Abstract
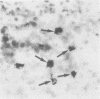
Biotin-avidin systems can be used as an alternative to indirect antibody sandwich methods in the detection of T lymphocyte subsets in cryostat sections of human lymphoid tissue. Appreciable endogenous avidin binding activity (EABA) has been found, however, in human lymph nodes and tonsils. Such EABA can be a source of false positive staining when biotin-avidin detection systems are used to identify cells in cryostat sections. The finding that avidin binding cells may also contain endogenous peroxidase activity and are morphologically similar to histiocytes suggests that such cells may be of histiocytic lineage. EABA is not seen in intrafollicular "tingible body" macrophages, however, and only rarely in medullary sinus histiocytes. Thus further studies are necessary to identify the lineage of avidin binding cells in lymphoid tissues. EABA can be effectively blocked by treatment of cryostat sections with 1% avidin followed by 0.01% biotin before specific staining with biotinylated antibodies and avidin-peroxidase or avidin-fluorochrome conjugates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee D., Thibert R. F. Natural killer-like cells found in B-cell compartments of human lymphoid tissues. Nature. 1983 Jul 21;304(5923):270–272. doi: 10.1038/304270a0. [DOI] [PubMed] [Google Scholar]
- Burns J. Background staining and sensitivity of the unlabelled antibody-enzyme (PAP) method. Comparison with the peroxidase labelled antibody sandwich method using formalin fixed paraffin embedded material. Histochemistry. 1975 Jun 5;43(3):291–294. doi: 10.1007/BF00499711. [DOI] [PubMed] [Google Scholar]
- DAKSHINAMURTI K., MISTRY S. P. Tissue and intracellular distribution of biotin-C-1400H in rats and chicks. J Biol Chem. 1963 Jan;238:294–296. [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Moss J., Lane M. D. The biotin-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]