Abstract
OBJECTIVES
Sutureless and rapid-deployment valves were recently introduced into clinical practice. The Edwards INTUITY valve system is a combination of the Edwards Magna pericardial valve and a subvalvular stent-frame to enable rapid deployment. We performed a parallel cohort study for comparison of the two valve types.
METHODS
All patients receiving either an Edwards Magna Ease valve or an Edwards INTUITY valve system due to aortic stenosis from May 2010 until July 2014 were included. Patients undergoing bypass surgery, an additional valve procedure, atrial ablation surgery or replacement of the ascending aorta were excluded. Preoperative characteristics, operative specifications, survival, valve-related adverse events and transvalvulvar gradients were compared.
RESULTS
One hundred sixteen patients underwent rapid-deployment aortic valve replacement [mean age 75 years (SD: 8); 62% female] and 132 patients underwent conventional aortic valve replacement [70 years (SD: 9); 31% female; P < 0.001]. Conventional valve patients were taller and heavier. The mean EuroSCORE II was 3.1% (SD: 2.7) and 4.4% (SD: 6.0) for rapid-deployment and conventional valve patients, respectively (P = 0.085). The mean implanted valve size was higher in the conventional group [23.2 mm (SD: 2.0) vs 22.5 mm (SD: 2.2); P = 0.007], but postoperative transvalvular mean gradients were comparable [15 mmHg (SD: 6) vs 14 mmHg (SD: 5); P = 0.457]. A subgroup analysis of the most common valve sizes (21 and 23 mm; implanted in 63% of patients) revealed significantly reduced mean postoperative transvalvular gradients in the rapid-deployment group [14 mmHg (SD: 4) vs 16 mmHg (SD: 5); P = 0.025]. A significantly higher percentage received minimally invasive procedures in the rapid-deployment group (59 vs 39%; P < 0.001). The 1- and 3-year survival rate was 96 and 90% in the rapid-deployment group and 95 and 89% in the conventional group (P = 0.521), respectively. Valve-related pacemaker implantations were more common in the rapid-deployment group (9 vs 2%; P = 0.014) and postoperative stroke was more common in the conventional group (1.6 vs 0% per patient year; P = 0.044).
CONCLUSIONS
We conclude that this rapid-deployment valve probably facilitates minimally invasive surgery. Furthermore, a subgroup analysis showed reduced transvalvular gradients in smaller valve sizes compared with the conventionally implanted valve of the same type. The favourable haemodynamic profile and the potentially different spectrum of valve-related adverse events should be addressed in further clinical trials.
Keywords: Aortic valve replacement, Rapid deployment, Transvalvular gradient, Sutureless valve, Minimally invasive surgery
INTRODUCTION
Aortic valve replacement (AVR) is one of the most common procedures in cardiovascular medicine. The range of available prostheses changed significantly during the last decades in favour of biological valve substitutes. This is partly due to the ageing patient population, which reveals excellent survival with current biological prostheses [1]. Biological valve substitutes are also increasingly implanted in younger patients due to a higher durability enabled by improved anti-calcification treatment and the adverse events associated with mechanical prostheses [2–4]. A major advance in the surgical technique was the introduction of minimally invasive procedures for valve surgery [5]. Isolated AVR can be performed with minor procedural adaptations through an upper hemi-sternotomy or with advanced surgical techniques via anterior right thoracotomy (ART). However, minimally invasive AVR has been associated with longer aortic cross-clamp times compared with conventional surgery due to demanding valve exposure and time-consuming suture placement [6].
Recently, rapid-deployment biological aortic valves were approved for routine clinical use [7, 8]. These rapid-deployment systems offer several potential advantages over standard biological prostheses, including reduced procedural time and facilitated implantation in minimally invasive procedures [9]. Our centre participated in the market release trial of the Edwards INTUITY valve system, which was subsequently standardized at our department and the majority of staff surgeons was trained for valve implantation [10]. We hypothesized that the rapid-deployment valves are faster to implant and may also have a reduced transvalvular gradient due to a valve fixation system without pledgets. This hypothesis was pre-specified prior to the analysis. We report here a direct, single-centre comparison of the Edwards Magna Ease valve and its rapid-deployment successor regarding survival, reoperation rate, valve-related adverse events and echocardiographic data.
MATERIALS AND METHODS
Study population
All consecutive patients undergoing isolated AVR with either an Edwards rapid-deployment valve system (all generations) or a Carpentier-Edwards Magna Ease pericardial prosthesis (Edwards Lifesciences, Irvine, CA, USA) during the same time period starting after the introduction of the rapid-deployment valve system between May 2010 and July 2014 at a university hospital were included in this analysis. Patients receiving the rapid-deployment valve were initially included in the TRITON market release trial (Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve, clinical trial number: NCT01445171 on http://clinicaltrial.gov) and thereafter part of the FOUNDATION post-market release registry (Assessing standard of care and clinical Outcomes using the EDWARDS INTUITY valve system in a European multicentre, active, post‐market surveillance study, clinical trial number: NCT02338154 on http://clinicaltrial.gov). The inclusion and exclusion criteria of both trials are provided in Supplementary Material. Furthermore, 26 patients who received the rapid-deployment valve outside the TRITON or FOUNDATION trial were included in this analysis. Patients received a conventional valve as the standard of care at our department. Only some of them were contraindicated for one of the clinical trials because they either did not meet the inclusion criteria of the clinical trials or preferred having a conventional prosthesis implanted. The surgeon decided on the type of the conventional valve prosthesis based on his or her preference independent of this analysis. However, only patients receiving the Edwards Magna Ease pericardial prosthesis were included in this analysis. All patients requiring concomitant coronary bypass, valve, aortic replacement or atrial ablation surgery were excluded. Patients with root and/or annular enlargement or aortic reduction plasty were included in the analysis. Patients in TRITON and FOUNDATION were followed according to the study protocol [7]. Patients who were not part of these studies were followed in our institutional prospective INTUITY registry and in case of the conventional valve, by our institutional routine protocol.
Surgical techniques
Surgical techniques have been described previously [7]. In brief, the majority of our patients underwent 64-slice computed tomography as part of routine preoperative evaluation at our centre to identify anatomical features that may increase the risk of stroke (calcifications and soft plaques) and to determine whether the patient was suitable for the ART approach. An upper hemi-sternotomy incision angled into the right third intercostal space was performed in a routine fashion. ART was performed through a 7- to 8-cm skin incision at the level of the second or third intercostal space with medial detachment of the third rib from the sternum. Direct aortic and venous cannulation was favoured in minimally invasive procedures. Custodiol solution was applied in these patients for cardioplegia (Custodiol HTK, Dr Köhler Pharma, Vienna, Austria). A hockey-stick aortotomy extending into the non-coronary sinus was used to access the valve, and calcium debridement and excision of the diseased aortic valve leaflets were performed. For the INTUITY valve, three guiding sutures without pledgets placed in a 120° orientation were passed through the nadir of the aortic annulus and the corresponding part of the prosthesis' sewing ring. The INTUITY valve was deployed and its correct position confirmed under a direct vision. The delivery system was removed and the three guiding sutures were tied. Conventional valves were implanted by multiple, braided multifilament 2-0 pledgeted U-sutures by a non-everting technique. Every patient in both groups received an intraoperative transoesophageal echocardiogram prior to and after valve implantation.
Data management
The internal review board approved the clinical and observational studies reported here (TRITON: EK 887/2009, FOUNDATION: EK 2052/2012, Conventional Valves EK 955/2011). Informed patient consent was obtained for clinical studies and waived for the postoperative observational cohort. Patients' characteristics and risk factors were documented prospectively in the electronic data capture system of our institution (Cardiac, S2-Engineering, Steyr, Austria). Risk scores (additive and logistic EuroSCORE as well as the EuroSCORE II after its introduction) were calculated and stored. The annular diameter was measured in the subgroup of patients with a preoperative CT scan. A mean diameter was calculated out of four rectangular and diagonal measurements for each patient. Follow-up was performed in accordance with current guidelines for reporting mortality and morbidity after heart valve surgery [11]. All patients were routinely required to perform a postoperative follow-up visit after the rehabilitation process. Patients in a clinical study protocol were followed accordingly. In addition, all postoperative clinical contacts in public hospitals were assessed. Every patient was contacted for study purposes by telephone to complete follow-up. The follow-up time was in the range of 0–5.1 years in both groups, with a mean time of survival follow-up of 2.0 years (SD: 1.3) in the rapid-deployment AVR (RD-AVR) group and 2.9 years (SD: 1.2) in the conventional AVR (C-AVR) group (P = 0.005). The databank's closing interval was from July 2015 to August 2015 (8 weeks).
Mortality
We included all deaths after valve implantation regardless of the cause for the calculation of overall mortality. Early mortality was defined as every death during the first 30 days after the procedure. Furthermore, cardiac- and valve-related deaths were analysed. Patient survival status was also cross-checked with the countrywide database maintained by the national statistical institute (Statistics Austria, Vienna, Austria).
Morbidity
Valve-related adverse events including structural valve deterioration, non-structural valve deterioration, endocarditis, bleeding, valve thrombosis, thromboembolism (stroke, transient ischaemic attack and peripheral emboli), pacemaker implantation and myocardial infarction were assessed during follow-up according to the current guidelines [11]. Reoperations were categorized according to the underlying pathology into reoperations for structural valve disease, non-structural valve disease, valve thrombosis and endocarditis. Early surgical exploration was separated into revision for bleeding (intrathoracic bleeding or haematoma requiring re-thoracotomy or subxiphoidal drainage) and revision for myocardial ischaemia (ischaemic event leading to acute bypass surgery). Three (rapid-deployment) and nine (conventional) percent of patients were lost to follow-up for valve-related complications after the early postoperative period (P = 0.121).
Statistical analysis
Descriptive statistical methods were applied to depict the study population regarding preoperative risk factors. Continuous variables were presented as mean and standard deviation (SD) and compared by the independent samples t-test between valve types. Total numbers and proportions were reported for categorical outcomes and compared with the χ2 test between treatment groups. The Kaplan–Meier method with a log-rank test was performed to compare survival and valve-related events. The average linearized event rates per patient year of adverse event follow-up were calculated for valve-related events. To assess a potential independent effect of the novel valve prosthesis on postoperative gradients, a multiple linear regression model was applied comprising body surface area, valve size and valve type. The residuals were inspected and there was no violation of the assumptions required for linear regression analysis. Scatterplots were added to Supplementary Material. IBM SPSS Statistics 21 (IBM Corp., Released 2012, IBM SPSS Statistics for Mac, Version 21.0, Armonk, NY, USA) was used for statistical analysis. A P-value less than 0.05 was considered as significant.
RESULTS
The study population consisted of 132 patients who underwent C-AVR and 116 patients who underwent RD-AVR. The study populations differed considerably regarding baseline characteristics, due to the inclusion and exclusion criteria of the TRITON trial and, during the early study period, the unavailability of large valve sizes for the rapid-deployment valve (Table 1). Significantly more male patients were included in the conventional group. Patients in the C-AVR group were significantly taller [171 cm (SD: 8) vs 168 cm (SD: 8); P = 0.003] and heavier [84 kg (SD: 15) vs 79 kg (SD: 16); P = 0.008], which resulted in an increased valve size [23.2 mm (SD: 2.0) vs 22.5 mm (SD: 2.2); P = 0.007].
Table 1:
Preoperative patient characteristics
| Factor | RD-AVR | C-AVR | P-value |
|---|---|---|---|
| Age [(SD), years] | 75 (8) | 70 (9) | <0.001 |
| Sex (f/m) | 71 (62%)/45(38%) | 41 (31%)/91(69%) | <0.001 |
| Height [(SD), cm] | 168 (8) | 171 (8) | 0.003 |
| Weight [(SD), kg] | 79 (16) | 84 (15) | 0.008 |
| Body mass index [(SD), kg/m2] | 27.86 (5.48) | 28.64 (4.86) | 0.234 |
| Body surface area [(SD), m2] | 1.88 (0.20) | 1.96 (0.19) | 0.001 |
| NYHA III and IV | 76 (66%) | 90 (68%) | 0.726 |
| Additive EuroSCORE [(SD), %] | 7 (2) | 7 (3) | 0.217 |
| Logistic EuroSCORE [(SD), %] | 9.3 (9.1) | 9.3 (10.1) | 0.987 |
| EuroSCORE II [(SD), %] | 3.1 (2.7) (n = 91) | 4.4 (6.0) (n = 69) | 0.085 |
| Ejection fraction [(SD), %] | 57 (10) | 55 (11) | 0.107 |
| Ejection fraction > 50%, n (%) | 100 (90%) | 102 (82%) | 0.170 |
| Mean preoperative gradient [(SD), mmHg] | 62 (17) | 56 (16) | 0.005 |
| Smoking (all time) | 24 (21%) | 35 (27%) | 0.282 |
| Diabetes | 34 (29%) | 41 (31%) | 0.311 |
| Dyslipidaemia | 72 (62%) | 64 (49%) | 0.032 |
| Dialysis | 0 (0%) | 2 (2%) | 0.183 |
| Cerebrovascular disease | 12 (10%) | 19 (14%) | 0.336 |
| Cerebrovascular event | 7 (6%) | 10 (8%) | 0.508 |
| Endocarditis | 0 (0%) | 3 (2%) | 0.102 |
| Peripheral vascular disease | 6 (5%) | 9 (7%) | 0.587 |
| Previous cardiovascular interventions | 14 (12%) | 18 (14%) | 0.713 |
| Previous valve surgery | 1 (1%) | 5 (4%) | 0.135 |
| Previous bypass surgery | 0 (0%) | 3 (2%) | 0.102 |
| Previous pacemaker implantation | 7 (6%) | 5 (4%) | 0.411 |
Continuous data are presented as the mean and standard deviation (SD); categorical data as total number and percentage.
RD-AVR: rapid-deployment aortic valve replacement; C-AVR: conventional aortic valve replacement.
We measured the annular diameter in a subgroup of patients with a preoperative CT scan (n = 103) and were able to show a trend towards a larger annular diameter in the conventional group [24.3 mm (SD: 2.1) vs 23.7 mm (SD: 1.7); P = 0.082]. The implanted valve size showed a strong correlation with the annular diameter (Pearson's correlation coefficient 0.674; P < 0.001).
Minimally invasive procedures were significantly more common in the RD-AVR group (59 vs 39%; Fig. 1; P < 0.001). Overall cross-clamp, cardiopulmonary bypass or procedural times were comparable between groups (Table 2). A subgroup analysis of patients operated through a full sternotomy revealed significantly reduced aortic cross-clamp time, perfusion time and procedural time in the RD-AVR group (Table 2). Other subgroups, periprocedural specifications and outcomes are also reported in Table 2. A second deployment attempt was necessary in 8% of patients in the rapid-deployment group. No patient required a second pump run; however, 1 patient was reoperated due to severe paravalvular regurgitation on the day after valve implantation (non-structural valve disease; Table 3).
Figure 1:
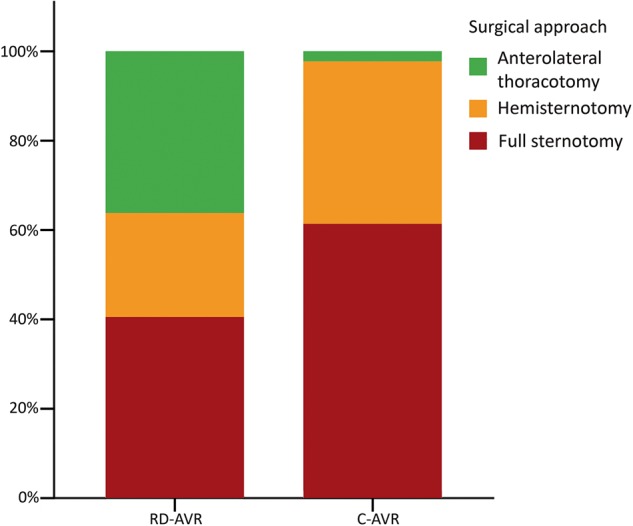
Surgical approach for aortic valve replacement. RD-AVR: rapid-deployment aortic valve replacement; C-AVR: conventional aortic valve replacement; P < 0.001.
Table 2:
Procedural specifications and early follow-up
| Factor | RD-AVR | C-AVR | P-value |
|---|---|---|---|
| Elective procedure | 101 (87%) | 117 (89%) | 0.280 |
| Access | |||
| Full sternotomy | 47 (41%) | 81 (61%) | <0.001 |
| Hemi-sternotomy | 27 (23%) | 48 (36%) | |
| Thoracotomy | 42 (36%) | 3 (2%) | |
| Access conversion | 2 (2%) | 1 (1%) | 0.487 |
| Aortic reduction plasty | 9 (8%) | 5 (4%) | 0.176 |
| Annular enlargement | 0 (0%) | 3 (2%) | 0.102 |
| Cross-clamp time [(SD), min] | 68 (23) | 69 (17) | 0.641 |
| Full sternotomy | 52 (17) | 66 (18) | <0.001 |
| Hemi-sternotomy | 72 (21) | 72 (13) | 0.912 |
| Thoracotomy | 84 (16) | 99 (24) | 0.140 |
| Cardiopulmonary bypass time [(SD), min] | 100 (30) | 101 (28) | 0.827 |
| Full sternotomy | 81 (23) | 98 (27) | <0.001 |
| Hemi-sternotomy | 106 (31) | 101 (23) | 0.427 |
| Thoracotomy | 118 (24) | 180 (52) | <0.001 |
| Procedural time [(SD), min] | 226 (54) | 225 (59) | 0.926 |
| Full sternotomy | 199 (40) | 232 (62) | 0.001 |
| Hemi-sternotomy | 228 (46) | 206 (41) | 0.040 |
| Thoracotomy | 256 (59) | 325 (94) | 0.066 |
| Valve size [(SD), mm] | 22.5 (2.2) | 23.2 (2.0) | 0.007 |
| Paravalvular leak (trivial, mild, moderate, severe) | 6%, 3%, 2%, 0% | 2%, 0%, 0%, 0% | 0.155 |
| Revision for bleeding | 2 (2%) | 3 (2%) | 0.759 |
| Revision for myocardial ischaemia | 0 (0%) | 2 (2%) | 0.183 |
| Early pacemaker implantation | 11 (9%) | 3 (2%) | 0.014 |
Continuous data are presented as the mean and standard deviation (SD); categorical data as total number and percentage.
RD-AVR: rapid-deployment aortic valve replacement; C-AVR: conventional aortic valve replacement.
Table 3:
Overall valve-related outcome regarding adverse events (total number and events per patient year)
| Factor | RD-AVR | C-AVR | P-value |
|---|---|---|---|
| Structural valve dysfunction (reoperation) | 0 (0%) | 0 (0%) | |
| Non-structural valve dysfunction (reoperation) | 1 (0.5%) | 0 (0%) | 0.285 |
| Embolism | |||
| Stroke | 0 (0%) | 5 (1.6%) | 0.044 |
| TIA | 1 (0.5%) | 3 (1.0%) | 0.377 |
| Emboli | 0 (0%) | 0 (0%) | |
| Myocardial infarction | 2 (1.0%) | 0 (0%) | 0.138 |
| Valve thrombosis | 0 (0%) | 0 (0%) | |
| Bleeding event | 1 (0.5%) | 0 (0%) | 0.214 |
| Endocarditis | 1 (0.5%) | 1 (0.3%) | 0.684 |
| Endocarditis (reoperation) | 1 (0.5%) | 0 (0%) | 0.286 |
Total number of events and (%/year) are reported; a Kaplan–Meier analysis with a log-rank test was performed to compare groups.
RD-AVR: rapid-deployment aortic valve replacement; C-AVR: conventional aortic valve replacement; TIA: transient ischemic attack.
One patient died in each study group during the first 30 days (0.9% in the RD-AVR and 0.8% in the C-AVR group; P = 1.000), which was considerably below the predicted surgical risk. The long-term survival rate was, respectively, 96, 90 and 90% at 1, 3 and 5 years after surgery in the RD-AVR group, which was comparable to the C-AVR group (95, 89 and 81%; Fig. 2; P = 0.521). Overall, valve-related and cardiac mortality rates were 6.9% (n = 8), 3.4% (n = 4) and 4.3% (n = 5) in the RD-AVR group and 11.4% (n = 15), 6.1% (n = 8) and 6.1% (n = 8) in the C-AVR group, respectively (P = 0.226).
Figure 2:
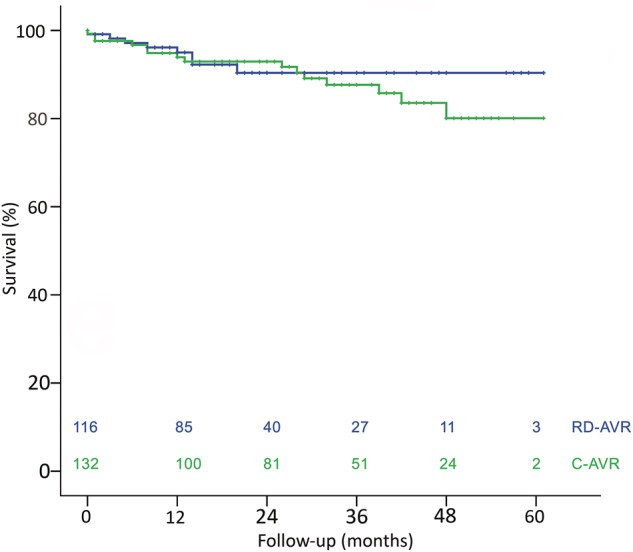
Overall survival (Kaplan–Meier). RD-AVR (blue): rapid-deployment aortic valve replacement; C-AVR (green): conventional aortic valve replacement; numbers at risk are provided for each time period; log-rank test P = 0.521.
Although the mean implanted valve size was larger in the C-AVR group, postoperative mean gradients were comparable between groups [15 mmHg (SD: 6) in the conventional group vs 14 mmHg (SD: 5) in the rapid-deployment group; P = 0.457]. The specific distribution of valve sizes is depicted in Fig. 3 and was also significantly different (χ2 test; P = 0.028). A subgroup analysis of the most common valve sizes was performed (21 and 23 mm; 64% of all implants) and revealed an increased transvalvular gradient in the C-AVR group [16 mmHg (SD: 5) vs 14 mmHg (SD: 4); P = 0.025]. A multiple linear regression analysis including patients' body surface area, valve type and valve size was performed in this subgroup and revealed valve size [regression coefficient = −1.128 (95% CI −2.078 to −0.178); P = 0.020] and valve type [regression coefficient = 1.967 (95% CI 0.034–3.899); P = 0.046], favouring the INTUITY prosthesis, as the relevant independent factors for the observed reduction in mean gradients. The body surface area had no significant effect [regression coefficient = 3.751 (95% CI −1.259 to 8.761); P = 0.141].
Figure 3:
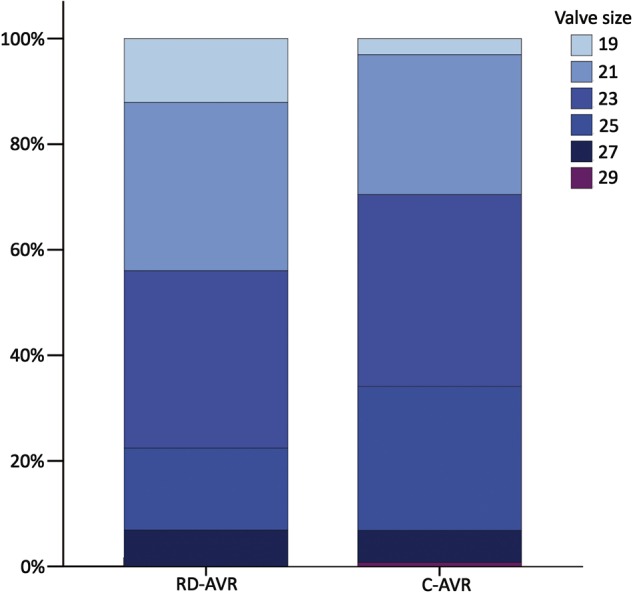
Percentage of patients with a specific valve size per group. RD-AVR: rapid-deployment aortic valve replacement; C-AVR: conventional aortic valve replacement; P = 0.028.
DISCUSSION
This is the first direct comparison of a rapid-deployment biological heart valve to its conventional counterpart. One prior randomized trial (CADENCE-MIS) compared the rapid-deployment system with a range of different conventional prostheses [9]. The Edwards INTUITY valve system combines a new rapid-deployment, stent-based fixation system with an established biological valve prosthesis known for proven long-term durability [4]. Therefore, good early results are a strong predictor of excellent long-term data. Clinical data from the pre-market TRITON trial are promising [7, 10]. A special benefit in addition to the fast delivery may be a low transvalvular gradient in smaller valve sizes. The direct comparison between the two identical valve components but different anchoring mechanisms excludes potential bias regarding valve-specific leaflet tissue or commissural design. Thus, the isolated effect of the new stent-based fixation system can be evaluated and reported in vivo. Furthermore, patients with additional procedures were excluded to improve the outcome comparability. This is of specific interest in analysis of surgical access, procedural times and valve-related outcome. As previously reported, long-term survival and adverse event rates are improved for isolated AVR compared with AVR with concomitant procedures such as coronary bypass grafting because of the additional disease burden in these patients [1]. Thus, we can report two main findings from this institutional trial.
Surgical access
Minimally invasive surgical procedures were significantly more common in the rapid-deployment group. This suggests that the rapid-deployment valve system facilitates a minimally invasive surgical approach. The rapid-deployment system design was enhanced during the study period. For example, a flexible valve delivery handle became available for the later generation that further improves the ease of implantation and may increase the adoption of minimally invasive procedures. The higher ratio of minimally invasive techniques did not negatively impact the overall procedural times (Table 2). Furthermore, we performed a subgroup analysis to compare procedural times according to surgical access. The rapid-deployment valve showed decreased cross-clamp, perfusion and procedural times in full sternotomy cases (Table 2). Individual subgroups with minimally invasive access were smaller and results have to be interpreted with caution. Patients with an ART had a reduced cross-clamp time (which was not significant—but only 3 patients in the C-AVR group) and also a significantly reduced perfusion time. However, cross-clamp time and perfusion time did not differ in the hemi-sternotomy group. We have to state that the handle of the first generation was not flexible, which was a distinct disadvantage for hemi-sternotomy cases. Full sternotomy allows compensation for a stiff handle and ART allows rectangular positioning of a stiff handle to the annular plane and thus parallel alignment to the outflow tract supporting an easier placement of the prosthesis, which is not the case through a hemi-sternotomy. This problem was solved with the introduction of a malleable handle. Furthermore, the learning curve with this new system is also included in the procedural times reported here.
Transvalvular gradient
This study indicates that transvalvular gradients are reduced in the rapid-deployment valve. This was also suggested by previous reports. However, this is the first ever study—although limited by study design—directly comparing these two valves [9, 10]. The difference may, at first, seem counterintuitive given the identical valvular components of the Magna Ease valve and its rapid-deployment successor. But the stent-based fixation system may be the reason for a reduced transvalvular gradient. It is conceivable that the subvalvular stent-frame reshapes the left ventricular outflow tract, which may reduce turbulent flow and optimize the haemodynamic performance of the valve prosthesis. Turbulent flow and subclinical obstruction at the valve inlet may be induced by protrusion of bulky pledget material used to fixate the conventional valve or ventricular septal hypertrophy. Tabata et al. [12] previously demonstrated the negative effect of pledgeted mattress sutures on transvalvular gradients compared with single interrupted sutures. This could also be confirmed in a recent in vitro study [13].
A third potential benefit of the INTUITY valve system is that it allows the introduction of a larger valve size in a comparable annular diameter because of the recommended ‘snug fit’. In contrast, when implanting a conventional valve, the surgeon may be compelled to use a smaller-sized valve in these cases or may even have to perform annular enlargement to avoid prosthesis–patient mismatch [9]. Although we were not able to confirm this hypothesis in our subgroup analysis with a rather small sample size of patients with small prosthesis and a preoperative CT scan, we will address this relevant question in a further analysis at our department.
Most periprocedural adverse events were comparable. The rapid-deployment system had a significantly reduced number of postoperative strokes. However, the rate of pacemaker implantations was increased. Patients with pre-existing bundle branch block or a pre-existing AV block greater than first degree are also reported in this number, which might overestimate the valve-induced component. Surgical details during the implantation of the rapid-deployment valve may alter the positioning of the stent and by that influence the perioperative pacemaker rate. We are therefore currently examining anatomical preparations to further elucidate this question.
Limitations
This study was non-randomized and patient groups differed regarding gender, height, weight and body surface area due to inclusion/exclusion criteria of the clinical trials. The subgroup analyses performed here were not pre-specified and were performed to adjust for the differences between patient groups by applying a multiple linear regression for data interpretation.
Follow-up was more structured in the clinical studies, but a cross-sectional follow-up was performed in all patients for this analysis. The sample size and follow-up time of this trial are probably not sufficient to analyse potential differences of long-term valve-related adverse events. A preoperative computed tomography was performed in the majority of patients to identify aortic calcification preoperatively. The advantages and potential drawbacks of this approach in comparison with preoperative echocardiography of the ascending aorta (when possible) or other strategies are currently under study and were not evaluated in this trial.
CONCLUSION
In conclusion, this rapid-deployment valve probably facilitates minimally invasive surgery. Furthermore, a subgroup analysis showed reduced transvalvular gradients in smaller valve sizes compared with the conventionally implanted valve of the same type. The favourable haemodynamic profile and the potentially different spectrum of valve-related adverse events should be addressed in further clinical trials.
SUPPLEMENTARY MATERIAL
Funding
This study was not funded. However, patients from clinical trials funded by Edwards Lifesciences (Irvine, CA, USA) were included in this analysis.
Conflict of interest: M Andreas and G Laufer received speaking fees from Edwards in 2015. A Kocher is a proctor for Edwards and received speaking fees from Edwards and Medtronic.
Supplementary Material
APPENDIX. CONFERENCE DISCUSSION
Dr M. Vola (St. Etienne, France): From the paper, I saw that all the Magna were sutured with W patched stitches. So probably the 2 mm of gradient that you gain with this sutureless version of the valve may be impacted by these pledgets. Do you think that it is possible, or in your clinical practice you use also the Magna without pledgets or it is 10% of the valve with the pledgets? This is the first question.
Secondly, one concerns one of the most interesting accesses for the future of minimally invasive surgery, I mean, the right anterior minithoracotomy. I would like to know what was the cross-clamping time of the subgroup of the minithoracotomy? Also how do you feel with this valve, how many pop-ups did you have. If you had some pop-ups, if you could see properly the landing zone of the valve? If you think basically then the Intuity was the only way to do a minithoracotomy to begin that program or if firstly you have really to master the minithoracotomy with a sutured valve?
Dr Andreas: First, the pledgets of the pledgeted sutures we use have round edges. The figure in the presentation has other pledgets. We already used stitches with pledgets having a lower profile to reduce the gradient and still we have found this difference. If we would use conventional pledgets I have shown in the picture, which are from a different company, there might be an even higher gradient. I am not so sure if it is a good idea to implant a conventional valve without pledgets, because you may have more para valvular leakage. Our standard is to just use sutures with pledgets.
Now the second question, the minimally invasive program was started before. So we started with conventional valves to do minimally invasive surgery, but it is much easier to gain better views if you have a valve like this which is better to implant, especially if you have big patients with deep thoraxes where it is hard to put a valve in.
What we did observe is, that the first generation was in some patients rather complicated to implant because the handle was not steerable. There was a risk that the handle is not in the right angle to the annular plane in patients operated through a hemi-sternotomy, and this may cause pop-up, as you said. But with the new handle you may easily arrange the valve directly to the annular plane, and this is very important for good placement of the valve.
Dr Vola: I want to know if you can tell us the cross-clamping time in minithoracotomy with this new technology in the subgroup of the minithoracotomy of your series.
Dr Andreas: In the subgroup analysis there is reduced cross clamp time.
Dr J. Seeburger (Leipzig, Germany): I might be a little bit too critical, but you are mixing something up. You are talking about first generation, second generation; you are talking about this approach, that approach; you are talking about haemodynamics felts, or pledgets or no pledgets. So overall you showed actually no difference between the valves. Would you please give us a good argument to use that valve instead of a normal standard valve?
Dr Andreas: Well, I think there are several arguments. First of all, it is faster to implant.
Dr Seeburger: That's not true. It is 68 versus 69 minutes.
Dr Andreas: You have to do a subgroup analysis, which is in the paper. If you do minimally invasive approaches, it takes longer, as we have heard from the first presentation. So we have comparable times.
Dr Seeburger: But the cross-clamp times are still the same.
Dr Andreas: If you use this valve for minimal-invasive surgery, you can do it in the same time compared to a conventional valve in full sternotomy. You can do a higher amount of minimally invasive procedures with the overall same procedural time compared to a conventional valve.
Dr Seeburger: It is not very convincing, I have to say, but that is my opinion.
Dr B. Meuris (Leuven, Belgium): But you did have a much higher proportion of right anterior thoracotomies in your rapid deployment group, so that corrects it a little bit. Is this now your policy? When you want to do a right anterior minithoracotomy you use these rapid deployment valves as a first choice?
Dr Andreas: Yes.
Dr G. Laufer (Vienna, Austria): I did the majority of the anterolateral cases, and I want to correct something. The message from this paper with the methodology used is that there is a difference in gradients. So when you comment that there is no difference, that is not true, because you saw the P-values, and you can now raise questions with this retrospective analysis if that is true. But that is something which is hypothesis generating, and our hypothesis that we want to submit to you is that the sutureless valves have a lower gradient at discharge and also in the long term, as well as there is a marked reduction in cross-clamp time. I think you cannot discuss that away. I think that is a clear message from the manuscript, and if you go into the details of the manuscript, you will be able to see that.
Dr Seeburger: I was just discussing the data he presented. I am not trying to offend you. I wanted to comment that 68 versus 69 minutes, there is not much of a difference, and 21 versus 22 is also not much of a difference. That is all I tried to point out.
Dr Laufer: My final reply to that is you have seen on the slide that there were much more cases done in the anterolateral fashion with the Intuity than with the conventional valves, and if you know that, anterolateral thoracotomy is associated with prolonged cross-clamping time and prolonged bypass time compared to conventional valves, it is clear that the same cross-clamp time does mean different things.
REFERENCES
- 1.Andreas M, Wallner S, Ruetzler K, Wiedemann D, Ehrlich M, Heinze G et al. . Comparable long-term results for porcine and pericardial prostheses after isolated aortic valve replacement. Eur J Cardiothorac Surg 2014;48:557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H et al. . Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1–44. [DOI] [PubMed] [Google Scholar]
- 3.Andreas M, Wiedemann D, Seebacher G, Rath C, Aref T, Rosenhek R et al. . The Ross procedure offers excellent survival compared with mechanical aortic valve replacement in a real-world setting. Eur J Cardiothorac Surg 2014;46:409–13; discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 4.Forcillo J, Pellerin M, Perrault LP, Cartier R, Bouchard D, Demers P et al. . Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg 2013;96:486–93. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove DM III, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596–7. [PubMed] [Google Scholar]
- 6.Brown ML, McKellar SH, Sundt TM, Schaff HV. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2009;137:670–9. e5. [DOI] [PubMed] [Google Scholar]
- 7.Kocher AA, Laufer G, Haverich A, Shrestha M, Walther T, Misfeld M et al. . One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110–5; discussion 15–6. [DOI] [PubMed] [Google Scholar]
- 8.Shrestha M, Folliguet TA, Pfeiffer S, Meuris B, Carrel T, Bechtel M et al. . Aortic valve replacement and concomitant procedures with the Perceval valve: results of European trials. Ann Thorac Surg 2014;98:1294–300. [DOI] [PubMed] [Google Scholar]
- 9.Borger MA, Moustafine V, Conradi L, Knosalla C, Richter M, Merk DR et al. . A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17–25. [DOI] [PubMed] [Google Scholar]
- 10.Haverich A, Wahlers TC, Borger MA, Shrestha M, Kocher AA, Walther T et al. . Three-year hemodynamic performance, left ventricular mass regression, and prosthetic-patient mismatch after rapid deployment aortic valve replacement in 287 patients. J Thorac Cardiovasc Surg 2014;148:2854–60. [DOI] [PubMed] [Google Scholar]
- 11.Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL et al. . Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523–8. [DOI] [PubMed] [Google Scholar]
- 12.Tabata M, Shibayama K, Watanabe H, Sato Y, Fukui T, Takanashi S. Simple interrupted suturing increases valve performance after aortic valve replacement with a small supra-annular bioprosthesis. J Thorac Cardiovasc Surg 2014;147:321–5. [DOI] [PubMed] [Google Scholar]
- 13.Tasca G, Vismara R, Fiore GB, Romagnoni C, Redaelli A, Antona C et al. . Does the type of suture technique affect the fluid-dynamic performance of bioprostheses implanted in small aortic roots? Results from an in vitro study. J Thorac Cardiovasc Surg 2015;149:912–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


