Abstract
Extracellular matrix (ECM) derived from small intestinal submucosa (SIS) is widely used in clinical applications as a scaffold for tissue repair. Recently, CorMatrix® porcine SIS-ECM (CorMatrix Cardiovascular, Inc., Roswell, GA, USA) has gained popularity for ‘next-generation’ cardiovascular tissue engineering due to its ease of use, remodelling properties, lack of immunogenicity, absorbability and potential to promote native tissue growth. Here, we provide an overview of the biology of porcine SIS-ECM and systematically review the preclinical and clinical literature on its use in cardiovascular surgery. CorMatrix® has been used in a variety of cardiovascular surgical applications, and since it is the most widely used SIS-ECM, this material is the focus of this review. Since CorMatrix® is a relatively new product for cardiovascular surgery, some clinical and preclinical studies published lack systematic reporting of functional and pathological findings in sufficient numbers of subjects. There are also emerging reports to suggest that, contrary to expectations, an undesirable inflammatory response may occur in CorMatrix® implants in humans and longer-term outcomes at particular sites, such as the heart valves, may be suboptimal. Large-scale clinical studies are needed driven by robust protocols that aim to quantify the pathological process of tissue repair.
Keywords: Cardiovascular surgery, CorMatrix, Small intestinal submucosa, Extracellular matrix, Tissue engineering
INTRODUCTION
Cardiovascular disease (CVD) is a global health challenge and the leading cause of death worldwide [1]. CVD represents a heterogeneous group of diseases; treatment is, therefore, disease- and patient-specific, but surgical options remain at the forefront of the therapeutic armamentarium in both acquired and congenital pathologies. Regardless of aetiology, the surgical treatment of CVD, particularly following the reconstructive approach, frequently requires additional biological or prosthetic tissue to act as an anatomical substitute. Reconstruction with patches, conduits and valves forms the bedrock of congenital cardiac surgery, while synthetic grafts are used to replace damaged, occluded, ruptured, aneurysmal or atherosclerotic vessels. As a result, a wide range of autologous, heterologous and synthetic materials have been developed to meet the needs of different surgical applications, each with their advantages and disadvantages. Traditionally, either autologous or cross-linked xenopericardium has been used for patch repair in cardiac surgery [2], but these are particularly susceptible to fibrosis, thickening, calcification and retraction over time and do not have the capacity to facilitate tissue growth [3]. Homografts of entire valves or a valve leaflet have been used for decades for valve repair, their advantages being favourable haemodynamics, a low incidence of thromboembolic complications, suitability in the presence of infection and no requirement for anticoagulation. However, the long-term outcomes are age-dependent and the homograft supply cannot always meet demand [4]. A synthetic material such as woven nylon (Dacron; Koch Industries, Inc., Wichita, KS, USA) is not intrinsically biocompatible, is fairly rigid and promotes reactive inflammation and endocarditis [5]. In addition, the paediatric population has particular needs since bioprostheses, homografts and xenografts are susceptible to accelerated degeneration in this age group [6]. For all the aforementioned reasons, there is a need for improved materials for use in cardiovascular surgery to overcome these limitations.
One such material that has recently shown promise in experimental and clinical cardiovascular surgery is CorMatrix®, a biological scaffold derived from decellularized porcine small intestinal submucosa (SIS) [7]. CorMatrix® has emerged as the leading commercially available SIS-ECM scaffold for cardiovascular use and is the most widely used SIS-ECM product in cardiovascular surgery; this review, therefore, focuses on CorMatrix® as the exemplar since it is the material that cardiovascular surgeons are most likely to encounter. In general, SIS-ECM may (i) possess a three-dimensional (3D) architecture to support the ingrowth of host cells (termed ‘bioinduction’); (ii) be sufficiently biologically active to initiate and maintain the molecular control of cell proliferation and differentiation (and hence have growth potential); (iii) be absorbable and (iv) lack the immunogenicity to stimulate a host immunological response [8]. These properties would make the material suitable for a wide range of surgical procedures and might ensure longevity of repair of cardiac defects in children. Furthermore, large amounts of the material can be manufactured to quality-assured standards, thereby ensuring a continuous and standardized supply of material to surgeons.
Here, we review the basic biology, preclinical and clinical studies reporting the use of porcine SIS, and particularly CorMatrix®.
SEARCH STRATEGY
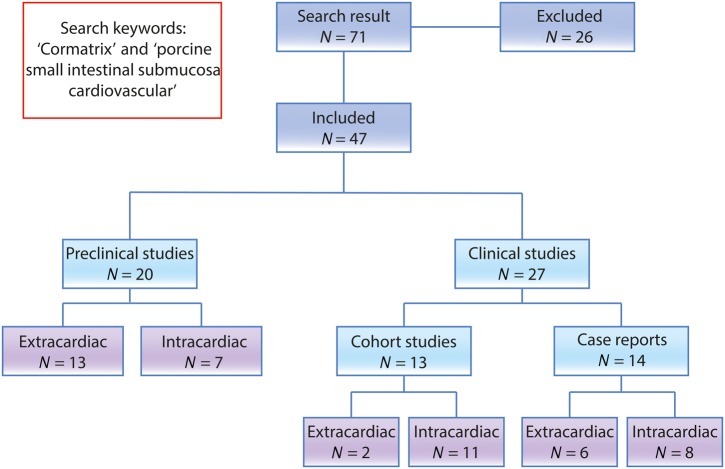
We conducted a comprehensive literature search up to October 2015 using the search terms ‘CorMatrix’ and ‘porcine small intestinal submucosa cardiovascular’ in the PubMed database; a flowchart of the literature search is shown in Fig. 1. All studies relating to these materials were reviewed. The abstract of the articles was reviewed only if the title of the article and/or keywords were relevant. The full text of all potentially relevant articles was read for inclusion in the study, and the reference lists of included studies were manually searched along with company websites. A total of 47 articles were included and tabulated (Tables 1–5).
Figure 1:
Flow chart demonstrating the study design.
Table 1:
Experimental/animal studies of porcine SIS and SIS-ECM used at extracardiac sites or for cardiac regeneration
| Study | Year | Indication | Animal model | Number | Follow-up | Outcome measure or method | Result(s) | Reported remodelling process |
|---|---|---|---|---|---|---|---|---|
| Sandusky et al. [9] | 1992 | Carotid artery grafting | Dog | n = 24 | Up to 180 days | Histology, Doppler | SIS thrombosis Day 2, 90 and 180 SIS graft outcome was similar to SVG grafts |
Smooth muscle infiltration, neovascularization, endothelialization |
| Hiles et al. [10] | 1995 | Aortic grafts | Dog | n = 8 | Up to 60 days | Mechanical testing and histology | Stronger than normal artery, thicker | Remodelling with graft resorption |
| Robotin-Johnson et al. [11] | 1998 | Superior vena cava graft | Pig | n = 11 | 90 days | Physical properties and histology | No graft material-related deaths. Patent thrombus free grafts. Anastomotic stricture and aneurysm in 2 of 9 | Endothelialization, neoangiogenesis |
| Pavcnik et al. [12] | 2002 | Bicuspid venous valve | Sheep | n = 12 | Up to 6 months | Venogram, histology | 88% good function without leak, one thrombosis. Thickened SIS membrane | Remodeled collagen, fibroblast infiltration with neoangiogenesis |
| Badylak et al. [8] | 2003 | Myocardial repair | Pigs and dogs |
n = 6 (pigs) n = 4 (dogs) |
Up to 24 weeks | Histology, in vitro contractility assessment (n = 2 dogs) | Scaffold replacement with a mixed cellular infiltrate, including myocytes. 70% contractility of normal | Complete replacement of matrix with connective tissue, cartilage, adipose and myocardium with neoangiogenesis |
| Yavuz et al. [13] | 2006 | High-pressure implantation in the abdominal aorta | Sheep | n = 12 each of SIS, Dacron and ePTFE | Up to 18 weeks | Aortogram, histology | Patent suspended devices without thrombosis or aortic wall contact. | Neointimal formation with endothelialization Dacron > SIS > ePTFE |
| Pavcnik et al. [14] | 2009 | Carotid artery grafts | Sheep | n = 13 | Up to 4 months | Doppler, angiography, histology | 90% patency at 1 week decreasing to 30% at 3–4 months | Thickened graft wall. Variable endothelialisation: partial in medsection and complete distally |
| Boni et al. [15] | 2012 | Pulmonary artery reconstruction | Lambs | n = 6 | Up to 6 months | CT angiography and histology with IHC and electron microscopy | No failures. No stenosis or aneurysm. Endothelialization and smooth muscle infiltration. Patch resorption by 6 months | Neoangiogenesis, c-kit-positive cell infiltration raising the possibility of multipotent cells |
| Fallon et al. [16] | 2012 | Carotid artery repair | Sheep | n = 15 | Up to 6 months | Mechanical testing, angiography, histology | Mild stenosis in ECM implant sites at 30 days, resolving by 90 days | Graft resorption, neoangiogenesis, endothelialization |
| Padalino et al. [17] | 2012 | Vascular patch | Rat |
n = 3 (sham) n = 3 (control) n = 20 (treatment) |
Up to 6 months | Histology with IHC/IF | Intact aortic wall, no aneurysms. Almost complete remodeled graft by 6 months | Complete graft resorption, neoangiogenesis and endothelialisation, the new intima and media layers found to be of donor origin |
| Mewhort et al. [18] | 2014 | Ischaemic heart failure | Rat |
n = 13 (normal) n = 15 (sham) n = 28 (treatment) |
16 weeks | Histology with IHC, echocardiography, invasive hemodynamic assessments | Integration of CorMatrix®, increased FGF expression, enhanced ejection fraction in treatment versus sham (P < 0.001) | Patch integration with host myocardium with patch being free from scaring or inflammatory reactivity |
| Slaughter et al. [19] | 2014 | Ischaemic heart failure | Cow | n = 11 | N/A | N/A | N/A | Feasibility study of injecting particulate CorMatrix® into ischaemic myocardium |
| Soucy et al. [20] | 2014 | Ischaemic heart failure | Calves | n = 12 | 60 days (n = 6) 90 days (n = 6) |
Echocardiography, cell proliferation, regional blood flow | P-ECM and HVAD largest functional and biological gains | N/A |
SIS: small intestinal submucosa; ECM: extracellular matrix; IHC: immunohistochemistry; SVG: saphenous vein graft; CT: computed tomography; ePTFE: polytetrafluoroethylene, Dacron; IF: immunoflurescence; P-ECM: particulate extracellular matrix; HVAD: HeartWare ventricular assist devices; FGF: fibroblast growth factor.
Table 5:
Clinical case reports of porcine SIS-ECM used at intra- and extracardiac sites
| Study | Year | Indication | Site | Follow-up | Outcome measure or method | Result |
|---|---|---|---|---|---|---|
| Gilbert et al. [41] | 2011 | Tri-leaflet pulmonary valved conduit | IC | 5 months | Echocardiogram | No flow gradient and trivial valvular insufficiency |
| Eckhauser et al. [42] | 2013 | Repair of innominate artery | EC | 8 days | MRI | Repair intact on Day 8 |
| Stelly and Stelly [43] | 2013 | Pericardial closure | EC | 5 years | Redo surgery | Neo-pericardium |
| Yeen et al. [44] | 2013 | Anomalous pulmonary vein reconstruction | EC | N/A | Postoperative CT | No reported failure |
| Poulin et al. [45] | 2013 | Atrioventricular continuity reconstruction | IC | N/A | Echocardiogram | Patch dehiscence |
| Deorsola et al. [46] | 2014 | Aortic coarctation repair | EC | 11 months | Serial echocardiograms | Stenosis at 4–5 months |
| DuBose and Azizzadeh [47] | 2014 | Repair of arterio-venous fistula aneurysm | EC | 4 months | Clinical | Patent AVF |
| Cua et al. [48] | 2014 | Tricuspid valve replacement | IC | N/A | Postoperative echocardiogram | Moderate regurgitation |
| Slachman [49] | 2014 | Aortic root repair | IC | 34 months | Autopsy | Death not patch related (myelodysplastic syndrome) |
| Szczeklik et al. [50] | 2014 | Reconstruction of right atrium and superior vena cava | IC | 8 weeks | Echocardiography | Successful repair |
| Wallen and Rao [51] | 2014 | Tricuspid valve repair after endocarditis | IC | 3 months | Echocardiography and clinical | Mild residual TR |
| Yanagawa et al. [52] | 2014 | Myocardial regeneration | IC | 1 year | Echocardiography | Baseline LVF restored |
| Holubec et al. [53] | 2014 | Post-MI free wall rupture repair | IC | 3 months | Echocardiography | Successful repair |
| Bibevski and Scholl [54] | 2015 | Atrioventricular valve | EC | N/A | Echocardiography | Excellent function noted |
SIS: small intestinal submucosa; ECM: extracellular matrix; IC: intracardiac; EC: extracardiac; AVF: arterio-venous fistula; TR: tricuspid valve; MI: myocardial infarction; LVF: left ventricular function.
WHAT ARE THE IDEAL PROPERTIES OF A CARDIOVASCULAR BIOSCAFFOLD?
The concept of ‘inertness’—an absence of antigenicity and toxicity in the grafted material—has traditionally underpinned graft design and development. Indeed, an uncontrolled inflammatory reaction to the implanted graft material might be expected to result in pathological consequences in the form of thrombosis and hyperplasia (in the case of vascular grafts) or calcification, retraction and scarring (in the case of cardiac patches, valves and conduits). However, inertness must be balanced against the need for a scaffold to facilitate controlled (or ‘constructive’ [55]) remodelling, which is a highly complex and tightly regulated process that produces site-appropriate functional tissue [56]. Furthermore, in the case of congenital heart repairs, an ideal scaffold should promote controlled healing and native tissue formation and have the potential to facilitate native tissue growth over time to avoid or minimize the need for repeat surgery; materials such as Dacron do not change size or shape over time and, therefore, further operations are likely to be needed to accommodate patient growth [36]. Given the complex nature of these endogenous and exogenous interactions (see below), the ‘optimal’ biological scaffold has yet to be found. An ideal biological scaffold should, therefore, meet a number of important criteria: (i) resist tissue calcification, thickening, retraction or degradation; (ii) be biocompatible and should not promote intense inflammation, fibrosis or be susceptible to infection; (iii) be pliable and easy to handle, while being strong and durable to resist mechanical failure; (iv) have the potential to undergo remodelling and regeneration, ideally with the capacity for adaptive growth and (v) be able to be manufactured in sufficient quantities of standardized quality to meet the demands of a global marketplace. For a comparison of SIS-ECM and other patch materials, we refer the interested reader to the review by Holubec et al. [7].
EXTRACELLULAR MATRIX AS A BIOLOGICAL SCAFFOLD
The use of extracellular matrix (ECM) as a biological scaffold for tissue repair and regeneration is not new. SIS was first used in its native form as a large vascular autograft in a dog in 1989 [57], and inverted SIS had been used even earlier in the 1960s in early vascular grafting experiments [58]. ECM has been harvested from a variety of organs and repurposed as a biological scaffold, including from the liver [59], pancreas [60] and urinary bladder [61]. SIS-ECM represents the exemplar ECM for tissue engineering, its biochemical and biomechanical properties have been comprehensively characterized, and SIS-ECM has been used in more than 1 million patients for a variety of reconstructions at multiple sites including the skin [62], rotator cuff [63], urinary tract [64] and intestine [65]. Although clinical results have been variable, ECM materials have gained widespread acceptance within the clinical community. As a result, commercial scaffold materials manufactured from a range of ECM materials from different animals are available, including several porcine SIS products (e.g. Surgisis®, Durasis® and Stratasis®; Cook Biotech, Lafayette, IN, USA; Restore®; DuPuy, West Chester, PA, USA).
However, ECM scaffolds were only recently introduced to cardiovascular surgery in spite of the putative benefits of the material. Of the commercially available SIS-ECM products, CorMatrix® (CorMatrix Cardiovascular, Inc., Roswell, GA, USA) has Food and Drug Administration clearance as a device and a European CE mark for pericardial patch repair and reconstruction, cardiac tissue repair, carotid repair and enveloping implantable electronic devices. It is a decellularized four-ply sheet material made from porcine SIS, which theoretically contains the necessary structural proteins (such as collagens), adhesion molecules and matricellular proteins to promote ‘constructive’ remodelling. CorMatrix® is also available in ‘envelope’ form (CorMatrix Cangaroo®) to hold implantable electronic devices in vivo to restrict migration, impede infection and improve comfort for the patient. Although other similar commercial ECM products exist for cardiovascular use [such as Autotissue MatrixP®, Germany (decellularized equine pericardial matrix) and CardioCel® (decellularized bovine pericardium; Admedus, Perth, Australia)], these have only recently been introduced to the marketplace and therefore data on their use are very limited.
PHYSICAL AND BIOLOGICAL CHARACTERISTICS OF EXTRACELLULAR MATRIX
ECM represents a complex 3D structural framework that supports cells to provide biological, physical and mechanical properties that dictate cellular and, ultimately, tissue function [66]. Manufacturers of commercial ECM scaffolds therefore aim to remove the cellular components while retaining the intact ECM meshwork and its biomechanical functions to support host cells. Decellularization may involve a combination of physical, ionic, chemical and enzymatic methods tailored to the tissue of interest [67]. It is known that excessive decellularization can impair the release of endogenous growth factors that promote constructive remodelling [67]. Similarly, excessive chemical crosslinking can alter the profile of peptide release during the remodelling process, thereby hampering it [67]. Commercially available ECM products are available in a variety of forms, most frequently multilaminated sheets but also as powders and injectable gels.
ECM can be considered in both mechanical and functional terms. As a mechanical substrate, ECM is a dynamic structure with topologically distinct areas. For instance, the basement membrane is a specialized collagen-rich ECM structure that forms a physical barrier and primarily supports the mucosal epithelium, and the migration of cells through the ECM requires focal remodelling [68]. As a functional substrate, the main ECM components are collagens, glycoproteins, proteoglycans, mucins, elastic fibres and growth factors [69] which, as well as providing the structural support to the tissue, interact with the cell surface via numerous receptors that mediate intracellular signalling pathways that dictate tissue homeostasis and function. The ECM is also tissue-specific, the more obvious examples being the greater quantity of type II collagen and glycosaminoglycans (GAGs) in articular cartilage to confer high resistance to deformation forces [70] or the type I collagen in tendons to resist tensile loading [71].
With these properties in mind, SIS may be considered an ECM well suited to a broad range of applications. Specifically, 90% of the SIS-ECM is collagen (predominantly type I), with minor amounts of type III, IV, V and VI collagens, GAGs, fibronectin and laminin as well as growth factors [72]. Urinary bladder matrix is similar in composition, but with greater amounts of type III collagen and type VII collagen originating from the endothelial basement membrane [72]. In addition to its chemical composition, SIS-ECM has a collagen fibre alignment suited to the mechanical requirements of cardiovascular tissue engineering: there are two distinct populations of collagen fibres orientated ∼30° from the longitudinal axis of the small intestine (the ‘global preferred fibre alignment’) that confer greater strength and stiffness to SIS-ECM than other sources of ECM such as urinary bladder submucosa [55]. This mechanical advantage is further enhanced by lamination.
The mechanisms by which implanted ECM scaffolds undergo remodelling are imperfectly understood, and the mechanical and physical properties of ECM are insufficient to explain all the observed remodelling effects. One useful framework for understanding the remodelling process is the ‘bioinduction’ model: degradation of the non-native matrix triggers host cell responses that give rise to mature tissue. Bioinduction may occur via several mechanisms: (i) degradation of the ECM scaffold by circulating enzymes and/or early infiltrating cells, particularly M2-type macrophages [73]; (ii) release of growth factors during scaffold degradation; these include vascular endothelial growth factor [74], transforming growth factor beta (TGF-β) [75] and other ‘cryptic’ peptides (such as endostatin and angiostatin; see ref. [55]); (iii) host cell infiltration, including circulating bone marrow-derived cells that sustain long-term tissue remodelling (notably endothelial and mesenchymal progenitor cells; [73]) and (iv) sustained tissue formation with neoangiogenesis. In this way, ECM degradation may initiate a physiological remodelling process that suppresses an inflammatory response and its resulting fibrous (scar) tissue formation, with rapid replacement of the SIS-ECM (e.g. 60% resorption after 1 month, complete resorption after 3 months in a canine model [76]). Since ECM is thought to provide a stem cell niche (i.e. one suitable for the recruitment and differentiation of stem cells), it also provides the ideal environment to sustain and promote multipotent stem cell development and remodelling of complex tissue structures [66]. ECM scaffolds are not completely immunologically inert and monocytes are recruited to the material as part of the bioinductive process; however, these are thought to differentiate into M2-phenotype macrophages that secrete an anti-inflammatory or ‘healing’ cytokine profile (IL-10 and TGF-β), rather than M1-phenotype macrophages that promote an inflammatory cascade and scarring [73, 77].
PRECLINICAL STUDIES OF PORCINE SMALL INTESTINAL SUBMUCOSA AND SMALL INTESTINAL SUBMUCOSA-EXTRACELLULAR MATRIX FOR CARDIOVASCULAR USE
As noted above, SIS has been used in experimental models of cardiovascular surgery since the late 1980s, when autologous SIS was used as both small [78] and large [57] diameter vascular grafts in dogs. Follow-on studies in the early 1990s utilized similar models to examine porcine (xenogeneic) SIS [9, 10]. Over the last decade, porcine SIS and the newer SIS-ECM have been used in a variety of cardiovascular applications in a number of different animals, including pigs [8, 11, 21, 23], sheep [12–14, 24] and cows [22], and for a variety of purposes such as arterial or venous grafting [9–11, 14–17], valve replacement [12, 21, 23–26] and myocardial repair or patching [8, 22].
Although the number of animals used in these studies was often small, these were important studies that together laid the ground for the clinical studies using SIS-ECM. The results of these studies are illustrated in Table 1 (extracardiac use) and Table 2 (intracardiac use). In general, results of porcine SIS used under a range of different experimental models, conditions and purposes provided enough evidence to warrant further clinical testing. In contrast to clinical studies of porcine SIS (see below), the material has been tested more frequently in the extracardiac setting although results at all sites appear to be equivalent. In those studies where functional investigations were carried out to assess patency (e.g. by venography or angiography), thrombosis was the main cause of failure (in refs [12] and [9]). Of all the preclinical studies published, only one study by Pavcnik et al. [14] reported a high failure rate of 70% at 3–4 months in an ovine model of carotid artery grafting due to dilatation, stenosis, dissections and aneurysm formation. It is uncertain why the failure rate was particularly high in this study: the authors suggested that the animal model used, the graft length (10 cm), the maintenance of anticoagulation or surgical technique could all have accounted for the failures.
Table 2:
Experimental/animal studies of porcine SIS and SIS-ECM used at intracardiac sites
| Study | Year | Indication | Animal model | Number | Follow-up | Outcome measure or method | Result(s) | Reported remodelling process |
|---|---|---|---|---|---|---|---|---|
| Matheny et al. [21] | 2000 | Pulmonary valve leaflets | Pig | n = 4 | Up to 111 days | Echocardiography and histology | Normal valve competence | Replacement with fibrous connective tissue, neoangiogenesis, endothelialization |
| Rosen et al. [22] | 2005 | Intracardiac patch | Calves | n = 5 | Up to 6 months | Histology | Macroscopically unremarkable, neointima formation | Thick neointima with collagen and smooth muscle |
| Ruiz et al. [23] | 2005 | Pulmonary valve replacement | Pig | n = 12 | Up to 12 months | Echocardiography and histology | No valve misplacements, embolizations or regurgitation | Absent inflammation, fibroblastic infiltration, endothelialization |
| White et al. [24] | 2005 | Pulmonary valve replacement | Sheep | n = 4 | Immediate postoperative analysis | Echocardiography and histology | Good function | Red blood cell infiltration |
| Fallon et al. [25] | 2014 | Prosthetic tricuspid valve | Sheep | n = 4 | up to 12 months | Echocardiography and histology | Normal gross morphology with coaptation. Structural reorganization, elastin, and GAGs by 5 months | Host cell infiltration with endothelialization |
| Toeg et al. [26] | 2014 | Aortic valve repair (ex vivo porcine aortic roots) | Pig (ex vivo) | CorMatrix®, bovine pericardial and Dacron grafts | N/A | Haemodynamic and pressurization studies | Reduced orifice area in CorMatrix® grafts (P = 0.0001) and largest quantitative profile difference | N/A |
| Zafar et al. [27] | 2015 | Tricuspid valve bioprosthesis | Sheep | n = 8 (n = 4 controls) | Up to 8 months | Echocardiography, ventricular function and histology | One severe regurgitation. Normal ventricular function. Close to normal architecture by 8 months, with separation of collagen, elastin and GAGs layers of the proximal part of the leaflet | Resident mesenchymal cell infiltration and trilaminar ECM organization; no inflammation |
SIS: small intestinal submucosa; ECM: extracellular matrix; GAGs: glycosaminoglycans.
Supporting the expectation that porcine SIS-ECM has low immunogenicity, inflammatory reactions were rarely observed at graft sites, even in the xenogeneic setting. Inflammation was observed in the grafts in two studies: a chronic inflammatory infiltrate in bicuspid venous valves [12], and moderate chronic inflammation in carotid artery grafts [16]. The observation of inflammation in sheep and not other animals does not necessarily mean that the ovine model is unsuitable for preclinical testing because (i) inflammation was absent in several other ovine studies and (ii) in general, the physiological arterial pressures reached in sheep closely mimic those found in humans [16]. Thus, sheep can still be considered suitable models for vascular studies. Furthermore, calcification is accelerated in vascular prostheses in sheep [16], and therefore the absence of calcification in any of the ovine SIS-ECM studies positively supports the notion that this material resists calcification during the remodelling process. Indeed, one very recent study in which tricuspid valve bioprostheses were implanted into lambs for 3 or 8 months reported only one failure (regurgitation) in eight experimental grafts [27]. Furthermore, the explanted valves were grossly normal with microscopic features similar to mature native tricuspid valves and evidence of ‘growth’ of the annular ring, which, as noted above, would be particularly beneficial for the paediatric population.
These animal studies have some limitations including small sample sizes, short follow-up and a lack of standardized functional and histological assessment. However, these preclinical data have the advantage of availability of explant tissue for detailed histopathological analysis, in contrast to most of the reported clinical studies. These results shed light on the remodelling process: the majority of porcine SIS and SIS-ECM explants show at least some degree of matrix repopulation with new cells (fibroblasts and smooth muscle cells), neoangiogenesis and surface endothelializtion. At least one study provides direct evidence that infiltrating cells are of host origin using a genetically engineered rat model [17], and there is indirect evidence that the remodelling process may result from repopulation with pluripotent cells: in one study of myocardial repair in pigs and dogs, the infarcted myocardium was replaced with not just myocardium but also adipose tissue and cartilage [8], whereas another study identified a c-kit-positive population of cells within remodelling ECM, raising the possibility that multipotent progenitors participate in the remodelling process [15]. Of note, this principle of native tissue replacement via the inductive properties of CorMatrix® has recently been exploited in a bovine model of cardiac ischaemia, in which particulate (rather than sheet) CorMatrix® was successfully injected into ischaemic myocardium to restore contractility, with the highest levels of cell proliferation and end-organ perfusion in the group receiving particulate ECM ([19, 20] and Table 1).
CLINICAL STUDIES OF CORMATRIX® IN CARDIOVASCULAR SURGERY: EMERGING CONTROVERSIES
CorMatrix® has been used in clinical cardiovascular surgery since 2010. Summaries of clinical studies published to date are presented in Table 3 (extracardiac use) and Table 4 (intracardiac use) [79]. A similar number of case reports describing the use of CorMatrix® are presented in Table 5.
Table 3:
Clinical studies of porcine SIS-ECM used at extracardiac sites
| Study | Indication | Level of evidence | Patients | Follow-up | Outcome measure or method | Result |
|---|---|---|---|---|---|---|
| Boyd et al. 2010 [28] | Pericardial reconstruction | III |
n = 111 (treatment) n = 111 (control) |
N/A | Postoperative AF | 54% reduction in relative risk (P < 0.001) |
| Quarti et al. 2011 [29] | Vascular repair at different sites or valve reconstruction | IV | n = 26 total | Mean 13.2 months, range (4–25 months) | Echocardiogram | No serious patch-related complications or deaths |
SIS: small intestinal submucosa; ECM: extracellular matrix; AF: atrial fibrillation.
Table 4:
Clinical studies of porcine SIS-ECM used at intracardiac sites
| Study | Indication | Level of evidence | Patients | Follow-up | Outcome measure or method | Results |
|---|---|---|---|---|---|---|
| Witt et al. [30] | Congenital CV reconstructions: septal defects (n = 13), vascular augmentation (n = 26), outflow tract augmentation (n = 7), valve reconstruction (n = 3) | IV | n = 37 in 48 locations | 411 days CorMatrix® in situ: 555 and 23 days, and a biopsy from ASD patch at 336 days |
Retrospective review of procedure, implant location, echocardiogram, reintervention and pathology |
Clinical: 4 deaths not related to the patch. Three reoperations, 2 for patch failure; progressive vascular stenosis (1 RVOT patch and 1 PA patch). Histological: thickening and chronic inflammation (eosinophils) |
| Yanagawa et al. [31] | Pericardial patch repair after post-MI complications (n = 7 aneurysm; n = 3 VSD; n = 1 both) | IV | n = 11 | Mean 207 days (clinical) Mean 176 days (echocardiography) |
Echocardiography | One death and 2 reoperations not related to CorMatrix®. No repair failures |
| Brinster and Patel [32] | Aortic root enlargement | IV | n = 7 | N/A | Postoperative echocardiograms | No reported failures |
| Gerdisch et al. [33] | Tricuspid valve endocarditis repair | IV | n = 18 | 1–18 months | Echocardiogram | No deaths. Four reoperations for: disruption of papillary attachment in n = 3, tricuspid regurgitation an = 1, fungal infection n = 1 |
| Gerdisch et al. [34] | Mitral valve repair | IV | n = 19 | Mean 10.9 months echocardiography | Echocardiogram | Three deaths, not MV-related. Three MV reoperations, 1 for early tear. Repaired valves showed good function and no evidence of calcification. In 1 patient reported incomplete healing of A2–A3. Histology showed regions of vascularized patch at 18 months |
| Sundermann et al. [35] | Endocarditis (mitral valve) | IV | n = 2 | 34 days and 3 months | Echocardiography | Successful repair |
| Zaidi et al. [36] | Valve reconstruction in congenital heart disease (17 mitral and 26 aortic) | III | n = 57 patients, n = 9 available for histology | N/A CorMatrix® in situ: MV: median 64 days (range 5–261). AV: median 63 days (range 49–198) |
Explant histology |
Clinical: 8 of 17 MV reoperations for valve dysfunction, 6 of 8 patch related. Three of 26 post AV repair, 1 of 3 death, 2 of 3 reoperations for failure Histological: 8 of 9 explants; dense chronic inflammatory infiltrates (eosinophils and giant cells). No remodelling or reabsorption of CorMatrix®. In many cases, a thick neointima had formed |
| Rosario-Quinones et al. [37] | Congenital cardiac surgery, various sites | III | n = 25 patients, (n = 6 explants; MV, aortic arch, PV, PA, RVOT and pericardium) | N/A CorMatrix® in situ: range: 9 weeks to 13 months |
Explant histology |
Clinical: all patients had significant haemodynamic lesions at the implantation site. Most reoperations related to patch failure. Histological: intense inflammation (eosinophils, histiocytes, plasma cells, with granulation tissue and fibrosis). In the patient with reconstructed PV, the scaffold was not grossly identified 13 months after implantation |
| Luk et al. [38] | Endocarditis (1 = MV, 2 = AV and MV) | IV | n = 2 both MV | N/A CorMatrix® in situ: 10 and 18 months |
Explant histology |
Clinical: delayed postoperative infection and perforation of MV leaflet Histological: intact patches with no resorption of the material. No cellular infiltrates in the tissue. Connective tissue and endothelial cell deposition on the surface. More thickening was observed at 18 months |
| Woo et al. [39] | Paediatric heart reconstructions | III | n = 532 patients, n = 12 explants from 11 patients (2 mitral, 2 aortic and 8 outflow/septal/conduit patches) | 2.5 years CorMatrix® in situ: mean 518.6 days (range 77–1294) |
Explant histology |
Clinical: 6 cases had clinical evidence of graft failure before surgery. Histological: no evidence of native cells deposition or organized collagenization of CorMatrix. Grade 3 chronic inflammation (giant cells) in 8 of 12 explants and acute inflammation (eosinophil and giant cells) in 3 of 12. Degeneration of material in 9 of 12 but no resorption. Calcification in surrounding tissues in 3 of 12 but not on the CorMatrix® |
| Padalino et al. [40] | Congenital cardiac surgery, various sites | II | n = 103 patients, n = 132 implants (38 valves; 16 septal reconstructions; 71 arterioplasties; 7 other) | Median 23.3 months (range 0.3–55.23) CorMatrix® in situ: median 25.2 months (range: 2.5–34.1) |
(i) Reoperation; (ii) interventional procedure and (iii) functional ECM failure. Explant histology also assessed |
Clinical: short term: no deaths or immediate postoperative complications related to scaffold failure. Mid-term: 6 reoperations and 8 interventional procedures for scaffold failure. Histological: intact patch, mild stiffness, mild inflammation (lymphocytes and giant cells) and no calcification was noted in explants |
SIS: small intestinal submucosa; ECM: extracellular matrix; CV: cardiovascular; ASD: atrio-septal defect; RVOT: right ventricular outflow tract; PA: pulmonary artery; MI: myocardial infarction; VSD: ventriculo-septal defect; MV; mitral valve; AV: aortic valve; PV: pulmonary valve.
This patient also had disruption of papillary attachment.
CorMatrix® has been used in congenital cardiac and vascular surgery [29, 30, 36, 41], pericardial reconstruction [28, 31, 43], valve reconstruction in both adults and children [28, 33, 34, 36, 48, 51], endocarditis [33, 35, 51], acquired vascular defects at different sites [42, 47] and to repair damaged myocardium after infarction [52]: more studies have been conducted in the intracardiac setting (Table 4) than the extracardiac setting (Table 3). The first clinical report of CorMatrix® was as a pericardial substitute [28], which was a retrospective case–control study of 222 patients undergoing pericardial reconstruction after primary isolated coronary artery bypass grafting. The treatment group showed a 54% relative risk reduction of developing postoperative AF although it is uncertain how or why the graft reduced AF and the study was limited by being retrospective, non-randomized and underpowered. Nevertheless, the study provided early evidence that the material might be suitable for cardiac use, prompting greater adoption of the material in a range of cardiovascular applications. As a vascular substitute, one extracardiac study reported a complication of stenosis at the site of coarctation repair treated with a CorMatrix® patch, which was subsequently remedied with balloon angioplasty [46]. However, as noted below, more recent prospective clinical studies have been less than favourable, casting doubt on its use, particularly in the paediatric population.
Extracardiac sites tend to be low mechanical force environments that are likely to facilitate (or at least not hinder) remodelling; results may therefore be expected to be favourable at these sites. However, even at intracardiac sites frequently exposed to high shear forces and mechanical pressures, only minor failures were noted in a limited number of cases in early studies (Table 4). For instance, in a study by Quarti et al. [29], 9 patients underwent surgery using CorMatrix® for valve repair (5 aortic, 2 tricuspid, 1 mitral and 1 pulmonary). There were no serious patch-related complications or deaths, and trivial aortic valve regurgitation was only noted in four AV repairs and mild pulmonary valve regurgitation in one PV repair. However, this study was limited by the fact that follow-up was only up to 25 months (mean 13 months), which is far too short to fully assess the functional success of valve repair. Some uncontrolled, and therefore methodologically limited, studies report isolated major complications: patch dehiscence after atrioventricular continuity reconstruction following massive posterior annulus decalcification and mitral valve replacement for mitral stenosis due to dystrophic calcification [45], progressive vascular stenosis in 2 of 37 paediatric patients with CorMatrix® patch repairs for a variety of congenital defects [30] and one fungally infected tricuspid valve 6 months after repair for infective endocarditis (the other three failures in this study being attributed to the surgical technique rather than material failure) [33].
These early results must be considered with caution. To date, there has been only one level II study [40] and only four studies that can reasonably be classified as level III studies [28, 36, 37, 39], the remainder representing level IV studies that are, for the most part, case reports or small case series (Table 5). The majority of published studies only report immediate or very early postoperative findings although a handful of case reports examine outcomes past a year or more.
The most robust study to date is a very recent prospective multicentre (but non-randomized) clinical study of 103 paediatric and adult patients receiving 132 CorMatrix® implants at a variety of sites: 38 valve repairs, 16 septal reconstructions, 71 arterioplasties and 7 at ‘other’ sites [40]. The surgical experience was regarded as ‘positive’ by the operating surgeons with good handling characteristics although prolonged washing of the material was sometimes associated with delamination. No immediate postoperative events were attributable to the ECM scaffold, but 6 patients required reoperation due to ECM scaffold failure at a median follow-up of 25.2 months (range 2.5–34.1 months): 5 of 38 were valve replacements for failing aortic valve plasty and 1 for a failed mitral valve plasty. Although no calcifications were seen in the explants, there was a mild chronic inflammatory infiltrate in the explant tissue without signs of regeneration. Eight patients required interventional cardiology procedures at CorMatrix® sites, all of which were on the pulmonary arteries. Surgery on the semilunar valves was a predictor of functional failure in multivariate analysis. These are important data that highlight that there is heterogeneity in clinical responses to SIS-ECM use at different sites, and that further work is required to determine optimal indications for SIS-ECM use.
Recent results have raised some doubt on the in vivo biology of CorMatrix® [80]. One level III clinical study on CorMatrix® reports the histological findings from 71 mitral or aortic valvuloplasties using either CorMatrix® or autologous pericardium [36]. Of these, nine CorMatrix® explants subsequently (5–261 days in situ) became available at the time of reoperation for valve replacement or valvuloplasty for systematic histopathological analysis although this may have been itself due to a failure of the material in this setting, particularly since the failures were associated with intense chronic inflammation. Contrary to expectations and the manufacturer's claims that the material does not elicit an inflammatory response, 8 of the 9 cases did exhibit an intense chronic inflammatory infiltrate involving the matrix without significant resorption of the implanted material, with little or no remodelling into a structure resembling native valve tissue. Furthermore, these features were apparent as late as 9 months after implantation. These results have prompted recent histopathological studies that have also reported similar findings [37, 80]: in 6 of 25 paediatric patients undergoing reoperation after CorMatrix® implantation, the explanted specimens exhibited dense, mainly eosinophilic inflammatory tissue infiltrates that were frequently accompanied by granulation tissue and fibrosis [37]. In the second study of 532 patients in total (mean follow-up of 2.5 years), 12 CorMatrix® implants were obtained from 11 paediatric patients from a range of sites (4 valves, 2 mitral and 2 aortic; 8 outflow, septal or conduit patches) [39]. Chronic inflammation was observed in adjacent tissue in 11 of 12 explants and, in addition, acute inflammation was seen in 3 cases and tissue necrosis in 5. Notably, these acute inflammation cases were associated with short in situ duration (only 103 days on average). Although the CorMatrix® was degraded in 9 of 12 cases, it was not totally resorbed in any case and remodelling was not associated with organized collagen. It is clear that some failures are associated with adverse inflammatory responses although whether these tissue reactions are causative remains to be determined.
One very recent report of the histology of CorMatrix® explants from 2 adult patients describes a multilaminar neo-valvular structure, albeit with one of the layers being composed of non-resorbed biomaterial. However, there was little inflammation except at the anastomosis [38]. Two other case reports describe the histological appearances of a clinical CorMatrix® explants [43, 49]: one mentions the presence of mild chronic inflammation in the explant [49] and both report that calcifications were present within the material examined, a feature reported not to occur with CorMatrix® grafts in contrast to autologous pericardium. Taken together, these studies suggest that CorMatrix® may elicit tissue reactions in human subjects after implantation; Rosario-Quinones et al. [37] attributed the intense eosinophilia seen in their explants to a hypersensitivity reaction, perhaps to α-gal epitopes present in the porcine, but not in the human, intestine. As noted in this review, it is also noteworthy that of the preclinical studies (listed in Tables 1 and 2), only xenogeneic implants elicited measurable inflammatory responses to porcine SIS and SIS-ECM.
In summary, there are few reports of complications when CorMatrix® is used in the low pressure, usually extracardiac environment (i.e. veins), but when used at higher pressure intracardiac sites such as the aortic valve or in semilunar valves, complications are more likely to occur. However, given recent data suggesting that CorMatrix® may elicit significant inflammatory reactions, there is a need to conduct more histopathological evaluations of explant material in order to shed light on this controversy. Further prospective randomized clinical trials to compare patch materials at different sites are needed, and pathological evaluation of explant material in cases of reoperation or failure should be undertaken to better understand how CorMatrix® really behaves in the complex milieu of the human cardiovascular system.
CONCLUSIONS
Porcine SIS-ECM has been used for over 20 years in a range of cardiovascular applications. The preclinical and clinical results support the notion that CorMatrix® may possess many features of an ‘ideal’ biological scaffold: its intrinsic biological properties render it strong and durable; it has been used in a wide range of clinical applications at different sites and it is easy to manipulate during surgery; the clinical data that are favourable (by no means all) suggest that it does not undergo undue calcification, thickening or retraction; and it has successfully been used in the paediatric population, albeit with the caveat that the explanted tissue did not have a native three-layer structure and inflammation was present.
Although some results have been positive, significant uncertainties still remain—not least from recent prospective data—about whether its clinical performance really meets the expectations set by theory and in vitro and in vivo preclinical studies. This is mainly due to a lack of large-scale clinical studies, which has been exacerbated by poor systematic reporting of functional and pathological findings in both human and animal studies. Uncertainties also remain about the remodelling process, particularly in the clinical setting and with regard to implant resorption and immunogenicity. This doubt about clinical efficacy raises questions about whether larger clinical studies in the paediatric population are ethically justifiable, given that the reoperation rate for CorMatrix® failure is up to, or even exceeds, 10% in this population in some studies.
ACKNOWLEDGEMENTS
The authors thank Pascale Segers, our scientific secretary, for her support with bibliography, reference management and formatting.
Conflict of interest: none declared.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Guo Y, Ziegler KR, Model LS, Eghbalieh SD, Brenes RA et al. Current usage and future directions for the bovine pericardial patch. Ann Vasc Surg 2011;25:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Heever JJ, Neethling WM, Smit FE, Litthauer D, Joubert G. The effect of different treatment modalities on the calcification potential and cross-linking stability of bovine pericardium. Cell Tissue Bank 2013;14:53–63. [DOI] [PubMed] [Google Scholar]
- 4.Talwar S, Mohapatra R, Saxena A, Singh R, Kumar AS. Aortic homograft: a suitable substitute for aortic valve replacement. Ann Thorac Surg 2005;80:832–8. [DOI] [PubMed] [Google Scholar]
- 5.Vaideeswar P, Mishra P, Nimbalkar M. Infective endocarditis of the Dacron patch—a report of 13 cases at autopsy. Cardiovasc Pathol 2011;20:e169–75. [DOI] [PubMed] [Google Scholar]
- 6.Henaine R, Roubertie F, Vergnat M, Ninet J. Valve replacement in children: a challenge for a whole life. Arch Cardiovasc Dis 2012;105:517–28. [DOI] [PubMed] [Google Scholar]
- 7.Holubec T, Caliskan E, Sundermann SH, Starck CT, Plass A, Bettex D et al. The use of extracellular matrix patches in cardiac surgery. J Card Surg 2015;30:145–8. [DOI] [PubMed] [Google Scholar]
- 8.Badylak S, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. Heart Surg Forum 2003;6:E20–6. [DOI] [PubMed] [Google Scholar]
- 9.Sandusky GE Jr, Badylak SF, Morff RJ, Johnson WD, Lantz G. Histologic findings after in vivo placement of small intestine submucosal vascular grafts and saphenous vein grafts in the carotid artery in dogs. Am J Pathol 1992;140:317–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Hiles MC, Badylak SF, Lantz GC, Kokini K, Geddes LA, Morff RJ. Mechanical properties of xenogeneic small-intestinal submucosa when used as an aortic graft in the dog. J Biomed Mater Res 1995;29:883–91. [DOI] [PubMed] [Google Scholar]
- 11.Robotin-Johnson MC, Swanson PE, Johnson DC, Schuessler RB, Cox JL. An experimental model of small intestinal submucosa as a growing vascular graft. J Thorac Cardiovasc Surg 1998;116:805–11. [DOI] [PubMed] [Google Scholar]
- 12.Pavcnik D, Uchida BT, Timmermans HA, Corless CL, O'Hara M, Toyota N et al. Percutaneous bioprosthetic venous valve: a long-term study in sheep. J Vasc Surg 2002;35:598–602. [DOI] [PubMed] [Google Scholar]
- 13.Yavuz K, Geyik S, Pavcnik D, Uchida BT, Corless CL, Hartley DE et al. Comparison of the endothelialization of small intestinal submucosa, Dacron, and expanded polytetrafluoroethylene suspended in the thoracoabdominal aorta in sheep. J Vasc Interv Radiol 2006;17:873–82. [DOI] [PubMed] [Google Scholar]
- 14.Pavcnik D, Obermiller J, Uchida BT, Van Alstine WG, Edwards JM, Landry GJ et al. Angiographic evaluation of carotid artery grafting with prefabricated small-diameter, small-intestinal submucosa grafts in sheep. Cardiovasc Intervent Radiol 2009;32:106–13. [DOI] [PubMed] [Google Scholar]
- 15.Boni L, Chalajour F, Sasaki T, Snyder RL, Boyd WD, Riemer RK et al. Reconstruction of pulmonary artery with porcine small intestinal submucosa in a lamb surgical model: viability and growth potential. J Thorac Cardiovasc Surg 2012;144:963–9. [DOI] [PubMed] [Google Scholar]
- 16.Fallon A, Goodchild T, Wang R, Matheny RG. Remodeling of extracellular matrix patch used for carotid artery repair. J Surg Res 2012;175:e25–34. [DOI] [PubMed] [Google Scholar]
- 17.Padalino MA, Castellani C, Dedja A, Fedrigo M, Vida VL, Thiene G et al. Extracellular matrix graft for vascular reconstructive surgery: evidence of autologous regeneration of the neoaorta in a murine model. Eur J Cardiothorac Surg 2012;42:e128–35. [DOI] [PubMed] [Google Scholar]
- 18.Mewhort HE, Turnbull JD, Meijndert HC, Ngu JM, Fedak PW. Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J Thorac Cardiovasc Surg 2014;147:1650–9. [DOI] [PubMed] [Google Scholar]
- 19.Slaughter MS, Soucy KG, Matheny RG, Lewis BC, Hennick MF, Choi Y et al. Development of an extracellular matrix delivery system for effective intramyocardial injection in ischemic tissue. ASAIO J 2014;60:730–6. [DOI] [PubMed] [Google Scholar]
- 20.Soucy KG, Smith EF, Monreal G, Rokosh G, Keller BB, Yuan F et al. Feasibility study of particulate extracellular matrix (P-ECM) and left ventricular assist device (HVAD) therapy in chronic ischemic heart failure bovine model. ASAIO J 2015;61:161–9. [DOI] [PubMed] [Google Scholar]
- 21.Matheny RG, Hutchison ML, Dryden PE, Hiles MD, Shaar CJ. Porcine small intestine submucosa as a pulmonary valve leaflet substitute. J Heart Valve Dis 2000;9:769–74. [PubMed] [Google Scholar]
- 22.Rosen M, Roselli EE, Faber C, Ratliff NB, Ponsky JL, Smedira NG. Small intestinal submucosa intracardiac patch: an experimental study. Surg Innov 2005;12:227–31. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz CE, Iemura M, Medie S, Varga P, Van Alstine WG, Mack S et al. Transcatheter placement of a low-profile biodegradable pulmonary valve made of small intestinal submucosa: a long-term study in a swine model. J Thorac Cardiovasc Surg 2005;130:477–84. [DOI] [PubMed] [Google Scholar]
- 24.White JK, Agnihotri AK, Titus JS, Torchiana DF. A stentless trileaflet valve from a sheet of decellularized porcine small intestinal submucosa. Ann Thorac Surg 2005;80:704–7. [DOI] [PubMed] [Google Scholar]
- 25.Fallon AM, Goodchild TT, Cox JL, Matheny RG. In vivo remodeling potential of a novel bioprosthetic tricuspid valve in an ovine model. J Thorac Cardiovasc Surg 2014;148:333–40. [DOI] [PubMed] [Google Scholar]
- 26.Toeg HD, Abessi O, Al-Atassi T, de Kerchove L, El Khoury G, Labrosse M et al. Finding the ideal biomaterial for aortic valve repair with ex vivo porcine left heart simulator and finite element modeling. J Thorac Cardiovasc Surg 2014;148:1739–45. [DOI] [PubMed] [Google Scholar]
- 27.Zafar F, Hinton RB, Moore RA, Baker RS, Bryant R III, Narmoneva DA et al. Physiological growth, remodeling potential, and preserved function of a novel bioprosthetic tricuspid valve: tubular bioprosthesis made of small intestinal submucosa-derived extracellular matrix. J Am Coll Cardiol 2015;66:877–88. [DOI] [PubMed] [Google Scholar]
- 28.Boyd WD, Johnson WE III, Sultan PK, Deering TF, Matheny RG. Pericardial reconstruction using an extracellular matrix implant correlates with reduced risk of postoperative atrial fibrillation in coronary artery bypass surgery patients. Heart Surg Forum 2010;13:E311–6. [DOI] [PubMed] [Google Scholar]
- 29.Quarti A, Nardone S, Colaneri M, Santoro G, Pozzi M. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interact CardioVasc Thorac Surg 2011;13:569–72. [DOI] [PubMed] [Google Scholar]
- 30.Witt RG, Raff G, Van Gundy J, Rodgers-Ohlau M, Si MS. Short-term experience of porcine small intestinal submucosa patches in paediatric cardiovascular surgery. Eur J Cardiothorac Surg 2013;44:72–6. [DOI] [PubMed] [Google Scholar]
- 31.Yanagawa B, Rao V, Yau TM, Cusimano RJ. Initial experience with intraventricular repair using CorMatrix extracellular matrix. Innovations (Phila) 2013;8:348–52. [DOI] [PubMed] [Google Scholar]
- 32.Brinster DR, Patel JA. The use of CorMatrix extracellular matrix for aortic root enlargement. J Cardiothorac Surg 2014;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerdisch MW, Boyd WD, Harlan JL, Richardson JB Jr, Flack JE III, Palafox BA et al. Early experience treating tricuspid valve endocarditis with a novel extracellular matrix cylinder reconstruction. J Thorac Cardiovasc Surg 2014;148:3042–8. [DOI] [PubMed] [Google Scholar]
- 34.Gerdisch MW, Shea RJ, Barron MD. Clinical experience with CorMatrix extracellular matrix in the surgical treatment of mitral valve disease. J Thorac Cardiovasc Surg 2014;148:1370–8. [DOI] [PubMed] [Google Scholar]
- 35.Sundermann SH, Rodriguez Cetina BH, Emmert MY, Falk V. Use of extracellular matrix materials in patients with endocarditis. Thorac Cardiovasc Surg 2014;62:76–9. [DOI] [PubMed] [Google Scholar]
- 36.Zaidi AH, Nathan M, Emani S, Baird C, del Nido PJ, Gauvreau K et al. Preliminary experience with porcine intestinal submucosa (CorMatrix) for valve reconstruction in congenital heart disease: histologic evaluation of explanted valves. J Thorac Cardiovasc Surg 2014;148:2216-4, 2225e1. [DOI] [PubMed] [Google Scholar]
- 37.Rosario-Quinones F, Magid MS, Yau J, Pawale A, Nguyen K. Tissue reaction to porcine intestinal submucosa (CorMatrix) implants in pediatric cardiac patients: a single-center experience. Ann Thorac Surg 2015;99:1373–7. [DOI] [PubMed] [Google Scholar]
- 38.Luk A, Rao V, Cusimano RJ, David TE, Butany J. CorMatrix extracellular matrix used for valve repair in the adult: is there de novo valvular tissue seen? Ann Thorac Surg 2015;99:2205–7. [DOI] [PubMed] [Google Scholar]
- 39.Woo JS, Fishbein MC, Reemtsen B. Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc Pathol 2016;25:12–17. [DOI] [PubMed] [Google Scholar]
- 40.Padalino MA, Quarti A, Angeli E, Frigo AC, Vida VL, Pozzi M et al. Early and mid-term clinical experience with extracellular matrix scaffold for congenital cardiac and vascular reconstructive surgery: a multicentric Italian study. Interact CardioVasc Thorac Surg 2015;21:40–9. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert CL, Gnanapragasam J, Benhaggen R, Novick WM. Novel use of extracellular matrix graft for creation of pulmonary valved conduit. World J Pediatr Congenit Heart Surg 2011;2:495–501. [DOI] [PubMed] [Google Scholar]
- 42.Eckhauser AW, Hannon D, Molitor M, Scaife E, Gruber PJ. Repair of traumatic aortoinnominate disruption using CorMatrix. Ann Thorac Surg 2013;95:e99–e101. [DOI] [PubMed] [Google Scholar]
- 43.Stelly M, Stelly TC. Histology of CorMatrix bioscaffold 5 years after pericardial closure. Ann Thorac Surg 2013;96:e127–9. [DOI] [PubMed] [Google Scholar]
- 44.Yeen WC, Faber C, Caldeira C, Nallamshetty L, Haddad T, Rolfe M. Reconstruction of pulmonary venous conduit with CorMatrix in lung transplant. Asian Cardiovasc Thorac Ann 2013;21:360–2. [DOI] [PubMed] [Google Scholar]
- 45.Poulin F, Horlick EM, David T, Woo A, Thavendiranathan P. 3-Dimensional transesophageal echocardiography-guided closure of a Gerbode shunt due to CorMatrix patch dehiscence. J Am Coll Cardiol 2013;62:e5. [DOI] [PubMed] [Google Scholar]
- 46.Deorsola L, Pace NC, Abbruzzese PA. Repair of an unusual aortic coarctation using an extracellular matrix patch. Ann Thorac Surg 2014;97:1059–61. [DOI] [PubMed] [Google Scholar]
- 47.DuBose JJ, Azizzadeh A. Utilization of a tubularized CorMatrix extracellular matrix for repair of an arteriovenous fistula aneurysm. Ann Vasc Surg 2015;29:366.e1–4. [DOI] [PubMed] [Google Scholar]
- 48.Cua CL, Kollins K, McConnell PI. Echocardiographic analysis of an extracellular matrix tricuspid valve. Echocardiography 2014;31:E264–6. [DOI] [PubMed] [Google Scholar]
- 49.Slachman FN. Constructive remodeling of CorMatrix extracellular matrix after aortic root repair in a 90-year-old woman. Ann Thorac Surg 2014;97:e129–31. [DOI] [PubMed] [Google Scholar]
- 50.Szczeklik M, Gupta P, Amersey R, Lall KS. Reconstruction of the right atrium and superior vena cava with extracellular matrix. J Card Surg 2015;30:351–4. [DOI] [PubMed] [Google Scholar]
- 51.Wallen J, Rao V. Extensive tricuspid valve repair after endocarditis using CorMatrix extracellular matrix. Ann Thorac Surg 2014;97:1048–50. [DOI] [PubMed] [Google Scholar]
- 52.Yanagawa B, Rao V, Yau TM, Cusimano RJ. Potential myocardial regeneration with CorMatrix ECM: a case report. J Thorac Cardiovasc Surg 2014;147:e41–3. [DOI] [PubMed] [Google Scholar]
- 53.Holubec T, Caliskan E, Bettex D, Maisano F. Repair of post-infarction left ventricular free wall rupture using an extracellular matrix patch. Eur J Cardiothorac Surg 2015;48:800–3. [DOI] [PubMed] [Google Scholar]
- 54.Bibevski S, Scholl FG. Feasibility and early effectiveness of a custom, hand-made systemic atrioventricular valve using porcine extracellular matrix (CorMatrix) in a 4-month-old infant. Ann Thorac Surg 2015;99:710–2. [DOI] [PubMed] [Google Scholar]
- 55.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials 2007;28:3587–93. [DOI] [PubMed] [Google Scholar]
- 56.Badylak SF, Brown BN, Gilbert TW, Daly KA, Huber A, Turner NJ. Biologic scaffolds for constructive tissue remodeling. Biomaterials 2011;32:316–9. [DOI] [PubMed] [Google Scholar]
- 57.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 1989;47:74–80. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto T, Holmes RH, Burdick CO, Metzger JF, Heisterkamp CA III, O'Connell TJ Jr. A study of inverted intestinal graft in the major veins. Angiology 1966;17:842–50. [DOI] [PubMed] [Google Scholar]
- 59.Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood 2003;101:2973–82. [DOI] [PubMed] [Google Scholar]
- 60.Meyer T, Chodnewska I, Czub S, Hamelmann W, Beutner U, Otto C et al. Extracellular matrix proteins in the porcine pancreas: a structural analysis for directed pancreatic islet isolation. Transplant Proc 1998;30:354. [DOI] [PubMed] [Google Scholar]
- 61.Dahms SE, Piechota HJ, Dahiya R, Lue TF, Tanagho EA. Composition and biomechanical properties of the bladder acellular matrix graft: comparative analysis in rat, pig and human. Br J Urol 1998;82:411–9. [DOI] [PubMed] [Google Scholar]
- 62.Macleod TM, Sarathchandra P, Williams G, Sanders R, Green CJ. Evaluation of a porcine origin acellular dermal matrix and small intestinal submucosa as dermal replacements in preventing secondary skin graft contraction. Burns 2004;30:431–7. [DOI] [PubMed] [Google Scholar]
- 63.Phipatanakul WP, Petersen SA. Porcine small intestine submucosa xenograft augmentation in repair of massive rotator cuff tears. Am J Orthop (Belle Mead NJ) 2009;38:572–5. [PubMed] [Google Scholar]
- 64.Alpert SA, Cheng EY, Kaplan WE, Snodgrass WT, Wilcox DT, Kropp BP. Bladder neck fistula after the complete primary repair of exstrophy: a multi-institutional experience. J Urol 2005;174:1687–9. [DOI] [PubMed] [Google Scholar]
- 65.Sardeli C, Axelsen SM, Bek KM. Use of porcine small intestinal submucosa in the surgical treatment of recurrent rectocele in a patient with Ehlers-Danlos syndrome type III. Int Urogynecol J Pelvic Floor Dysfunct 2005;16:504–5. [DOI] [PubMed] [Google Scholar]
- 66.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res 2014;163:268–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011;32:3233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng 2006;12:519–26. [DOI] [PubMed] [Google Scholar]
- 69.Hynes RO, Naba A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 2012;4:a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leyh M, Seitz A, Durselen L, Springorum HR, Angele P, Ignatius A et al. Osteoarthritic cartilage explants affect extracellular matrix production and composition in cocultured bone marrow-derived mesenchymal stem cells and articular chondrocytes. Stem Cell Res Ther 2014;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol 2007;88:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 2009;5:1–13. [DOI] [PubMed] [Google Scholar]
- 73.Piterina AV, Cloonan AJ, Meaney CL, Davis LM, Callanan A, Walsh MT et al. ECM-based materials in cardiovascular applications: inherent healing potential and augmentation of native regenerative processes. Int J Mol Sci 2009;10:4375–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium 2001;8:11–24. [DOI] [PubMed] [Google Scholar]
- 75.McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A 2003;67:637–40. [DOI] [PubMed] [Google Scholar]
- 76.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am 2007;89:621–30. [DOI] [PubMed] [Google Scholar]
- 77.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol 2008;20:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lantz GC, Badylak SF, Coffey AC, Geddes LA, Blevins WE. Small intestinal submucosa as a small-diameter arterial graft in the dog. J Invest Surg 1990;3:217–27. [DOI] [PubMed] [Google Scholar]
- 79.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wells WJ. Responsible innovation. J Thorac Cardiovasc Surg 2014;148:2225–6. [DOI] [PubMed] [Google Scholar]