Abstract
OBJECTIVES
Surgical resection of pulmonary metastases is considered as a therapeutic procedure in selected cases. However, many patients are unable to tolerate surgical intervention due to comorbidities and/or poor pulmonary reserve, also related to repeated parenchymal resections. Considering this scenario, we decided to investigate the role of radiofrequency ablation (RFA).
METHODS
The outcomes of all patients that underwent RFA for lung metastases, during the period 2003–2013, were analysed. The primary end-points were overall survival (OS) and local progression-free survival (LPFS). Secondary end-point was the analysis of possible risk factors affecting OS and LPFS.
RESULTS
Ninety-nine RFAs were performed on 61 patients (38 men, 23 women, median age of 74 years). Fourteen patients were treated for two or more lesions, for a total of 86 lesions. Twelve lesions were treated up to three times. The median lesion diameter was 2 cm. The majority of patients were affected by lung metastases from colorectal cancer (47.5%). All procedures were successfully completed. One death occurred, whereas the morbidity rate was 11% (8% pneumothorax requiring chest drainage). At a median follow-up of 28 months, the 1-, 3-, 5-year OS (LPFS) rates were 94.8% (86.3%), 49.0% (70.3%) and 44.5% (68.3%), respectively. No significant correlation was found, using univariate and multivariate analysis, between OS and age, gender, histology of primary cancer (colon versus others), type of approach (computed tomography versus ultrasonography guidance), number of treated lesions (1 vs >1), disease-free interval (from primary tumour to first lung metastases) (1-35 vs >35 months), previous lung resections (yes versus no), whereas a tendency towards better OS was observed, by applying univariate analysis, for a lesion of <3 cm (P = 0.051) and for the presence of local disease 1 month after treatment (P = 0.056), however, without a statistically significant difference. With regard to LPFS, lesion dimensions (P = 0.005) and the presence of local disease 1 month after treatment (P < 0.001) were found to be significant risk factors, in both univariate and multivariate analyses.
CONCLUSIONS
RFA appears as a feasible and safe procedure, with an acceptable morbidity, offering the possibility to safely repeat the treatment on the same lesion. RFA can be considered a valid option for the local control of lung metastases, in patients not eligible for surgery, especially those with lesions smaller than 3 cm.
Keywords: Lung metastases, Radiofrequency ablation, Minimally invasive therapy, High-risk patients, Non-surgical patients, Percutaneous ablation
INTRODUCTION
The lungs are one of the most common sites of metastases, and ∼30% of patients suffering from malignant solid tumour will develop pulmonary metastases. Surgical resection is reserved for selected patients, including those with complete control of primary tumour, no extrathoracic disease, long disease-free interval (DFI) and limited pulmonary metastases [1]. However, the efficacy of metastasectomy is variable, ranging from a 5-year survival rate of 26–69%, depending on different factors, such as the histology of the primary tumour, the complete resection and the number of lesions [2–5]. Therefore, sometimes, surgical resection could appear too aggressive for patients that are affected by Stage IV cancer, and often the practice is driven by the feeling of a need to ‘do something’, especially for young patients [3]. Additionally, the number of patients with resectable lung metastases, though not suitable for surgery, is unknown.
For these reasons, in the last decades, several non-surgical treatments were developed. One of these being radiofrequency ablation (RFA) that in an international study of 2004 has been defined as a minimally invasive tool for local disease control, with negligible mortality, low morbidity, short hospital stay and a gain in quality of life [6]. Several studies have shown that RFA is feasible, but few long-term outcomes have been reported [7–9].
The primary end-points of this study were overall survival (OS) and local progression-free survival (LPFS). Secondary end-point was the analysis of possible risk factors affecting OS and LPFS.
MATERIALS AND METHODS
Sixty-one patients underwent RFA of a total of 86 lung metastasis, during a 10-year period from 2003 to 2013. All patients signed a written informed consent, after adequate explanation of risks and possible benefits of the procedure.
Preoperative assessment and selection criteria
All patients underwent chest and abdominal computed tomography (CT) scan. Additional examinations (i.e. brain CT scan, bone scintigraphy and positron emission tomography (PET) were used to assess the complete control of the primary tumour, the absence of extrathoracic disease or the presence of extrathoracic disease controlled or being treated. Patients with lymph node involvement (observed at CT scan or PET scan) were not selected for the treatment. Functional assessment consisted in lung function test and in cardiological evaluation. All patients considered unsuitable for surgical resection were evaluated, case by case, by an experienced thoracic surgeon, together with a pneumologist, an oncologist and also an anaesthesiologist. The decision to exclude the surgical therapy was based not only on respiratory function tests (lung functions that made the patient unable to tolerate a wedge resection or excessive parenchymal loss required to resect a nodule sited deeply in the parenchyma or previous repeated wedge resections), but also on medical comorbidities that elevated the surgery at an excessively high-risk level.
Other main selection criteria were: lesions smaller than 5 cm; distance from large vessels and airways more than 1 cm; platelet count greater than 50 × 103/µl, as reported in a previous paper [10]. Demographic and clinical data of each patient were recorded, including age, gender, comorbidity and (DFI, defined as the time between the primary tumour and the first lung metastasis. The adult comorbidity evaluation scoring system (ACE-27) was used to stratify population based on comorbidities [11].
Device and technique
All procedures were performed with curative intent, under conscious sedation and local anaesthesia, utilizing a radiofrequency generator (RITA-Model 1500/1500X, AngioDynamics, Latham, NY, USA). In the majority of cases, CT guidance was used, while, more recently, lesions in contact with parietal pleura underwent RFA by ultrasonography (US) guidance. Before 2007, an employable array (StarBurst XL, AngioDynamics) was utilized to reach the target temperature of 90°C maintained for a predeterminate period ranging between 15 and 27 min based on the size of the lesion. Subsequently, a new employable array (StarBurst Talon, AngioDynamics) with a perfusion system (Intelliflow pump, AngioDynamics) that operates at 105°C was applied, reducing the ablation time to the target temperature to 5–9 min, again according to the size of the tumour, as reported in a previous paper [10]. When technically possible, the purpose of the RFA protocol was to create a coagulation necrosis 1 cm larger than the target tumour. At the end of the procedure, after the automatic cooling of the radiofrequency system, the expandable tines were retracted and the generator was shifted to ‘track ablation mode’, allowing the ablation of the pathway from the lesion to the subcutaneous tissue to prevent bleeding or tumour cell dissemination. Complications were recorded and stratified according to the Common Terminology Criteria for Adverse Events, version 4.03 [12]. Morbidity rate and mean hospital stay were calculated on the 99 RFA procedures.
Follow-up
Patients were evaluated at our day hospital with a contrast-enhanced chest and abdomen CT and tumour-specific markers at 1, 3 and 6 months after the procedure, and then with an interval of every 6 months with the same examinations. The assessment of target tumour response was based on the CT analysis of lesion size, lesion shape and lesion enhancement contrast. Considering that the objective of the RFA protocol was to encompass the tumour with an ablation zone thickness of at least 1 cm, the 1-month follow-up CT scan (in which the high-density area representing the ablation zone was usually larger than the native tumour) was considered a term of reference, as reported in a previous paper [10]. Nodules showing at least a 30% decrease in longest diameter compared with the diameter measured at 1-month CT, no evidence of tumour growth from the zone of ablation and no evidence of contrast enhancement, were assumed to have undergone complete ablation. The threshold of 30% is consistent with the new response evaluation criteria in solid tumours (RECIST) and was utilized to prevent overestimation of treatment response due to measurement variability [13, 14]. Conversely, nodules showing at least a 20% increase in longest diameter in any of the follow-up CT studies (as per RECIST, taking as reference the smallest diameter measured at any time point), evidence of tumour growth from the zone of ablation or intratumour contrast uptake (equal to or greater than preoperative, in any case greater than 25 Hounsfield units) were assumed to have disease recurrence. During the follow-up, if considered necessary, a PET scan was also performed.
Local recurrence (LR) was defined as evidence of tumour recurrence near to, in the site of thermal ablation or within the same lobe. OS was calculated on 61 patients and was defined as the interval between RFA and last follow-up or death. LPFS was calculated on 86 treated lesions and was defined as the interval between RFA treatment and evidence of disease LR. In case of multiple RFAs, the follow-up analysis for OS and LPFS was calculated starting from the last treatment. In case of LR during the follow-up period, if the patients underwent a treatment other than RFA, LPFS was calculated as the time between the RFA and the date of LR evidence.
Statistical analysis
Statistical analysis was performed using the software SPSS version 18.0 for Windows (SPSS, Chicago, USA). Continuous variables were expressed in terms of mean and standard deviation, whereas categorical variables were expressed in terms of frequency. Differences and similarities between two cohorts were assessed using unpaired t-test and the χ2 test or Fisher's exact, when appropriate, for continuous data and categorical measures, respectively. OS and LPFS were estimated with Kaplan–Meier method.
We investigated possible correlations between morbidity and the size of the lesion (<3 vs >3 cm), the type of approach (CT versus US), the site of the lesions (unilateral versus bilateral), the number of treated lesions per patients (patients treated for 1 lesion versus more than 1) and the number of repeated treatment on the same lesion (1 vs more than 1).
We investigated using univariate analysis, possible correlations between OS and LPFS with age, gender, type of approach (CT versus US), size of the lesion (>3 vs <3 cm), histology of primary cancer (colon versus others), number of treated lesions (1 vs more than 1), DFI (from 0 to 11 months vs more than 11 months, from 0 to 23 months vs more than 23 months, from 0 to 35 months vs more than 35 months), previous lung resections (yes versus no), the presence of local disease 1 month after the treatment (yes versus no). With the multivariate analysis, we were able to investigate possible correlation between OS and LPFS with age, lesion dimension (>3 vs >3 cm), histology (colon versus others), DFI (from 0 to 35 months vs more than 35 months) and the presence of local disease 1 month after the treatment (yes versus no).
For the univariate analysis, categorical variables were analysed with the log-rank test, and continuous variables were analysed using Cox regression. Multivariate analysis was performed using Cox regression. The odds ratio and corresponding 95% confidence intervals (CIs) were reported for covariates. A P-value of <0.05 was considered statistically significant.
RESULTS
Ninety-nine RFAs were performed on 61 patients (38 men, 23 women, with a median age of 74 years, range 40–86). All patients, except three that refused surgical intervention, were evaluated as unfit for surgery due to comorbidities or insufficient cardiopulmonary reserve. Demographic and clinical data are summarised in Table 1. The ACE-27 grade was 3 in 30 cases and 2 in 31 cases. The total number of treated lesions was 86 (median size 2 cm, range 0.7–5). Fourteen patients underwent RFA of two or more metastases (seven for two metastases, five for three metastases, one for four metastases and one for six metastases). The mean number of metastases per patient was 1.4. In 10 cases, the lesions were bilateral. In 12 cases, the same nodule was treated twice and in 1 case thrice, due to incomplete ablation or disease recurrence. The median interval between RFA procedures was 9 months (range 1–26). The primary cancer was colorectal in 29 (47.5%) cases, head and neck in 8 (13.11%), renal in 4 (6.55%), other in 20 (32.78%). The median DFI was 3 years (range 0–27). Twenty-four patients underwent previous resection for other metastatic lung lesions (21 wedge resections and 3 lobectomies followed by 3 wedge resections), whereas 37 patients had no prior lung resection.
Table 1:
Demographic and clinical characteristics of the study population
| Characteristics | n | % |
|---|---|---|
| Patients | 61 | −100 |
| Gender | ||
| Male | 38 | −62 |
| Female | 23 | −38 |
| Median age (range, years) | 74 | 40–86 |
| Primary cancer | ||
| Colon | 29 | −48 |
| Head and neck | 8 | −13 |
| Renal | 4 | −7 |
| Sarcoma | 5 | −8 |
| Other | 15 | −4 |
| Median lesion size (range, cm) | 2 | 0.7–5 |
| Site of lung metastases | ||
| RUL | 25 | −25.3 |
| ML | 3 | −3 |
| RLL | 20 | −20.2 |
| LUL | 23 | −23.2 |
| LLL | 28 | −28.3 |
| RFA guidance | ||
| CT | 90 | −89 |
| US | 9 | −11 |
| Previous lung resection | ||
| No | 37 | −61 |
| Yes | 24 | −39 |
| DFI (months) | ||
| 0–11 | 11 | −18 |
| 12–35 | 19 | −31 |
| >36 | 31 | −51 |
RUL: right upper lobe; ML: middle lobe; RLL: right lower lobe; LUL: left upper lobe; LLL: left lower lobe; CT: computed tomography; US: ultrasonography; DFI: disease-free interval, defined as the time between the primary tumour and the first lung metastasis; RFA: radiofrequency ablation.
Of the 99 procedures, 90 (90.9%) were performed under CT guidance and 9 (9.1%) under US guidance. All procedures were completed successfully. The 30-day mortality rate was 0%, whereas the 90-day mortality rate was 1%. One death occurred in a patient affected by severe COPD, ischaemic heart disease, paroxysmal atrial fibrillation, who was suffering from two lung metastases of bladder's carcinoma. The first RFA of the lesion sited in the left upper lobe was uneventful. After 1 month, during the second RFA procedure of the lesion sited in the left lower lobe, a pneumothorax requiring a chest drainage occurred. Subsequently, a progressive respiratory failure arose, causing the death of the patient, on 45 days post-treatment.
The morbidity rate was 11.0%. The most common complication was pneumothorax that occurred in 9 cases (8.7%): 8 patients (7.7%) required a chest drainage (AE Grade 2), of which 1 was treated with a talc pleurodesis by VATS, after 10 days of air leakage (AE Grade 3b). The pleural effusion occurred in 2 cases (2.0%), (AE Grade 2) in subpleural metastases ablated under US guidance. The median post-procedural hospital stay was 1 day (range 1–45). Three patients underwent wedge resection of the nodule after performing RFA due to disease recurrence, at a mean distance of 20 months (range 12–34) from the RFA treatment. These patients were those who initially refused surgery, even if were evaluated as operable. The pathological results of the specimens showed in all cases coagulative necrosis associated with residual area of vital tumour. Nine patients underwent stereotactic radiotherapy after performing RFA at a mean distance of 20 months (range 9–40) from the ablative treatment. Three of them were lost at follow-up, for the others local control of the disease was obtained.
Analysis of survival and progression
Three patients were lost at follow-up. The median follow-up period was 28 months (range 2–126). At the time of analysis, 27 patients were alive. Of these patients, 7 had LR of the disease, 1 had distant recurrence and 19 were disease free. Thirty-one patients died during the follow-up, of which 25 had cancer (15 patients with LR) and 6 were disease free. The actuarial mean OS was 65 months (95% CI 51–79). The 1-year OS was 94.8% (SE 0.029), whereas 3- and 5-year OS were 49.0% (SE 0.070) and 44.5% (SE 0.070), respectively. The 1-, 3-, 5-year LPFS rates were 86.3% (SE 0.038), 70.3% (SE 0.056) and 68.0% (SE 0.059), respectively. The actuarial mean LPFS was 96 months (95% CI 82–110).
Risk factor analysis
With regard to possible factors affecting morbidity, no association was observed with the lesion dimension (P = 0.262), the type of approach (P = 0.262), the sidedness of the lesions (P = 0.186), the number of treatments per patient (P = 0.594) and the number of repeated treatments on the same lesion (P = 0.524).
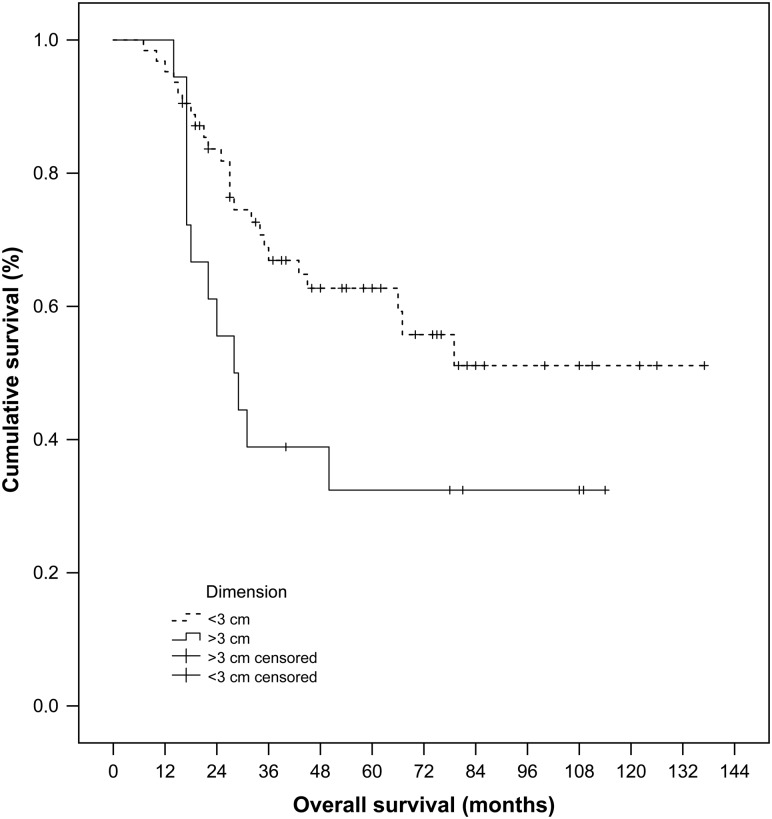
With regard to the possible risk factors affecting OS, in both univariate (Table 2) and multivariate (Table 3) analyses, no correlation was observed between OS and age, gender, type of approach, histology of primary cancer, number of treated lesions, DFI, previous lung resection. But, an important tendency towards better OS was observed, using univariate analysis, for lesions smaller than 3 cm, however, with a borderline statistically significant P-value (P = 0.051) (Fig. 1): the 1-, 3-, 5-year OS was 94.9, 55.7, 52.0% for lesion smaller than 3 cm, and 94.4, 30.9, 30.9% for lesion larger than 3 cm, respectively. Furthermore, the presence of local disease 1 month after treatment seemed to affect OS, even if without statistical significance (P = 0.056).
Table 2:
Univariate analysis of factors correlating with OS and LPFS
| Risk factor | OS P-value | LPFS P-value |
|---|---|---|
| Age (years)a | 0.53 | 0.33 |
| Genderb (male versus female) | 0.14 | 0.69 |
| Type of approachb (CT versus US) | 0.990 | 0.310 |
| Lesion dimensionb (<3 vs ≥3 cm) | 0.051 | 0.005 |
| Cancer typeb (colon versus other type) | 0.84 | 0.23 |
| Previous lung resectionb (yes versus no) | 0.37 | 0.35 |
| DFI-1b | 0.66 | 0.94 |
| DFI-2b | 0.44 | 0.96 |
| DFI-3b | 0.29 | 0.96 |
| Number of treated lesionb (one versus more lesions) | 0.35 | 0.16 |
| One-month LDb (yes versus no) | 0.056 | <0.001 |
CT: computed tomography; US: ultrasonography; DFI-1: disease-free interval (0–11 months vs more than 11 months); DFI-2: disease-free interval (0–23 months vs more than 24 months); DFI-3: disease-free interval (0–35 months vs more than 36 months); one-month LD: presence of local disease at 1 month after RFA; OS: overall survival; LPFS: local progression-free survival; RFA: radiofrequency ablation.
aContinuous variable.
bCategorical variables.
Table 3:
Multivariate analysis of factors correlating with OS and LPFS
| Risk factors | OS |
LPFS |
||||
|---|---|---|---|---|---|---|
| P-value | OR | 95% CI | P-value | OR | 95% CI | |
| Agea | 0.598 | 1.0101 | 0.974–1.046 | 0.172 | 1.031 | 0.987–1.077 |
| Dimensionb (<3 vs ≥3 cm) | 0.333 | 1.617 | 0.609–4.312 | 0.010 | 3.174 | 1.314–7.666 |
| DFIb (<3 vs ≥3 years) | 0.206 | 0.617 | 0.292–1.304 | 0.600 | 1.296 | 0.492–3.414 |
| Histologyb (colon versus other type) | 0.924 | 1.038 | 0.482–2.237 | 0.652 | 0.800 | 0.303–2.112 |
| One-month LDb | 0.507 | 1.504 | 0.451–5.014 | 0.001 | 5.289 | 2.011–13.913 |
OR: odd ratio; 95% CI: 95% confidential interval; DFI: disease-free interval, defined as the time between the primary tumour and the first lung metastasis; one-month LD: presence of local disease at 1 month after RFA; OS: overall survival; LPFS: local progression-free survival; RFA: radiofrequency ablation.
aContinuous variable.
bCategorical variables.
Figure 1:
Cumulative overall survival for the lesions of <3 and >3 cm, estimated from the latest RFA procedure, on 61 patients, with Kaplan–Meier method and log-rank test (P = 0.051). RFA: radiofrequency ablation.
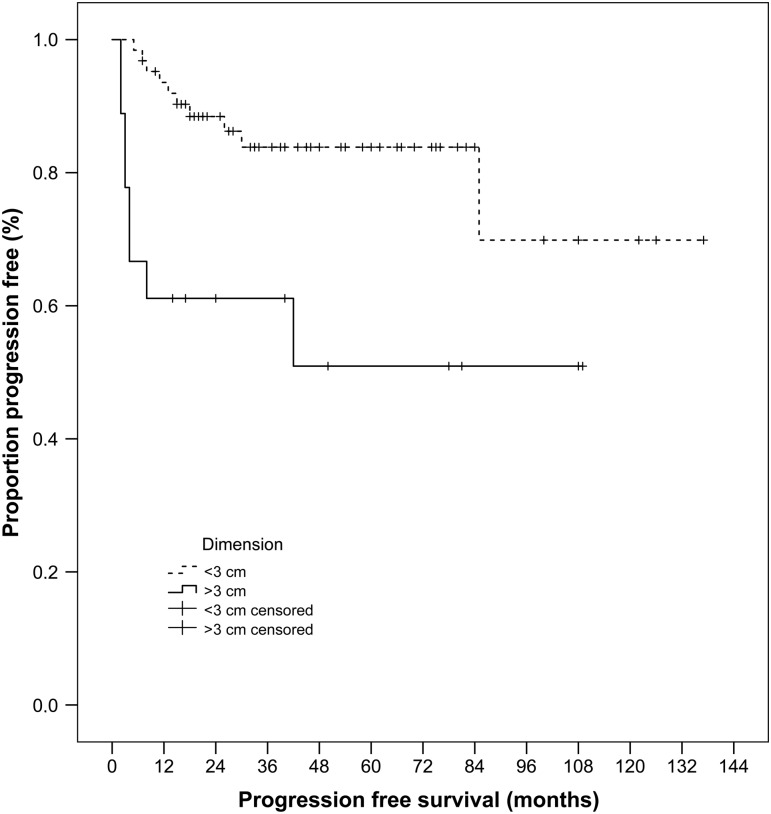
With regard to LPFS analysis (Tables 2 and 3), none of the above-mentioned parameters resulted as a significant risk factor, with the exception of the presence of local disease 1 month after treatment (P < 0.001) and of the lesion dimensions (P = 0.005) (Fig. 2): the 1-, 3-, 5-year LPFS was 93.5, 75.1, 70.1% for lesion smaller than 3 cm, and 61.1, 53.5, 42.8% for lesion larger than 3 cm, respectively. Local disease 1month after treatment and lesion dimensions were found to significantly affect LPFS also by multivariate analysis.
Figure 2:
Cumulative local progression-free survival for the lesions of <3 and >3 cm, estimated from the latest RFA treatment, on 86 lesions, with Kaplan–Meier method and log-rank test (P = 0.005). RFA: radiofrequency ablation.
DISCUSSION
The lung is one of the most frequent sites for tumour spread. The surgical resection of pulmonary metastases is now considered a standard therapeutic procedure in properly selected patients [1–5]. Additionally, during the last years, different techniques have been developed to improve the application of VATS for nodule resection [15–17]. Furthermore, recent papers have evaluated the role of VATS, showing no less favourable outcomes with respect to thoracotomy in terms of survival and disease recurrence [4, 5, 18]. In spite of these improvements, many patients, affected by lung metastasis, are unable to tolerate any kind of surgical intervention.
In this scenario, we investigated the efficacy of RFA in non-surgical patients. In our series, the most frequent complication was pneumothorax that occurred in 8.7% of cases. Our percentage is lower than that reported in the literature: Gillams et al. [19] reported an incidence of 15% of pneumothorax requiring chest tube, whereas in the study by Chua et al. [20], the percentage of pneumothorax requiring chest tube was 30, and in the study by de Baère et al. it rose to 58 [21]. This can probably be explained by the high incidence of previous lung resection that may create pleural adhesions (in our series 39%, in respect to 16 and 13% in Gillams and Chua et al. papers, respectively [19, 20]).
Additionally, Gillams et al. [19] observed major complications in 3.9% of patients, including two nerve injuries (recurrent laryngeal nerve and brachial plexus), but the severity of these adverse events was not specified. Such events were not experienced in our study, probably due to the minimum distance of 1 cm between the tumour and vital structures/mediastinum, which was always respected as per inclusion criteria.
In our series, we observed one case of pneumothorax, requiring surgical intervention due to the prolonged air leaking, and also in the series of Chua et al. [20] surgical intervention was necessary to treat a pneumothorax complicated by an abscess associated with empyema.
With regard to mortality, in our study, one death occurred, due to respiratory failure, 45 days after the treatment. Also in the study of Gillams et al. [19], one (1/122) death occurred after delayed infection resulting in a fatal haemoptysis, whereas de Baère et al. [21] reported two deaths (0.4%) related to cardiopulmonary failure and cerebral stroke, respectively.
On the basis of these data (Table 4), RFA can be evaluated as a safe procedure with an acceptable morbidity. Additionally, these studies as well as our series included also patients treated for more than one lesion, bilateral or unilateral [7, 19, 20] and up to eight [21], and in some cases, patients were treated for the same lesions up to four times [21, 22]. Chua et al. found that the number of the lesions treated (1 or 2 vs more than 2) and the sidedness (unilateral versus bilateral) significantly affected complications and required a chest tube. On the contrary, this was not observed in our analysis: no correlation was found between the morbidity and the sidedness of the lesions (unilateral versus bilateral), the number of treatments per patient and the number of repeated treatments of the same lesion. Probably, it is due to the low incidence of pneumothorax observed in our study. However, these data suggest that RFA can be applied not only for the treatment of a single lesion such as in case of early stage of NSCLC, but it can be also repeated safely, on different unilateral and/or contralateral nodules and on the same lesion. Evidence like these should be taken into consideration when approaching patients with metastatic disease, who suffer or probably will suffer from multiple lung nodules.
Table 4:
Recent studies regarding RFA for the treatment of lung metastases
| First author, year | Pts | Mts | Median diameter (cm), range | Histology | 90-day mortality | Morbidity | Median follow-up (months), range | 1-y OS | 3-y OS | 5-y OS |
|---|---|---|---|---|---|---|---|---|---|---|
| Gillams, 2013 | 122 | 398 | 1.7a (0.5–4) | Colon | 1/122 | 15% pnx drained 3.9% major complications |
12 (6–102) | NR | 57%b | NR |
| Chua, 2010 | 188 | 148 | 4a (SD ± 1) | Various | 0 | 30% pnx drained (1 pt operated for lung abscess) 11% pleural effusion |
29 (2–103) | NR | 60% | 45% |
| Yan, 2006 | 55 | 70c | 2.1a (SD ± 1.1) | Colon | 0 | 16% pnx drained 7% pleural effusion |
24 (6–40) | 85% | 46% | NR |
| Petre, 2013 | 45 | 69 | NR (0.4–3.5) | Colon | 0 | 19% pnx drained 4% pleural effusion |
18 (7–43) | 95% | 50% | NR |
| de Baère, 2015 | 566 | 642 | 1.5 (0.4–7) | Various | 2/566 | 58% pnx drained | 35 (20–53)d | 92% | 68% | 52% |
| Present report | 61 | 86 | 2 (0.7–5) | Various | 1/61 | 7.7% pnx drained (1 pt operated for prolonged air leak) 2% pleural effusion |
28 (2–126) | 95% | 49% | 45% |
Pts: number of treated patients; Mts: number of treated metastases; 1-y OS: 1-year overall survival; 3-y OS: 3-year overall survival; 5-y OS: 5-year overall survival; NR: not reported; RFA: radiofrequency ablation; SD: standard deviation.
aMean.
bCalculated from the first RFA treatment.
cTotal RFA.
dInterquartile.
With regard to survival, few studies evaluated the role of RFA for the treatment of lung metastases with an adequate follow-up period (Table 4). Gillams et al. reported a 3-year OS of 57% on 122 patients affected by colorectal lung metastases with a mean total number of lesions ablated of 3.3. However, the authors calculated the survival from the first treatment and, in cases of multiple RFAs, they did not specify the mean interval between the first and last RFA [19]. In the study of Chua et al. [20], which included different histologies, 19% of patients had multiple RFAs, obtaining a 3- and 5-year OS of 60 and 45%, respectively, with a median follow-up period of 29 months. The other study that included different histologies is the multi-institutional French study by de Baère et al. [21], in which the authors obtained a 5-year OS of 52%, with a follow-up period of 35 months. In our series, we obtained a 1-, 3- and 5-year OS of 95, 49 and 45% with a median follow-up of 28 months, whereas LPFS rates at 1-, 3- and 5-year were 86.3, 70.3 and 68.0%, respectively. The local control of disease at 5 years reached 70.1% for lesion smaller than 3 cm. In fact, concerning the risk factor analysis, in our series, a weighty tendency towards better OS was observed for smaller lesions (P = 0.051), but without reaching statistical significance, probably due to the small sample size. None of the other investigated parameters influenced OS. With regard to LPFS, we found that it was significantly associated with the lesion size and with the presence of disease 1 month after the treatment, by both univariate and multivariate analyses. These data are similar to the data reported in the paper of Yan et al. [7], where the dimension of >3 cm and repeated RFA treatments for disease recurrence were found to be significant prognostic factors for OS. Likewise, the study of Gillams et al. [19], on colorectal cancer metastases, showed a tendency towards better OS for lesion smaller than 2 cm. These data suggest that one of the most significant factors affecting RFA outcomes is the dimension of the lesion, as confirmed in a larger series of de Baère et al. [21], where nodule diameter significantly affected both survival and progression-free survival. In this paper, in addition to lesion size, DFI also significantly affected survival, as reported in the paper of Chua et al. [20], but these data were found in analysis series.
Despite our interesting results, we acknowledge some of the limitations of this research. The first one is the limited number of treated patients, which resulted from precise and strictly followed inclusion criteria, a single-centre experience and the heterogeneous histological type of lung metastases. Additionally, no confirmatory biopsy was routinely performed: history of previous cancer with PET-positive, newly discovered or enlarging lung nodule was considered diagnostic for malignant disease. Secondly, the population of this study included different kinds of patients, who were treated with RFA for several reasons: surgical refusal and poor lung function, thus making the patient unable to tolerate even a limited resection, the presence of coexisting comorbidities that make surgical risk unacceptable. Thirdly, despite the strict criteria adopted for the definition of LR, effectiveness of the procedure was confirmed by radiological means alone. However, CT imaging was evaluated always by the same multidisciplinary team that has decennial experience with RFA procedures and their follow-up imaging.
In addition, we have to consider the recent results of another non-surgical therapy: stereotactic body radiation therapy (SBRT) that appeared to be more effective than RFA for the treatment of early-stage NSCLC. The comparison between RFA and SBRT is probably more appropriate because they are both conservative and local therapies. Takahashi et al. [23], recently reported their results on 42 lung metastases (single nodule in 76% of cases, predominantly from lung and colon cancers, median maximum diameter 19 mm) treated with SBRT, showing a 1-year OS and local control rate of 81 and 91%, respectively, at a median follow-up of 20 months. In a similar series on 61 oligometastatic lung tumours (single nodule 74%) of Ricardi et al. [24], 2-year OS and local control rates were 66.5 and 89%, respectively, at a median follow-up of 20 months.
However, we have to consider three different aspects: firstly, lung metastases are frequently discovered as multiple lung nodules, and not only as a single nodule; secondly, some patients might have been subjected to previous radiotherapy for a prior lung cancer; thirdly, the most frequent side effect of SBRT is lung toxicity, which can occur also as late event, and that is dose dependent. Currently, few studies have analysed the outcomes and the side effects of SBRT on multiple lung metastases in a single treatment course or as a retreatment modality for new isolated lung nodules. Owen et al. [25] investigated 63 patients (128 nodules, with a mean size of 1.8 cm), who received multiple courses of lung SBRT or SBRT following high-dose external beam radiotherapy (EBRT) to the mediastinum. They reported a 1-year OS and LPFS of 85 and 91.9%, respectively, and an acute toxicity of 51% (most frequently Grades 1 and 2), which was significantly associated with previous EBRT and with high biologically effective dose. Late toxicity (most frequently related to the damage to normal lung tissue) was found in 29/63 (46%) patients (2 of whom became oxygen dependent and 1 of whom died due to pneumonitis) and it was associated with the number of SBRT sites treated. Evidence like this should be taken into consideration when approaching a patient with multiple lung metastases. In this sense, it should be underlined that RFA would be safely repeated on the same lesion and on other lesions in the same or contralateral lung. This can be explained by the fact that radiofrequency energy spreads less in normal lung tissue, which has a higher level of impedance related to the presence of air, in respect to the solid lesion [6, 14]. Additionally, the approach for SBRT is not uniform across centres: the optimal radiation dose and number of fractions required are still not clearly defined. Considering these preliminary data, further studies are necessary to clearly delineate the role of RFA for the treatment of lung metastases. As suggested by Treasure et al. [3], comparative randomized trials, evaluating different therapeutic options, are desirable to define the risks and benefits of each therapy and to understand, on a case by case basis, the best choice for a single patient. However, the comparison between different therapies for the treatment of lung metastases is difficult due to the heterogeneity of the analysed populations, the different histology and the multiplicity and repetition of treatments [3].
However, the results of our study confirm that RFA is a safe minimally invasive procedure, with an acceptable morbidity, offering the possibility to safely repeat the treatment on the same lesion and on other lung nodules. In patients not eligible for surgery, RFA offers good local control of lung metastases, also in the long-term period, particularly for lesions smaller than 3 cm. It can be considered a safe and effective therapeutic option, for treatment of small lung metastases, and made available when approaching a patient with Stage IV disease.
Funding
This study is part of a project that won, in 2000, a fund by the Italian Ministry of University and Research (MIUR). MIUR was not involved in any of the following activities: the study design; the collection, analysis, and interpretation of data; the writing of the report; the decision to submit the work for publication.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We thank Teresa Hung Key for checking English in this manuscript.
APPENDIX. CONFERENCE DISCUSSION
Dr P. Van Schil (Antwerp, Belgium): In the present series, 61 patients who were unfit for surgery or who refused an intervention are analysed, and they underwent radiofrequency ablation for a total of 86 lung metastases during a 10-year period. The primary tumor was mainly colorectal cancer. Twenty-four patients previously had undergone resection of lung metastases. The 90-day mortality was only 1% with an overall survival at 5 years of 45% and a local progression-free survival of 68%.
Were all patients discussed at the multidisciplinary team meeting, and which precise criteria were then used to decide whether to apply RFA, surgery, or stereotactic radiotherapy?
Dr Aprile: All patients were discussed in a multidisciplinary setting, including a thoracic surgeon, an oncologist, a pulmonologist, and an anaesthesiologist.
The preferred treatment for lung metastasis is surgical treatment, but not all patients are suitable for surgery.
When we started to treat lung metastasis in patients unsuitable for surgery, stereotactic radiotherapy was not available in our department.
Radiofrequency ablation, can be performed in one session. It can be repeated on the same nodule and impairs the pulmonary reserve less than stereotactic radiotherapy. So we prefer to treat lung metastasis in the patient with a pulmonary vessel with radiofrequency ablation.
However, not all the lesions can be treated with radiofrequency ablation due to the dimensions and localization. We couldn't treat lesions bigger than 5 cm, we couldn't treat lesions near the main airways or vessels, in the apix of the lungs, posterior lesions and lesions close to the scapula for the difficulty to position the needle. So the role of multidisciplinary setting is very important to decide the best treatment for patient unsuitable for surgery.
Dr Van Schil: Yes, I agree.
Secondly, yesterday I presented a case of lung metastasis when in fact we found five nodules in one lung with four different histologists.
So my second question is in how many cases was the pathological diagnosis obtained at the initial radiofrequency ablation treatment, and also secondly, at the diagnosis of recurrent disease?
Dr Aprile: About 17% of patients had pathological diagnosis. For the other patients, the oncologists didn't require a biopsy. For these patients, the diagnosis was clinical and radiological.
With regard to recurrences, the assessment of the tumor response was based on the CT analysis of lesion dimension, geometry, and the constant enhancement according to modified response evaluation criteria in solid tumor.
Dr Van Schil: But as with stereotactic radiotherapy after radiofrequency ablation treatment, you have a lot of inflammation surrounding the nodule. So how can you make the distinction sometimes between recurrent disease and just inflammatory reaction?
Dr Aprile: It's very difficult to recognize the inflammatory lesion surrounding the lesion treated with radiofrequency ablation and recurrences. We use the CT scan routinely, but in cases of doubt, we can perform a PET-CT never in the early post-treatment period.
Dr P. Tcherveniakov (Leeds, UK): Who actually does the procedure? Do you get the radiologist involved or do you do everything yourself?
Dr Aprile: No, the radiologists, the radiologists with the thoracic surgeon, we work together during the treatment.
Dr Tcherveniakov: Secondly, what are your technical limitations in terms of application of radiofrequency ablation? When do you consider a lesion and unsuitable for radiofrequency ablation. What stops you?
Dr Aprile: For a small apical lesion, for a lesion located posteriorly, for a lesion close to the scapula, it's very difficult to treat these lesions for the difficulty of the position of the needle. So in these cases, we propose the stereotactic radiotherapy.
REFERENCES
- 1.Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37–49. [DOI] [PubMed] [Google Scholar]
- 2.Rolle A, Pereszlenyi A, Koch R, Richard M, Baier B. Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1318-nm Nd:YAG laser. J Thorac Cardiovasc Surg 2006;131:1236–42. [DOI] [PubMed] [Google Scholar]
- 3.Treasure T, Milošević M, Fiorentino F, Macbeth F. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carballo M, Maish MS, Jaroszewski DE, Holmes CE. Video-assisted surgery (VATS) as a safe alternative for resection of pulmonary metastases: a retrospective cohort study. J Cardiothorac Surg 2009;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossot D, Radu C, Girard P, Le Cense A, Bonvalot S, Boudaya MS et al. Resection of pulmonary metastases from sarcoma: can some patients benefit from less invasive approach. Ann Thorac Surg 2009;87:238–43. [DOI] [PubMed] [Google Scholar]
- 6.Steinke K, Sewell PE, Dupuy D, Lencioni R, Helmberger T, Kee ST et al. Pulmonary radiofrequency ablation-an international study survey. Anticancer Res 2004;24:339–44. [PubMed] [Google Scholar]
- 7.Yan TD, King J, Sjarif A, Glenn D, Steinke K, Morris DL. Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol 2006;13:1529–37. [DOI] [PubMed] [Google Scholar]
- 8.Ketchedjian A, Daly B, Luketich J, Fernando HC. Minimally invasive techniques for managing pulmonary metastases: video-assisted thoracic surgery and radiofrequency ablation. Thorac Surg Clin 2006;16:157–65. [DOI] [PubMed] [Google Scholar]
- 9.Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CH, Nq T et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007;243:268–75. [DOI] [PubMed] [Google Scholar]
- 10.Ambrogi MC, Fanucchi O, Cioni R, Dini P, DeLiperi A, Cappelli C et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol 2011;6:2044–51. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291.20:2441–7. [DOI] [PubMed] [Google Scholar]
- 12.Common Terminology Criteria for Adverse Events version 4.03. US Department of Health and Human Services. National Institutes of Health, National Cancer Institute, 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]
- 13.Eisenhauer E, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 14.Ambrogi MC, Fontanini G, Cioni R, Faviana P, Fanucchi O, Mussi A et al. Biologic effects of radiofrequency thermal ablation on non-small cell lung cancer: results of a pilot study. J Thorac Cardiovasc Surg 2006;131.5:1002–6. [DOI] [PubMed] [Google Scholar]
- 15.Ciriaco P, Negri G, Puglisi A, Nicoletti R, Del Maschio A, Zannini P. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429–33. [DOI] [PubMed] [Google Scholar]
- 16.Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576–85. [DOI] [PubMed] [Google Scholar]
- 17.Ambrogi MC, Melfi F, Zirafa C, Lucchi M, De Liperi A, Mariani G et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc 2012;26:914–9;11. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Interact CardioVasc Thorac Surg 2013;17:720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillams A, Khan Z, Osborn P, Lees W. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol 2013;36:724–30. [DOI] [PubMed] [Google Scholar]
- 20.Chua TC, Sarkar A, Saxena A, Glenn D, Zhao J, Morris DL. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol 2010;21:2017–22. [DOI] [PubMed] [Google Scholar]
- 21.de Baère T, Aupérin A, Deschamps F, Chevallier P, Gaubert Y, Boige V et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petre EN, Jia X, Thornton RH, Sofocleous CT, Alago W, Kemeny NE et al. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer 2013;12:37–44. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi W, Yamashita H, Niibe Y, Shiraishi K, Hayakawa K, Nakagawa K. Stereotactic body radiotherapy for metastatic lung cancer as oligo-recurrence: an analysis of 42 cases. Pulm Med 2012;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricardi U, Filippi AR, Guarneri A, Ragona R, Mantovani C, Giglioli F et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012;75.1:77–81. [DOI] [PubMed] [Google Scholar]
- 25.Owen D, Olivier KR, Mayo CS, Miller RC, Nelson K, Bauer H et al. Outcomes of stereotactic body radiotherapy (SBRT) treatment of multiple synchronous and recurrent lung nodules. Radiat Oncol 2015;10.1:43. [DOI] [PMC free article] [PubMed] [Google Scholar]