Abstract
OBJECTIVES
To study the characteristics of patients with Propionibacterium acnes prosthetic valve endocarditis (PVE) who required surgery.
METHODS
A single-centre retrospective cohort study was conducted during a 7-year period. Patients with definite infective P. acnes endocarditis, according to the modified Duke criteria, were included. An extended culture protocol was applied. Information on medical health status, surgery, antibiotic treatment and mortality was obtained.
RESULTS
Thirteen patients fulfilled the criteria for P. acnes endocarditis (0.53% of 2466 patients with valve replacement in a 7-year period). All patients were male and had a previous valve replacement. The health status of patients was poor at diagnosis of P. acnes PVE. Most patients (11 of 13, 85%) were admitted with signs of heart failure due to a significant paravalvular leak; 2 of 13 (15%) patients presented with septic emboli. Twelve patients needed redo surgery, whereas one could be treated with antibiotic therapy only. The time between the index surgery and presentation with P. acnes PVE varied between 5 and 135 months (median 26.5 months). Replacement and reconstruction of the dysfunctional valve and affected anatomical structures was mainly performed with a mechanical valve (n = 5, 42%) or a (bio-) Bentall prosthesis (n = 6, 50%). Antibiotic therapy consisted of penicillin with or without rifampicin for 6 weeks after surgery. The mortality in this series was low (n = 1, 8%) and no recurrent endocarditis was found during a median follow-up of 38 months.
CONCLUSIONS
Propionibacterium acnes PVE is a rare complication after valve surgery. Redo surgery is often required. Treatment of the dysfunctional prosthetic aortic valve most often consists of root replacement, in combination with antibiotic therapy.
Keywords: Cardiac surgery, Prosthetic valve endocarditis, Propionibacterium acnes
INTRODUCTION
With an incidence of 30–100 episodes per million patient-years [1, 2], endocarditis might be a rare disease, but the risk of an adverse outcome is high. The in-hospital mortality rate is ∼20% and more than one-third of patients die within the first year of diagnosis [3–6], despite advances in diagnostic options, treatment and follow-up.
Patients who are especially at risk for developing endocarditis are those who have undergone previous implantation of a prosthetic valve, device or lead. For this reason, prosthetic cardiac valves have been labelled as a predisposing cardiac condition for infectious endocarditis in all major guidelines [7].
Prosthetic valve endocarditis (PVE) is a complication with an even higher rate of mortality and morbidity than endocarditis in the absence of prosthetic material. In the literature, it is described as a relatively uncommon clinical entity. The reported incidence of PVE is between 0.3 and 1.2 cases per patient-year, affecting 1–6% of patients with a cardiac valve prosthesis [8]. The mortality rate ranges between 21 and 74% [9].
This paper focuses at Propionibacterium acnes (P. acnes) as the agent responsible for PVE. Propionibacterium acnes is a facultative anaerobic, non-spore-forming Gram-positive rod. It is considered to be normal flora of the human skin, but can also be found on conjunctive tissue, and in the oral cavity, intestinal tract and the external auditory canal [10]. This micro-organism tolerates oxygen for several hours and is able to survive in anaerobic conditions up to 8 months in vitro. It can also survive for long periods in human tissues with low oxidation potential [11]. Furthermore, it can resist phagocytosis and persist in macrophages [12]. Propionibacterium acnes is most known as the causative agent of acne. It is generally considered to have a low level of virulence but can cause cerebral, ocular, spinal and postsurgical infections [13]. In cardiac surgery, it is an extremely uncommon substrate for primary valve, prosthetic valve or conduit infection, with a description of only around 70 cases in English cardiac surgery literature to date [14]. Among these papers, there are several case reports and case series reporting on this bacterium in relation to endocarditis. Propionibacterium acnes seems to have a predilection for prosthetic valves [15].
We report on our case series of 13 patients in a 7-year period with a proven P. acnes prosthetic valve infective endocarditis. To the best of our knowledge, it is the largest single-centre case series described in the literature so far.
PATIENTS AND METHODS
Study setting and design
A retrospective study design was used. All patients operated at the Department of Cardio-thoracic Surgery at the Erasmus Medical Centre, a large academic teaching hospital in the Netherlands, between January 2008 and August 2015 were evaluated for the possibility of having P. acnes endocarditis. To define infective endocarditis, modified Duke criteria were used [16]. Patients or their legal representatives provided their written informed consent and the local Medical Ethics Committee of the Erasmus MC approved the study (MEC-2015-232).
Baseline characteristics
Only cases that fulfilled the modified Duke criteria for the diagnosis of definite infective endocarditis were further investigated for index surgery, presentation at readmission, redo surgery characteristics, treatment modalities, mortality and outcome parameters such as a new episode of endocarditis, new valvular dehiscence or paravalvular leakage.
RESULTS
Patients
Within the period of investigation, no patients with a native valve P. acnes endocarditis were found. In the cohort of patients who underwent valve replacement (N = 2466, male 60%), 14 patients (0.54%) with an implanted cardiac valve prosthesis or conduit were eligible for the diagnosis P. acnes PVE. All but one patient fulfilled modified Duke criteria for definite infective endocarditis (11 patients based on pathological criteria with or without clinical criteria; 2 patients based on clinical criteria). The 13 patients underwent their index cardiac surgery between 2002 and 2014. All but one patient underwent redo surgery in the same centre, while one was treated with antibiotics. Characteristics of these patients and their surgery can be found in Table 1 and in depth in (Supplementary Table 1).
Table 1:
Demographics finding of patients at index surgery and redo surgery
| n = 13 | n = 12 | |
|---|---|---|
| Index surgery | Redo surgery | |
| Clinical presentation | ||
| Age, mean [SD] (range) (years) | 53.4 [12.4] (29–70) | 58.2 [13.3] (30–71) |
| Male | 13 (100%) | 12 (100%) |
| Renal insufficiency (not requiring dialysis)a | 2 (16%) | 5 (42%) |
| Left ventricular function | ||
| Good | 10 (77%) | 6 (50%) |
| Impaired | 2 (15%) | 5 (42%) |
| Poor | 1 (8%) | 1 (8) |
| Cerebrovascular accident history | 0 (0%) | 2 (17%) |
| Diabetes mellitus | 1 (8%) | 1 (8%) |
| Hypertension | 5 (38%) | 5 (42%) |
| Logistic EUROSCORE®, mean [SD] (range) | 4.9 [3.7] (10.2) | 28.4 [23.6] (77.4) |
| Type of surgery | ||
| Aortic valve replacement, bioprosthesis | 1 (8%) | |
| Aortic valve replacement, mechanical valve | 9 (69%) | 4 (33%) |
| Aortic valve replacement bioprosthesis and CABG | 1 (8%) | |
| Mechanical aortic valve replacement and CABG | 1 (8%) | |
| Mechanical aortic valve and root replacement (Bentall) | 1 (8%) | 4 (33%) |
| Aortic valve replacement, bioprosthesis, aortic root replacement (bio-Bentall) and CABG | 1 (8%) | |
| Bentall and mitral valve plasty | 1 (8%) | |
| Aortic and mitral valve replacement, mechanical valves | 1 (8%) | |
| Mitral valve replacement composite valve | 1 (8%) | |
| Surgical characteristics | ||
| Cardiopulmonary bypass time, mean [SD] (min–max time) | 2:15 [0:52] (1:19–4:10) | 3:55 [2:05] (1:47–9:07) |
| Aortic clamp time, mean [SD] (min–max time) (h:min) | 1:43 [0:40] (1:02–3:00) | 2:35 [1:04] (1:27–5:16) |
| Perioperative antibiotic regime (protocol followed)b | 13 (100%) | 7 (58%) |
| Continuation of antibiotic regime during surgery | 0 (0%) | 5 (42%) |
| Re-exploration for bleeding | 0 (0%) | 5 (42%) |
| Follow-up data | ||
| Follow-up time since redo surgery in months, mean [SD] (range) | 35 [26] (1–81) | |
| Confirmed new paravalvular leak | 2 (17%) | |
| Confirmed re-endocarditis | 0 (0%) | |
| Left ventricular function | ||
| Good | 8 (67%) | |
| Impaired | 3 (25%) | |
| Poor | 1 (8%) | |
SD: standard deviation; CABG: coronary artery bypass grafting.
aRenal insufficiency (not requiring dialysis) is a glomerular filtration rate of <60 ml/min/1.73 m2
bProtocol consists of a total of 4 g of cefazoline during a 24-h period.
Patient characteristics at redo surgery
The 13 patients with definite infective P. acnes endocarditis were all male. The average age was 53 years (range 29–70 years) (Table 1). All patients had valve replacement in their history (11 aortic, 1 Bentall, and 1 combined aortic and mitral valve replacement): in 2 patients for a congenital defect and in 11 for acquired valvular dysfunction. The time between index surgery and redo surgery varied greatly (ranging from 5 to 135 months, Table 1 and as depicted in Fig. 1). Heart failure based on massive paravalvular leakage of the prosthetic valve was the clinical presentation of the majority of patients (n = 11, 85%). All patients were in poor clinical condition and most of the patients (n = 10, 77%) were admitted to a referring hospital before readmission at our department. The majority had an NYHA classification of III or IV at presentation (n = 10, 77%). The left ventricular function was also poorer than before. Of the 9 patients with a previously good left ventricular function, 3 had a poorer function at the time of reoperation for PVE (Table 1). Comparing the groups based on index surgery logistic EUROSCORE [17] and redo logistic EUROSCORE reveals a large difference in the calculated risk of mortality. The mean score was 4.9% at the index surgery and 28.4% at the redo surgery. At redo surgery, in all but 1 patient the aortic valve prosthesis was infected. Of these 12 patients, 5 received a Bentall (42%), 1 a bio-Bentall, 4 a mechanical aortic valve and 1 a bioprosthesis. One patient underwent replacement of the mitral valve mechanical prosthesis by a new mechanical prosthesis. Information on concomitant surgery can be found in Table 1. One patient did not undergo redo surgery. The index surgery of this patient was an aortic valve replacement by a mechanical valve. There was no paravalvular leak at the diagnosis of PVE. Treatment consisted of a 6-week regimen of penicillin and the patient recovered well. When comparing patients with endocarditis due to P. acnes and due to other microorganisms, who were operated between 2008 and 2015 at our centre, there were no significant differences between patient characteristics and 30-day mortality (Table 2).
Figure 1:
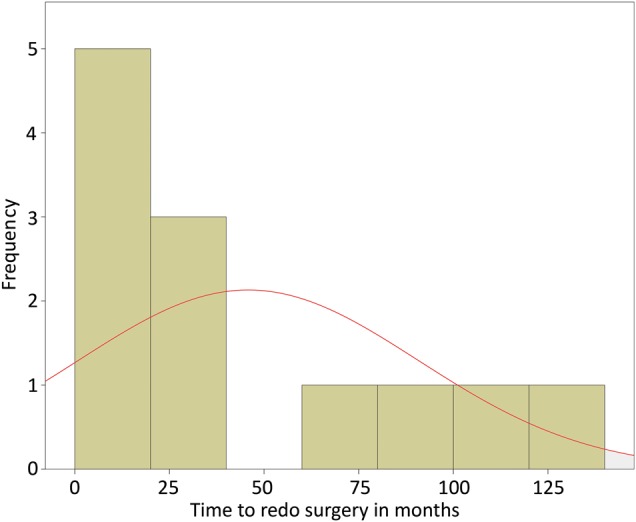
Time between index operation and redo surgery in months.
Table 2:
Demographics finding of patients with PVE between 2008 and 2015
| Clinical presentation | N = 91 | N = 12 |
|---|---|---|
| PVE complete population | PVE P. Acnes | |
| Age, mean [SD] (range) (years) | 56.3 [13] (18–81) | 58.2 [13.3] (30–71) |
| Male | 72 (79%) | 12 (100%) |
| Renal insufficiency (not requiring dialysis)a | 29 (32%) | 5 (42%) |
| Left ventricular function | ||
| Good | 55 (60%) | 6 (50%) |
| Impaired | 31 (34%) | 5 (42%) |
| Poor | 5 (6%) | 1 (8%) |
| Cerebrovascular accident history | 14 (15%) | 2 (17%) |
| Diabetes mellitus | 7 (7%) | 1 (8%) |
| Logistic EUROSCORE® mean [SD] (range) | 27.3 [21.2] (82) | 28.4 [23.6] (77.4) |
| 30-day mortality | 4 (4.4%) | 0 (0%) |
SD: standard deviation.
aRenal insufficiency (not requiring dialysis) is a glomerular filtration rate of <60 ml/min/1.73 m2.
Surgical findings
In 6 patients, the surgeon classified his findings as fitting with active endocarditis. In 2 patients, surgeons reported mainly calcifications but no signs of endocarditis. In 4 patients, no obvious signs of endocarditis were observed. Valvular dehiscence was objectified in 11 of the 12 reoperated patients. The one patient without valvular dehiscence presented relatively shortly after the index surgery (10 months). Another frequent finding was the formation of cavities; 75% of patients had small crypts or true aneurysm (7 patients with crypts and 2 patients with a true aneurysm). The latter two required Bentall procedures as did 4 of the 7 patients with small crypts. Two patients had failure to wean from bypass. In one patient, coronary flow was impaired after placement of a Bentall prosthesis, requiring additional coronary bypass surgery and placement of a veno-arterial extracorporeal membrane oxygenation (ECMO) device. This patient could be successfully weaned from ECMO on the 6th postoperative day. The second patient developed heart failure with failure to wean from bypass, requiring placement of an intra-aortic balloon pump, and had high levels of inotropic and vasopressive support.
Antibiotic regime during and after redo surgery
Nine patients (75%) received antibiotic treatment before surgery because of positive blood cultures with gram-positive rods. Six patients were treated with a vancomycin-, 2 patients with a penicillin-containing regime and 1 patient with a ceftriaxone-containing regime. After surgery, all patients were treated with antibiotics for a minimum period of 6 weeks. Different antibiotic regimes were used. Of the 13 patients, 4 (31%) were treated with penicillin alone (2–3 million units 6 times daily) and 7 (54%) were treated with a combination of penicillin (6 times daily 2–3 million units) and rifampicin 450 mg 2 times daily. The other 2 patients received vancomycin (due to allergy) or penicillin in combination with gentamycin.
Follow-up
The median follow-up after redo surgery was 36 months (range: 1–71 months). Only one patient died due to pneumosepsis, 2 years after surgery, not related to endocarditis. At the time of analysis, all other patients were alive and showed no signs of endocarditis. Ventricular function at follow-up was improved compared with before redo surgery, implying ventricular remodelling after repair of the paravalvular leak. Two patients showed a small paravalvular leak on ultrasound in the follow-up. Both patients had received a prosthetic valve in the aortic position after developing PVE. Haemolysis was minimal in both patients and no reintervention was required. In both patients, no signs of recurrent prosthetic valve endocarditis were found as proved by negative blood cultures.
DISCUSSION
In this study, patients with a proven PVE with P. acnes were evaluated. All patients were male and they all had previous valve replacement. Time between index surgery and presentation with endocarditis varied between 5 and 135 months. Twelve of 13 (85%) patients with P. acnes endocarditis presented with severe paravalvular leak. Crypts or true aneurysms were present in 75% (9 of 12) of patients reoperated on. Decreased left ventricular function was present in 40% of patients with a previous good left ventricular function. At presentation, patients were severely ill, but none of them died in the perioperative phase. Only one patient died during long-term follow-up.
The reason why P. acnes endocarditis is predominantly found in the male population, as also shown in other studies, is unknown. The difference might be explained by the fact that the absolute numbers of men receiving cardiac valves are higher; this is not only reported in the literature but also in our own institute [18]. It is interesting that the same gender difference phenomenon has been described in studies on osteo-articular prosthesis infections with P. acnes [19], especially in patients who have had shoulder surgery. Still, no clear explanation has been provided why men are affected more frequently than women.
What is the source of the P. acnes PVE in these men? P. acnes is considered as an omnipresent and usual commensal skin flora, but recently it has been shown that it could be considered a potentially opportunistic pathogen [20]. Propionibacterium acnes carries components on its surface that can trigger or mediate inflammatory processes or that can exhibit cell-adherent properties (dermatan-sulphate adhesion, thrombospondin type 3 repeat protein) [20]. Contamination of prosthetic material at the time of implantation due to the presence of P. acnes from either skin flora of the patient, or from an exogenous source, as well as virulence factors may be responsible for the increased risk of infection among patients with prosthetic valves. The long period between index surgery and redo surgery might be explained by the low virulence of this micro-organism. In patients with a long time interval between index surgery and redo surgery (e.g. >11 years in this series and >23 years in known literature [21]), virulence will most likely have occurred due to bacteraemia from a distant focus and secondary metastasis to prosthetic valves.
The diagnosis of P. acnes PVE can be difficult. One of the major problems is the prolonged incubation time of this micro-organism. Prolonged aerobic and anaerobic cultures of blood and tissue for up to 2 weeks may be required to detect the organism [21]. In addition, diagnosis of P. acnes PVE is difficult because of non-specific clinical symptoms. In addition, the use of the modified Duke criteria is more complex, because of this particular micro-organism not being included in the list of typical bacteria causing endocarditis.
Treatment of P. acnes PVE usually consists of surgical reintervention in combination with antibiotics. Therapeutic strategies can be a challenge, since surgical reintervention can be associated with a high risk of an unfavourable outcome and antibiotic regimens are hindered by the potential of P. acnes to form a biofilm.
In the past, it was common practice to replace an infected prosthetic valve with an allograft with an aortic root [9]. Even today the debate continues whether allograft or valve prosthesis is the best choice. Allografts have better reconstructive abilities in destroyed tissue and durability is no problem in the short and intermediate term; however, long-term durability is limited [22]. This small series showed that results of reoperation where a new prosthetic valve and root is placed are good. Those patients presenting with the formation of crypts or true aneurysms often required the placement of a conduit in the aortic root position after precise and complete debridement of the affected area. There was no topical usage of betadine. The surgical treatment needs to be combined with an adequate antibiotic treatment.
Surgery times for the redo operation were significantly longer than for index surgery and the peri- and postoperative course was complicated by the need for mechanical support devices due to heart failure in 2 patients and the need for re-exploration for bleeding in 5 of 12 (42%) patients. Both can be attributed to the more complex reoperation with adhesions, and longer extracorporeal circulation and cross-clamp times. The preoperative condition of patients was also worse compared with the condition of patients at the start of the index surgery.
Patients in this series were predominantly treated with penicillin in combination with rifampicin, as has been proposed in the literature [23]. In animal studies, rifampicin has shown to play a unique role in complete sterilization of foreign bodies (in that case with Staphylococcus aureus) because of its potential to penetrate biofilms [24]. Resistance of pathogens can vary per country, but for now, this treatment regime seems adequate for patients with a P. acnes PVE [20]. Patients with a proven allergy to penicillin (1 patient in this series) can be treated with vancomycin. The duration of 6 weeks from redo surgery seemed adequate and was also propagated in other series [21]. In contrast to previous studies, the mortality in this study was low. The literature reports a mortality rate of between 15 and 27% [25] fitting the calculated mortality risk using the logistic EUROSCORE. This series had a 0% 30-day mortality and 7.7% late mortality. This was, however, 2 years after the redo surgery and was due to pneumosepsis. This overall very low mortality after PVE might be explained by advances in postoperative mechanical support. Furthermore, mortality in our centre for PVE is low. Table 2 presents the complete cohort of PVE patients in the same time period compared with P. acnes PVE patients, showing similar high logistic EUROSCORE® scores and a low 30-day mortality.
A limitation of this study is the low number of patients. Still, this series is the largest single-centre series described in the literature. Overall, some 70 cases were described in the literature before this series. The reason why our centre has such a large series to describe is open for debate. The general rate of PVE is no higher than in other centres. Most likely, the attention for this pathogen as a substrate for new paravalvular leakage plays a role. This presumption would imply that P. acnes PVE does occur more often, but would be undetected in many cases. This could be attributed to both the latency of this micro-organism in clinical features and the prolonged culture time it needs.
CONCLUSION
Patients with prosthetic valve surgery in their medical history are at risk for P. acnes endocarditis. Therefore, if such a patient presents with a new paravalvular leak, P. acnes PVE should be in the differential diagnosis and it should be checked with the laboratory where the cultures are incubated for an appropriate time of 14 days. Therapy generally consists of redo surgery, combined with antimicrobial therapy consisting of penicillin with or without rifampicin. Follow-up of the patients shows favourable outcomes, with improved ventricular function, no valvular revisions and low mortality. Good cooperation between the cardiologist, surgeon and microbiologist is important to treat these patients appropriately.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
REFERENCES
- 1.Selton-Suty C, Celard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B et al. Pre-eminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012;54:1230–9. [DOI] [PubMed] [Google Scholar]
- 2.Tleyjeh IM, Abdel-Latif A, Rahbi H, Scott CG, Bailey KR, Steckelberg JM et al. A systematic review of population-based studies of infective endocarditis. Chest 2007;132:1025–35. [DOI] [PubMed] [Google Scholar]
- 3.Thuny F, Grisoli D, Collart F, Habib G, Raoult D. Management of infective endocarditis: challenges and perspectives. Lancet 2012;379:965–75. [DOI] [PubMed] [Google Scholar]
- 4.Cabell CH, Pond KK, Peterson GE, Durack DT, Corey GR, Anderson DJ et al. The risk of stroke and death in patients with aortic and mitral valve endocarditis. Am Heart J 2001;142:75–80. [DOI] [PubMed] [Google Scholar]
- 5.Mokhles MM, Ciampichetti I, Head SJ, Takkenberg JJ, Bogers AJ. Survival of surgically treated infective endocarditis: a comparison with the general Dutch population. Ann Thorac Surg 2011;91:1407–12. [DOI] [PubMed] [Google Scholar]
- 6.Head SJ, Mokhles MM, Osnabrugge RL, Bogers AJ, Kappetein AP. Surgery in current therapy for infective endocarditis. Vasc Health Risk Manag 2011;7:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2007;138:739–45, 47–60. [DOI] [PubMed] [Google Scholar]
- 8.Lalani T, Chu VH, Park LP, Cecchi E, Corey GR, Durante-Mangoni E et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013;173:1495–504. [DOI] [PubMed] [Google Scholar]
- 9.Habib G, Thuny F, Avierinos JF. Prosthetic valve endocarditis: current approach and therapeutic options. Prog Cardiovasc Dis 2008;50:274–81. [DOI] [PubMed] [Google Scholar]
- 10.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011;9:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csukas Z, Banizs B, Rozgonyi F. Studies on the cytotoxic effects of Propionibacterium acnes strains isolated from cornea. Microb Pathog 2004;36:171–4. [DOI] [PubMed] [Google Scholar]
- 12.Webster GF, Leyden JJ, Musson RA, Douglas SD. Susceptibility of Propionibacterium acnes to killing and degradation by human neutrophils and monocytes in vitro. Infect Immun 1985;49:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy O, Iyer S, Atoun E, Peter N, Hous N, Cash D et al. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 2013;22:505–11. [DOI] [PubMed] [Google Scholar]
- 14.Sohail MR, Gray AL, Baddour LM, Tleyjeh IM, Virk A. Infective endocarditis due to Propionibacterium species. Clin Microbiol Infect 2009;15:387–94. [DOI] [PubMed] [Google Scholar]
- 15.Durupt S, Boibieux A, Ballet-Mechain M, Chaumentin G, Tremeau G, Roure C et al. [Propionibacterium acnes infectious endocarditis]. Presse Med 1998;27:1839–41. [PubMed] [Google Scholar]
- 16.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 17.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 18.Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999;15:816–22; discussion 22–3. [DOI] [PubMed] [Google Scholar]
- 19.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol 2009;47:1878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Med Mal Infect 2014;44:241–50. [DOI] [PubMed] [Google Scholar]
- 21.Lalani T, Person AK, Hedayati SS, Moore L, Murdoch DR, Hoen B et al. Propionibacterium endocarditis: a case series from the International Collaboration on Endocarditis Merged Database and Prospective Cohort Study. Scand J Infect Dis 2007;39:840–8. [DOI] [PubMed] [Google Scholar]
- 22.Neely RC, Leacche M, Shah J, Byrne JG. Current readings: status of surgical treatment for endocarditis. Semin ThoracCardiovasc Surg 2014;26:53–66. [DOI] [PubMed] [Google Scholar]
- 23.Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 2012;56:1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuard C, Herrmann M, Vaudaux P, Waldvogel FA, Lew DP. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob Agents Chemother 1991;35:2611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delahaye F, Fol S, Celard M, Vandenesch F, Beaune J, Bozio A et al. Propionibacterium acnes infective endocarditis. Study of 11 cases and review of literature. Arch Mal Coeur Vaiss 2005;98:1212–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


