Abstract
Several forms of long-term depression (LTD) of glutamatergic synaptic transmission have been identified in the dorsal striatum and in the nucleus accumbens (NAc). Such experience-dependent synaptic plasticity might play important roles in reward-related learning. The GABAA receptor agonist muscimol was recently found to trigger a long-lasting depression of glutamatergic synaptic transmission in the NAc of adolescent mice, but the mechanisms that underlie this novel form of LTD were not studied. Here we examined the effect of muscimol applied in the perfusion solution on the amplitude of field excitatory postsynaptic potentials/population spikes (fEPSP/PSs) in mouse brain slices. We found that muscimol depressed the fEPSP/PS in the NAc of adolescent mice but not adult mice, through both postsynaptic and presynaptic mechanisms. Indeed, muscimol altered the fEPSP/PS paired-pulse ratio, depolarized the membrane of projection neurons, and decreased the frequency, but not amplitude, of spontaneous excitatory postsynaptic currents in the NAc of adolescent mice. The LTD induced by muscimol likely involved endocannabinoids, metabotropic glutamate receptors (mGluRs), but not TRPV1 receptors. Muscimol-LTD was occluded by prior induction of LTD through low-frequency stimulation (LFS) of the slice, demonstrating a common pathway in the induction of LFS-LTD and muscimol-LTD. We also found that muscimol induced a form of LTD in the dorsolateral striatum of adult but not adolescent mice. This LTD was mediated by endocannabinoids but did not involve mGluRs or TRPV1 receptors. These results identify a novel form of synaptic plasticity, and its mechanisms of induction, which is age and region dependent. These findings may contribute to a better understanding of the increased susceptibility of the adolescent brain to long-term synaptic changes in regions associated with reward mechanisms.
The nucleus accumbens (NAc) and the dorsal striatum play key roles in reward-motivated behaviors and in motor learning. The NAc integrates motivational information while the dorsolateral part of the striatum (DLS) is involved in habit formation. Drugs of abuse, such as alcohol and psychostimulants, are suggested to mediate their reinforcing effects through alterations of glutamatergic synaptic transmission and plasticity in the NAc and in the dorsal striatum (Hyman et al. 2006; Vengeliene et al. 2008; Everitt and Robbins 2013). A well-described form of long-term depression (LTD) of glutamatergic neurotransmission in the striatal complex involves the production and release of endocannabinoids such as anandamide and 2-arachidonylglycerol. Endocannabinoids were shown to inhibit glutamate release in several brain regions including the NAc (Robbe et al. 2002; Grueter et al. 2010). Several neurotransmitters, such as glutamate, dopamine, adenosine, and serotonin contribute to the induction of endocannabinoid-dependent LTD in the striatum (Lovinger 2010; Tozzi et al. 2011; Lerner and Kreitzer 2012; Burattini et al. 2014). Although GABA is the most important inhibitory neurotransmitter in the brain, few studies have examined its ability to induce synaptic plasticity at glutamatergic synapses (Akhondzadeh and Stone 1995). We recently found that the GABAA receptor agonist muscimol induced a long-lasting inhibition of glutamatergic synaptic transmission in the NAc in adolescent but not adult mice (Mishra and Chergui 2013). Because adolescents might be more sensitive to drug-induced plasticity than adults, further studies were needed to clearly identify age-related differences in the mechanisms that lead to LTD induction in the NAc and possibly also in the striatum. The first aim of our study was to examine the contribution of endocannabinoids as a possible mechanism for the ability of muscimol to induce LTD in the NAc of adolescent mice. Our second aim was to determine if the age-related ability of muscimol to induce LTD was specific for the NAc or if LTD was also triggered in the DLS.
Results
Muscimol induces a form of LTD in the NAc of adolescent, but not adult, mice
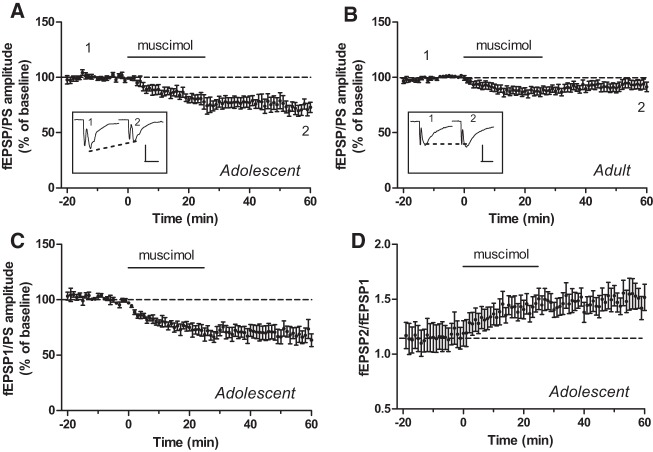
We have examined glutamatergic synaptic transmission in the core region of the NAc in mouse brain slices by recording extracellular field excitatory postsynaptic potentials/population spikes (fEPSP/PSs), as described previously (Schotanus et al. 2006; Schotanus and Chergui 2008). Adolescent mice were 22–30 d old, and adult mice were 5–8 mo old. After a stable baseline fEPSP/PS was recorded for at least 20 min, we applied muscimol in the perfusion solution. We conducted pilot experiments to determine the concentration of muscimol and the duration of its perfusion that induced a stable and reproducible effect on the fEPSP/PS amplitude. We found that 1 µM muscimol applied for 25 min in the perfusion solution produced a long-lasting inhibitory effect on glutamatergic neurotransmission in the NAc of adolescent mice. The amplitude of the fEPSP/PS was decreased to 71.2 ± 4.9% of baseline 55–60 min after the start of the perfusion with muscimol (P < 0.001; n = 8 slices; Figs. 1A, 3F). In contrast, in the NAc of adult mice, the same treatment did not induce a long-lasting depression (92.5 ± 3.8% of baseline; P = 0.08; n = 20 slices; Fig. 1B). To determine whether muscimol had a presynaptic locus of action in the NAc of adolescent mice, we used a paired-pulse stimulation protocol in which two stimuli were delivered at a 20-msec interval. We found that the amplitude of the fEPSP/PS evoked by the first stimulation pulse decreased to a degree similar to that seen in single-pulse experiments (68.8 ± 6.8% of baseline; P < 0.01; n = 7 slices; Fig. 1C). The ratio between the amplitude of the second and the first fEPSP/PS increased (from 1.18 ± 0.11 before muscimol to 1.51 ± 0.11 after muscimol; P < 0.05; n = 7 slices; Fig. 1D) with a similar time course as the effect of muscimol on the first fEPSP/PS. This result suggests that muscimol presynaptically decreases glutamate release in the NAc of adolescent mice.
Figure 1.
The GABAA receptor agonist muscimol induces a form of LTD in the NAc of adolescent but not adult mice. (A,B) The graphs show the time course of the effect of muscimol (1 µM), applied for 25 min in the perfusion solution at the time indicated by the black bar, on the mean (±SEM) amplitude of the fEPSP/PS measured in the NAc of adolescent mice (A; n = 8 slices) and adult mice (B; n = 20 slices). Insets show records of fEPSP/PSs measured in two slices at the time points indicated on the graphs (i.e., before [1] and 50–60 min after [2] the start of perfusion with muscimol). Scale bars: 0.6 mV/10 msec (A) and 0.3 mV/10 msec (B). (C,D) Time course of the effect of muscimol in the NAc of adolescent mice on the amplitude of the first fEPSP/PS (C) and on the ratio between the amplitude of the second fEPSP/PS and the amplitude of the first fEPSP/PS (D) in paired-pulse experiments (n = 7 slices). Data are expressed as a percentage of baseline fEPSP/PS amplitude in A–C.
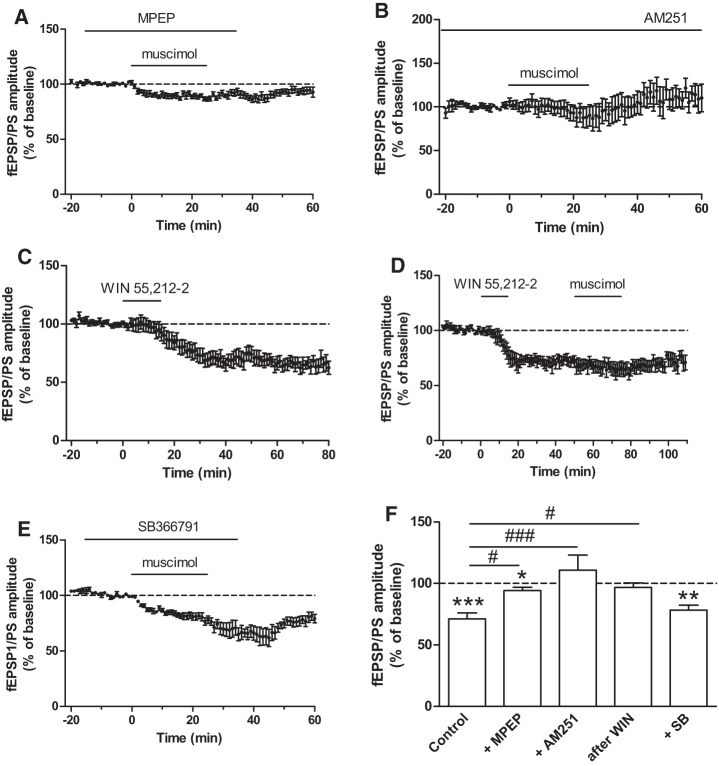
Figure 3.
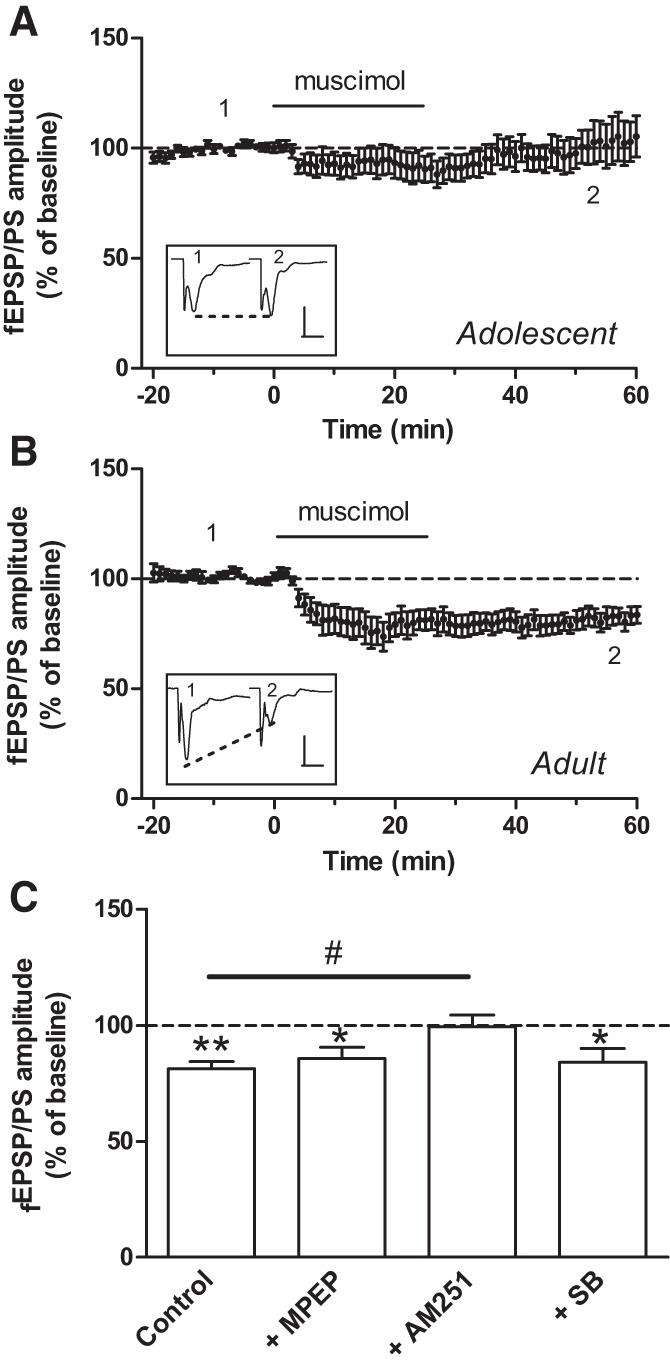
Muscimol-induced LTD in the NAc of adolescent mice is mediated by metabotropic glutamate receptors and endocannabinoids. (A,B) Time course of the effect of muscimol in the NAc of adolescent mice on the amplitude of the fEPSP/PS in the presence of the mGluR5 receptor antagonist MPEP (20 µM, n = 9 slices, graph A) and in the presence of the cannabinoid receptor antagonist AM251 (2 µM, n = 6 slices, graph B). (C) The cannabinoid receptor agonist WIN 55,212-2 (1 µM, for 15 min) induces an LTD in the NAc of adolescent mice (n = 10 slices). (D) LTD induced by WIN 55,212-2 occludes LTD induced by muscimol (n = 7 slices). (E) Time course of the effect of muscimol in the NAc of adolescent mice on the amplitude of the fEPSP/PS in the presence of the TRPV1 receptor antagonist SB366791 (20 µM, n = 8 slices). (F) Averaged effect of muscimol on the fEPSP/PS amplitude in the NAc of adolescent mice in control slices, in the presence of MPEP, AM251, or SB366791 and following LTD induced by WIN 55,212-2 (after WIN). (*) P < 0.05, (**) P < 0.01, (***) P < 0.001 compared with baseline (paired Student's t-test); (#) P < 0.05; (###) P < 0.001 (Dunnett).
Muscimol has pre- and postsynaptic effects in the NAc of adolescent mice
To further identify the locus of action of muscimol, we performed whole-cell patch-clamp recordings from medium spiny projection neurons in the NAc of adolescent mice as done previously (Chergui 2011; Feng et al. 2014). In these neurons, muscimol induced a membrane depolarization (from a resting membrane potential of −85.0 ± 1.5 mV to −71.2 ± 0.7 mV; P < 0.001; n = 5; Fig. 2A) and an inward current (81.8 ± 9.7 pA; n = 6). We measured spontaneous excitatory postsynaptic currents (sEPSCs) in neurons voltage-clamped at −80 mV before and after bath application of muscimol. Muscimol decreased the frequency of sEPSCs (from 3.14 ± 0.3 Hz to 1.76 ± 0.3 Hz; P < 0.001; n = 6; Fig. 2B,D) but did not alter their amplitude (Fig. 2C,D). Taken together with our field recordings, these results show that muscimol has both post- and presynaptic actions on the principal neurons of the NAc of adolescent mice.
Figure 2.
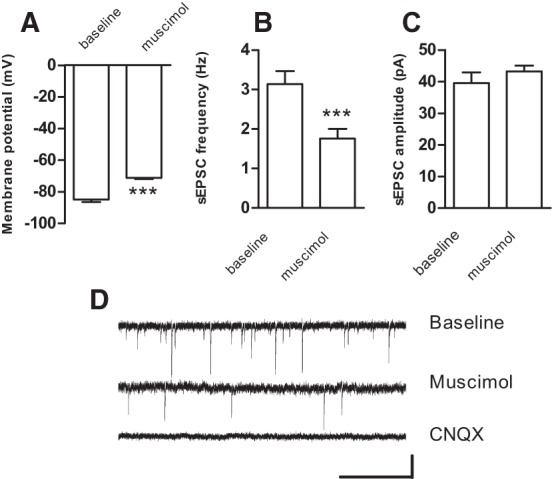
Muscimol has post- and presynaptic effects on medium spiny projection neurons in the NAc of adolescent mice. (A) Membrane potential (mean ± SEM) before and after muscimol (1 µM) measured in five medium spiny projection neurons in the NAc of adolescent mice in whole-cell current-clamp mode. (B,C) Average frequency (B) and amplitude (C) of sEPSCs measured in six medium spiny projection neurons in the NAc of adolescent mice before (baseline) and after muscimol application. (***) P < 0.001 compared with baseline (paired Student's t-test). (D) sEPSCs recorded in whole-cell voltage-clamp mode (at −80 mV) in one medium spiny projection neuron before (upper trace) and after perfusion with muscimol (1 µM, middle trace). Bath application of the AMPA receptor antagonist CNQX (10 µM, lower trace) eliminates all sEPSCs, demonstrating that the events measured are mediated by spontaneous release of glutamate. Scale bars: 20 pA/1 sec.
LTD induced by muscimol is mediated by metabotropic glutamate receptors and endocannabinoids
The long-lasting inhibitory action of muscimol on glutamatergic synaptic transmission resembles endocannabinoid-dependent LTD previously demonstrated in the NAc, following the activation of, for example, group I metabotropic glutamate receptors (mGluRs) (Lovinger et al. 2003; Robbe et al. 2003; Thomas and Malenka 2003). We examined whether the muscimol-induced depression observed in the NAc of adolescent mice involved group I mGluRs and endocannabinoids. We found that muscimol-induced LTD was significantly reduced in the presence of the mGluR5 antagonist MPEP (20 µM; 94.2 ± 2.7% of baseline; n = 9 slices; P < 0.05 compared with control slices; Fig. 3A,F). In the presence of the cannabinoid receptor 1 (CB1) antagonist/inverse agonist AM251 (2 µM), muscimol did not depress synaptic transmission (110.8 ± 12.4% of baseline; P < 0.001 compared with control slices; n = 6 slices; Fig. 3B,F). These results suggest that muscimol induces an mGluR5- and endocannabinoid-dependent form of LTD in the NAc of adolescent mice.
To further determine the involvement of endocannabinoids in muscimol-induced LTD, we examined whether the cannabinoid receptor agonist WIN 55,212-2 mimicked and occluded the muscimol-induced depression. We found that WIN 55,212-2 (1 µM, applied for 15 min in the perfusion solution) induced LTD in the NAc of adolescent mice. Indeed, WIN 55,212-2 produced a long-lasting depression of the fEPSP/PS amplitude (% of baseline: 66.5 ± 6.3; P < 0.001; n = 10 slices; Fig. 3C). In a separate set of experiments, we found that muscimol bath applied during the synaptic depression induced by WIN 55,212-2 did not further decrease the amplitude of the fEPSP/PS (96.7 ± 3.8% of baseline before muscimol; P < 0.05 compared with control slices; n = 7 slices; Fig. 3D,F). These results show that the activation of cannabinoid receptors by WIN 55,212-2 induces LTD, which occludes muscimol-induced LTD. The effect of WIN 55,212-2 was age dependent because, as shown recently (Zhang et al. 2015), this compound failed to induce LTD in the NAc of adult mice (percent of baseline: 90.6 ± 16.2; P = 0.68; n = 7 slices, not shown).
We have examined the contribution of the vanilloid receptor member of the transient receptor potential superfamily of ion channels (TRPV1) in muscimol-induced LTD given that these receptors mediate a form of LTD in a subpopulation of medium spiny projection neurons in the NAc (Grueter et al. 2010). We found that in the presence of the TRPV1 receptor antagonist SB366791 (20 µM), muscimol was still able to induce LTD in the NAc of adolescent mice (78.4 ± 4.0% of baseline; P = 0.78 compared with control slices; n = 8 slices; Fig. 3E,F), showing that TRPV1 receptors do not play a role in muscimol-induced LTD.
LTD induced by low-frequency stimulation occludes muscimol-induced LTD
We tested the possibility that the LTD induced by muscimol involved similar pathways as LTD induced by electrical stimulation of the slice, which leads to neurotransmitter release. Several reports have identified stimulation protocols that can elicit endocannabinoid-dependent LTD in the NAc. We used a low-frequency stimulation (LFS) that consisted of a 20 min-long 4 Hz train as described earlier (Burattini et al. 2014). We confirmed that this protocol induced an LTD in the NAc of adolescent mice (61.7 ± 12.1% of baseline; P < 0.01 compared with baseline; n = 8 slices; Fig. 4A). We then tested whether this form of LTD could occlude the LTD induced by muscimol. We found that after LFS-LTD was established, muscimol failed to produce a long-lasting decrease in the fEPSP/PS amplitude (102.4 ± 10.0% of baseline during LFS-LTD; P = 0.94; n = 5 slices; Fig. 4B,C). Thus, muscimol-induced LTD observed in control slices is occluded by LFS-LTD. This result suggests that the LTD triggered by LFS and the LTD induced by muscimol share similar mechanisms of induction.
Figure 4.
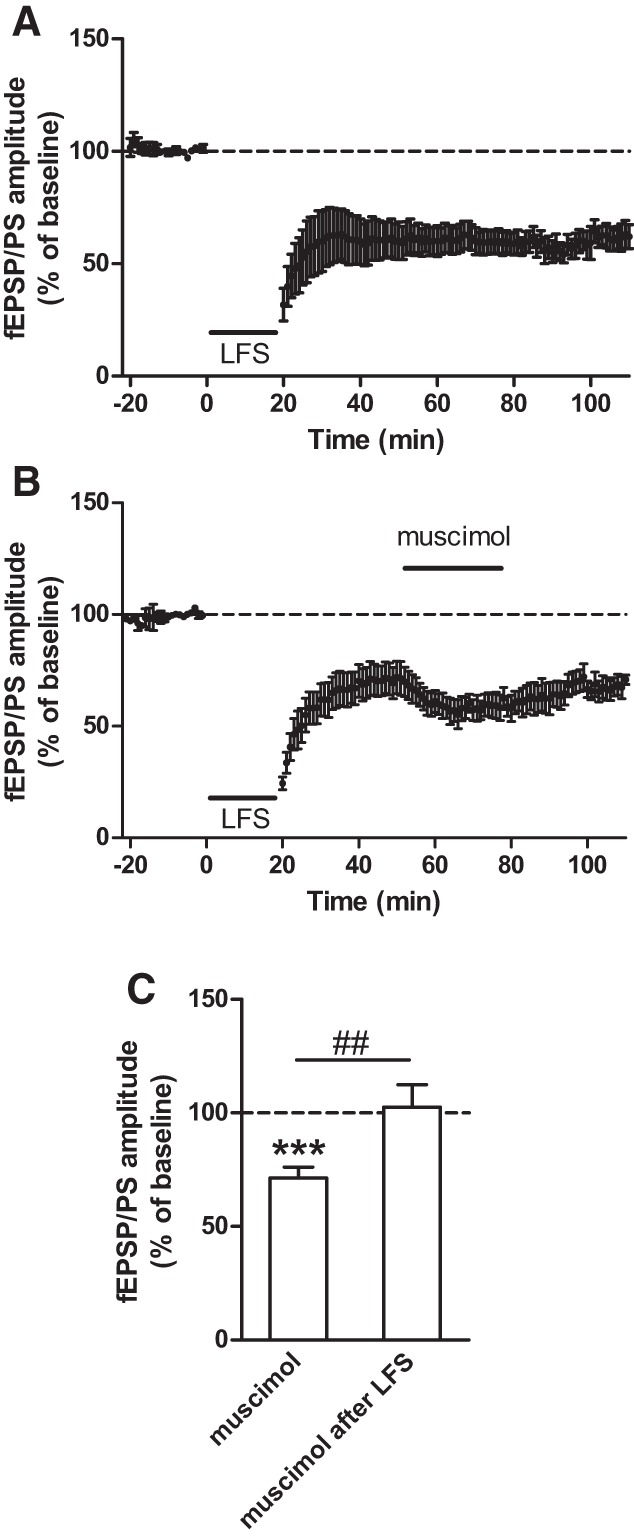
LTD induced by low-frequency stimulation occludes muscimol-induced LTD in the NAc of adolescent mice. (A) LTD induced in the NAc of adolescent mice by low-frequency stimulation (LFS), applied at the time indicated by the black horizontal bar (n = 8 slices). (B) LTD induced by LFS occludes LTD induced by muscimol (n = 5 slices). (C) Averaged effect of muscimol on the fEPSP/PS amplitude in the NAc of adolescent mice in control slices (from Fig. 3F) and following LTD induced by LFS. (***) P < 0.001 compared with baseline (paired Student's t-test); (##) P < 0.01 (unpaired Student's t-test).
Muscimol-LTD is induced in the DLS of adult but not adolescent mice
We asked whether muscimol-induced LTD was region specific, and we examined the ability of muscimol to affect glutamatergic synaptic transmission in the DLS. In the DLS of adolescent mice, muscimol failed to induce LTD (103.8 ± 10.0% of baseline; P = 0.83; n = 10 slices; Fig. 5A). However, in the DLS of adult mice, muscimol induced a long-lasting inhibition of the fEPSP/PS (81.3 ± 3.1% of baseline; P < 0.01; n = 13 slices; Fig. 5B,C). This inhibition was not observed in the DLS of adult mice in the presence of the CB1 receptor antagonist AM251 (2 µM; 99.5 ± 5.1% of baseline; P = 0.82; n = 6 slices; Fig. 5C). However, LTD was still induced by muscimol in the presence of the mGluR5 receptor antagonist MPEP (20 µM; 85.8 ± 4.7% of baseline; P < 0.05; n = 5 slices; Fig. 5C) and of the TRPV1 receptor antagonist SB366791 (20 µM; 84.1 ± 5.9% of baseline; P < 0.05; n = 5 slices; Fig. 5C). As shown recently (Zhang et al. 2015), the cannabinoid receptor agonist WIN 55,212-2 failed to induce LTD in the DLS of adult mice. Indeed, the amplitude of the fEPSP/PS remained unchanged 80 min after perfusion with WIN 55,212-2 (1 µM; % of baseline: 93.5 ± 6.3; P = 0.60; n = 6 slices; not shown). WIN 55,212-2 did not significantly affect the amplitude of the fEPSP/PS in the DLS of adolescent mice either (% of baseline: 107.7 ± 14.1; P = 0.66; n = 9 slices; not shown).
Figure 5.
In the dorsolateral striatum, muscimol fails to induce LTD in adolescent mice but induces a form of LTD in adult mice. (A,B) Time course of the effect of muscimol (1 µM) on the mean (±SEM) amplitude of the fEPSP/PS measured in the DLS of adolescent mice (A, n = 10 slices) and adult mice (B, n = 13 slices). Insets show records of fEPSP/PSs measured in two slices at the time points indicated on the graphs, i.e., before (1) and 50–60 min after (2) the start of perfusion with muscimol. Scales bars: 0.6 mV/10 msec (A) and 0.3 mV/10 msec (B). (C) Averaged effect of muscimol on the fEPSP/PS amplitude in the DLS of adult mice in control slices (n = 13) and in the presence of MPEP (20 µM, n = 5 slices), AM251 (2 µM, n = 6 slices), and SB366791 (20 µM, n = 5 slices). (*) P < 0.05, (**) P < 0.01 compared with baseline (paired Student's t-test), and (#) P < 0.05 (Dunnett).
Discussion
Our results demonstrate that activation of GABAA receptors with muscimol induces long-lasting synaptic changes at glutamatergic synapses in the striatal complex, in an age- and region-dependent manner. Thus, muscimol triggers a form of LTD in the NAc of adolescent but not adult mice, and in the DLS of adult but not adolescent mice. The mechanism involved in muscimol-induced LTD likely includes the release of endocannabinoids that act as retrograde signaling molecule to presynaptically reduce glutamate release.
GABA is released by spontaneously active neurons in the striatum, even in brain slices. Indeed, around 98% of the total striatal neuronal population is made of GABAergic neurons and there are strong and frequent inhibitory responses in projection neurons mediated by tonically released GABA acting on GABAA receptors in the striatum (Chergui et al. 2000; Feng et al. 2014). Activation of GABAA receptors by muscimol applied in the perfusion solution might mimic the tonic activation of these receptors by GABA released from GABAergic interneurons or projection neurons. Our results identify a new form of LTD in the NAc that is induced by muscimol and is age dependent. The basic properties of glutamatergic synaptic transmission in the NAc were shown to be similar in adolescent and adult mice (Kasanetz and Manzoni 2009), which suggests that the differences observed in our study are not due to an altered transmission. The age-related differences in the ability of muscimol to induce synaptic plasticity might be attributable to several mechanisms. Our previous study suggested that an increased inhibitory tone mediated by GABA in adult NAc, when compared with adolescent NAc, might contribute to the inability of muscimol to induce LTD (Mishra and Chergui 2013). Furthermore, the level of expression of GABAA receptors or differences in the subunit composition or channel properties of these receptors (Laurie et al. 1992) might underlie the differences observed in this study.
In the present work, we demonstrate that synaptic depression induced by muscimol is due to both post- and presynaptic mechanisms. Muscimol depolarizes the membrane of medium spiny neurons, which at rest is much hyperpolarized (∼90 mV) and negative to the reversal potential of GABAA inhibition (Wilson 2007). Such a depolarization might trigger the release of endocannabinoid that inhibit glutamate release. Presynaptic inhibition is demonstrated by a decrease in the sEPSC frequency and an increase in paired-pulse ratio of fEPSP/PSs. In several brain regions, including the NAc, activation of group I mGluRs induces LTD, which is mediated by endocannabinoids (Lovinger et al. 2003; Robbe et al. 2003; Grueter et al. 2010). Here, we also demonstrate that muscimol-induced LTD in the NAc is dependent on mGluR5 and endocannabinoids. The involvement of mGluRs demonstrates that glutamate released upon synaptic stimulation also contributes to muscimol-induced LTD. The mechanism remains to be determined but concurrent stimulation of mGluRs and depolarization induced by muscimol might be necessary to induce the release of endocannabinoids.
We found that muscimol-induced LTD is not induced in the presence of the CB1 receptor antagonist AM251. However, this compound and endocannabinoids might modify synaptic transmission and induce synaptic plasticity through CB1 receptor-independent actions (Edwards et al. 2012; Golovko et al. 2015). We have performed several sets of experiments to examine this possibility. We found that a TRPV1 receptors antagonist does not block LTD induced by muscimol in the NAc of adolescent mice, which suggests that TRPV1 receptors are not involved in muscimol-induced LTD. In addition, muscimol-induced LTD is occluded by prior LTD induced by either the CB1 receptor agonist WIN 55,212-2 or by a low-frequency stimulation protocol that was shown to induce endocannabinoids-mediated LTD in the NAc (Burattini et al. 2014). These findings show that a common mechanism mediates LTD and likely involves the release of endocannabinoids and presynaptic depression of glutamate release. Furthermore, the age-related difference in muscimol-induced LTD correlates with the age-dependent ability of WIN 55,212-2 to induce LTD in the NAc and with a 50% reduction in the levels of CB1 receptor in the adult NAc when compared with the adolescent NAc (Zhang et al. 2015). Finally, the involvement of CB1 receptors in LTD in the NAc was unequivocally demonstrated through the use of CB1 null mice in which the inhibition induced by WIN 55,212-2 and LFS-LTD were absent (Robbe et al. 2002). Such forms of LTD were also reliably prevented by CB1 receptors antagonists such as the one we have used in our study. Taken together, these findings support our hypothesis that muscimol-induced LTD in the NAc of adolescent mice is mediated by endocannabinoids, likely acting on CB1 receptors.
In the DLS, muscimol triggered an LTD in adult mice but not in adolescent mice, while the opposite was observed in the NAc. Both forms of LTD are blocked by AM251, suggesting that they involve endocannabinoids. However, the mechanism that led to endocannabinoid production and release might differ between the two brain regions. In particular, muscimol-induced LTD in the DLS of adult mice does not seem to involve mGluR5, and it does not involve TRPV1 receptors either. In the DLS, synaptic depression induced by muscimol is not mimicked by WIN 55,212-2 at a concentration that induces LTD in the NAc of adolescent mice. The ability of this cannabinoid receptor agonist to induce LTD in the DLS is likely influenced by the experimental conditions, as described in previous studies (Huang et al. 2001; Kreitzer and Malenka 2005; Sergeeva et al. 2007; Chepkova et al. 2009; Clarke and Adermark 2010; Zhang et al. 2015).
Conclusions
Our findings identify the mechanisms of a form of synaptic plasticity mediated by endocannabinoids released following the activation of GABAA receptors. In addition, GABA-induced synaptic plasticity shifts from the NAc in adolescent mice to the DLS in adult mice. A comparable switch might constitute a cellular substrate for the transition from motivation and initial drug use to compulsive intake described for drug addiction (Everitt and Robbins 2013). Our observations emphasize the importance of the age of the animals used for electrophysiological studies of synaptic plasticity. They provide further evidence for an increased sensitivity of the NAc of adolescent mice, when compared with adult mice, to cannabinoid receptor-dependent synaptic plasticity. Our study might contribute to a better understanding of the increased sensitivity of the adolescent limbic system to the effects of abused drugs.
Materials and Methods
Animals and brain slice preparation
Experiments were approved by our local ethical committee (the Stockholm's north animal experimentation ethics committee) and were performed as described previously (Schotanus et al. 2006; Zhang et al. 2014). All efforts were made to minimize animal suffering. We used male C57BL/6 mice (Harlan Laboratories) aged 22–30 d (adolescent) and 5–8 mo (adult). Mice were maintained on a 12:12 h light–dark cycle and had free access to food and water. Mice were anesthetized with isoflurane and underwent cervical dislocation followed by decapitation. Their brains were rapidly removed and coronal brain slices (400 µm thick) containing the NAc, dorsal striatum, and the overlying cortex were prepared with a microslicer (VT 1000S, Leica Microsystem). Slices were incubated, for at least 1 h, at 32°C in oxygenated (95% O2 + 5% CO2) artificial cerebrospinal fluid (aCSF) containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.3 MgCl2, 2.4 CaCl2, 10 glucose, and 26 NaHCO3, pH 7.4. Slices were transferred to a recording chamber and were continuously perfused with oxygenated aCSF at 28°C.
Electrophysiology in brain slices
Extracellular field potentials were recorded using a glass micropipette filled with aCSF positioned on the slice surface in the NAc or in the DLS. These synaptic responses were evoked by stimulation pulses applied every 15 sec to the brain slice through a concentric bipolar stimulating electrode (FHC) placed near the recording electrode on the surface of the slice. Single stimuli (0.1 msec duration) were applied at an intensity yielding 50%–60% maximal response as assessed by a stimulus–response curve established, for each slice, at the beginning of the recording session, by measuring the amplitude of the field excitatory postsynaptic potentials/population spikes (fEPSP/PSs) evoked by increasing stimulation intensities. Paired-pulse stimulations consisted of two stimulation pulses separated by a 20-msec interval. Low-frequency stimulation was used to induce LTD of the fEPSP/PS and consisted of a 4 Hz train of 20 min duration. The stimulation intensity used for baseline fEPSP/PS recording was doubled during the train. Signals were amplified 500 or 1000 times via an Axopatch 200B or a GeneClamp 500B amplifier (Axon Instruments), acquired at 10 kHz, and filtered at 2 kHz.
Whole-cell patch-clamp recordings of medium spiny projection neurons in the NAc were made with the help of infrared-differential interference contrast video microscopy as done previously (Chergui 2011; Feng et al. 2014). Patch electrodes were filled with a solution containing, in mM: 120 d-gluconic acid, 20 KCl, 2 MgCl2, 1 CaCl2, 10 HEPES, 10 EGTA, 2 MgATP, 0.3 Na3GTP, pH adjusted to 7.3 with KOH. Whole-cell membrane currents and potentials were recorded with an Axopatch 200B (Axon Instruments), acquired at 10 kHz and filtered at 2 kHz.
Data acquisition and analysis
Data were acquired and analyzed with the pClamp 9 or pClamp 10 software (Axon Instruments). Numerical values are shown as means with S.E.M., with n indicating the number of slices or neurons tested. For fEPSP/PSs, data are expressed as percent of the baseline response measured for each slice during the 5–10 min preceding start of perfusion with muscimol or with WIN 55,212-2, or before the delivery of low-frequency stimulation. We used the Mini Analysis program Synaptosoft (Synaptosft, Inc.) to analyze the frequency and amplitude of sEPSCs in individual medium spiny neurons of the NAc. Statistical significance of the results was assessed by using the Student's t-test for paired and unpaired observations or one-way ANOVA followed by Dunnett's multiple comparison test.
Chemicals and drugs
Chemicals and drugs were purchased from Sigma-Aldrich, Tocris Bioscience, and AbcamBiochemicals. All compounds were prepared in stock solutions, diluted in aCSF to the desired final concentration and applied in the perfusion solution. The following compounds were used (final concentrations in µM): AM251 (2), CNQX (10), MPEP (20), muscimol (1), SB366791 (20), and WIN 55,212-2 (1).
Acknowledgments
This work was supported by the Swedish Research Council (grant 2014-3254) and the Swedish Society of Medicine.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.043190.116.
References
- Akhondzadeh S, Stone T. 1995. Induction of a novel form of hippocampal long-term depression by muscimol: involvement of GABAA but not glutamate receptors. Br J Pharmacol 115: 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burattini C, Battistini G, Tamagnini F, Aicardi G. 2014. Low-frequency stimulation evokes serotonin release in the nucleus accumbens and induces long-term depression via production of endocannabinoid. J Neurophysiol 111: 1046–1055. [DOI] [PubMed] [Google Scholar]
- Chepkova AN, Fleischer W, Kazmierczak T, Doreulee N, Haas HL, Sergeeva OA. 2009. Developmental alterations of DHPG-induced long-term depression of corticostriatal synaptic transmission: switch from NMDA receptor-dependent towards CB1 receptor-dependent plasticity. Pflugers Arch 459: 131–141. [DOI] [PubMed] [Google Scholar]
- Chergui K. 2011. Dopamine induces a GluN2A-dependent form of long-term depression of NMDA synaptic responses in the nucleus accumbens. Neuropharmacology 60: 975–981. [DOI] [PubMed] [Google Scholar]
- Chergui K, Bouron A, Normand E, Mulle C. 2000. Functional GluR6 kainate receptors in the striatum: indirect downregulation of synaptic transmission. J Neurosci 20: 2175–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB, Adermark L. 2010. Acute ethanol treatment prevents endocannabinoid-mediated long-lasting disinhibition of striatal output. Neuropharmacology 58: 799–805. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Gibson HE, Jensen T, Nugent F, Walther C, Blickenstaff J, Kauer JA. 2012. A novel non-CB1/TRPV1 endocannabinoid-mediated mechanism depresses excitatory synapses on hippocampal CA1 interneurons. Hippocampus 22: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. 2013. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37: 1946–1954. [DOI] [PubMed] [Google Scholar]
- Feng ZJ, Zhang X, Chergui K. 2014. Allosteric modulation of NMDA receptors alters neurotransmission in the striatum of a mouse model of Parkinson's disease. Exp Neurol 255: 154–160. [DOI] [PubMed] [Google Scholar]
- Golovko T, Min R, Lozovaya N, Falconer C, Yatsenko N, Tsintsadze T, Tsintsadze V, Ledent C, Harvey RJ, Belelli D, et al. 2015. Control of inhibition by the direct action of Cannabinoids on GABAA receptors. Cereb Cortex 25: 2440–2455. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. 2010. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13: 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. 2001. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol 532: 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Manzoni OJ. 2009. Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. J Neurophysiol 101: 2516–2527. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. 2005. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci 25: 10537–10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie D, Wisden W, Seeburg P. 1992. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci 12: 4151–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Talia N, Kreitzer Anatol C. 2012. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron 73: 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. 2010. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC. 2003. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann NY Acad Sci 1003: 226–240. [DOI] [PubMed] [Google Scholar]
- Mishra D, Chergui K. 2013. Ethanol inhibits excitatory neurotransmission in the nucleus accumbens of adolescent mice through GABAA and GABAB receptors. Addict Biol 18: 605–613. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. 2002. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci 99: 8384–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Manzoni OJ. 2003. Exogenous and endogenous cannabinoids control synaptic transmission in mice nucleus accumbens. Ann NY Acad Sci 1003: 212–225. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. 2008. NR2A-containing NMDA receptors depress glutamatergic synaptic transmission and evoked-dopamine release in the mouse striatum. J Neurochem 106: 1758–1765. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Fredholm BB, Chergui K. 2006. NMDA depresses glutamatergic synaptic transmission in the striatum through the activation of adenosine A1 receptors: evidence from knockout mice. Neuropharmacology 51: 272–282. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Doreulee N, Chepkova AN, Kazmierczak T, Haas HL. 2007. Long-term depression of cortico-striatal synaptic transmission by DHPG depends on endocannabinoid release and nitric oxide synthesis. Eur J Neurosci 26: 1889–1894. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC. 2003. Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond B Biol Sci 358: 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Di Filippo M, Tantucci M, Costa C, Borsini F, Ghiglieri V, Giampa C, Fusco FR, Picconi B, et al. 2011. The distinct role of medium spiny neurons and cholinergic interneurons in the D2/A2A receptor interaction in the striatum: implications for Parkinson's disease. J Neurosci 31: 1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. 2008. Neuropharmacology of alcohol addiction. Br J Pharmacol 154: 299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. 2007. GABAergic inhibition in the neostriatum. In Progress in brain research (ed. James EDA, Tepper M, Bolam JP), Vol. 160, pp. 91–110. Elsevier, New York. [DOI] [PubMed] [Google Scholar]
- Zhang X, Feng ZJ, Chergui K. 2014. GluN2D-containing NMDA receptors inhibit neurotransmission in the mouse striatum through a cholinergic mechanism: implication for Parkinson's disease. J Neurochem 129: 581–590. [DOI] [PubMed] [Google Scholar]
- Zhang X, Feng Z-J, Chergui K. 2015. Induction of cannabinoid- and N-methyl-d-aspartate receptor-mediated long-term depression in the nucleus accumbens and dorsolateral striatum is region and age dependent. IntJ Neuropsychopharmacol 18 10.1093/ijnp/pyu052. [DOI] [PMC free article] [PubMed] [Google Scholar]