Abstract
OBJECTIVES
The purpose of this study was to investigate the prognostic factors for repeat lung metastasectomy in patients with colorectal cancer, which may be clinically helpful in defining a subset of patients who are most likely to benefit from repeat lung metastasectomy.
METHODS
In total, 138 patients underwent complete lung resection for the first time due to metastases of colorectal cancer between January 2004 and December 2013 at Fujita Health University School of Medicine. Among them, 33 underwent repeat pulmonary metastasectomy for lung tumour recurrence. Kaplan–Meier survival curves and log-rank tests were used to analyse the survival rates.
RESULTS
No patient died as a direct result of surgery, and all patients were discharged after the repeat pulmonary metastasectomy. The 5-year survival rate after the initial pulmonary resection of the 33 patients who underwent repeat lung resection was 64%, which was not significantly different from that of the 105 patients who did not undergo repeat lung resection (5-year survival rate, 61%; P = 0.779). Univariate analysis identified only one significant prognostic factor: preoperative serum carcinoembryonic antigen (CEA) level (P = 0.002). The 5-year survival rates of patients with high preoperative CEA levels and normal CEA levels after repeat metastasectomy were significantly different at 47 and 90%, respectively.
CONCLUSIONS
Prethoracotomy serum CEA levels affect survival rates after repeat pulmonary resection. The preoperative assessment of serum CEA levels before repeat metastasectomy is important when considering repeat pulmonary resection, and prethoracotomy CEA levels should be taken into account when selecting patients for repeat lung resection.
Keywords: Colorectal cancer, Lung metastasis, Repeat metastasectomy
INTRODUCTION
Colorectal cancer is one of the most common cancers worldwide [1], and it frequently metastasizes to the liver and lungs [2, 3]. Recently, the development of chemotherapy for metastatic colorectal cancer has been reported [4], but surgical resection for lung metastasis is still considered to be the optimal treatment where possible [5]. However, the main cause of death after pulmonary metastasectomy for colorectal carcinoma is tumour recurrence, which involves the lungs in approximately half of the patients [6, 7]. Although many studies have reported on the survival and prognostic factors for patients undergoing pulmonary metastasectomy for colorectal carcinoma [8–15], few studies have investigated prognostic factors after repeat pulmonary metastasectomy for recurrent lung metastases from colorectal carcinoma [7, 16–19]. Therefore, we reviewed a recent series of consecutive patients who underwent repeat pulmonary metastasectomy at Fujita Health University School of Medicine. The main purpose of this study was to investigate the prognostic factors of repeat lung metastasectomy in patients with colorectal cancer, which may be clinically helpful in defining a subset of patients who are most likely to benefit from repeat lung metastasectomy.
MATERIALS AND METHODS
One hundred and thirty-eight patients underwent lung resection for the first time due to the metastases of colorectal cancer between January 2004 and December 2013 at our institution. Among these 138 patients, 33 underwent repeat pulmonary metastasectomy for lung tumour recurrence following colorectal cancer.
All patients who underwent resection of their pulmonary metastases met the following criteria: (i) the primary tumour was controlled; (ii) there was no evidence of extrathoracic metastasis except liver metastasis; (iii) complete resection of recurrent lung disease was considered to be possible at presentation regardless of the number of lesions; (iv) the patient was in a good general condition and had adequate respiratory function to tolerate lung resection. The detailed regimens of chemotherapy were different among patients; however, all 33 patients underwent pre- or postoperative chemotherapy. The patients were followed up until August 2015.
We reviewed each patient's medical records to obtain clinicopathological information, which included the following: (i) age at the repeat metastasectomy (dichotomized at the median age of 65), (ii) gender, (iii) smoking history (never- or ever-smoker), (iv) prethoracotomy serum carcinoembryonic antigen (CEA) level before repeat pulmonary metastasectomy (cut-off at the normal upper limit of 5 ng/ml), (v) primary site (colon or rectum), (vi) prior resection of liver metastasis (yes or no), (vii) Dukes' stage of the primary tumour (A-B or C-D), (viii) histological differentiation of the primary tumour (well differentiated or moderately/poorly differentiated), (ix) number of pulmonary metastases (≤2 or >2), (x) largest diameter of the resected tumour (≤1 or >1 cm), (xi) the disease-free interval (DFI) between the colorectal resection and repeat pulmonary resection (≤36 or >36 months) and (xii) the DFI between the initial pulmonary resection and the repeat pulmonary resection (≤12 or >12 months).
The duration of the overall survival rate was calculated in months from the date of initial or repeat pulmonary metastasectomy to the date of death due to any aetiology or the date of the last follow-up. All cumulative survival curves were estimated using the Kaplan–Meier method, and differences between groups were evaluated using log-rank tests. The significance level was set at <0.05. Analyses were performed using the statistical software SPSS 11.0 (Dr SPSS II for Windows, Standard Version 11.0, SPSS, Inc., Chicago, IL, USA).
RESULTS
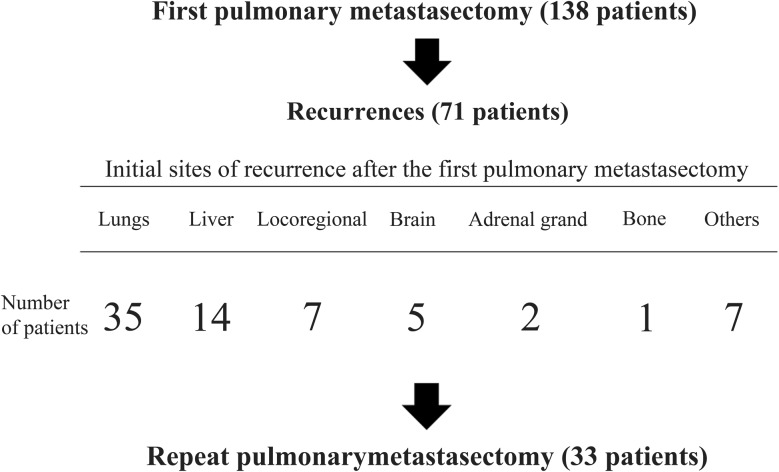
The 5-year survival rate of all 138 patients who underwent complete lung resection was 61.7%. Of the 138 patients, 71 had recurrence after the first lung resection, with 35 developing recurrence in the lungs. Of these patients, 33 underwent repeat lung resection for recurrent lung metastases (Fig. 1). Postoperative morbidities were observed in 6 patients: prolonged air leak in 4 patients, pleural effusion in 1 patient and arrhythmia in 1 patient. No patient died as a direct result of the repeat surgery, and all patients were discharged after the repeat pulmonary metastasectomy. Patient characteristics are presented in Table 1. The cohort consisted of 14 women and 19 men. The ages ranged from 28 to 82 years with a median of 65 years. Video-assisted thoracic surgery (VATS) for the initial metastasectomy was performed in 31/33 (94%) patients and for the repeat metastasectomy in 21/31 (68%) patients. The number of metastases ranged from 1 to 6 with a median of 2. The surgical method for repeat pulmonary resection was a wedge resection in 20 patients, segmentectomy in 5, lobectomy in 7 and completion pneumonectomy in 1. All repeat pulmonary metastasectomies were curative resections. The median time interval between the initial and repeat pulmonary resections was 12 months (range: 5–37 months). The median follow-up period after repeat metastasectomy was 51 months (range: 8–127 months). Of the 33 patients, 9 were alive with no evidence of disease and 9 were alive with disease at the end of the follow-up period of the study. One had died of another disease, and 14 had developed recurrences and died of the disease.
Figure 1:
Patients who underwent complete lung resection for the first time due to metastases of colorectal cancer.
Table 1:
Patient characteristics
| Characteristics | Number of patients |
|---|---|
| Overall | 33 |
| Age at the second pulmonary resection | |
| Median | 65 |
| Range | 28–82 |
| Gender | |
| Women | 14 |
| Men | 19 |
| Smoking habits | |
| Non-smoker | 13 |
| Current or former smoker | 10 |
| Unknown | 10 |
| CEA before the second pulmonary resection | |
| Within normal range | 16 |
| Elevated | 16 |
| Unknown | 1 |
| Primary site | |
| Colon | 12 |
| Rectum | 21 |
| Prior resection of liver metastasis | |
| Yes | 10 |
| No | 23 |
| Dukes' stage | |
| A-B | 6 |
| C-D | 18 |
| Unknown | 9 |
| Histological differentiation | |
| Well differentiated | 8 |
| Moderately/poorly differentiated | 19 |
| Unknown | 6 |
| Number of pulmonary metastases | |
| 1 | 12 |
| 2 | 10 |
| 3–6 | 11 |
| Maximum tumour size (cm) | |
| Median | 1 |
| Range | 0.2–5 |
| Interval between the primary resection and the second pulmonary resection (months) | |
| Median | 36 |
| Range | 13–92 |
| Interval between the first pulmonary resection and the second pulmonary resection (months) | |
| Median | 12 |
| Range | 5–37 |
CEA: preoperative serum carcinoembryonic antigen levels, normal upper limit at 5 ng/ml.
The 5-year survival rate after the initial pulmonary resection for the 33 patients who underwent repeat lung resection was 64.3%, which was not significantly different from that for the 105 patients who did not undergo the repeat lung resection (5-year survival rate, 61.3%; P = 0.779). Table 2 lists the 5-year survival rates after the repeat pulmonary resection according to clinicopathological features for all 33 patients. Univariate analysis (log-rank test) identified only one significant prognostic factor: preoperative serum CEA levels prior to repeat thoracotomy (Table 2). The 5-year survival rates for patients with a high preoperative CEA level and normal CEA level were significantly different at 46.9 and 90.0%, respectively (P = 0.002, Fig. 2).
Table 2:
Five-year survival rates according to clinicopathological features
| Characteristic | No. of patients | 5-year overall survival after the second pulmonary resection (%) | Univariate analysis |
||
|---|---|---|---|---|---|
| P-value† | HR | CI | |||
| Overall | 33 | 55 | |||
| Age (years) | |||||
| ≤65 | 16 | 72 | |||
| >65 | 17 | 46 | 0.108 | 1.931 | 0.629–5.933 |
| Gender | |||||
| Women | 14 | 53 | |||
| Men | 19 | 66 | 0.831 | 0.738 | 0.248–2.203 |
| Smoking habits | |||||
| Non-smoker | 13 | 61 | |||
| Current or former smoker | 10 | 50 | 0.806 | 1.226 | 0.342–4.388 |
| Unknown | 10 | ||||
| CEA | |||||
| Within normal range | 16 | 90 | |||
| Elevated | 16 | 47 | 0.002* | 3.749 | 1.031–13.632 |
| Unknown | 1 | ||||
| Primary site | |||||
| Colon | 12 | 44 | |||
| Rectum | 21 | 79 | 0.221 | 0.544 | 0.181–1.632 |
| Prior resection of liver metastasis | |||||
| Yes | 23 | 76 | |||
| No | 10 | 39 | 0.128 | 2.261 | 0.748–6.832 |
| Dukes' stage | |||||
| A-B | 6 | 72 | |||
| C-D | 18 | 58 | 0.298 | 2.293 | 0.366–23.376 |
| Unknown | 9 | ||||
| Histological differentiation | |||||
| Well differentiated | 8 | 82 | |||
| Moderately/poorly differentiated | 19 | 56 | 0.183 | 2.764 | 0.550–13.878 |
| Unknown | 6 | ||||
| Number of pulmonary metastases | |||||
| ≤2 | 22 | 53 | |||
| >2 | 11 | 59 | 0.298 | 0.556 | 0.121–2.552 |
| Maximum tumour size (cm) | |||||
| ≤1 | 17 | 58 | |||
| >1 | 16 | 52 | 0.531 | 1.19 | 0.376–3.772 |
| Interval between the primary resection and the second pulmonary resection (months) | |||||
| ≤36 | 15 | 47 | |||
| >36 | 18 | 59 | 0.478 | 0.606 | 0.201–1.825 |
| Interval between the first pulmonary resection and the second pulmonary resection (months) | |||||
| ≤12 | 18 | 49 | |||
| >12 | 15 | 60 | 0.554 | 0.706 | 0.236–2.114 |
CEA: preoperative serum carcinoembryonic antigen levels, normal upper limit at 5 ng/ml; HR: hazard ratio for death; CI: confidence interval.
Log-rank test.
Significance.
Figure 2:
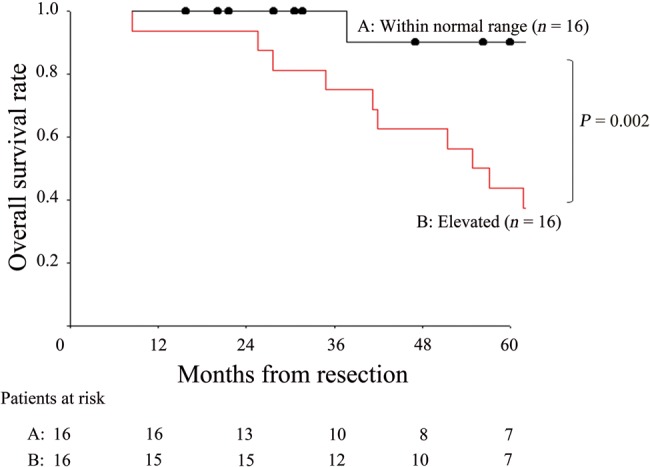
Overall survival curves after the second lung resection for the patients according to prethoracotomy serum CEA levels before the second thoracotomy. CEA: carcinoembryonic antigen.
DISCUSSION
The lungs are one of the most frequently affected metastatic sites in patients with colorectal cancer [2, 3]. Lung metastases are sequentially or simultaneously detected in ∼10% of patients with colorectal cancer [20]. Several studies have demonstrated the efficacy of lung metastasectomy in colorectal cancer patients [5, 8–15]. Various factors associated with prolonged survival after surgery for lung metastases from colorectal cancer have been identified, including (i) a single isolated metastasis <3 cm in size [8–10], (ii) a long DFI [11–13], (iii) the absence of thoracic lymph node invasion [14, 15] and (iv) prethoracotomy CEA level [5, 14]. This knowledge is clinically helpful for defining a subset of patients who are most likely to benefit from surgical resection. Although approximately half of the patients developed lung tumours after pulmonary metastasectomy for colorectal carcinoma [6, 7], there are few studies investigating the prognostic factors after repeat pulmonary metastasectomy for recurrent lung metastases from colorectal carcinoma. Because there is no consensus on appropriate indications for the resection of repeat lung metastases, we investigated a recent series of patients with repeat resected lung metastases from colorectal cancer in our current study. The main purpose of this study was to investigate the prognostic factors of repeat metastasectomy in patients with previously resected lung metastases, which may be clinically helpful in defining a subset of patients who are most likely to benefit from repeat pulmonary metastasectomy.
In the current study, a high CEA level before the repeat thoracotomy was shown to be the only poor prognostic factor. Earlier studies have also shown that a high preoperative CEA level is associated with poorer survival in patients with pulmonary metastases from colorectal cancer [5, 14]. The elevation of serum CEA is considered to be an indication of increased malignancy and rapid, aggressive growth of the tumour [21, 22], which leads to multiple lesions and a poorer prognosis. CEA levels may therefore reflect the highly malignant nature of cancer cells that undergo systemic dissemination. We concluded that the group with high CEA levels prior to repeat thoracotomy should be carefully selected for the resection of recurrent lesions. If we apply appropriate surgical treatment for recurrent lesions, careful postoperative follow-up with frequent CEA measurement and periodic computed tomography (CT) scans to check for early recurrence may be the key to improved survival in some patients with high preoperative CEA levels.
In the current study, there were no occurrences of operative major morbidity or mortality regardless of whether the patient underwent repeat thoracotomy. Our results may be a result of VATS because 31/33 (94%) of initial metastasectomy procedures were performed using VATS. Recently, VATS has become a very popular method for minimally invasive surgery, and it is increasingly being used for pulmonary metastasectomy [23]. Although its efficacy for pulmonary metastasectomy is controversial, in our study, 94% of the patients underwent VATS metastasectomy and showed a comparable survival rate to those undergoing open surgery [5, 8–15]. The main disadvantages of VATS metastasectomy are establishing the localization of small nodules and the loss of non-visualized additional nodules. However, in terms of the loss of non-visualized nodules, Nakas et al. [24] reported no difference in the incidence of missed lesions and concluded that VATS metastasectomy in conjunction with multidetector CT was justified. Therefore, if complete resection of pulmonary metastasectomies using VATS is promising and no additional detection of nodules during open surgery is required due to precise CT results, VATS can certainly be the ideal approach for lung metastasectomy from colorectal cancer, especially for future repeat lung metastasectomy.
There are several limitations to our analysis. Firstly, we studied a small sample size. Secondly, because this is a retrospective study on surgical cases, the patients included in the analysis were carefully selected, and our sample may not represent a cross section of all patients with recurrent pulmonary metastases from colorectal cancer. A prospective, large-scale study with multiple institutions would be necessary to confirm the current results.
CONCLUSION
Prethoracotomy serum CEA levels affect survival after the second pulmonary resection. Preoperative assessment of serum CEA levels before repeat thoracotomy is important for repeat pulmonary resection. Furthermore, prethoracotomy CEA levels should be taken into consideration when selecting patients for repeat lung resection. In a carefully selected subset of patients who develop resectable recurrence in the lungs following initial metastasectomy of colorectal cancer lung metastasis, repeat resection is feasible.
Conflict of interest: none declared.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10-year results. Arch Surg 1992;127:1403–6. [DOI] [PubMed] [Google Scholar]
- 3.Goya T, Miyazawa N, Kondo H, Tsuchiya R, Naruke T, Suemasu K. Surgical resection of pulmonary metastases from colorectal cancer. 10-year follow-up. Cancer 1989;64:1418–21. [DOI] [PubMed] [Google Scholar]
- 4.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539–44. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Kiyoshima M, Kitahara M, Asato Y, Amemiya R. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2015;99:435–40. [DOI] [PubMed] [Google Scholar]
- 6.McAfee MK, Allen MS, Trastek VF, Ilstrup DM, Deschamps C, Pairolero PC. Colorectal lung metastases: results of surgical excision. Ann Thorac Surg 1992;53:780–6. [DOI] [PubMed] [Google Scholar]
- 7.Ogata Y, Matono K, Hayashi A, Takamor S, Miwa K, Sasatomi T et al. Repeat pulmonary resection for isolated recurrent lung metastases yields results comparable to those after first pulmonary resection in colorectal cancer. World J Surg 2005;29:363–8. [DOI] [PubMed] [Google Scholar]
- 8.Pfannschmidt J, Muley T, Dienemann H, Hoffmann H. Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg 2003;126:732–9. [DOI] [PubMed] [Google Scholar]
- 9.Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Prauer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg 2004;91:1066–71. [DOI] [PubMed] [Google Scholar]
- 10.Koga R, Yamamoto J, Saiura A, Yamaguchi T, Hata E, Sakamoto M. Surgical resection of pulmonary metastases from colorectal cancer: four favourable prognostic factors. Jpn J Clin Oncol 2006;36:643–8. [DOI] [PubMed] [Google Scholar]
- 11.Rena O, Casadio C, Viano F, Cristofori R, Ruffini E, Filosso PL et al. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg 2002;21:906–12. [DOI] [PubMed] [Google Scholar]
- 12.Takakura Y, Miyata Y, Okajima M, Okada M, Ohdan H. Short disease-free interval is a significant risk factor for intrapulmonary recurrence after resection of pulmonary metastases in colorectal cancer. Colorectal Dis 2010;12:68–75. [DOI] [PubMed] [Google Scholar]
- 13.Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol 2009;16:1026–32. [DOI] [PubMed] [Google Scholar]
- 14.Zampino MG, Maisonneuve P, Ravenda PS, Magni E, Casiraghi M, Solli P et al. Lung metastases from colorectal cancer: analysis of prognostic factors in a single institution study. Ann Thorac Surg 2014;98:1238–45. [DOI] [PubMed] [Google Scholar]
- 15.Welter S, Jacobs J, Krbek T, Poettgen C, Stamatis G. Prognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2007;31:167–72. [DOI] [PubMed] [Google Scholar]
- 16.Kanzaki R, Higashiyama M, Oda K, Fujiwara A, Tokunaga T, Maeda J et al. Outcome of surgical resection for recurrent pulmonary metastasis from colorectal carcinoma. Am J Surg 2011;202:419–26. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Sakai H, Miyahara R, Bando T, Okubo K, Date H. Repeat resection of pulmonary metastasis is beneficial for patients with colorectal carcinoma. World J Surg 2010;34:2373–8. [DOI] [PubMed] [Google Scholar]
- 18.Kim AW, Faber LP, Warren WH, Saclarides TJ, Carhill AA, Basu S et al. Repeat pulmonary resection for metachronous colorectal carcinoma is beneficial. Surgery 2008;144:712–7. [DOI] [PubMed] [Google Scholar]
- 19.Welter S, Jacobs J, Krbek T, Krebs B, Stamatis G. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2007;84:203–10. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K, Saito N, Sugito M, Ito M, Kobayashi A, Nishizawa Y. Incidence and predictive factors for pulmonary metastases after curative resection of colon cancer. Ann Surg Oncol 2013;20:1374–80. [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal RD, Hansen HJ, Goldenberg DM. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res 2005;65:8809–17. [DOI] [PubMed] [Google Scholar]
- 22.Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 2013;32:643–71. [DOI] [PubMed] [Google Scholar]
- 23.Landreneau RJ, De Giacomo T, Mack MJ, Hazelrigg SR, Ferson PF, Keenan RJ et al. Therapeutic video-assisted thoracoscopic surgical resection of colorectal pulmonary metastases. Eur J Cardiothorac Surg 2000;18:671–6. [DOI] [PubMed] [Google Scholar]
- 24.Nakas A, Klimatsidas MN, Entwisle J, Martin-Ucar AE, Waller DA. Video-assisted versus open pulmonary metastasectomy: the surgeon's finger or the radiologist's eye? Eur J Cardiothorac Surg 2009;36:469–74. [DOI] [PubMed] [Google Scholar]