further demonstrate a correlation between the TTFM variables and the degree of ischaemia. Instead, we used the regional wall motion score, which cannot perfectly distinguish hibernated ischaemic myocardium from non-viable myocardium. Therefore, we could not accurately assess the influence of myocardial viability on early graft failure.
CONCLUSIONS
TTFM values were found to predict the early graft failure of conduits anastomosed to CTOs. We should evaluate bypass grafts using the cut-off values of TTFM variables for graft failure differently from the methods that have previously been reported in the literature; a Qmean value of <11.5 ml/min is a good predictor of arterial graft failure, while a PI value of >5.85 is a good predictor of venous graft failure. The collateral grade to CTOs was not associated with graft failure; however, akinetic or dyskinetic wall motion in the CTO territory was a potential risk factor for early graft failure.
Conflict of interest: none declared.
REFERENCES
- 1.Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S et al. . Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol 2012;59:991–7. [DOI] [PubMed] [Google Scholar]
- 2.Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol 2005;95:1088–91. [DOI] [PubMed] [Google Scholar]
- 3.Masuda M, Kuwano H, Okumura M, Amano J, Arai H, Endo S et al. . Thoracic and cardiovascular surgery in Japan during 2012: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:734–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widimsky P, Straka Z, Stros P, Jirasek K, Dvorak J, Votava J et al. . One-year coronary bypass graft patency: a randomized comparison between off-pump and on-pump surgery angiographic results of the PRAGUE-4 trial. Circulation 2004;110:3418–23. [DOI] [PubMed] [Google Scholar]
- 5.Fefer P, Gannot S, Kochkina K, Maor E, Matetzky S, Raanani E et al. . Impact of coronary chronic total occlusions on long-term mortality in patients undergoing coronary artery bypass grafting. Interact CardioVasc Thorac Surg 2014;18:713–6. [DOI] [PubMed] [Google Scholar]
- 6.D'Ancona G, Karamanoukian HL, Salerno TA, Schmid S, Bergsland J. Flow measurement in coronary surgery. Heart Surg Forum 1999;2:121–4. [PubMed] [Google Scholar]
- 7.Kim KB, Kang CH, Lim C. Prediction of graft flow impairment by intraoperative transit time flow measurement in off-pump coronary artery bypass using arterial grafts. Ann Thorac Surg 2005;80:594–8. [DOI] [PubMed] [Google Scholar]
- 8.Di Giammarco G, Pano M, Cirmeni S, Pelini P, Vitolla G, Di Mauro M. Predictive value of intraoperative transit-time flow measurement for short-term graft patency in coronary surgery. J Thorac Cardiovasc Surg 2006;132:468–74. [DOI] [PubMed] [Google Scholar]
- 9.Tokuda Y, Song MH, Ueda Y, Usui A, Akita T. Predicting early coronary artery bypass graft failure by intraoperative transit time flow measurement. Ann Thorac Surg 2007;84:1928–33. [DOI] [PubMed] [Google Scholar]
- 10.Di Giammarco G, Rabozzi R. Can transit-time flow measurement improve graft patency and clinical outcome in patients undergoing coronary artery bypass grafting? Interact CardioVasc Thorac Surg 2010;11:635–40. [DOI] [PubMed] [Google Scholar]
- 11.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5:587–92. [DOI] [PubMed] [Google Scholar]
- 12.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al. . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, Chang SA, Choi JO, Song YB, Hahn JY, Choi SH et al. . Frequency of myocardial infarction and its relationship to angiographic collateral flow in territories supplied by chronically occluded coronary arteries. Circulation 2013;127:703–9. [DOI] [PubMed] [Google Scholar]
- 14.FitzGibbon GM, Burton JR, Leach AJ. Coronary bypass graft fate: angiographic grading of 1400 consecutive grafts early after operation and of 1132 after one year. Circulation 1978;57:1070–4. [DOI] [PubMed] [Google Scholar]
- 15.Takami Y, Masumoto H. Angiographic fate of collateral vessels after surgical revascularization of the totally occluded left anterior descending artery. Ann Thorac Surg 2007;83:120–5. [DOI] [PubMed] [Google Scholar]
- 16.Kaku D, Nakahira A, Hirai H, Sasaki Y, Hosono M, Bito Y et al. . Does rich coronary collateral circulation distal to chronically occluded left anterior descending artery compete with graft flow? Interact CardioVasc Thorac Surg 2013;17:944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhoye JP, Abouliatim I, Drochon A, de Latour B, Leclercq C, Leguerrier A et al. . Collateral blood flow between left coronary artery bypass grafts and chronically occluded right coronary circulation in patients with triple vessel disease. Observations during complete revascularisation of beating hearts. Eur J Cardiothorac Surg 2007;31:49–54. [DOI] [PubMed] [Google Scholar]
- 18.Murashita T, Makino Y, Kamikubo Y, Yasuda K, Mabuchi M, Tamaki N. Quantitative gated myocardial perfusion single photon emission computed tomography improves the prediction of regional functional recovery in akinetic areas after coronary bypass surgery: useful tool for evaluation of myocardial viability. J Thorac Cardiovasc Surg 2003;126:1328–34. [DOI] [PubMed] [Google Scholar]
- 19.Kieser TM, Rose S, Kowalewski R, Belenkie I. Transit-time flow predicts outcomes in coronary artery bypass graft patients: a series of 1000 consecutive arterial grafts. Eur J Cardiothorac Surg 2010;38:155–62. [DOI] [PubMed] [Google Scholar]