Abstract
OBJECTIVES
Surgery for prosthetic valve endocarditis (PVE) is associated with significant morbidity and mortality as well as with increased resource utilization and costs. For risk and cost reduction, an understanding of contributing factors and interrelations is essential.
METHODS
Out of 1080 heart valve procedures performed between January 2010 and December 2012, 41 patients underwent surgery for PVE. Complete economic data were available for 30 of them (study cohort). The patients' mean age was 64 ± 12 years (range 37–79 years), and 73% were men. The clinical course was reviewed and morbidity, mortality and costs as well as associations between them were analysed. The cost matrix for each individual patient was obtained from the Institute for the Hospital Remuneration System (InEK GmbH, Germany). The median follow-up was 2.6 years [interquartile range (IQR) 3.7 years; 100% complete].
RESULTS
Preoperative status was critical (EuroSCORE II >20%) in 43% of patients. Staphylococci were the most common infecting micro-organisms (27%). The operative mortality rate (≤30 days) was 17%. At 1 year, the overall survival rate was 71 ± 9%. At least one disease- or surgery-related complication affected 21 patients (early morbidity 70%), >1 complication affected 12 patients (40%). There was neither a recurrence of endocarditis, nor was a reoperation required. The mean total hospital costs were 42.6 ± 37.4 Thousand Euro (T€), median 25.7 T€, IQR 28.4 T€ and >100 T€ in 10% of cases. Intensive care unit/intermediate care (ICU/IMC) and operation accounted for 40.4 ± 18.6 and 25.7 ± 12.1% of costs, respectively. There was a significant correlation (Pearson's sample correlation coefficient) between total costs and duration of hospital stay (r = 0.83, P < 0.001) and between ICU/IMC costs and duration of ICU/IMC stay (r = 0.97, P < 0.001). The median daily hospital costs were 1.8 T€/day, but >2.4 T€/day in 25% of patients (upper quartile). The following pattern of associations was identified (P < 0.05). Early mortality was related to preoperative morbidity and postoperative renal failure. Early morbidity was associated with preoperative morbidity and urgency. Total costs were mainly explained by preoperative morbidity, postoperative morbidity and urgency. High EuroSCORE II, complex surgery, need for mechanical circulatory support as well as postoperative mortality and morbidity increased daily costs.
CONCLUSIONS
The timely diagnosis and treatment of these patients must be a priority, as preoperative morbidity is the major contributor towards mortality, morbidity and costs after surgery for PVE.
Keywords: Aortic valve replacement, Prosthetic valve endocarditis, Reoperation, Mortality, Morbidity, Costs
INTRODUCTION
Prosthetic valve endocarditis (PVE), the most severe form of infective endocarditis (IE), accounts for 10–30% of all cases of IE and occurs in 1–6% of patients with valve prostheses [1–6]. Surgical treatment of PVE, which is frequently required, is not only often extremely challenging, but also associated with significant morbidity and mortality [1–10]. Furthermore, it was demonstrated that surgery for native and prosthetic valve IE requires substantial health care resources with significant economic impact [11–13]. For reduction of risks and costs, an understanding of contributing factors and interrelations is essential. Therefore, the objective of this study was to investigate morbidity, mortality and costs after surgery for PVE primarily to identify associations between them.
PATIENTS AND METHODS
Patients
Out of 1080 isolated and combined heart valve procedures performed at the Department of Cardiovascular Surgery at Charité Campus Mitte between January 2010 and December 2012, 41 patients underwent surgery for PVE. Economic data could be obtained from the Institute for the Hospital Remuneration System (InEK GmbH, Siegburg, Germany) for 30 of them, representing the study cohort. Retrospective individual assignation of costs failed in 11 patients. There was no evidence for differences of these patients from the study cohort regarding age, EuroSCORE II, 30-day mortality, 30-day morbidity and hospital stay. The patient's mean age was 64 ± 12 years (range 37–79 years), and 73% were men. With approval from our institutional Ethics Committee (EA1/032/13), we performed a retrospective review of their clinical course and analysed morbidity, mortality and costs as well as interrelated factors.
Definitions
Diagnosis of PVE was based on clinical findings (fever, inflammatory syndromes), laboratory testing (blood cultures, leucocytosis, levels of C-reactive protein and procalcitonin), results of transthoracic/transoesophageal echocardiography and intraoperative findings [6, 14]. According to Guidelines of the European Society of Cardiology (ESC), PVE occurring within 1 year of primary valve surgery was classified as early and beyond 1 year as late [6]. Culture-negative endocarditis was present when no micro-organism could be identified, neither in serial blood cultures, nor in cultures from the explanted material despite the clinical presence of characteristic signs for IE as vegetations, periprosthetic destruction or pus. For assessment of perioperative risk, logistic EuroSCORE II was calculated [15]. PVE predispositions were classified following the criteria proposed by Grinda et al. [16]. Endocarditis was considered locally uncontrolled, when the infectious pathology extended beyond the prosthetic valve (i.e. paravalvular dehiscence, destruction and/or purulent deformation of adjacent tissue, periprosthetic abscesses and fistulas into a cardiac chamber or the pericardium). Concomitant procedures were all surgical procedures performed to correct associated cardiac diseases. Operations were classified as emergency if surgery was performed within 48 h and urgent if performed within 5 days after definite diagnosis, clearly indicating surgical treatment. All other procedures were classified as elective.
Surgery and postoperative treatment
All operations were performed through a median sternotomy, using an oscillating saw. Cardiopulmonary bypass, installed via cannulation of the distal ascending aorta, the aortic arch or femoral artery and the right atrium or femoral vein, was used with systemic normothermia or mild hypothermia (32°C) if a patent mammary artery bypass was present. Myocardial protection was achieved with intermittent antegrade blood cardioplegia. Previously implanted prostheses were removed in total and abscesses and fistulas were thoroughly debrided. The remaining tissue was disinfected using povidone–iodine solution. In the presence of large abscess cavities, fistulas or tissue defects, a pericardial (autologous, bovine or equine) patch repair was performed. The choice for the new prosthesis was at the discretion of the surgeon. Concomitant procedures, if needed, were performed according to standard techniques. Infected intravascular catheters were removed before surgery.
All patients underwent intravenous antibiotic/antimycotic treatment for at least 6 weeks postoperatively. Antibiotic regimen was directed by microbiological findings and based on guidelines (ESC Guidelines). In the case of culture-negative PVE, an empirical, broad range, antibiotic treatment, usually consisting of vancomycin, rifampicin and gentamycin, was initiated.
Outcomes
Any death and postoperative complication occurring within 30 days after surgery (30-day mortality and 30-day morbidity) were regarded as early outcome. Long-term outcomes were assessed by death, recurrent endocarditis and reoperation occurring after 30 days.
Hospital costs
Cost matrix for each individual patient was obtained from the Institute for the Hospital Remuneration System (InEK GmbH). Since the Charité is one of the hospitals providing source data to InEK, real costs of each individual case were available. Moreover, a breakdown of overall hospital costs into costs of intensive care unit (ICU) or intermediate care (IMC) ward, general ward, operation, anaesthesia, dialysis, laboratory, diagnostics and miscellaneous costs was obtained as well. Time-related costs, in particular daily hospital costs, daily ICU/IMC costs and operation costs per minute, were calculated in relation to the corresponding time span.
Follow-up
Follow-up was obtained by registry office data, mail questionnaire and/or telephone interview. Complications were confirmed by contact with the patient's cardiologist or family physician. In case of rehospitalizations, copies of the medical reports were used.
Statistical analysis
Categorical variables are reported as absolute and relative frequencies. For continuous data, means and standard deviations and/or medians with the lower and upper quartile and inter-quartile ranges were calculated, respectively. Boxplots were used for visualization of data distribution where appropriate. The significance of the association between categorical data was examined by Fisher's exact test. Continuous variables were compared between two groups using the Mann–Whitney U-test. Correlation between continuous variables was tested according to Pearson's sample correlation statistic. Scatterplots were used to depict the relation between continuous variables. Survival was analysed using the Kaplan–Meier estimator. A P-value <0.05 was considered to be statistically significant. All the statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Preoperative status, microbiological findings and indications for surgery
Baseline characteristics are listed in Table 1. Extracardiac infection was present in 12 patients (40%) and one or more predispositions for endocarditis were found in 17 patients (57%). In total, there was 1 patient with a history but no evidence of current intravenous drug abuse. Owing to the presence of shock (two septic, one cardiogenic) and acute renal failure as well as the need for ventilatory and pharmacological circulatory support, 33% of patients were in a critical preoperative state and 43% of patients were at high risk for early death (EuroSCORE II >20%). Staphylococcus species were the most common causative pathogens (n = 8, 27%), followed by Streptococcus (n = 4, 13%) and Enterococcus species (n = 3, 10%). Problematic micro-organisms (Staphylococcus species, gram-negative bacteria, multiple and/or drug-resistant germs) affected 11 patients (37%), while 12 cases (40%) were culture-negative. Locally uncontrolled infection (n = 21, 70%) and vegetations (n = 19, 63%) were the most common indications for surgery, whereas heart failure due to severe prosthetic valve dysfunction and/or fistulas was present in 16 patients (53%). Twelve patients (40%) presented with more than one indication for surgery.
Table 1:
Baseline characteristics
| Parameter | n (%) | Mean ± SD |
|---|---|---|
| Age (years) | 64 ± 12 | |
| Age >70 years | 11 (37) | |
| Male gender | 22 (73) | |
| BSA (m2) | 2.0 ± 0.2 | |
| Early PVE | 16 (53) | |
| Late PVE | 14 (47) | |
| EuroSCORE II (%) | 18.7 ± 13.7 | |
| EuroSCORE II >20% | 13 (43) | |
| Heart failure NYHA class III–IV | 19 (63) | |
| LVEF | 0.52 ± 0.12 | |
| LVEF <0.40 | 4 (13) | |
| Critical preoperative state | 10 (33) | |
| Shock | 3 (10) | |
| Preoperative ventilatory support | 4 (13) | |
| Preoperative circulatory support (catecholamines) | 5 (17) | |
| Preoperative acute renal failure | 9 (30) | |
| Recurrent thromboembolic events | 6 (20) | |
| Preoperative neurological deficits | 2 (7) | |
| Cerebrovascular disease | 2 (7) | |
| Peripheral arterial disease | 4 (13) | |
| Pulmonary hypertension | 4 (13) | |
| Chronic renal dysfunction | 11 (37) | |
| Diabetes | 7 (23) | |
| COPD | 3 (10) | |
| Arterial hypertension | 13 (43) |
SD: standard deviation; BSA: body surface area; PVE: prosthetic valve endocarditis; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease.
Operation and perioperative course
Previous valvular surgery comprised two mitral valve repairs as well as 21 aortic, 1 mitral and 6 double valve replacements including 25 bioprostheses, 7 mechanical valves and 1 Ross procedure. The mean time from primary valve surgery to surgery for PVE was 3 ± 5 years (median 0.9 years, lower quartile 0.4 years, upper quartile 2.6 years). For 21 patients (70%), it was the first redo surgery, whereas 8 (27%) and 1 (3%) patients underwent their third and fourth cardiac operation, respectively. Detailed information of the recent procedures is given in Table 2. Only 27% were elective. In the majority of patients (91%), bioprostheses were used, in the aortic position predominantly stentless valves (90%). Five patients underwent aortic root reconstruction or replacement. In 6 cases, for reconstruction of perivalvular tissue defects pericardial patch repair was performed.
Table 2:
Operative data
| Parameter | n (%) | Mean ± SD |
|---|---|---|
| Operative priority | ||
| Emergency | 4 (13) | |
| Urgent | 18 (60) | |
| Elective | 8 (27) | |
| Procedural figures | ||
| Operation time (min) | 280 ± 88 | |
| CPB time (min) | 164 ± 75 | |
| ACC time (min) | 118 ± 55 | |
| Surgical procedures | ||
| Aortic valve surgery | 21 (70) | |
| Mitral valve surgery | 5 (17) | |
| Double valve surgery | 3 (10) | |
| Triple valve surgery | 1 (3) | |
| Mechanical prostheses | 3 (9) | |
| Biological prostheses | 32 (91) | |
| Pericardial patch repair | 6 (20) | |
| Concomitant procedures | 3 (1) | |
| Mitral valve repair | 2 (7) | |
| CABG | 1 (3) | |
| Mechanical circulatory support | 3 (10) | |
| IABP | 1 (3) | |
| ECMO | 3 (10) | |
| Complex surgerya | 17 (57) | |
SD: standard deviation; CPB time: cardiopulmonary bypass time; ACC time: aortic cross-clamp time; CABG: coronary artery bypass grafting; IABP: intra-aortic balloon pump; ECMO: extracorporeal membrane oxygenation.
Definition of complex surgery: aortic root reconstruction/replacement (n = 5), surgery on more than one valve (n = 6), concomitant procedures (n = 3) and/or mechanical circulatory support (n = 3).
The median duration of postoperative mechanical ventilation was 2 days (lower quartile 1 day, upper quartile 4). The median ICU and hospital stay were 7.5 days (lower quartile 5 days, upper quartile 17 days) and 16 days (lower quartile 9.8 days, upper quartile 34.5 days), respectively. With respect to mechanical ventilation of 3 or more days and ICU stay of 7 or more days, 8 (27%) and 13 (43%) patients required increased intensive care resources, respectively.
Mortality and morbidity
Within 30 days after surgery, 5 patients died (a 30-day mortality rate of 17%). Causes of death are given in Table 3. During follow-up (median 2.6 years, lower quartile 0.2 years, upper quartile 3.9 years, 100% complete), 3 patients died (1 candida sepsis, 1 sudden death, 1 unknown), 1 of them (candida sepsis) during the same hospitalization (an in-hospital mortality rate of 20%). The overall survival rate was 71 ± 9% at 1 year and unchanged thereafter. In total, 21 patients experienced at least one disease- or surgery-related complication, resulting in an early morbidity rate of 70% (Table 3). More than one morbidity-defining event occurred in 12 patients (40%). During follow-up, there was neither a recurrence of endocarditis, nor was a reoperation required.
Table 3:
Causes of perioperative mortality and morbidity (≤30 days)
| n | |
|---|---|
| 30-day mortality: 17% | |
| Septic multiple organ failure | 2 |
| Heart failure | 1 |
| Pneumonia | 1 |
| Cerebral haemorrhage | 1 |
| 30-day morbidity: 70% at least 1 event; 40% >1 event | |
| Re-exploration | 4 |
| Bleeding | 3 |
| Pericardial tamponade | 1 |
| Resuscitation | 1 |
| Postoperative renal failure (haemodialysis) | 10 |
| Pulmonary failure | 4 |
| Atrial fibrillation | 7 |
| Pneumonia | 3 |
| Permanent pacemaker implantation | 1 |
| Neurological events | 2 |
| Permanent | 1 |
| Transient | 1 |
| Delirium | 8 |
| Tracheotomy | 5 |
Costs
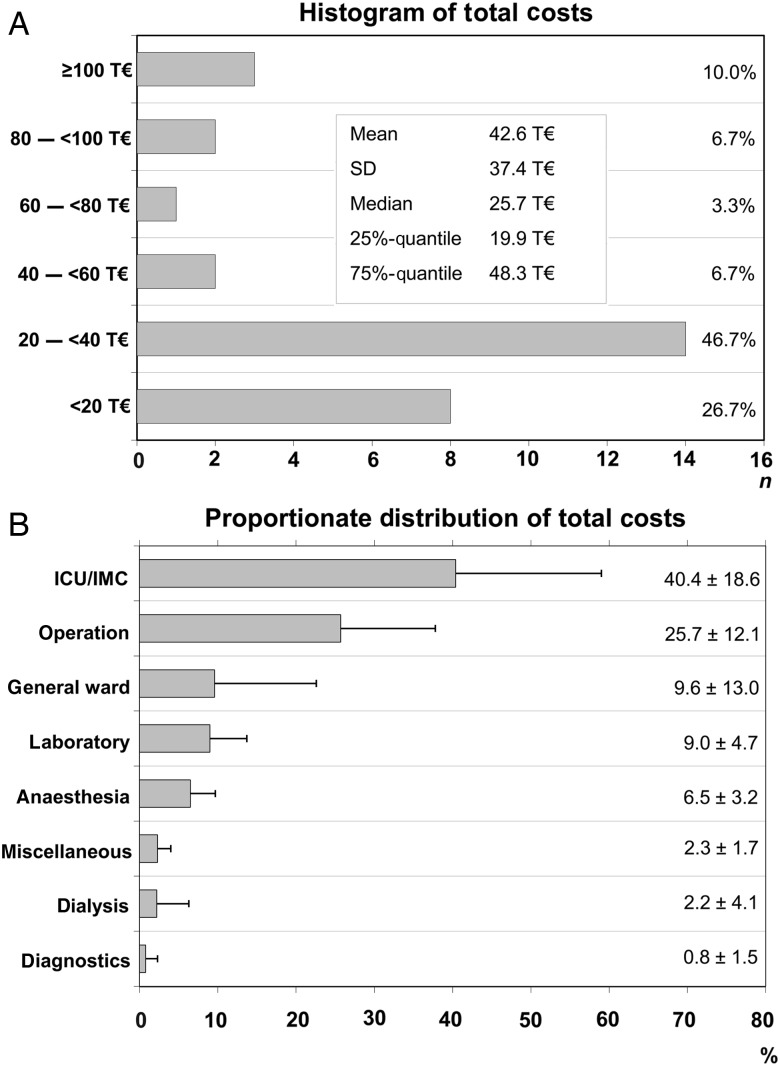
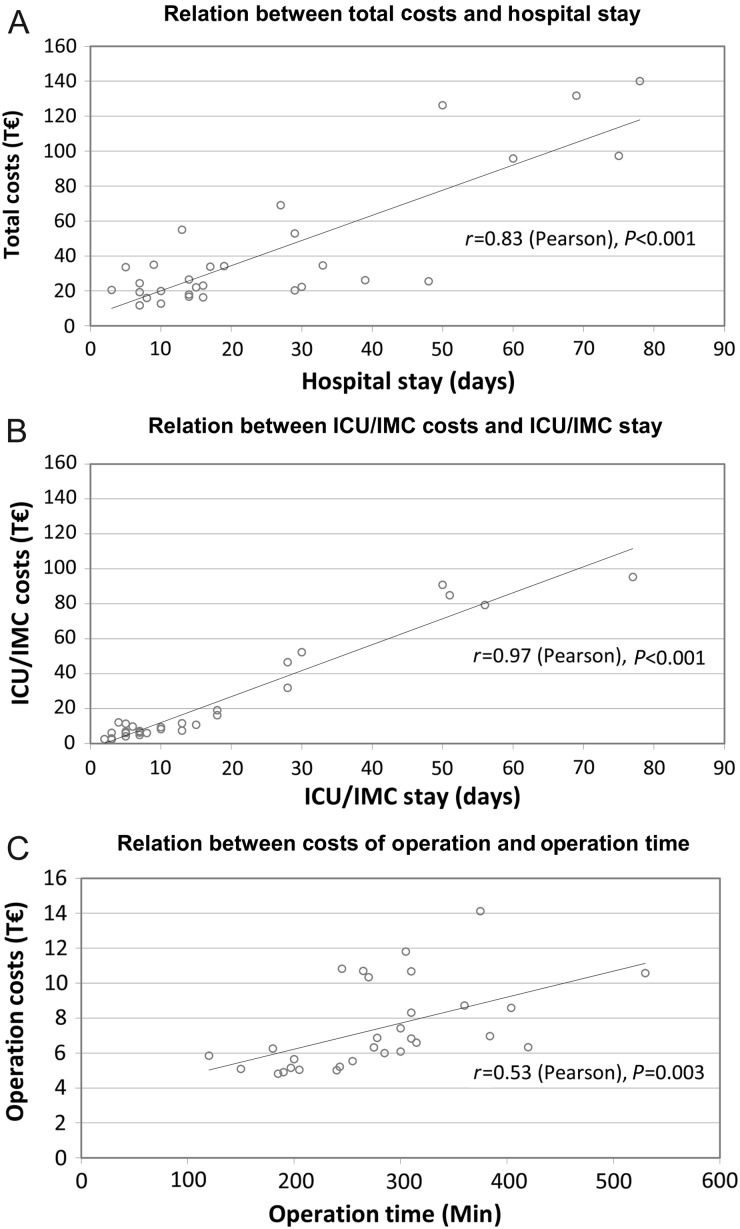
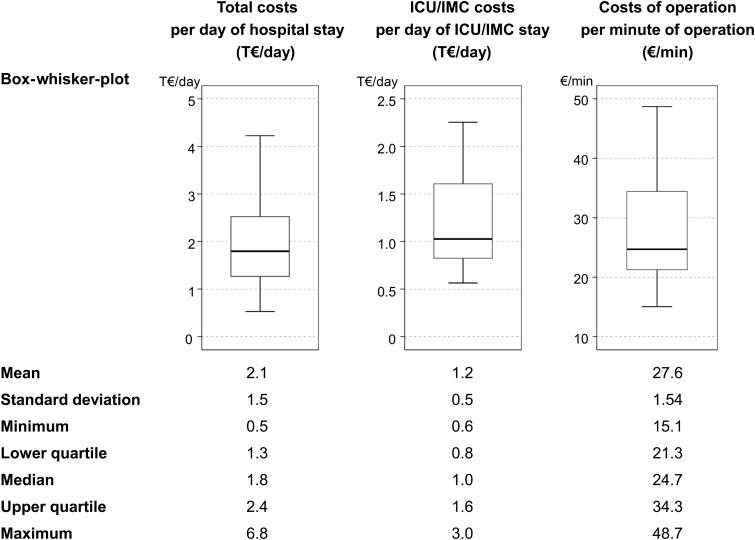
Although the mean total hospital costs for surgical treatment of PVE were 42.6 ± 37.4 Thousand Euro (T€)—median 25.7 T€, lower quartile 19.9 T€, upper quartile 48.3 T€—costs exceed by 48 and 100 T€ in 25% (upper quartile) and 10% of cases, respectively (Fig. 1). With 40.4 ± 18.6%, intensive care medicine (ICU/IMC) occupies the leading proportion of hospital expenses, followed by costs of operation. However, the standard deviation reflects considerable interindividual variability. As shown in Fig. 2, there was a strong correlation between total costs and duration of hospital stay and between ICU/IMC costs and duration of ICU/IMC stay. Costs of operation and duration of operation were weakly correlated. Despite the linear relationship, also time-related costs showed marked variance (Fig. 3). The median total costs per day of hospital stay and median ICU/IMC costs per day of ICU/IMC stay were 1.8 and 1.0 T€/day, respectively, whereas 25% of cases (upper quartile) cost more than 2.4 T€/day (total) and 1.6 T€/day (ICU/IMC).
Figure 1:
Hospital costs of surgery for prosthetic valve endocarditis. Distribution of total hospital costs within the study cohort (A) and relative breakdown of costs (B) are depicted. Error bars in the lower graph denote standard deviation. T€: thousand €; SD: standard deviation; ICU/IMC: intensive care unit/intermediate care.
Figure 2:
Correlations between costs and hospital time. Correlations between total hospital costs (A), costs of ICU/IMC (B), costs of operation (C) and the respective time intervals were analysed according to Pearson's method. T€: thousand €.
Figure 3:
Variability of time-related costs. Descriptive statistics of time-related costs including box-whisker plots illustrating their variability. T€, thousand €; ICU/IMC: intensive care unit/intermediate care.
Associations
Table 4 summarizes early outcomes in subgroups. Early mortality (≤30 days) was mainly related to preoperative variables like EuroSCORE II >20%, recurrent embolic events and problematic micro-organisms. Furthermore, mortality was associated with the need for mechanical circulatory support (Table 4) and the presence of postoperative renal failure (40% mortality if present versus 5% if not present, P = 0.031). Mortality was not associated with age, early PVE, locally uncontrolled infection and left ventricular dysfunction (LVEF <0.40) as well as performance of complex surgery. Early morbidity (≤30 days) was related to EuroSCORE II >20%, critical preoperative state and heart failure greater than or equal to New York Heart Association (NYHA) class III not only regarding the occurrence of >1 event (Table 4), but also regarding the incidence of postoperative renal failure [62% if EuroSCORE >20% versus 12% if EuroSCORE II ≤20% (P = 0.007); 60% if preoperative state was critical versus 20% if not (P = 0.045); 50% if heart failure greater than or equal to NYHA III was present versus 8% if not (P = 0.024)] and the requirement of tracheotomy [39% if EuroSCORE II >20 versus 0% if EuroSCORE II ≤20% (P = 0.009); 40% if preoperative state was critical versus 5% if not (P = 0.031); 2% if heart failure greater than or equal to NYHA III was present versus 0% if not (P = 0.066)]. Also the urgency of surgery and the need for mechanical circulatory support increased the incidence of morbidity-defining events (Table 4). However, 30-day morbidity was not associated with age, early PVE, locally uncontrolled infection, left ventricular dysfunction, problematic micro-organisms and recurrent embolic events as well as performance of complex surgery.
Table 4:
Early outcomes in subgroups
| Variable | n | 30-day morbidity (40% >1 event) |
30-day mortality (17%) |
||||
|---|---|---|---|---|---|---|---|
| Variable present | Variable not present | Variable present | Variable not present | ||||
| % | % | P-value | % | % | P-value | ||
| Preoperative | |||||||
| Age >70 years | 11 | 55 | 32 | 0.27 | 27 | 11 | 0.33 |
| EuroSCORE II >20% | 13 | 77 | 12 | 0.001 | 39 | 0 | 0.009 |
| Critical state | 10 | 80 | 20 | 0.004 | 30 | 10 | 0.30 |
| Problematic micro-organisms | 10 | 40 | 40 | 1.0 | 40 | 5 | 0.031 |
| Recurrent embolic events | 6 | 67 | 33 | 0.18 | 67 | 4 | 0.003 |
| Heart failure greater than or equal to NYHA III | 18 | 61 | 8 | 0.007 | 28 | 0 | 0.066 |
| Operative | |||||||
| Urgent/emergency surgery | 22 | 55 | 0 | 0.01 | 23 | 0 | 0.29 |
| Complex surgery | 17 | 43 | 35 | 0.71 | 23 | 12 | 0.63 |
| Mechanical support | 3 | 100 | 33 | 0.054 | 67 | 11 | 0.064 |
NYHA: New York Heart Association.
Clinical variables and respective costs are listed in Table 5. It shows that total costs were mainly explained by pre- and postoperative morbidity. Costs were not influenced by the presence of early PVE or locally uncontrolled infection. Age >70 years increased ICU/IMC costs significantly, but demonstrated only a moderate and not significant effect on total costs. Remarkably, the highest daily hospital costs were related to mechanical circulatory support and 30-day mortality (Table 5). Increased costs of operation were marginally significantly associated with EuroSCORE II >20% (8.4 ± 2.8 vs 6.6 ± 1.9 T€, P = 0.053) and critical preoperative state (8.8 ± 2.9 vs 6.7 ± 2.0 T€, P = 0.061), but significantly associated with LVEF <40% (9.7 ± 1.9 vs 7.1 ± 2.4 T€, P = 0.044) and complex surgery (9.1 ± 2.5 vs 6.1 ± 1.5 T€, P < 0.001).
Table 5:
Associations between costs and preoperative, operative and postoperative variables
| Variable | Total costs |
Total costs per day of hospital stay |
ICU/IMC costs |
ICU/IMC costs per day of ICU/IMC stay |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 42.6 ± 37.4 T€ |
2.1 ± 1.5 T€/day |
22.1 ± 28.7 T€ |
1.2 ± 0.6 T€/day |
|||||
| Median (LQ, UQ) | 25.7 (19.9, 48.3) T€ |
1.8 (1.3, 2.4) T€/day |
8.7 (6.0, 18.3) T€ |
1.0 (0.8, 1.6) T€/day |
|||||
| n | T€ | P-value | T€/day | P-value | T€ | P-value | T€/day | P-value | |
| Preoperative | |||||||||
| Age >70 years | 11 | 56.8 ± 43.2 | 0.16 | 2.4 ± 1.6 | 0.25 | 33.3 ± 31.4 | 0.033 | 1.5 ± 0.6 | 0.057 |
| EuroSCORE II >20% | 13 | 65.0 ± 46.9 | 0.003 | 2.9 ± 1.9 | 0.022 | 39.6 ± 36.5 | 0.002 | 1.6 ± 0.6 | <0.001 |
| Critical state | 10 | 72.9 ± 47.1 | <0.001 | 2.2 ± 1.0 | 0.27 | 44.0 ± 35.7 | 0.002 | 1.5 ± 0.5 | 0.024 |
| Recurrent embolic events | 6 | 43.1 ± 39.8 | 0.40 | 3.4 ± 2.8 | 0.53 | 23.7 ± 30.9 | 0.86 | 1.8 ± 0.9 | 0.044 |
| Operative | |||||||||
| Urgent/emergency surgery | 22 | 50.0 ± 41.3 | 0.056 | 2.4 ± 1.8 | 0.34 | 27.3 ± 31.9 | 0.070 | 1.3 ± 0.6 | 0.24 |
| Complex surgery | 17 | 53.9 ± 46.0 | 0.094 | 2.7 ± 1.8 | 0.004 | 28.7 ± 35.4 | 0.059 | 1.4 ± 0.6 | 0.15 |
| Mechanical support | 3 | 60.1 ± 57.6 | 0.56 | 5.4 ± 2.5 | 0.008 | 36.3 ± 47.3 | 0.38 | 2.3 ± 0.6 | 0.002 |
| Postoperative | |||||||||
| Early death (≤30 days) | 5 | 42.1 ± 31.4 | 0.48 | 4.4 ± 2.4 | 0.011 | 18.2 ± 19.1 | 0.45 | 2.1 ± 0.6 | <0.001 |
| Tracheotomy | 5 | 105.6 ± 27.9 | <0.001 | 2.2 ± 1.7 | 0.71 | 72.8 ± 22.3 | <0.001 | 1.6 ± 0.2 | 0.019 |
| Renal failure | 10 | 61.8 ± 46.0 | 0.049 | 3.3 ± 2.0 | 0.003 | 38.3 ± 35.7 | 0.011 | 2.1 ± 0.6 | <0.001 |
| Morbidity (>1 event) | 12 | 70.8 ± 45.5 | <0.001 | 2.9 ± 2.0 | 0.031 | 44.0 ± 35.6 | <0.001 | 1.6 ± 0.6 | <0.001 |
ICU/IMC: intensive care unit/intermediate care; SD: standard deviation; T€: thousand Euro; LQ: lower quartile; UQ: upper quartile.
COMMENT
In Germany, payment to hospitals is based on the system of diagnosis-related groups (DRG), which is a cornerstone of the healthcare system, which refers mainly to the principle that healthcare is funded by a statutory contribution system. The DRG system implies that hospitals are paid the same for the same ‘type’ of patient. By entering the respective hospital data annually, the German DRG system is a continuously revised and self-adjusting system. Although it permanently puts pressure on hospitals to perform procedures quickly and at minimal costs, it accounts for changes in real costs (personnel costs, costs of new technology, …) and allows their scientific investigation.
Surgery for PVE is associated not only with relevant mortality and morbidity, but also with significant costs. This study shows that average total hospital cost for surgical treatment of PVE was 42.6 T€ per case, but total costs exceeded 100 T€ in 10% of cases. However, it has to be considered that overall costs of PVE are higher, as patients usually need further hospital care before as well as after hospitalization for surgical treatment. Accordingly, it was estimated that the cost for PVE is ∼72 T€ per case in Germany [11]. As reported previously [11] and confirmed herein, the paramount costs result from treatment at the ICU and/or the IMC ward amounting to around 40% of total costs. They could even be higher as costs for laboratory tests, dialysis, diagnostics and blood products frequently arise during the ICU/IMC stay. By comparison, ∼26% of costs account for the operation per se.
Preoperative morbidity has an established role for postoperative outcomes after surgery for PVE. Besides high EuroSCORE II, critical state, problematic pathogens, recurrent embolism and congestive heart failure being associated with early morbidity and/or mortality in this study, also age, staphylococcal infection, early PVE, complicated PVE, renal failure, etc. have been shown to increase early mortality [4, 5, 10, 17–20]. Demonstrating that costs of surgical treatment for PVE are significantly associated with preoperative factors (Table 5), this study pointed out a further role of preoperative morbidity. It is important to understand that morbidity, mortality and costs are not necessarily associated with the endocarditis of the prosthetic valve per se, but with preoperative comorbidity resulting from PVE (typically occurring in the circumstances of late diagnosis) or concomitant diseases. Regarding this relation, indirect effects of preoperative factors acting on costs via increased postoperative morbidity and mortality have to be considered as well (Tables 4 and 5).
With respect to postoperative mechanical ventilation, ICU and hospital stay, we could recently demonstrate that one-fourth to one-third of patients undergoing surgical treatment for PVE require increased hospital resources [13]. Regarding real costs, a strong linear relation between length of hospital stay and total hospital costs and in particular between length of ICU/IMC stay and ICU/IMC costs exists (Fig. 2). Therefore, it is obvious that the longer the necessity for hospitalization and/or intensive care, the higher will be the costs. Despite this linear relation, time-related costs—especially daily hospital and daily ICU/IMC costs—vary remarkably (Fig. 3), reflecting considerable interindividual variability and suggesting the impact of further cost-related factors. Increased resource utilization in a number of patients certainly causes higher expenses for treatment and can explain the observed variability [13]. As several conditions require specific medical treatment, e.g. renal failure may require haemodialysis, the association between increased time-related costs and patient- or surgery-related factors defining morbidity becomes evident. In this respect, increased resource utilization was shown to be determined by critical preoperative status [13]. Most notably, mechanical circulatory support results in highest costs per day of hospital and ICU/IMC stay, respectively (Table 5). Regarding early mortality as a cost-increasing factor, it has to be considered that 2 of 5 patients who died within 30 days after surgery in this study were on extracorporeal membrane oxygenation treatment, which may overestimate the impact of mortality per se. Thus, mechanical circulatory support was reported to be the strongest predictor for early mortality (<30 days) after surgery for PVE [19]. However, it was recently shown in patients undergoing surgery for native valve IE in the USA, that among other preoperative, intraoperative and postoperative factors, in-hospital mortality was associated with significantly increased hospital charges [12].
Study limitations
The present study has limitations owing to its retrospective nature and the limited number of patients. However, the study period of three consecutive years was intentionally chosen to reduce the impact of fluctuating hospital costs which can arise due to variable costs of materials, administrative changes and/or adjustments of medical professionals' salary over the years. The contribution of variations in perioperative treatment was thereby reduced as well. We report results from a single surgical centre of a large university hospital. Therefore, caution has to be exercised regarding generalization of absolute costs.
CONCLUSIONS
Surgery for PVE is not only associated with relevant morbidity and mortality, but also with significant costs. For reduction of mortality, morbidity and costs, it is, above all, preoperative morbidity which has to be addressed by timely diagnostic assessment and immediate treatment. It is reasonable that patients with suspected PVE should be transferred to a tertiary care hospital where diagnostics and treatment can be provided by a multidisciplinary team including not only cardiologists and cardiac surgeons, but also specialists in infectious diseases, microbiologists and specialists of intensive care medicine. As guidelines clearly indicate surgery for heart failure due to valve dysfunction, for uncontrolled infection and/or for prevention of systemic embolism in case of large and mobile vegetations, early or immediate surgery has to be preferred if these are present.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr T. Folliguet (Nancy, France): Thank you for the manuscript that you sent to me. Obviously this is a very difficult problem. I work also in a tertiary centre so I know the problem of getting all these endocarditis who are very late.
Obviously your group is very high risk since you have 33% of critical state, and we see that you placed ECMO in 3 patients. So these are extremely sick patients. The results are somewhat good because you only have 16% mortality with 71% survival, but we see that 20% of them die within the first month. So I mean, you're telling us, of course, that these patients should be sent earlier, but actually it should not be to us that you should say this, you should say this to the cardiologists. I think the problem is that we all have to talk cardiologists into sending them earlier.
However, if we don't control this, when you read your manuscript it's very good, but what would you do now to try to improve the mortality? Would you delay a little bit the operation in terms of getting the patient somewhat less critical, like in heart failure? Do you use ultrafiltration? Do you put them on echo pre-operation? Reading the manuscript, we all know that if you operate on them critically in emergency it costs money and they stay in the ward. But the question is, because we're faced with this every day, you have somebody who is in renal failure, you have to operate on him, of course, otherwise he's going to die, but how can you improve him to decrease the morbidity of the surgery? Also there is nothing in the manuscript unfortunately on this. Otherwise it's a very good study.
Dr Grubitzsch: I completely agree with you, and I think all of us would have this answer. So to be honest, there is no single solution for this problem. Because if we see the patient coming in with stroke, it is already too late and we cannot improve their critical state and cannot improve the results, therefore. So it is right, we have to discuss not only with the cardiologists, but also with infectologists or GPs, because it is very complex. We saw this kind of time delay in prosthetic valve endocarditis to get a timely diagnosis and consequently to get a timely treatment. Many patients with a delayed diagnosis have no treatment at all, that means even no antibiotics. Frequently, they don't have a clear indication for surgery when there are signs for prosthetic valve endocarditis, but they need further follow-up and antibiotic treatment.
Dr Folliguet: I know. But how would you improve it–you? Let's say you're in the ICU.
Dr Grubitzsch: I cannot by myself. Because, yes, there is a problem with it. We all cannot improve this pre-operative field by ourselves because all of us see the patients, not in this period of time. So we have to communicate these problems to our colleagues and have to discuss, if we have the opportunity, to build multidisciplinary teams.
Dr M. Musci (Berlin, Germany): Let's change the situation. You have a very nice cardiologist and he calls you in time, the first week after diagnosis. You have a stable patient with prosthetic valve endocarditis and he asks you, “Do you want to operate on him?”
Dr Grubitzsch: The question is what the valve function is, what is the ––
Dr Musci: We have only the diagnosis of, prosthetic valve endocarditis.
Dr Grubitzsch: With a perivalvular ––
Dr Musci: No, just the first week of diagnosis and you have no complication. Because you say you don't want to delay surgery, so he asks you, “Do you want to operate now?”
Dr Grubitzsch: What is the microorganism? If it is Staphylococcus, I would re-operate, go for surgery ––
Dr Musci: Okay, it's staphylococcus.
Dr Grubitzsch: If it is early, I mean occurring within 1 year after first operation, I would rather go for surgery. If it is a late prosthetic valve endocarditis with no perivalvular involvement, I would go for antibiotics and watch the patient.
Dr Musci: So you would delay the operation if you do.
Dr Grubitzsch: No. If it is clearly indicated, if it is a destroyed valve, even with a streptococcus, I would go for surgery. I mean, we have to look, is there a clear indication for surgery though? If there is one, I wouldn't delay the surgery. If there is, let's say, a small vegetation, 2 years after primary aortic valve replacement, and he found maybe streptococci, I would say: Please start antibiotics and look 1 week later. If it is okay, we don't need the surgery. If it is progressing, he will need surgery.
Dr G. El Khoury (Brussels, Belgium): I mean, he will not need surgery after maybe 1, 2, 3 weeks, so everything is clear; but 6 months later he comes back with abscess. That's the problem of prosthetic endocarditis. I mean, we are never sure that we can cure the endocarditis with antibiotics. The question is should we go really when the patient is okay or not? And I agree that we are facing this every day. So should we operate every prosthetic endocarditis or not?
Dr R. Klautz (Leiden, Netherlands): I agree with the previous speaker. And to make it even more complicated, is that if patients are referred early, many centres still delay surgery. So there is a problem in our community as well.
The second problem is, if they send in patients with a prosthetic valve and fever, it's like the tip of a pyramid. You'll get hundreds of patients sent to your clinic. So from the cardiology perspective, they think, well, let's figure it out first before we send it out to the tertiary centre. So there is an understandable delay to some extent. In the perfect world, it wouldn't be like that. But a patient with a prosthetic valve and fever, do you want to have them and see them all? Because many of them have something else.
Dr El Khoury: I don't understand why we're always talking about our colleague cardiologists. They don't like those patients. They call us immediately, as soon as they see those patients. That's our problem, not really the problem of our colleagues. I'm sorry, I mean, they call us immediately with this because they don't like those patients.
Dr Grubitzsch: I want to be involved in these patients, of course, but I think it's not necessary to operate on every patient with just fever and a prosthetic valve.
REFERENCES
- 1.Piper C, Körfer R, Horstkotte D. Prosthetic valve endocarditis. Heart 2001;85:590–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akowuah EF, Davies W, Oliver S, Stephens J, Riaz I, Zadik P et al. Prosthetic valve endocarditis: early and late outcome following medical or surgical treatment. Heart 2003;89:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Paré C et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007;297:1354–61. [DOI] [PubMed] [Google Scholar]
- 4.Habib G, Thuny F, Avierinos JF. Prosthetic valve endocarditis: current approach and therapeutic options. Prog Cardiovasc Dis 2008;50:274–81. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Valle H, Fariñas-Alvarez C, García-Palomo JD, Bernal JM, Martin-Durán R, Gutiérrez Diez JF et al. Clinical course and predictors of death in prosthetic valve endocarditis over a 20-year period. J Thorac Cardiovasc Surg 2010;139:887–93. [DOI] [PubMed] [Google Scholar]
- 6.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). The task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC). Eur Heart J 2009;30:2369–413. [DOI] [PubMed] [Google Scholar]
- 7.Lytle BW, Priest BP, Taylor PC, Loop FD, Sapp SK, Stewart RW et al. Surgical treatment of prosthetic valve endocarditis. J Thorac Cardiovasc Surg 1996;111:198–210. [DOI] [PubMed] [Google Scholar]
- 8.Manne MB, Shrestha NK, Lytle BW, Nowicki ER, Blackstone E, Gordon SM et al. Outcomes after surgical treatment of native and prosthetic valve infective endocarditis. Ann Thorac Surg 2012;93:489–93. [DOI] [PubMed] [Google Scholar]
- 9.Edlin P, Westling K, Sartipy U. Long-term survival after operations for native and prosthetic valve endocarditis. Ann Thorac Surg 2013;95:1551–6. [DOI] [PubMed] [Google Scholar]
- 10.Grubitzsch H, Schaefer A, Melzer C, Wernecke KD, Gabbieri D, Konertz W. Outcome after surgery for prosthetic valve endocarditis and the impact of preoperative treatment. J Thorac Cardiovasc Surg 2014;148:2052–9. [DOI] [PubMed] [Google Scholar]
- 11.Kuehn C, Graf K, Heuer W, Hilfiker A, Chaberny IF, Stiesch M et al. Economic implications of infections of implantable cardiac devices in a single institution. Eur J Cardiothorac Surg 2010;37:875–9. [DOI] [PubMed] [Google Scholar]
- 12.Kemp CD, Arnaoutakis GJ, George TJ, Smith MA, Patel ND, Cameron DE et al. Valve surgery for infective endocarditis is associated with high hospital charges. J Heart Valve Dis 2013;22:110–7. [PubMed] [Google Scholar]
- 13.Grubitzsch H, Schaefer A, Claus B, Treskatsch S, Sander M, Konertz W. Determinants for increased resource utilization after surgery for prosthetic valve endocarditis. J Heart Valve Dis 2014;23:752–8. [PubMed] [Google Scholar]
- 14.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 15.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–44. [DOI] [PubMed] [Google Scholar]
- 16.Grinda JM, Mainardi JL, D'Attellis N, Bricourt MO, Berrebi A, Fabiani JN et al. Cryopreserved aortic viable homograft for active aortic endocarditis. Ann Thorac Surg 2005;79:767–71. [DOI] [PubMed] [Google Scholar]
- 17.Grünenfelder J, Akins CW, Hilgenberg AD, Vlahakes GJ, Torchiana DF, Madsen JC et al. Long-term results and determinants of mortality after surgery for native and prosthetic valve endocarditis. J Heart Valve Dis 2001;10:694–702. [PubMed] [Google Scholar]
- 18.Gabbieri D, Dohmen PM, Linneweber J, Grubitzsch H, von Heymann C, Neumann K et al. Early outcome after surgery for active native and prosthetic aortic valve endocarditis. J Heart Valve Dis 2008;17:508–24; discussion 525. [PubMed] [Google Scholar]
- 19.Musci M, Hübler M, Amiri A, Stein J, Kosky S, Meyer R et al. Surgical treatment for active infective prosthetic valve endocarditis: 22-year single-centre experience. Eur J Cardiothorac Surg 2010;38:528–38. [DOI] [PubMed] [Google Scholar]
- 20.Gaca JG, Sheng S, Daneshmand MA, O'Brien S, Rankin JS, Brennan JM et al. Outcomes for endocarditis surgery in North America: a simplified risk scoring system. J Thorac Cardiovasc Surg 2011;141:98–106. [DOI] [PubMed] [Google Scholar]