Abstract
OBJECTIVES
The current consensus favours an open distal anastomosis for aortic dissection repair. A small number of experiences have compared early and long-term outcomes between closed and open distal anastomosis in the setting of acute aortic dissection.
METHODS
We reviewed our experience in 204 patients who underwent repair of spontaneous acute type A aortic dissection between January 2000 and December 2013. Open distal repair was performed in 109 patients, whereas 95 patients received a closed anastomosis. The clinical presentation, anatomical characteristics of aortic dissection, surgical techniques and the outcomes were analysed in the overall population and in the subgroup of patients (n = 100; open = 39, closed = 61) with Type 1 DeBakey dissection and a proximal intimal tear. Twenty-six preoperative and operative variables were studied to determine their impact on hospital mortality and postoperative neurological deficits. Imaging follow-up was available in 83 patients.
RESULTS
A more extensive involvement of the aortic arch characterized the open repair group. No differences in terms of mortality, morbidity and survival rates were observed between the two groups of patients. Open repair with cerebral perfusion was associated with a better neurological outcome. Patients who underwent an open distal anastomosis showed a significant higher rate of complete thrombosis of the false lumen.
CONCLUSIONS
An open repair does not increase the risk of early mortality and positively affect the evolution of the false lumen in distal unresected aortic segments. The use of cerebral perfusion reduces the risk of perioperative neurological injury.
Keywords: Aortic dissection, Aorta/aortic, Aortic operation
INTRODUCTION
The current consensus favours an open distal repair for aortic dissection [1]. This technique allows an excellent visualization for construction of the distal aortic anastomosis, the resection of additional distal tears in the aortic arch, the prevention of injuries of a fragile aortic wall at the clamp site or the avoidance of aortic cross-clamp and an extensive reapproximation of the false lumen. However, there is no strong evidence that this technique carries either an early [2, 3] or long-term survival benefit, neither has the current literature demonstrated a lower incidence rate of reoperations with an open distal technique [4–6]. A small number of series have compared the outcomes between closed and open distal anastomosis for acute aortic dissection repair [7]. These included small populations [8, 9] or very long periods of observation, thus introducing the possible bias related to the general improvement in care through the decades [4].
In this study, we have reviewed our experience in the surgical treatment of acute type A aortic dissection and evaluated the outcomes with open and closed distal anastomosis techniques.
PATIENTS AND METHODS
Population
The internal database of Wessex Cardiothoracic Centre at UHS was interrogated to find patients who had undergone emergency repair of spontaneous acute type A aortic dissection between January 2000 and December 2013. Two hundred and four consecutive patients were identified, 109 had an open distal anastomosis and 95 a closed distal repair.
In all the cases except for 4 patients, the diagnosis was made by computed tomography (CT) scan. Chronic (diagnosis over 14 days of onset of symptoms) and iatrogenic dissections were excluded; the data about interval time between symptoms onset and surgical operation were not retrieved, all the patients were operated on emergency basis soon after the diagnosis of acute type A aortic dissection had been notified. Stanford and DeBakey classifications were commonly used to define the extension of aortic dissection as it was established by preoperative imaging and at operation.
There were no generalized exclusion criteria and no information is available regarding patients who refused the operation, underwent a conservative management or died before the admission at the operative theatre.
Study design
We compared the preoperative and intraoperative characteristics of the two cohorts of patients considering the overall population and the subgroup of patients with Type 1 DeBakey dissection with a proximal intimal tear. We analysed early and long-term survival, the neurological outcomes and the evolution of the residual dissected aorta.
This evaluation is a retrospective cohort analysis of our database. The follow-up was completed by review of the online database system, Hospital Integrated Clinical Support System database (Ascribe Ltd, Bolton, U K) and patient records.
Imaging Follow-up
Follow-up CT or magnetic resonance aortograms were available in 83 patients who survived longer than 6 months (47% of the population). Images and/or reports were reviewed to characterize the patency of the false lumen and the presence of false aneurysm at the distal suture line.
Definitions
Myocardial ischaemia was defined as ST-T changes on electrocardiogram or positive troponin. Haemodynamic instability is referred to as the presence of hypotension (systolic blood pressure <80 mmHg) or the need for inotropes or vasopressor support at admission to our unit. Stroke was defined as the presence of a persistent loss, still present or resolved at the discharge, of neurological function with or without confirmation by CT scan. Temporary neurological deficit is defined as transient self-limiting dysfunction without evidence of acute brain damage on CT scan. Acute renal failure was defined as creatinine level >200 µmol/l, oligo/anuria or requirement of renal replacement therapy.
Operative techniques
The surgical techniques used in our institution have been previously reported [2]. Site for arterial cannulation was chosen according to the anatomical and pathological findings on CT scan. After cardiopulmonary bypass (CPB) institution, the circulation was initially cooled down to 32°C. The distal ascending aorta was clamped (200/204 patients) and the ascending aorta was transected to assess the presence of an intimal tear, the anatomy of the aortic root and the aortic valve. Cold blood cardioplegia was delivered intermittently directly into the coronary ostia. The decision to reconstruct rather than replace the aortic root and the aortic valve was made intraoperatively by the surgeon and supported by intraoperative evaluation of dynamic aortic valve and root properties at trans oesophageal echocardiogram. The ascending aorta distal to the sinotubular junction was replaced in all patients with a gelatine-impregnated Dacron graft of appropriate size. The presence of an intimal tear extending into the aortic arch or crossing the clamp area usually addressed the patient to an open distal anastomosis under hypothermic circulatory arrest (HCA). When the entry tear was limited to the aortic root or the ascending aorta, the distal repair was performed either with or without HCA depending on surgeon's preference, distal ascending aorta and aortic arch size, evidence of distal malperfusion on clamping the aorta. Three different strategies for cerebral protection were used for an open distal anastomosis: deep hypothermic circulatory arrest (DHCA), HCA with retrograde cerebral perfusion and HCA with antegrade cerebral perfusion. Antegrade cerebral perfusion is nowadays the first choice adjunct for brain protection during moderate HCA; since 2009, cerebral oximetry has been fully embedded during aortic procedures to detect cerebral malperfusion. All the surgeons throughout the study period equally performed open and closed distal anastomoses.
Statistical analysis
Continuous data are presented as mean ± standard deviation, and categorical variables are given as counts and percentages. Univariate comparisons of preoperative, operative and postoperative variables were performed between open distal anastomosis and closed anastomosis groups.
Eighteen preoperative variables (age >70 years, gender, myocardial ischaemia, tamponade, haemodynamic instability, cardiopulmonary resuscitation (CPR), pericardial drainage, new onset of stroke, mechanical ventilation, acute renal failure, limb ischaemia, abdominal ischaemia, Marfan syndrome, other connective tissue disorders, coronary dissection, Type I DeBakey dissection, intimal tear site, reoperation) and eight operative variables (open distal anastomosis, institution of cerebral perfusion, common femoral artery cannulation, aortic valve replacement, aortic root replacement, coronary artery bypass grafting, hemiarch replacement, total arch replacement) were studied to determine their influence on hospital mortality, the occurrence of postoperative neurological deficit (cerebral stroke+temporary neurological dysfunction) and postoperative cerebral stroke. Variables with a P-value <0.05 were entered into a logistic regression model to predict their independent significance. Similar statistical analysis design was applied for the subgroup of patients who presented with Type 1 DeBakey aortic dissection and an intimal tear limited to the aortic root and the ascending aorta (arch involvement and intimal tear site were not considered), and to study the subgroup of patients who underwent an open distal repair.
Survival rates were calculated using the Kaplan–Meier method. Univariate comparisons between groups for late deaths were performed with the log-rank test.
Statistical analyses were performed using the Stat-View Statistical Software Package 5.0 (SAS Institute, Inc., Cary, NC, USA), NCSS 2001 (Number Cruncher Statistical System, Kaysville, Utah).
RESULTS
Overall population
Demographic and presentation
The mean age of the patients was 64 ± 13 years with male gender predominating (n = 133, 65%). No differences were found between the group of patients who underwent open distal repair and the group who had a closed anastomosis (Table 1).
Table 1:
Preoperative data of patients who underwent emergency repair of acute type A aortic dissection
| Variables | Overall population, n = 204 | Open distal, n = 109 | Closed distal, n = 95 | P-value |
|---|---|---|---|---|
| Age (years ± SD) | 64 ± 13 | 63 ± 12 | 64 ± 14 | 0.68 |
| Gender (male/female) | 133/71 | 71/38 | 62/33 | 0.98 |
| Presentation | ||||
| Myocardial ischaemia | 36 (18%) | 22 (20%) | 14 (15%) | 0.31 |
| Tamponade | 36 (18%) | 17 (16%) | 19 (20%) | 0.41 |
| Pericardiocentesis | 2 (1%) | 0 | 2 (2%) | 0.42 |
| Haemodynamic instability | 42 (21%) | 24 (22%) | 18 (19%) | 0.59 |
| Resuscitation | 10 (5%) | 6 (6%) | 4 (4%) | 0.92 |
| New onset of cerebral stroke | 17 (8%) | 12 (11%) | 5 (5%) | 0.14 |
| Mechanical ventilation | 15 (7%) | 10 (9%) | 5 (5%) | 0.29 |
| Acute renal injury | 11 (5%) | 7 (6%) | 4 (4%) | 0.70 |
| Limb ischaemia | 11 (5%) | 4 (4%) | 7 (7%) | 0.39 |
| Abdominal ischaemia | 2 (1%) | 2 (2%) | 0 | 0.54 |
| Marfan syndrome | 6 (3%) | 4 (4%) | 2 (2%) | 0.80 |
| Other connective tissue disorders | 9 (5%) | 4 (4%) | 5 (5%) | 0.82 |
| Redo | 12 (6%) | 10 (9%) | 2 (2%) | 0.07 |
SD: standard deviation.
Anatomical findings and operative data
Type 2 DeBakey aortic dissection was more prevalent in the closed anastomosis group (P = 0.003); patients in open anastomosis group were more likely to present an intimal tear located in the aortic arch (46 vs 2%, P < 0.001). The two groups of patients received similar procedures for proximal repair. Significant longer myocardial ischaemia and CPB times were registered in the open anastomosis group. Table 2 summarizes anatomical and operative strategy data.
Table 2:
Anatomical findings and operative data
| Variables | Overall population, n = 204 | Open distal, n = 109 | Closed distal, n = 95 | P-value |
|---|---|---|---|---|
| Anatomy | ||||
| Type 2 DeBakey | 24 (12%) | 6 (6%) | 18 (19%) | 0.003 |
| Coronary artery (ies) dissection | 45 (23%) | 28 (26%) | 17 (18%) | 0.18 |
| Tear identifieda | 175 (88%) | 95 (89%) | 80 (87%) | 0.69 |
| Tear root-ascending aorta | 123 (70%) | 45 (42%) | 78 (85%) | <0.001 |
| Tear arch | 52 (30%) | 50 (46%) | 2 (2%) | <0.001 |
| Tear not founda | 24 (12%) | 12 (11%) | 12 (13%) | 0.72 |
| Arterial cannulationb | ||||
| Common femoral artery | 147 (74%) | 69 (65%) | 78 (84%) | 0.003 |
| Axillary artery | 39 (20%) | 26 (25%) | 13 (14%) | 0.06 |
| Other sites | 6 (2%) | 4 (3%) | 2 (2%) | 0.82 |
| Combined cannulation | 7 (4%) | 7 (7%) | 0 | 0.03 |
| Surgical strategies | ||||
| Open/closed distal anastomosis | 109/95 | 109 | 95 | |
| HCA time (min ± SD) | 30 ± 14 | |||
| HCA temperature (°C±SD) | 19 ± 4 | |||
| Antegrade cerebral perfusion | 28 (26%) | |||
| Retrograde cerebral perfusion | 24 (22%) | |||
| Aortic valve resuspension | 70 (34%) | 42 (39%) | 28 (29%) | 0.17 |
| Aortic valve replacement | 13 (6%) | 4 (3%) | 9 (10%) | 0.16 |
| Aortic root replacement | 59 (29%)c | 30 (28%) | 29 (31%) | 0.64 |
| Mechanical prosthesis | 45 (22%) | 22 (20%) | 23 (24%) | 0.49 |
| Biological prosthesis | 25 (12%) | 11 (10%) | 14 (15%) | 0.31 |
| Hemiarch replacement | 60 (29%) | 60 (55%) | ||
| Total arch replacement | 12 (6%) | 12 (11%) | ||
| Coronary artery bypass grafting | 19 (9%) | 11 (10%) | 8 (9%) | 0.68 |
| CPB time (min ± SD) | 185 ± 85 | 224 ± 86 | 142 ± 58 | <0.001 |
| Cross-clamp time (min ± SD) | 103 ± 39 | 109 ± 43 | 95 ± 34 | 0.015 |
HCA: hypothermic circulatory arrest; SD: standard deviation; CPB: cardiopulmonary bypass.
Data available in 199 patients (107 open distal; 92 closed distal).
Data available in 199 patients (106 open distal; 93 closed distal).
Two patients underwent David reimplantantion procedure (one open distal, one closed distal).
Mortality and morbidity
Overall in-hospital mortality was 11% (23/204 patients). Mortality, postoperative complication rate, ICU and overall hospital length of stay were comparable between the two groups of patients (Table 3).
Table 3:
Postoperative course and follow-up data
| Variables | Overall population, n = 204 | Open distal, n = 109 | Closed distal, n = 95 | P-value |
|---|---|---|---|---|
| Re-exploration | 35 (17%) | 19 (17%) | 16 (17%) | 0.91 |
| Mechanical ventilation (days ± SD) | 4.5 ± 9.2 | 4.0 ± 6.7 | 5.0 ± 11.4 | 0.48 |
| Tracheostomy | 23 (11%) | 12 (11%) | 11 (11%) | 0.90 |
| Neurological deficit | 41 (20%) | 20 (18%) | 21 (22%) | 0.50 |
| Cerebral stroke | 29 (14%) | 16 (14%) | 13 (14%) | 0.83 |
| Temporary neurological deficit | 12 (6%) | 4 (4%) | 8 (8%) | 0.25 |
| Acute renal failure | 48 (24%) | 27 (25%) | 21 (22%) | 0.65 |
| Renal replacement therapy | 17 (8%) | 12 (11%) | 5 (5%) | 0.14 |
| Sepsis | 43 (21%) | 27 (25%) | 16 (17%) | 0.17 |
| Deep sternal wound infection | 5 (2%) | 4 (4%) | 1 (1%) | 0.45 |
| Gastrointestinal complication | 2 (1%) | 2 (2%) | 0 | 0.54 |
| ITU length of stay (days ± SD) | 5.9 ± 7.3 | 6.0 ± 6.2 | 5.7 ± 8.5 | 0.72 |
| Overall length of stay (days ± SD) | 18.9 ± 23.6 | 16.5 ± 11.2 | 21.6 ± 32.5 | 0.16 |
| Intraoperative mortality | 3 (2%) | 2 (2%) | 1 (1%) | 0.90 |
| In-hospital mortality | 23 (11%) | 15 (14%) | 8 (8%) | 0.23 |
| Follow-up (months ± SD) | 67 ± 46 | 69 ± 44 | 67 ± 49 | 0.61 |
| Distal aortic reoperation | 9 (5%) | 7 (7%) | 2 (2%) | 0.21 |
SD: standard deviation; ITU: intensive therapy unit.
On univariate analysis, preoperative stroke and haemodynamic instability emerged as significant risk factors affecting early mortality [P = 0.002, odds ratio (OR) 5.45 (1.79–16.6) and P = 0.023, OR 2.88 (1.15–7.22), respectively]; preoperative cerebral stroke retained significance on multivariate analysis [P = 0.009, 4.59 (1.46–14.43)]. None of the operative variables including the choice for arterial cannulation site was found to significantly affect early survival.
Preoperative cardiac tamponade and preoperative CPR were associated with the occurrence of postoperative cerebral stroke, at univariate analysis P = 0.022, OR 2.77 (1.15–6.64) and P = 0.03, OR 4.36 (1.14–16.59), respectively (no significance after multivariate analysis). Closed anastomosis technique and the institution of DHCA without any form of cerebral perfusion were independent risk factors for postoperative neurological deficit [P = 0.041, OR 2.84 (1.04–7.74)].
Survival
The survival, at 1-, 5- and 10-years for the entire study population was 84, 78 and 68%, respectively. Univariate comparison between patients who underwent open versus closed distal anastomosis showed no difference in survival (log-rank test P = 0.19): open distal 1-, 5- and 10-year survival was 80, 7% and 65% respectively, closed distal 1-, 5- and 10-year survival was 88, 84 and 70%, respectively (Fig. 1).
Figure 1:
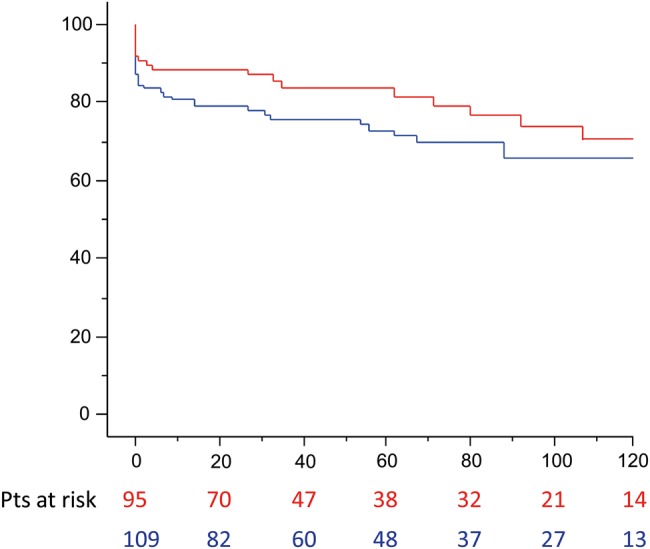
Survival (months) rate according to distal repair: open (blue line) versus closed (red line) anastomosis (log-rank P = 0.19).
Type 1 DeBakey aortic dissection with intimal tear limited to the aortic root and ascending aorta
One hundred patients presented with Type 1 DeBakey aortic dissection with a proximal intimal tear, of these 39 received open distal repair and 61 a closed anastomosis. Table 4 provides a complete summary and analysis of preoperative and operative data.
Table 4:
Preoperative, anatomical and operative data of patients with Type 1 DeBakey aortic dissection and intimal tear limited to the aortic root and the ascending aorta
| Variables | Overall population, n = 100 | Open distal, n = 39 | Closed distal, n = 61 | P-value |
|---|---|---|---|---|
| Age (years ± SD) | 62 ± 14 | 61 ± 12 | 63 ± 15 | 0.41 |
| Gender (male/female) | 69/31 | 26/13 | 43/18 | 0.69 |
| Presentation | ||||
| Myocardial ischaemia | 16 (16%) | 9 (23%) | 7 (11%) | 0.12 |
| Tamponade | 16 (16%) | 5 (13%) | 11 (18%) | 0.48 |
| Pericardiocentesis | 1 (1%) | 0 | 1 (2%) | 0.82 |
| Haemodynamic instability | 20 (20%) | 10 (26%) | 10 (16%) | 0.26 |
| Resuscitation | 7 (7%) | 3 (8%) | 4 (6%) | 0.85 |
| New onset of cerebral stroke | 4 (4%) | 2 (5%) | 2 (3%) | 0.95 |
| Mechanical ventilation | 5 (5%) | 4 (10%) | 1 (2%) | 0.15 |
| Acute renal injury | 4 (4%) | 1 (3%) | 3 (5%) | 0.95 |
| Limb ischaemia | 7 (7%) | 2 (5%) | 5 (8%) | 0.86 |
| Abdominal ischaemia | 0 | 0 | 0 | |
| Marfan syndrome | 4 (4%) | 2 (5%) | 2 (3%) | 0.95 |
| Other connective tissue disorders | 6 (6%) | 2 (5%) | 4 (6%) | 0.89 |
| Redo | 6 (6%) | 6 (15%) | 0 | 0.006 |
| Anatomy | ||||
| Coronary artery(ies) dissection | 28 (28%) | 14 (36%) | 14 (23%) | 0.16 |
| Arterial cannulationa | ||||
| Common femoral artery | 76 (76%) | 23 (61%) | 53 (86%) | 0.001 |
| Axillary artery | 19 (20%) | 11 (29%) | 8 (14%) | 0.06 |
| Other sites | 1 (1%) | 1 (2%) | 0 | 0.82 |
| Combined cannulation | 3 (3%) | 3 (8%) | 0 | 0.10 |
| Surgical strategies | ||||
| Open/closed distal anastomosis | 39/62 | 39 | 61 | |
| HCA time (min ± SD) | 40 ± 7 | |||
| HCA temperature (°C±SD) | 19 ± 3 | |||
| Antegrade cerebral perfusion | 9 (23%) | |||
| Retrograde cerebral perfusion | 8 (20%) | |||
| Aortic valve resuspension | 28 (28%) | 9 (23%) | 19 (31%) | 0.38 |
| Aortic valve replacement | 9 (9%) | 2 (5%) | 7 (11%) | 0.47 |
| Aortic root replacement | 40 (40%) | 17 (44%)b | 23 (38%) | 0.56 |
| Mechanical prosthesis | 32 (32%) | 11 (28%) | 21 (34%) | 0.51 |
| Biological prosthesis | 16 (16%) | 7 (18%) | 9 (15%) | 0.67 |
| Hemiarch replacement | 19 (19%) | 19 (49%) | ||
| Total arch replacement | 2 (2%) | 2 (5%) | ||
| Coronary artery bypass grafting | 9 (9%) | 2 (5%) | 7 (11%) | 0.47 |
| CPB time (min ± SD) | 176 ± 77 | 220 ± 77 | 149 ± 64 | <0.001 |
| Cross-clamp time (min ± SD) | 105 ± 37 | 116 ± 36 | 98 ± 36 | 0.016 |
HCA: hypothermic circulatory arrest; SD: standard deviation; CPB: cardiopulmonary bypass.
Data available in 99 patients (38 open distal; 61 closed distal).
One patient underwent David reimplantantion procedure.
There was no difference in early outcomes with regard to morbidity and mortality (Table 5).
Table 5:
Postoperative course and follow-up data in the subgroup with Type 1 DeBakey dissection and intimal tear limited to the aortic root and the ascending aorta
| Variables | Overall population, n = 100 | Open distal, n = 39 | Closed distal, n = 61 | P-value |
|---|---|---|---|---|
| Re-exploration | 17 (17%) | 6 (15%) | 11 (18%) | 0.73 |
| Mechanical ventilation (days ± SD) | 4.9 ± 11.0 | 4.4 ± 9.0 | 5.2 ± 12.2 | 0.72 |
| Tracheostomy | 12 (12%) | 4 (10%) | 8 (14%) | 0.57 |
| Neurological deficit | 22 (22%) | 7 (18%) | 15 (25%) | 0.43 |
| Cerebral stroke | 15 (15%) | 6 (15%) | 9 (14%) | 0.93 |
| Temporary neurological deficit | 7 (7%) | 1 (3%) | 6 (11%) | 0.32 |
| Acute renal failure | 25 (25%) | 8 (20%) | 17 (28%) | 0.40 |
| Renal replacement therapy | 9 (9%) | 5 (13%) | 4 (6%) | 0.48 |
| Sepsis | 18 (18%) | 8 (20%) | 10 (16%) | 0.60 |
| Deep sternal wound infection | 3 (3%) | 2 (5%) | 1 (3%) | 0.69 |
| Gastrointestinal complication | 0 | 0 | 0 | |
| ITU length of stay (days ± SD) | 5.9 ± 8.2 | 5.4 ± 5.5 | 6.3 ± 9.6 | 0.61 |
| Overall length of stay (days ± SD) | 17.9 ± 12.9 | 16.5 ± 10.9 | 18.8 ± 14.1 | 0.39 |
| Intraoperative mortality | 1 (1%) | 0 | 1 (2%) | 0.82 |
| In-hospital mortality | 11 (11%) | 4 (10%) | 7 (11%) | 0.89 |
| Follow-up (months ± SD) | 66 ± 48 | 73 ± 43 | 62 ± 50 | 0.26 |
| Distal aortic reoperation | 5 (5%) | 4 (11%) | 1 (2%) | 0.32 |
SD: standard deviation; ITU: intensive therapy unit.
Preoperative haemodynamic instability was the only predictor of in-hospital death on univariate analysis [P = 0.005, OR 6.42 (1.72–23.99)]; presentation with cardiac tamponade was the only risk factor associated with the occurrence of postoperative neurological deficit [P = 0.036, OR 3.37 (1.082–10.49)] and cerebral stroke [P = 0.013, OR 4.66 (1.36–15.92)].
The 1-, 5- and 10-year survival was 86, 80 and 68%, respectively. The survival between patients who underwent open and closed distal anastomosis was similar (log-rank test P = 0.79): open distal 90, 80 and 71% at 1, 5 and 10 years, respectively; closed distal 84, 81 and 66% at 1, 5 and 10 years, respectively (Fig. 2).
Figure 2:
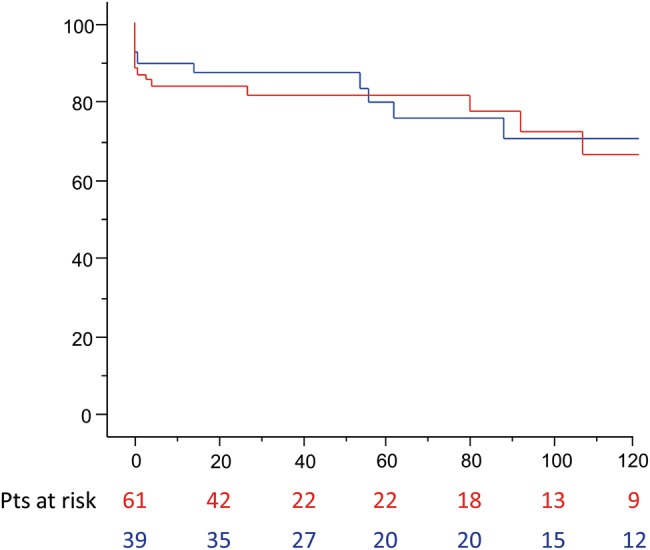
Survival of patients (months) with Type 1 DeBakey dissection and proximal intimal tear according to distal repair strategies (open: blue line; closed: red line; log-rank P = 0.79).
Open repair and neurological outcomes
In the open repair group (n = 109 patients), 52 patients underwent HCA with cerebral perfusion and 59 had DHCA. Preoperative presentation, extent of proximal or distal aortic repair, CPB duration (218 ± 87 vs 230 ± 89 min, P = 0.49), myocardial ischaemia time (104 ± 39 vs 114 ± 47 min, P = 0.23) and circulatory arrest time (29 ± 13 vs 32 ± 14 min, P = 0.24), were similar in both groups. DHCA correlated with a higher rate of postoperative neurological deficit (27 vs 10%, P = 0.02) and postoperative cerebral stroke (22 vs 8%, P = 0.048) when compared with HCA with cerebral perfusion. Furthermore, DHCA represented, among patients who had an open repair, the only significant factor predicting a worse postoperative neurological outcome.
Reoperations
In the open distal repair group, 14 aortic procedures were performed in 12 patients during the follow-up at a median interval time of 13 months (range 1–98 months). Three patients underwent elective thoraco-abdominal aortic aneurysm replacement and 1 patient abdominal aortic aneurysm repair because of progressive increase in aortic size. One patient had descending thoracic aorta replacement after a complicated redissection, 2 patients required urgent reoperation for arch replacement because of disruption of the distal anastomosis and development of distal aortic false aneurysm. The last case was completed with a thoracic endovascular aortic repair in proximal descending thoracic aorta. Proximal reoperations were performed in 4 patients for endocarditis (n = 2), development of aortic valve regurgitation (1 patient had AVR) and progressive dilatation of the aortic root.
Three patients who received a closed anastomosis required further aortic procedures. One patient had distal false aneurysm repair after 17 days from the first operation, 1 patient proximal false aneurysm repair after 1 month while 1 patient underwent 3 months later a redoroot and hemiarch replacement and subsequent surgical descending thoracic aorta repair.
Overall population freedom from distal aortic reoperations was at 1, 5 and 10 years, 96, 94 and 94%, respectively. No difference was found between the two groups of patients: open distal 95, 90 and 90% at 1-, 5- and 10-year follow-up; closed distal 99, 97 and 97% at 1-, 5- and 10-year follow-up (log-rank test P = 0.08).
Imaging follow-up
Table 6 summarizes the relevant results in terms of false lumen evolution and occurrence of aortic false aneurysms. Patients in the open distal repair group had a significant higher rate of complete thrombosis of the false lumen at the level of thoracic descending aorta (P = 0.036).
Table 6:
Imaging follow-up data
| Variables | Overall population | Open distal | Closed distal | P-value |
|---|---|---|---|---|
| Patients | 83 | 47 | 36 | |
| FU (months ± SD) | 34 ± 31 | 34 ± 29 | 35 ± 35 | 0.88 |
| Distal aortic false aneurysm | 4 (5%) | 2 (4%) | 2 (6%) | 0.80 |
| Type 1 DeBakey | 73 | 43 | 30 | 0.42 |
| FL DTA full thrombosis | 30/73 (40%) | 22/43 (50%) | 8/30 (27%) | 0.036 |
SD: standard deviation; FU: follow-up; FL: false lumen; DTA: descending thoracic aorta.
COMMENT
Open distal repair not only allows for complete resection of the entire intrapericardial aorta but also provides some technical advantages in the construction the anastomosis and in the reapproximation of the aortic layers. The possibility to extend the resection to the lesser curvature of the arch and to the clamp site may promote the thrombosis of the false lumen and reduce the risk of aortic false aneurysms. Furthermore, the possible avoidance of cross-clamp may prevent the pressurization of the false lumen, the creation of new tears and the risk of new malperfusion. A limited number of studies in literatures compared the results of open and closed distal anastomosis, usually small populations were evaluated and the use of historical controls could have introduced in some of these experiences a possible bias related to the substantial advances occurred in cardiac surgery through the decades [4, 7, 9]. A previously published study from our centre [2] did not reveal any difference in terms of early and mid-term outcomes between open and closed anastomosis. We aimed to update our results in a larger cohort of patients introducing the analysis of anatomical factors and presenting a longer follow-up supported by imaging assessment.
We found no difference in early and late survival according to the distal repair strategy; our results entirely support previous studies comparing open and closed distal anastomosis [3–6, 9, 10]. Despite the similar clinical profile at the presentation, the two groups of patients showed significant differences in the extension of the dissection flap and the location of the primary intimal tear; this is not surprising and reflects the tear resection-oriented approach in aortic dissection repair. On this basis, we analysed the subgroup of patients with Type 1 DeBakey dissection and an intimal tear proximal to the aortic arch. The rationale of this choice was the evaluation of a population that, based on our practice and as previously reported [3, 5, 6], could have been treated either with an open distal repair or with a closed anastomosis. In this subgroup, we found a lower in-hospital mortality rate and shorter intensive therapy unit and hospital stays in patients with an open repair; these differences, however, did not retain any statistical significance. Similarly, we were not able to demonstrate a consistent impact on survival of the different operative strategies. New onset of cerebral stroke and haemodynamic instability were predictors of in-hospital mortality and this confirmed the importance of preoperative presentation in determining the early and mid-term outcome in acute aortic dissection.
Twenty percent of our patients suffered a postoperative neurological deficit with a 14% rate of cerebral stroke. A lower rate of neurological complications was found in patients receiving an open distal anastomosis with the adjunct of cerebral perfusion. Recent evidence supports this finding and reports a significant advantage on neurological outcomes for patients who undergo moderate HCA with cerebral perfusion compared with DHCA [11–13]. Most of the experiences stating similar results between open distal repair and closed anastomosis came from populations who underwent open repair on DHCA without the adjunct of any cerebral perfusion [3, 5, 6, 10]. On the contrary, a significantly better early outcome after open distal repair compared with closed anastomosis has been reported in patients who had circulatory arrest with concomitant brain perfusion [8]. The strategy for cerebral protection should be always taken into account when analysing and comparing the outcomes between open and closed distal repair.
Another argument supporting the systematic adoption of the open distal repair is the possibility to avoid the cross-clamp on the dissected aorta, thus preventing the pressurization of the false lumen and consequently preserving an adequate flow to the neck vessels and cerebral embolism. Although some experiences did not report a significantly better neurological outcome with this strategy [14, 15], it can be argued that the avoidance of clamp could have reduced the occurrence of postoperative neurological deficits and allowed a lower mortality in our series of open repair. Our intraoperative monitoring protocol, pre- and post-aortic arch blood pressure measurements and continuous regional cerebral oxygen saturation using near infrared spectroscopy, revealed in 3 patients an asymmetric arterial pulse and regional cerebral oxygen saturation, which suggested an inadequate perfusion of the arch vessels. In all these cases, the signs of cerebral malperfusion became evident soon after CPB institution and we were able to restore the flow by changing to a new arterial inflow site. No cases with intraoperative clinical evidence of brain malperfusion after aorta cross-clamp were reported, obviously we cannot exclude embolic events from false lumen thrombus or debris. In a recent paper, Lawton et al. [16] compared early and long-term outcomes between patients who had acute type A aortic dissection repair according to a protocol based on open distal repair, no aortic cross-clamp use and antegrade perfusion during the rewarming phase (Group 1, 49 patients), and all the cases not fulfilling at least one of these criteria (Group 2, 147 patients). They reported a significant better survival in patients in Group 1; however, there was no difference in terms of postoperative complications, including neurological injuries and 30-day mortality.
Several studies have focused on the prognostic significance of a patent distal false lumen, which has been reported as a significant risk factor for progressive dilatation of the distal aortic segments [17–20], late aortic reoperation [17, 20, 21] and reduced survival [20–23]. However, the majority of these studies did not investigate the role of different operative techniques. We found a significant higher rate of complete false lumen thrombosis in the distal aorta of patients who underwent an open distal repair, and confirmed the results reported by Nguyen et al. [6] and Bernard et al. [24]. The possibility of having left in the closed anastomosis group an unresected primary tear behind the clamp is limited at 2 cases (Table 2) and it is not surprising that these patients showed a patent false lumen. Probably, the explanation of the positive evolution of the distal aortic false lumen in patients who had an open anastomosis can be more convincingly found in the systematic resection of the clamp area and in the possibility to extend the repair at the lesser curvature of the aortic arch or at the whole arch. Why this finding is not associated with a lower rate of distal aortic reoperations could be justified by other factors we were not able to characterize, such as clinical reasons for proposing or denying a reoperation and pre-existent extensive aneurysmatic disease.
An appropriate procedure in the comfort zone of the operating surgeon, guided by the clinical presentation and the evaluation of anatomical features should be always undertaken for patient safety. Our experience showed that a surgical treatment providing the resection of the primary tear and the proximal thoracic aorta is safe regardless of the approach for distal repair. Patient's survival remains undoubtedly the first goal in acute type A aortic dissection (ATAAD) surgery but, especially in younger patients, if we want to look ahead at a radical treatment of the aortic dissection and at a favourable evolution of the unresected aortic segments, a more extensive procedure with an open distal repair and the aid of the HCA are warranted. These findings were even more important in the presence of a dilated aortic arch, the involvement of the supra-aortic vessels or a complex distal tear. We are moving towards this direction and we have recently performed our first cases of ATAAD repair with the simultaneous antegrade stenting of the thoracic descending aorta. However, a closed anastomosis with potential shorter operative times will still represent in our armamentarium a safe and quick surgical option especially in frail or elderly patients who present an intimal tear in the ascending aorta.
Limitations of the study
This is a single institution retrospective study. Although the clinical presentation was similar between the two groups of patients, we reported significant differences in terms of extension of the aortic dissection and location of the primary tear. This finding obviously reflects the common policy of identifying and resecting the primary intimal tear. For this reason, we further analysed the population with Type 1 DeBakey dissection and a tear located at the level of the aortic root and the ascending aorta. The imaging follow-up was incomplete although it represents the largest actually available in literature focusing on the late evolution of unresected aortic segments according to the distal repair strategy.
CONCLUSIONS
There was no difference in early and late survival between patients receiving an open distal anastomosis and patients treated with a closed anastomosis. Although on the prognostic ground these results seemed suggesting similar safety and effectiveness for these two strategies, we should consider some important findings coming from our analysis:
The two groups of patients were undoubtedly characterized by different anatomical presentations of acute type A aortic dissection with a more extensive disease in patients who finally underwent an open repair.
There was a significant difference in terms of postoperative neurological complications according to the strategy for cerebral protection in the open distal group. The adjunct of the cerebral perfusion was associated with a reduced rate of postoperative neurological deficits.
Patients who received an open distal anastomosis showed a higher rate of complete false lumen thrombosis.
On the basis of patients' survival, a closed anastomosis represents a safe strategy for the repair of aortic dissection with a proximal intimal tear. The construction of the distal anastomosis during a period of HCA allows a more extensive resection of pathological tissue and satisfactory results in terms of survival and occurrence of postoperative complications even in cases that are more complex. The adjunct of cerebral perfusion guarantees a better cerebral protection. The resection of the clamp area, or the avoidance of the cross-clamp, may promote the thrombosis of the false lumen of the distal unresected aortic segments.
SUPPLEMENTARY MATERIAL
Conflict of interest: none declared.
Supplementary Material
REFERENCES
- 1.Bonser RS, Ranasinghe AM, Loubani M, Evans JD, Thalji NMA, Bachet JE et al. . Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol 2011;58:2455–74. [DOI] [PubMed] [Google Scholar]
- 2.Vohra HA, Modi A, Barlow CW, Ohri SK, Livesey SA, Tsang GMK. Repair of acute type A aortic dissection: results in 100 patients. Asian Cardiovasc Thorac Ann 2012;20:160–7. [DOI] [PubMed] [Google Scholar]
- 3.Danner BC, Natour E, Horst M, Dikov V, Ghosh PK, Dapunt OE. Comparison of operative techniques in acute type A aortic dissection performing the distal anastomosis. J Card Surg 2007;22:105–10. [DOI] [PubMed] [Google Scholar]
- 4.Lai DT, Robbins RC, Mitchell S, Moore KA, Oyer PE, Shumway NE et al. . Does profound hypothermic circulatory arrest improve survival in patients with acute type A aortic dissection. Circulation 2002;106:I-218-I-228. [PubMed] [Google Scholar]
- 5.Stamou SC, Kouchoukos NT, Hagberg RC, Khabbaz KR. Does the technique of distal anastomosis influence clinical outcome in acute type A aortic dissections? Interact CardioVasc Thorac Surg 2011;12:404–8. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen B, Muller M, Kipfer B, Berdat P, Walpoth B, Althaus U et al. . Different techniques of distal aortic repair in acute type A dissection: impact on late aortic morphology and reoperation. Eur J Cardiothorac Surg 1999;15:496–501. [DOI] [PubMed] [Google Scholar]
- 7.Myrmel T, Lai DTM, Miller DC. Can the principles of evidence-based medicine be applied to the treatment of aortic dissections? Eur J Cardiothorac Surg 2004;25:235–42. [DOI] [PubMed] [Google Scholar]
- 8.Bavaria JE, Woo YJ, Hall RA, Wahl PM, Acker MA, Gardner TJ. Circulatory management with retrograde cerebral perfusion for acute type A aortic dissection. Circulation 1996;94(9 Suppl):II173–6. [PubMed] [Google Scholar]
- 9.Yamashita C, Okada M, Ataka K, Yoshida M, Yoshimura N, Azami T et al. . Open distal anastomosis in retrograde cerebral perfusion for repair of ascending aortic dissection. Ann Thorac Surg 1997;64:665–9. [DOI] [PubMed] [Google Scholar]
- 10.Kipfer B, Striffeler H, Gersbach P, Mohadjer A, Gerber B, Schtipbach P et al. . Surgery for acute ascending aortic dissection: closed versus open distal aortic repair. Eur J Cardiothorac Surg 1995;9:248–52. [DOI] [PubMed] [Google Scholar]
- 11.Wiedemann D, Kocher A, Dorfmeister M, Vadehra A, Mahr S, Laufer G et al. . Effect of cerebral protection strategy on outcome of patients with Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2013;146:647–55. [DOI] [PubMed] [Google Scholar]
- 12.Etz CD, von Aspern K, da Rocha e Silva J, Girrbach FF, Leontyev S, Luehr M et al. . Impact of perfusion strategy on outcome after repair for acute type A aortic dissection. Ann Thorac Surg 2014;97:78–86. [DOI] [PubMed] [Google Scholar]
- 13.Algarni KD, Yanagawa B, Rao V, Yau TM. Profound hypothermia compared with moderate hypothermia in repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2888–94. [DOI] [PubMed] [Google Scholar]
- 14.Immer FF, Aydin NB, Lütolf M, Krähenbühl ES, Stalder M, Englberger L et al. . Does aortic crossclamping during the cooling phase affect the early clinical outcome of acute type A aortic dissection? J Thorac Cardiovasc Surg 2008;136:1536–40. [DOI] [PubMed] [Google Scholar]
- 15.Comas GN, Leshnower BG, Halkos ME, Thourani VH, Puskas JD, Guyton RA et al. . Acute type A dissection: impact of antegrade cerebral perfusion under moderate hypothermia. Ann Thorac Surg 2013;96:2135–41. [DOI] [PubMed] [Google Scholar]
- 16.Lawton JS, Liu J, Kulshrestha K, Moon MR, Damiano RJ, Maniar H et al. . The impact of surgical strategy on survival after repair of type A aortic dissection. J Thorac Cardiovasc Surg 2015;150:294–301. [DOI] [PubMed] [Google Scholar]
- 17.Immer FF, Hagen U, Berdat PA, Eckstein FS, Carrel TP. Risk factors for secondary dilatation of the aorta after acute type A aortic dissection. Eur J Cardiothorac Surg 2005;27:654–7. [DOI] [PubMed] [Google Scholar]
- 18.Zierer A, Voeller RK, Hill KE, Kouchoukos NT, Damiano RJ Jr, Moon MR. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg 2007;84:479–87. [DOI] [PubMed] [Google Scholar]
- 19.Fattouch K, Sampognaro R, Navarra E, Caruso M, Pisano C, Coppola G et al. . Long-term results after repair of type A acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244–50. [DOI] [PubMed] [Google Scholar]
- 20.Kimura N, Itoh S, Yuri K, Adachi K, Matsumoto H, Yamaguchi A et al. . Reoperation for enlargement of the distal aorta after initial surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2015;149:S91–8. [DOI] [PubMed] [Google Scholar]
- 21.Concistrè G, Casali G, Santaniello E, Montalto A, Fiorani B, Dell'Aquila A et al. . Reoperation after surgical correction of acute type A aortic dissection: risk factor analysis. Ann Thorac Surg 2012;93:450–5. [DOI] [PubMed] [Google Scholar]
- 22.Halstead JC, Meier M, Etz C, Spielvogel D, Bodian C, Wurm M et al. . The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127–35. [DOI] [PubMed] [Google Scholar]
- 23.Evangelista A, Salas A, Ribera A, Ferreira-González I, Cuellar H, Pineda V et al. . Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012;125:3133–41. [DOI] [PubMed] [Google Scholar]
- 24.Bernard Y, Zimmermann H, Chocron S, Litzler JF, Kastler B, Etievent JP et al. . False lumen patency as a predictor of late outcome in aortic dissection. Am J Cardiol 2001;87:1378–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


