Highlight
Two ubiquitin ligases control fumonisin B1-elicited programmed cell death by modulating jasmonate signalling transduction in Arabidopsis.
Key words: Arabidopsis, fumonisin B1, jasmonate, programmed cell death, RGLG3, RGLG4, salicylic acid.
Abstract
The mycotoxin fumonisin B1 (FB1) is a strong inducer of programmed cell death (PCD) in plants, but its underlying mechanism remains unclear. Here, we describe two ubiquitin ligases, RING DOMAIN LIGASE3 (RGLG3) and RGLG4, which control FB1-triggered PCD by modulating the jasmonate (JA) signalling pathway in Arabidopsis thaliana. RGLG3 and RGLG4 transcription was sensitive to FB1. Arabidopsis FB1 sensitivity was suppressed by loss of function of RGLG3 and RGLG4 and was increased by their overexpression. Thus RGLG3 and RGLG4 have coordinated and positive roles in FB1-elicited PCD. Mutated JA perception by coi1 disrupted the RGLG3- and RGLG4-related response to FB1 and interfered with their roles in cell death. Although FB1 induced JA-responsive defence genes, it repressed growth-related, as well as JA biosynthesis-related, genes. Consistently, FB1 application reduced JA content in wild-type plants. Furthermore, exogenously applied salicylic acid additively suppressed JA signalling with FB1 treatment, suggesting that FB1-induced salicylic acid inhibits the JA pathway during this process. All of these effects were attenuated in rglg3 rglg4 plants. Altogether, these data suggest that the JA pathway is hijacked by the toxin FB1 to elicit PCD, which is coordinated by Arabidopsis RGLG3 and RGLG4.
Introduction
Programmed cell death (PCD) is an essential biological process invoked during normal growth and development or under conditions of stress (Lam, 2004; Dickman and Fluhr, 2013). Several types of PCD exist in plants, and many PCD-related morphologies have been observed, such as cell volume reduction, chromatin condensation and nuclear segmentation (Reape and McCabe, 2008; van Doorn et al., 2011). A widely studied form of plant PCD is the hypersensitive response (HR), which takes place during incompatible pathogen-plant interactions and during which plant cells around the invasion site(s) actively and rapidly die to stop the supply of nutrients and limit pathogen growth, thereby preventing the disease from spreading throughout the whole plant (Coll et al., 2011).
To evade the host plant immune system, many pathogens, most of which are biotrophic, have evolved mechanisms to suppress the host plant HR by delivering specific effectors into the infected cell (Jamir et al., 2004; Bos et al., 2006; Kelley et al., 2010). However, a number of bacterial and fungal pathogens, most of which are necrotrophic, promote cell death as a source of nutrients from dead or dying cells by secreting toxins in the host plant (Howlett, 2006). Fumonisin B1 (FB1) is a potent sphingolipid-like PCD elicitor produced by the compatible fungal pathogen Fusarium moniliforme that causes serious disease symptoms in maize and other grains (Gilchrist, 1997, 1998). FB1 inhibits sphingolipid biosynthesis by competitive inhibition of ceramide synthase, which can lead to cell death (Stone et al., 2000; Desai et al., 2002), and, in Arabidopsis, depletes extracellular ATP (Chivasa et al., 2005). In addition to cell death, FB1 can elicit other HR-like responses including reactive oxygen species (ROS) generation, phenolic compound and callose deposition, phytoalexin accumulation and pathogenesis-related (PR) protein expression (Wolpert et al., 2002). FB1-induced cell death thus provides a simple pathogen-free system for elucidating the molecular basis of HR (Stone et al., 2000). However, the mechanism underlying FB1-triggered PCD is not well understood.
Jasmonates (JAs) are essential hormones that mainly mediate plant defence responses to necrotrophic pathogens (Glazebrook, 2005). In the inactivated state, jasmonate zim-domain (JAZ) proteins suppress the JA pathway by binding to various transcription factors (Boter et al., 2004; Lorenzo et al., 2004; Dombrecht et al., 2007; Fernandez-Calvo et al., 2011; Qi et al., 2011; Song et al., 2011; Kazan and Manners, 2012). Pathological stress promotes rapid JA production through the consecutive actions of diverse synthesis enzymes such as allene oxide synthase (AOS), OPDA reductase 3 (OPR3) and OPC-8:0 CoA ligase 1 (OPCL1) (Browse, 2009). JA can be further modified into numerous conjugates including the highly bioactive (+)-7-iso-jasmonoyl-l-isoleucine (JA-Ile) (Staswick and Tiryaki, 2004; Fonseca et al., 2009; Thines et al., 2007). JA-Ile is recognized by a receptor complex consisting of coronatine insensitive 1 (COI1), JAZs, and inositol pentakisphosphate (InsP5) (Sheard et al., 2010), which promotes COI1 to form a large SKP/CUL/F-box complex (SCFCOI1) with other partners (Devoto et al., 2002; Xu et al., 2002) and then targets JAZs for degradation by the 26S proteasome, thus activating downstream signalling (Chini et al., 2007; Thines et al., 2007).
The contribution of the JA pathway to PCD remains inconclusive, although several studies have provided conflicting results. For example, methyl jasmonate (MeJA) pretreatment of Arabidopsis ecotype Col-0 abolishes O3-induced cell death, and the JA-insensitive mutants jar1 and fad3/7/8 become more sensitive to O3 than wild type, suggesting that the JA pathway has a negative role in O3-induced PCD (Overmyer et al., 2000; Rao et al., 2000). In contrast, FB1-treated Arabidopsis protoplasts prepared from jar1 plants display enhanced viability (Asai et al., 2000), and, in Nicotiana benthamiana, interrupting the JA pathway can affect the positive role of NbHB1 in pathogen-induced cell death (Yoon et al., 2009), indicating a positive role for the JA pathway in PCD. Thus the molecular mechanism of JA in PCD awaits further studies.
Despite previous studies in Arabidopsis protoplasts indicating that FB1-induced PCD requires salicylic acid (SA), JA and ethylene (ET) signalling pathways (Asai et al., 2000), the actual roles of these hormone pathways have not been determined in plants. We have studied the functions of two recently identified JA pathway regulators, RGLG3 and RGLG4 (Zhang et al., 2012), in FB1-elicited PCD. Our in-plant analysis showed that a functional JA pathway was required for FB1 to trigger PCD. Despite that, FB1 suppressed JA production and part of the JA signalling pathway. Exogenous SA application promoted FB1 suppression of JA signalling, suggesting that FB1-induced SA could further inhibit the JA pathway. Significantly, these effects required the involvement of both RGLG3 and RGLG4. We propose that the JA pathway is hijacked by FB1 to initiate PCD, and RGLG3 and RGLG4 act as essential coordinators of this process.
Materials and methods
Plant materials and growth conditions
Arabidopsis ecotypes of Columbia-0 (Col-0) and Columbia glabrous (Col-gl) were used as wild type. Mutants of rglg3, rglg4, rglg3 rglg4, npr1-3, ein2-5, coi1-1, coi1-2, myc2-2, ein3-1 eil1-1, the overexpression plants and transgenic materials expressing promoter::GUS constructs of RGLG3 and RGLG4 were obtained as previously described (Zhang et al., 2012); eds5-1 (CS3735) was requested from the Arabidopsis Biological Resource Center. Seeds of Col-gl, NahG, NahG coi1-1, pad4, pad4 coi1-1 and sid2 were requested from Christiane Gatz, myc2 myc3 myc4 from Roberto Solano and npr1-1 ein2-1 jar1-1 from Xinnian Dong. All plants were grown under similar conditions, as described (Zhang et al., 2012).
Protein subcellular localization
Full-length RGLG3 and RGLG4 were amplified by PCR, and each was cloned into pRTL-GFP, generating a construct expressing an N-terminal GFP fusion protein. Arabidopsis mesophyll protoplast preparation, transfection and 4,6-diamidino-2-phenylindole (DAPI, 1mg/ml; Sigma, USA) staining were performed as described (He et al., 2011b). Confocal imaging was performed with a Zeiss LSM 710 NLO laser scanning confocal microscope (excitation 488nm; emission 505–550nm). Expression of GFP alone (from the control pRTL-GFP), GFP-RGLG3 and GFP-RGLG4 was confirmed by western blotting using anti-GFP (Abcam, Germany) as the primary antibody and horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Promega, USA) as the secondary antibody.
Fractionation of cytosolic and nuclear proteins
Cytosolic and nuclear protein extraction was carried out with CELLYTPN1 CelLytic PN isolation/Extraction Kit (Sigma, USA). Briefly, plant tissues were ground in liquid nitrogen and then gently resuspended with 1× nuclei isolation buffer supplemented with 1mM dithiothreitol (DTT), 2mM Na3VO4, 2mM NaF and 25mM phenylmethylsulfonyl fluoride (PMSF). The resulting suspension was filtered through five layers of Miracloth (Calbiochem, Germany) by centrifugation at 4°C. The pellet was resuspended with 1× nuclei isolation buffer supplemented with 0.3% (v/v) Triton X-100, 1mM DTT, 2mM Na3VO4, 2mM NaF, 25mM PMSF, 2.5mg/ml antipain, 2.5mg/ml chymostatin, 1mg/ml pepstatin, 5mg/ml leupeptin, 5mg/ml aprotinin and 100mM MG132 (Sigma, USA). After incubation on ice for 15min, extracts were centrifuged at 6800rpm for 5min. The resulting supernatant was transferred to a new tube containing 1× SDS buffer and designated as the cytosolic fraction. The pellet was resuspended in the above buffer and centrifuged and resuspended repeatedly until the pellet appeared slightly grey. The pellet was washed with 1× nuclei isolation buffer supplemented with 1mM DTT, 2mM Na3VO4, 2mM NaF and 25mM PMSF, dissolved in 1× SDS buffer and designated as the nuclear fraction. The cytosolic and nuclear proteins were loaded onto SDS-PAGE gels in proportion to the volume used in the initial extractions and the resuspension volume of each fraction. Western blotting was performed using anti-FLAG (Sigma, USA), anti-GDPase (Agrisera, Sweden) and anti-histone H3 (Abcam, USA) as the primary antibodies and HRP-conjugated anti-mouse/anti-rabbit IgG (Sigma, USA) as the secondary antibody.
Chemical treatment
For FB1 treatment, well-expanded leaves (~four weeks old) were infiltrated with 10 µM FB1 (Sigma, USA) in 10mM MgCl2 by a needleless syringe; mock-treated leaves were injected with 10mM MgCl2. Leaf samples were then collected at the indicated time points. To treat Arabidopsis seedlings, five-day-old plants were transferred to new MS plates containing 2 µM FB1 for another six days of growth, with MS plates as the control. FB1-induced phenotypes were classified into three categories (hypersensitive, sensitive and insensitive) and calculated as a percentage of the total plant population as reported (Lin et al., 2008). SA treatment was done by directly spraying 2mM SA (Sigma, USA) diluted in water onto four-week-old plants.
β-Glucuronidase (GUS) assay
GUS staining was performed as described (Zhang et al., 2012), and GUS activity was determined as reported (Jefferson et al., 1987). Briefly, total protein was extracted with GUS extraction buffer (50mM sodium phosphate buffer, pH 7.5; 10mM β-mercaptoethanol; 0.1% Triton X-100 and 10mM EDTA) and quantified using a protein assay kit (Bio-Rad, USA). Then 50mg total protein was incubated at 37°C for 5min before 1mM 4-methylumbelliferyl-β-d-glucuronide (4-MUG) (Sigma, USA) was added. After 60min, a 100-µl sample was taken and 2.4ml of 0.2M Na2CO3 was added to terminate the reaction. Each sample was then quantified for absorbance at excitation 365/emission 455 with a fluorospectrophotometer (Hitachi, Japan) to calculate 4-methylumbelliferone (4-MU) production. The final GUS activity was expressed as pmol (4-MU) min–1 µg–1 (fresh weight).
Ion conductivity measurements
Ion conductivity was measured as described (He et al., 2011a). Briefly, four-week-old Arabidopsis leaves grown under short-day conditions in soil were infiltrated with 10 μM FB1 (Sigma, USA) in 10mM MgCl2 or with 10mM MgCl2 as the mock treatment. Four leaf disks (6mm diameter) from four plants of each genotype were collected with a cork borer 72h after infiltration, immersed in 25ml deionized water for 45min and subsequently moved into a tube containing 6ml water for measuring ion conductivity using a conductivity meter (Hanna, Italy) at the indicated time points.
Plant hormone quantification
For JA and SA content measurement, four-week-old plant leaves were injected with 10 μM FB1 in l0mM MgCl2, and samples were collected at the indicated time points. Then, 200mg of plant tissues was used for JA and SA quantification as described (Fu et al., 2012).
Quantitative real-time PCR
Total RNA extraction, cDNA synthesis and real-time PCR were carried out as described (Zhang et al., 2012). Primers used for gene expression analysis are listed in Supplementary Table S1.
Results
RGLG3 and RGLG4 are localized in both the cytoplasm and nucleus
Our recent work has identified two ubiquitin ligases, RGLG3 and RGLG4, as important JA pathway regulators (Zhang et al., 2012). Despite this knowledge, additional properties and possible functions of these two proteins in Arabidopsis have awaited further analysis. First, their subcellular distributions remain unclear. We therefore expressed GFP-RGLG3 and GFP-RGLG4 in Arabidopsis protoplasts to observe their localization. Confocal images showed that GFP-RGLG3 and GFP-RGLG4 were present in both the cytoplasm and nucleus (indicated by DAPI staining), closely resembling the localization of GFP alone (Fig. 1A, B). To confirm this result, whole-cell extracts prepared from transgenic Arabidopsis constitutively expressing FLAG-tagged RGLG3 and RGLG4 (Zhang et al., 2012) were separated into cytosolic and nuclear fractions. With the subcellular compartment marker proteins H3 (nuclear) and GDPase (cytoplasmic) indicating effective separation, FLAG-RGLG3 and FLAG-RGLG4 were detected in both fractions (Fig. 1C).
Fig. 1.
Subcellular localization of RGLG3 and RGLG4. (A) Confocal microscopy images of GFP-tagged RGLG3 and RGLG4 compared with GFP alone expressed in Arabidopsis protoplasts. GFP, DAPI, bright field and the merged images of these three signals are shown. Bars, 10 μm. (B) Confirmed expression of GFP, GFP-RGLG3 and GFP-RGLG4 in protoplasts used in (A) by western blotting using GFP antibody. (C) Detection of Flag-tagged RGLG3 and RGLG4 in different subcellular fractions. T, total protein; S, soluble fraction; N, nuclear fraction. H3 antibody was used as a nuclear protein marker and GDPase as a cytoplasmic protein marker. Asterisks indicate positions of FLAG-tagged proteins.
RGLG3 and RGLG4 respond differently to mycotoxin FB1 treatment
To further explore the biological functions of RGLG3 and RGLG4 in Arabidopsis, transgenic plants expressing promoter::GUS constructs of RGLG3 and RGLG4 (Zhang et al., 2012) were assessed for promoter activity in the presence of several chemicals. Histochemical staining, GUS activity quantification and western blotting all indicated that FB1 stimulated RGLG3 promoter activity, whereas it suppressed that of RGLG4 (Fig. 2A, B, C). To confirm this conclusion, transcriptional responses of RGLG3 and RGLG4 to FB1 were examined by real-time PCR using adult leaves that were injected with FB1. As shown in Fig. 2D, the injection method in adult leaves may cause some kind of mechanical stimulation to expression of both RGLG3 and RGLG4, however, after FB1 was injected, this stimulation could be highly enhanced for RGLG3, but suppressed for RGLG4 compared to the mock treatment, consistent to the results in seedlings. Even though these responses varied, they both suggest that RGLG3 and RGLG4 might be involved in FB1-induced programmed cell death.
Fig. 2.
Promoter activities and transcriptional profiles of RGLG3 and RGLG4 after FB1 treatment. (A) Representative GUS staining of proRGLG3::GUS and proRGLG4::GUS transgenic plants after FB1 treatment. Five-day-old seedlings were treated with 2 μM FB1 for six days before GUS staining. Arrows indicate substantial changes in seedling leaves. (B) Quantified GUS activities in plants treated as in A. In each experiment, ~40 seedlings were treated and separated into four groups as four replicates for quantifying GUS activity. Bars indicate the mean +SD from the four replicates. Asterisks indicate a significant difference from mock (MS) treatment (Student’s t-test: *, P<0.05; **, P<0.01). (C) GUS protein levels assessed by western blot using GUS antibody in plants treated as in A. Actin was detected as a loading control. (D) Quantitative real-time PCR examination of RGLG3 and RGLG4 expression. RNA was extracted from 4-week-old Arabidopsis leaves at the indicated time points after infiltration with 10mM MgCl2 (mock) or 10 μM FB1 in 10mM MgCl2 (FB1). UBQ10 was used as an internal control for real-time PCR, and expression levels were normalized to that measured at time point 0. Error bars indicate the SD from the mean of four technical replicates. All experiments were repeated three times with similar results.
FB1-elicited PCD requires coordinated roles of RGLG3 and RGLG4
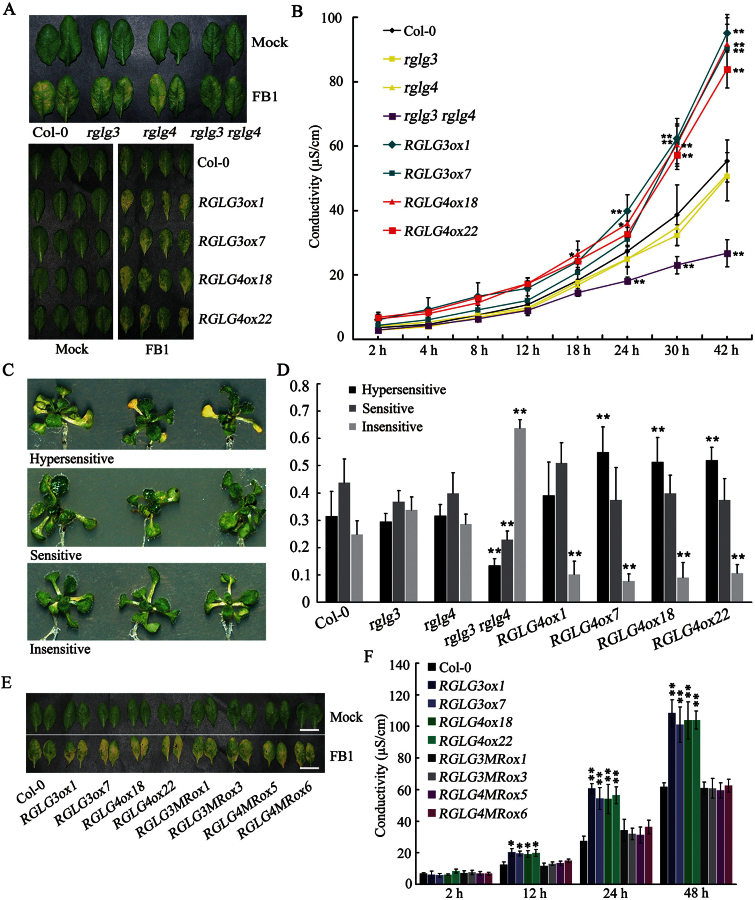
RGLG3 and RGLG4 mutants and transgenes (Zhang et al., 2012) were then used to study whether these genes regulate FB1-induced PCD. As shown in Fig. 3A, three days after four-week-old plants were injected with 10 µM FB1 to initiate PCD, the leaves of wild-type (WT), rglg3 and rglg4 plants all turned yellow except for those from the double mutant rglg3 rglg4; in contrast, all the overexpression lines of RGLG3 and RGLG4 became more sensitive to FB1 than WT, suggesting RGLG3 and RGLG4 positively and redundantly regulate PCD elicited by FB1 treatment. Consistent with these observations, we recorded changes in ion leakage at different time points after treatment. rglg3 rglg4 leaves underwent a less severe change in ion leakage compared with WT or either of the single mutants, whereas overexpressing either RGLG3 or RGLG4 promoted changes in ion leakage (Fig. 3B).
Fig. 3.
Roles of RGLG3 and RGLG4 in FB1-triggered cell death. (A) Lesion development indicating FB1-induced cell death in mutants rglg3, rglg4 and rglg3 rglg4 (upper panel) or in transgenic lines RGLG3ox1, RGLG3ox7, RGLG4ox18 and RGLG4ox22 (lower panel) compared with Col-0 plants. Four-week-old leaves were photographed 72h after they were infiltrated with 10mM MgCl2 (mock) or 10 μM FB1 in 10mM MgCl2 (FB1). (B) Ion leakage measurements (0–42h after sampling) in leaf disks from the plants used in A. Data indicate the mean ±SD from four replicates. Asterisks indicate a significant difference from the wild-type at the same time point (Student’s t-test: *, P<0.05; **, P<0.01). This representative experiment was repeated three times with similar results. (C, D) Altered FB1 sensitivity in mutant and transgenic seedlings of RGLG3 (C) and RGLG4 (D). One-week-old seedlings of each genotype were treated with 2 μM FB1 for six days and then classified as hypersensitive, sensitive or insensitive according to their responses to FB1 (left). The proportion of seedlings in each category is shown as the mean +SD (n>50) from three replicates. Asterisks indicate a significant difference from the wild-type (Student’s t-test: *, P<0.05; **, P<0.01). This experiment was repeated three times with similar results. (E) FB1-triggered lesion development on the leaves of transgenic Arabidopsis overexpressing RING domain-mutated RGLG3 or RGLG4 compared with those overexpressing wild-type RGLG3 or RGLG4. Two lines for each transgene were used, and four-week-old leaves were photographed 72h after infiltration with 10mM MgCl2 (mock) or 10 μM FB1 in 10mM MgCl2 (FB1). The experiments were repeated at least three times and yielded similar results. Bars (white), 1cm. (F) Ion leakage measurements for the plants used in E. Leaf discs were sampled for conductivity recording 72h after treatment, and the X-axis indicates the time after sampling. Data indicate the mean ±SD from four replicates. Asterisks indicate a significant difference from the wild type at the same time point (Student’s t-test: *, P<0.05; **, P<0.01). The experiments were repeated three times with similar results.
Similar results were observed in whole-seedling phenotypes classified according to their FB1 sensitivity (insensitive, sensitive, hypersensitive; Fig. 3C). Col-0, rglg3 and rglg4 showed no significant difference among the three sensitivity groups, whereas all overexpression lines had more hypersensitive and sensitive seedlings and rglg3 rglg4 had more insensitive seedlings (Fig. 3D). Taken together, these results demonstrate that RGLG3 and RGLG4 coordinately and positively regulate FB1-induced PCD.
Both RGLG3 and RGLG4 possess ubiquitin ligase (E3) activities (Zhang et al., 2012). To see if RGLG3 and RGLG4 regulate FB1-induced PCD by acting as E3s, transgenic plants overexpressing RING domain-mutated RGLG3 or RGLG4 were used for analysing cell death in comparison with unmutated RGLG3 and RGLG4. Both the visual observation of cell death in leaves and ion leakage measurements indicated that when the RING domain was mutated, RGLG3 and RGLG4 did not promote PCD (Fig. 3E, F), suggesting that E3 activities of both proteins are responsible for their functions and that they may target an unknown protein(s) for ubiquitination in regulating FB1-induced PCD.
Transcriptional responses of RGLG3 and RGLG4 to FB1 require an intact JA pathway
The JA, SA and ET signalling pathways are required for FB1-induced PCD (Asai et al., 2000), and they may be involved in RGLG3 and RGLG4 functions. We used hormone pathway mutants to determine which hormone pathway(s) controls the responsiveness of RGLG3 and RGLG4 to FB1. The following mutants were assessed: coi1-1 (Xie et al., 1998), coi1-2 (Xu et al., 2002), myc2-2 (Boter et al., 2004) and myc2 myc3 myc4 (Fernandez-Calvo et al., 2011) for JA; pad4 (Jirage et al., 1999), eds5-1 (Glazebrook et al., 1996), sid2 (Wildermuth et al., 2001), npr1-3 (Cao et al., 1994) and the transgenic line NahG (van Wees and Glazebrook, 2003) for SA and ein2-5 (Guzman and Ecker, 1990) and eil1-1 ein3-1 (Alonso et al., 2003) for ET. In addition, the double or triple crosses pad4 coi1-1, NahG coi1-1 and npr1-1 ein2-1 jar1-1 (Clarke et al., 2000), which disrupt two or three pathways, respectively, were analysed. Plants were treated with 2 µM FB1, and RGLG3 and RGLG4 expression levels were quantified. FB1 enhanced RGLG3 expression and suppressed RGLG4 expression in all genotypes except for mutants with severe JA pathway defects, including coi1, myc2 myc3 myc4 and npr1-1 ein2-1 jar1-1 (Fig. 4A). proRGLG3::GUS and proRGLG4::GUS reporter constructs introduced into the Col-0, coi1, npr1-3 and ein3-1 eil1-1 genetic backgrounds were then used to determine RGLG3 and RGLG4 promoter activities upon FB1 treatment. Both histochemical staining and quantification of enzymatic activity showed that FB1 caused obvious alteration in all the backgrounds but coi1 (Fig. 4B, C), consistent with the transcript quantification results. Together, these expression analyses indicate that the JA pathway is required for RGLG3 and RGLG4 responses to FB1.
Fig. 4.
JA pathway-dependent FB1 responsiveness of RGLG3 and RGLG4. (A) FB1-elicited expression changes of RGLG3 and RGLG4 in Col-0, coi1, myc2-2, myc2 myc3 myc4, NahG, NahG coi1-1, pad4, pad4 coi1-1, npr1-3, eds5-1, ein3-1 eil1-1, ein2-5 and npr1-1 ein2-1 jar1-1 plants. Five-day-old seedlings were treated with or without 2 μM FB1 and grown for six days. Expression levels were determined by real-time PCR. UBQ10 was used as an internal control, and expression in mock-treated plants was considered as 1.0. Bars indicate the mean ±SD from four technical replicates. Asterisks indicate a significant difference from mock (MS) treatment (Student’s t-test: *, P<0.05; **, P<0.01). (B) Representative GUS staining of transgenic plants in Col-0, coi1, npr1-3 and ein3-1 eil1-1 backgrounds expressing proRGLG3::GUS or proRGLG4::GUS. Seedlings were grown and treated as in A. Bars, 5mm. (C) Quantified GUS activity from plants as in B. In each experiment, ~30 seedlings were treated and separated into three groups as three replicates. Bars indicate the mean ±SD of these three replicates. Asterisks indicate a significant difference from mock (MS) treatment (Student’s t-test: *, P<0.05; **, P<0.01). All experiments were repeated three times with similar results.
Roles of RGLG3 and RGLG4 in FB1-induced cell death require an intact JA signalling pathway
To determine if the JA signalling pathway is critical for RGLG3 and RGLG4 functions in PCD, rglg3 rglg4, RGLG3ox1 and RGLG4ox18 plants, as well as those plants crossed with either coi1 or myc2 plants (rglg3 rglg4 coi1-2, rglg3 rglg4 myc2-2, RGLG3ox1 coi1-2, RGLG4ox18 coi1-2, RGLG3ox1 myc2-2 and RGLG4ox18 myc2-2) (Zhang et al., 2012) were used to investigate FB1-elicited PCD. Consistent with the finding that JA positively mediates FB1-induced cell death (Asai et al., 2000), visual lesion development in coi1-2 leaves was less severe than in Col-0 (Supplementary Fig. S1), and the increase in ion leakage rate was also lower in coi1-2 than in Col-0 after FB1 treatment (Fig. 5A). coi1-2 had a lower ion leakage rate and thus greater PCD suppression than rglg3 rglg4, but rglg3 rglg4 coi1-2 had similar conductivity to coi1-2 (Fig. 5A). Thus, although enhanced RGLG3 and RGLG4 expression promoted FB1-elicited ion leakage, these effects disappeared in the coi1-2 background (Fig. 5A). Similarly, promoted lesion development by RGLG3 or RGLG4 overexpression was repressed by coi1-2 mutation (Supplementary Fig. S1), suggesting a determinative role for COI1 in the positive effects of RGLG3 and RGLG4 on PCD. In myc2-2, lesion development was more severe and conductivity increased faster than in Col-0 (Supplementary Fig. S1 and Fig. 5A), implying a negative role for MYC2 in FB1-triggered cell death, which is similar to its role in pathogen defence responses (Lorenzo et al., 2004). Moreover, the attenuated ion leakage of rglg3 rglg4 was reversed and the increased ion leakage in the overexpression lines was further facilitated by myc2-2 (Fig. 5A), suggesting MYC2 involvement in RGLG3 and RGLG4 promotion of FB1-triggered cell death. Similar conclusion can also be drawn from lesion phenotypes (Supplementary Fig. S1).
Fig. 5.
Roles of the JA pathway in RGLG3- and RGLG4-mediated cell death. (A) Conductivity measurements in Col-0, rglg3 rglg4, coi1-2, myc2-2, rglg3 rglg4 coi1-2, rglg3 rglg4 myc2-2, RGLG3ox1, RGLG4ox18, RGLG3ox1 coi1-2, RGLG4ox18 coi1-2, RGLG3ox1 myc2-2 and RGLG4ox18 myc2-2 after FB1 treatment. Treatment and ion leakage quantification were as in Fig. 3B; bars show the mean ±SD from three independent replicates at different time points after sampling. Asterisks indicate a significant difference from the wild-type at the same time point (Student’s t-test: *, P<0.05; **, P<0.01). The experiments were repeated three times with similar results. (B) FB1-induced expression of PDF1.2 and PR5 in genotypes used in A. Five-day-old seedlings were treated as in Fig. 2A, and transcription levels were examined by real-time PCR. UBQ10 was used as an internal control, and expression levels were normalized to that of the mock treatment. Data represent the mean ±SD from four technical replicates. The experiments were repeated three times and had similar results.
FB1 stimulates expression of JA-responsive marker genes such as the PLANT DEFENSIN1.2 (PDF1.2) and PATHOGENESIS-RELATED5 (PR5) (Stone et al., 2000). In rglg3 rglg4, FB1-induced PDF1.2 and PR5 expression was attenuated, but in RGLG3ox1 and RGLG4ox18, their expression was enhanced compared with that in Col-0 (Fig. 5B), indicating that RGLG3 and RGLG4 can promote FB1-stimulated expression of JA marker genes. FB1-induced PDF1.2 and PR5 expression in the JA-insensitive coi1-2, rglg3 rglg4 coi1-2, RGLG3ox1 coi1-2 and RGLG4ox18 coi1-2 was similarly more down-regulated than in rglg3 rglg4 (Fig. 5B), suggesting that RGLG3 and RGLG4 effects on the FB1 responsiveness of PDF1.2 and PR5 were dependent on COI1. In myc2-2, PDF1.2 and PR5 expression was elevated compared with that in Col-0 (Fig. 5B), further confirming a negative role for MYC2 in FB1-triggered JA signalling. This is consistent with reports that MYC2 negatively regulates pathogen defence-related genes (Boter et al., 2004; Lorenzo et al., 2004). The suppressed induction of PDF1.2 and PR5 in rglg3 rglg4 could be reversed in rglg3 rglg4 myc2-2 to a level that was higher than that in Col-0 and was further enhanced in RGLG3ox1 myc2-2 and RGLG4ox18 myc2-2 (Fig. 5B), implying that MYC2 might act downstream of RGLG3 and RGLG4 in regulating these two FB1-responsive genes. Collectively, these data indicate that an intact JA pathway was important for RGLG3 and RGLG4 to perform their positive roles in FB1-induced PCD.
RGLG3 and RGLG4 control FB1 responses mainly by modulating the JA pathway versus the SA pathway
As FB1 activates both JA and SA signalling in eliciting PCD (Stone et al., 2000), we further examined the FB1 responsiveness of these two pathway genes in rglg3 rglg4 and Col-0 using mature Arabidopsis leaves. FB1 treatment induced expression of the defence-related genes, such as PR3, PR5 and PDF1.2 (Stone et al., 2000) compared to the mock treatment (Fig. 6A, B, C), and similar to the assays in young seedlings (Fig. 5B), FB1 induction was suppressed in rglg3 rglg4 compared with wild type (Fig. 6A, B, C). However, additional JA-responsive genes that are related to growth repression (Lorenzo et al., 2004), including JASMONIC ACID RESPONSIVE1 (JR1) (Rojo et al., 1999), JR2 and VEGETATIVE STORAGE PROTEIN2 (VSP2) (Staswick et al., 1991), and to JA biosynthesis, such as 12-OXOPHYTODIENOATE REDUCTASE3 (OPR3) (Stintzi and Browse, 2000) and LIPOXYGENASE2 (LOX2) (Bell and Mullet, 1993), were obviously suppressed by FB1 in Col-0 compared to the mock treatment, and this effect disappeared in rglg3 rglg4 plants, in which these genes even became inducible in response to FB1 (Fig. 6D, E, F, G, H). In contrast, FB1 induction of the typical SA-responsive genes PR1 and PR2 (Stone et al., 2000) was not apparently affected in rglg3 rglg4 versus Col-0 (Fig. 6I, J). Consistent with these expression profiles, hormone quantification showed FB1 indeed inhibited total JA production after FB1 infiltration in Col-0 compared to the mock treatment and this suppression was counteracted in rglg3 rglg4 (Fig. 6L). FB1-induced SA biosynthesis was not apparently affected in rglg3 rglg4 in comparison to that in Col-0 (Fig. 6K). Therefore, RGLG3 and RGLG4 mainly modulate the JA pathway during FB1 treatment.
Fig. 6.
Specific roles of RGLG3 and RGLG4 in the FB1-activated JA pathway. (A–J) Expression profiles of JA- and SA-responsive genes after FB1 treatment in Col-0 and rglg3 rglg4. RNA was extracted from four-week-old Arabidopsis leaves at the indicated time points after infiltration with 10mM MgCl2 (mock) or 10 μM FB1 in 10mM MgCl2 (FB1). UBQ10 was used as an internal control, and expression levels were normalized to that measured at time 0. Data show the mean ±SD from four technical replicates. (K, L) JA and SA quantification after FB1 treatment in Col-0 and rglg3 rglg4. Four-week-old Arabidopsis leaves were treated as in A, and then the samples were collected at the indicated times points for JA and SA measurement. Data represent the mean ±SD of three technical replicates. Asterisks indicate a significant difference from time point 0 (Student’s t-test: *, P<0.05; **, P<0.01).
RGLG3 and RGLG4 mediate SA suppression of JA signalling in FB1-induced responses
Crosstalk between SA and JA pathways has been widely documented in diverse pathogen defence responses (Pieterse et al., 2012), but it remains uncharacterized in the FB1 system. We examined how SA affected JA signalling after FB1 treatment and determined if RGLG3 and RGLG4 were involved. In Col-0, both exogenous SA application and FB1 injection promoted SA-responsive PR2 expression, and this induction was elevated by combined SA and FB1 treatment; in the SA-deficient NahG transgenic plant, PR2 induction by SA, FB1 or a combined treatment was attenuated; in both coi1-2 and rglg3 rglg4, PR2 induction by the three treatments was not substantially affected (Fig. 7). SA application suppressed all the checked JA-responsive genes in Col-0, consistent with previous reports (Leon-Reyes et al., 2010; Van der Does et al., 2013). This response was, however, different from that following FB1 treatment, which stimulated defence-related PDF1.2 while repressing growth-related VSP2 and biosynthesis-related OPR3. When SA and FB1 treatments were combined, PDF1.2 was only moderately inducible, and OPR3 and VSP2 were more severely inhibited in Col-0 (Fig. 7). Interestingly, SA repression was similarly reduced in rglg3 rglg4 and NahG, but remained unchanged in coi1-2. Moreover, the FB1 effect on PDF1.2 expression was promoted by the NahG transgene, whereas it was blocked in coi1-2 and rglg3 rglg4 plants; FB1 repression of OPR3 and VSP2 decreased in NahG plants, but in rglg3 rglg4 and coi1-2 plants these genes were induced (Fig. 7). The combined treatment with SA and FB1 in NahG mitigated FB1 induction of PDF1.2, whereas it promoted repression of OPR3 and VSP2, although not as severely as in Col-0 (Fig. 7). Altogether, these data indicate that RGLG3 and RGLG4 also mediate SA suppression of the JA pathway upon FB1 treatment.
Fig. 7.
Roles of RGLG3 and RGLG4 in SA suppression of the JA pathway. Four-week-old Arabidopsis leaves of the indicated genetic backgrounds were infiltrated with 10mM MgCl2 (mock) or 10 μM FB1 in 10mM MgCl2 (FB1), were sprayed directly with 2mM SA (SA) or were given a combined FB1 and SA treatment (FB1+SA). Expression of PDF1.2, VSP2, OPR3 and PR2 was assessed by real-time PCR. UBQ10 was used as an internal control, and expression levels were normalized to that measured at time 0. Data indicate the mean ±SD from four technical replicates. This experiment was repeated three times with similar results.
Discussion
The mycotoxin FB1 provides a useful pathogen-free system for studying PCD during the defence response (Stone et al., 2000). Our characterization of RGLG3 and RGLG4 in this process has underscored the contribution of the JA pathway to FB1-induced PCD.
Hormone pathways, including SA, JA and ET, have important roles in FB1-induced PCD (Asai et al., 2000), although the actual roles of these hormone pathways have not been determined in plants. Previous work using JA-resistant jar1 protoplast has indicated a positive role for the JA pathway in FB1-induced cell death (Asai et al., 2000). Here we show in adult plants that the JA receptor mutant coi1-2 was insensitive to FB1 (Fig. 5A), and FB1-induced PDF1.2 and PR5 expression was also suppressed in coi1-2 (Fig. 5B). Therefore, JA perception is required for FB1-triggered responses. In addition, similar to the effect in JA pathway-mediated defence responses (Dombrecht et al., 2007), mutating the essential regulator MYC2 facilitated FB1-induced cell death (Fig. 5A) and promoted FB1 induction of PDF1.2 and PR5 expression (Fig. 5B), suggesting a negative role for MYC2 in this process. Furthermore, both genetic evidence and molecular evidence provided here indicate that RGLG3 and RGLG4, two recently characterized upstream regulators of the JA pathway (Zhang et al., 2012), also coordinately regulate FB1-triggered responses in a COI1- and MYC2-dependent manner (Fig. 5). Their importance in FB1-induced PCD can be further supported by the expression profiling of lesion mimic genes (Supplementary Fig. S2), mutants of which display spontaneous PCD-like lesions, thus providing useful tools to study PCD pathways in plants (Lorrain et al., 2003). Consistently, RGLG3 and RGLG4 expression was similarly affected by FB1 and JA [Fig. 5 and Zhang et al. (2012)], suggesting that the mechanism used by JA to tightly regulate the protein levels of these genes also functions in the FB1-triggered response. Taken together, these findings strongly suggest that the JA pathway promotes FB1-induced PCD.
The JA pathway also promotes parasitism by the hemibiotrophic pathogen Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) (Thomma et al., 1998), whereas RGLG3 and RGLG4 support Pst DC3000 growth in infected Arabidopsis leaves (Zhang et al., 2012). These observations are contradictory to the dominant roles of JA in defence responses against necropathogenic pathogens, such as Alternaria brassicicola and Botrytis cinerea (Pieterse et al., 2009), and the molecular basis for these differences remains unclear. Interestingly, FB1-resistant 1 (fbr1) and fbr2 are insensitive to FB1 but also resistant to Pst DC3000 (Stone et al., 2000). Consideration of possible roles of the JA pathway may help to elucidate the underlying mechanism behind these effects. Two other FB1-resistant mutants, fbr11 (Teng et al., 2008) and fbr12 (Feng et al., 2007), have defects in pollen development and sterility, characteristics that are probably related to JA deficiency but have not been verified.
However, it is also possible that FB1 inhibited JA production (Fig. 6), probably resulting from feedback control, as JA biosynthesis undergoes positive feedback regulation (Turner et al., 2002), but growth-related genes and JA biosynthesis genes were suppressed by FB1 (Figs 6, 7). This suggests that new JA production (or high-level JA production) is disadvantageous for infection by FBI-producing pathogens. How FB1 can activate JA signalling is not clear. There may be a preexisting pool of inactive JA (Fonseca et al., 2009), and FB1 may stimulate this low-level pool and activate JA perception and downstream signalling. By this means, these pathogens may hijack the JA pathway to initiate cell death in favour of their invasion, meanwhile preventing host plants from producing high-level JA for host defence. A similar JA pathway-hijacking strategy is used by Pst DC3000, which secretes the JA-Ile mimic coronatine to activate JA signalling during the invasion process (Fonseca et al., 2009).
Another way to repress JA production by FB1 may come from SA suppression of JA signalling. Crosstalk between SA and JA pathways has been considered a mechanism for prioritizing limited resources for better defence according to the context of different stresses (Pieterse et al., 2012). FB1 activated both pathways, as disrupting either one in NahG or coi1-2 plants significantly attenuated the FB1-induced transcriptome (Fig. 7). Moreover, exogenous SA inhibited JA-responsive genes; FB1 induction of PDF1.2 was enhanced, and FB1 suppression of VSP2 and OPR3 was mitigated in NahG plants (Fig. 7). These results imply that activated SA signalling promotes FB1-suppressed JA production, which may further contribute to the defence response of the host plant upon FB1 injection. In contrast, Pst DC3000-activated JA signalling is accompanied by suppression of the SA pathway (Kloek et al., 2001).
RGLG3 and RGLG4 emerge as novel players mediating SA-JA crosstalk based on our results (Fig. 7). However, in the JA pathway RGLG3 and RGLG4 function upstream of COI1 (Zhang et al., 2012), and SA suppresses JA signalling downstream of COI1 (Van der Does et al., 2013). Therefore, RGLG3 and RGLG4 probably have another downstream target(s). Notably, RGLG3 and RGLG4 are different from previously identified factors, such as NPR1 (Spoel et al., 2003), GRX48 (Ndamukong et al., 2007), WRKY70 (Li et al., 2004) and MPK4 (Petersen et al., 2000), in that they have no effect on FB1-triggered SA production or signalling (Fig. 6) and the SA pathway is not required for their transcriptional regulation (Fig. 4). So far, most evidence indicates that SA repression targets JA transcriptional machinery (Pieterse et al., 2012; Van der Does et al., 2013). In addition, RGLG3- and RGLG4-modulated SA inhibition suggests that SA interferes with the turnover of certain transcription regulator(s) in the JA pathway.
Our data, together with previous reports, indicate that the four RGLGs in Arabidopsis have different spatial distributions in the cell, with RGLG1 and RGLG2 localized to the plasma membrane (Stone et al., 2005; Yin et al., 2007), whereas RGLG3 and RGLG4 are located in the plasma and nucleus (Fig. 1). The second glycine residue is important for the membrane location of RGLG2 (Yin et al., 2007), but RGLG3 and RGLG4 totally lack the N-terminal sequence that is present in RGLG1 and RGLG2 (Zhang et al., 2012), so the N-terminal differences in RGLG3 and RGLG4 probably account for their distinct localizations. A recent report indicates that RGLG2 translocates into the nucleus upon stress (Cheng et al., 2012). We did use exogenous FB1 to treat roots from transgenic Arabidopsis plants in our study, but no notable changes in RGLG3 and RGLG4 localization were observed (unpublished data), which suggests that they have different modes of action in the cell relative to RGLG1 and RGLG2. Protein ubiquitination has important roles in regulating cellular responses (Vierstra, 2009). For example, a membrane-localized Ring-type E3, RING1, negatively regulates FB1-triggered cell death (Lin et al., 2008), and other E3s, such as DAl1 and DAl2 (Basnayake et al., 2011), are also involved in FB1-triggered cell death. Therefore, the induction of PCD by FB1 is a complicated process involving multiple E3s with different roles at different locations in the cell. Substantially more work will be required to characterize the RGLG targets and other factors to fully appreciate the nature of FB1-induced cell death.
In summary, our data highlight the significance of the JA pathway in FB1-induced PCD and reveal the essential roles of two ubiquitin ligases, RGLG3 and RGLG4, in mediating this process. RGLG3 and RGLG4 may act as a regulatory cluster in the complex hormone network controlling cell death (Supplementary Fig. S3), and thus identifying their target(s) will no doubt further our understanding of the mechanisms of FB1-elicited PCD as well as JA signalling.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Representative leaves showing lesion development after FB1 treatment.
Supplementary Fig. S2. FB1 responsiveness of lesion mimic mutant (LMM) genes in rglg3 rglg4 plants versus Col-0 plants.
Supplementary Fig. S3. A model illustrating possible roles of RGLG3 and RGLG4 in FB1-triggered cell death.
Supplementary Table S1. List of primers used in this study.
Acknowledgements
We thank the Arabidopsis Biological Resource Center for Arabidopsis mutant lines (SALK_098983, SALK_096022C and CS3735) and Hongwei Guo, Christiane Gatz, Roberto Solano and Xinnian Dong for other mutants as indicated above. We also thank Shunong Bai for assistance in plant hormone measurements, Yi Li for the plasmid pRTL-GFP, Lijia Qu and Hongya Gu for plasmids pJim19 and PBI121-GUS and Lianfen Song and Qitao Zhang for technical assistance. This work was supported by grants from the National Key Basic Science ‘973’ Program (2012CB114006) and the National Natural Science Foundation (31272023, 31170231, 31101421 and 91117016) of the Chinese Government.
References
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. 2003. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis . Proceedings of the National Academy of Sciences, USA 100, 2992–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM. 2000. Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. The Plant Cell 12, 1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnayake BM, Li D, Zhang H, Li G, Virk N, Song F. 2011. Arabidopsis DAL1 and DAL2, two RING finger proteins homologous to Drosophila DIAP1, are involved in regulation of programmed cell death. Plant Cell Reports 30, 37–48. [DOI] [PubMed] [Google Scholar]
- Bell E, Mullet JE. 1993. Characterization of an Arabidopsis lipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiology 103, 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JI, Kanneganti TD, Young C, Cakir C, Huitema E, Win J, Armstrong MR, Birch PR, Kamoun S. 2006. The C-terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a-mediated hypersensitivity and suppress INF1-induced cell death in Nicotiana benthamiana . The Plant Journal 48, 165–176. [DOI] [PubMed] [Google Scholar]
- Boter M, Ruiz-Rivero O, Abdeen A, Prat S. 2004. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis . Genes & Development 18, 1577–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. 2009. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology 60, 183–205. [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. 1994. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. The Plant Cell 6, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MC, Hsieh EJ, Chen JH, Chen HY, Lin TP. 2012. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiology 158, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernandez G, et al. 2007. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671. [DOI] [PubMed] [Google Scholar]
- Chivasa S, Ndimba BK, Simon WJ, Lindsey K, Slabas AR. 2005. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. The Plant Cell 17, 3019–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. 2000. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis . The Plant Cell 12, 2175–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll NS, Epple P, Dangl JL. 2011. Programmed cell death in the plant immune system. Cell Death and Differentiation 18, 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai K, Sullards MC, Allegood J, et al. 2002. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochimica et Biophysica Acta 1585, 188–192. [DOI] [PubMed] [Google Scholar]
- Devoto A, Nieto-Rostro M, Xie D, et al. 2002. COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis . The Plant Journal 32, 457–466. [DOI] [PubMed] [Google Scholar]
- Dickman MB, Fluhr R. 2013. Centrality of host cell death in plant-microbe interactions. Annual Review of Phytopathology 51, 543–570. [DOI] [PubMed] [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, et al. 2007. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis . The Plant Cell 19, 2225–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Chen Q, Feng J, Zhang J, Yang X, Zuo J. 2007. Functional characterization of the Arabidopsis eukaryotic translation initiation factor 5A-2 that plays a crucial role in plant growth and development by regulating cell division, cell growth, and cell death. Plant Physiology 144, 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Calvo P, Chini A, Fernandez-Barbero G, et al. 2011. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. The Plant Cell 23, 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. 2009. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nature Chemical Biology 5, 344–350. [DOI] [PubMed] [Google Scholar]
- Fu J, Chu J, Sun X, Wang J, Yan C. 2012. Simple, rapid, and simultaneous assay of multiple carboxyl containing phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Analytical Sciences 28, 1081–1087. [DOI] [PubMed] [Google Scholar]
- Gilchrist DG. 1997. Mycotoxins reveal connections between plants and animals in apoptosis and ceramide signaling. Cell Death and Differentiation 4, 689–98. [DOI] [PubMed] [Google Scholar]
- Gilchrist DG. 1998. Programmed cell death in plant disease: the purpose and promise of cellular suicide. Annual Review of Phytopathology 36, 393–414. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM. 1996. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Tan G, Liu Q, Huang K, Ren J, Zhang X, Yu X, Huang P, An C. 2011. a . The LSD1-interacting protein GILP is a LITAF domain protein that negatively regulates hypersensitive cell death in Arabidopsis . PLoS One 6, e18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SP, Huang KW, Zhang X, Yu XC, Huang P, An CC. 2011. b . The LSD1-type zinc finger motifs of Pisum sativa LSD1 are a novel nuclear localization signal and interact with importin alpha. PLoS One 6, e22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett BJ. 2006. Secondary metabolite toxins and nutrition of plant pathogenic fungi. Current Opinion in Plant Biology 9, 371–375. [DOI] [PubMed] [Google Scholar]
- Jamir Y, Guo M, Oh HS, Petnicki-Ocwieja T, Chen S, Tang X, Dickman MB, Collmer A, Alfano JR. 2004. Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. The Plant Journal 37, 554–565. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. 1999. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proceedings of the National Academy of Sciences, USA 96, 13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM. 2012. JAZ repressors and the orchestration of phytohormone crosstalk. Trends in Plant Science 17, 22–31. [DOI] [PubMed] [Google Scholar]
- Kelley BS, Lee SJ, Damasceno CM, Chakravarthy S, Kim BD, Martin GB, Rose JK. 2010. A secreted effector protein (SNE1) from Phytophthora infestans is a broadly acting suppressor of programmed cell death. The Plant Journal 62, 357–366. [DOI] [PubMed] [Google Scholar]
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN. 2001. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. The Plant Journal 26, 509–522. [DOI] [PubMed] [Google Scholar]
- Lam E. 2004. Controlled cell death, plant survival and development. Nature Reviews Molecular Cell Biology 5, 305–315. [DOI] [PubMed] [Google Scholar]
- Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, Van Wees SC, Ritsema T, Pieterse CM. 2010. Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 232, 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET. 2004. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. The Plant Cell 16, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SS, Martin R, Mongrand S, Vandenabeele S, Chen KC, Jang IC, Chua NH. 2008. RING1 E3 ligase localizes to plasma membrane lipid rafts to trigger FB1-induced programmed cell death in Arabidopsis . The Plant Journal 56, 550–561. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R. 2004. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis . The Plant Cell 16, 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balague C, Roby D. 2003. Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends in Plant Science 8, 263–271. [DOI] [PubMed] [Google Scholar]
- Ndamukong I, Abdallat AA, Thurow C, Fode B, Zander M, Weigel R, Gatz C. 2007. SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. The Plant Journal 50, 128–139. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr., Kangasjarvi J. 2000. Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. The Plant Cell 12, 1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, et al. 2000. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 103, 1111–1120. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. 2009. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC. 2012. Hormonal modulation of plant immunity. Annual Review of Cell and Developmental Biology 28, 489–521. [DOI] [PubMed] [Google Scholar]
- Qi TC, Song SS, Ren QC, Wu DW, Huang H, Chen Y, Fan M, Peng W, Ren CM, Xie DX. 2011. The jasmonate-ZIM domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana . The Plant Cell 23, 1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR. 2000. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. The Plant Cell 12, 1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reape TJ, McCabe PF. 2008. Apoptotic-like programmed cell death in plants. New Phytologist 180, 13–26. [DOI] [PubMed] [Google Scholar]
- Rojo E, Leon J, Sanchez-Serrano JJ. 1999. Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana . The Plant Journal 20, 135–142. [DOI] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao HB, et al. 2010. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468, 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SS, Qi TC, Huang H, Ren QC, Wu DW, Chang CQ, Peng W, Liu YL, Peng JR, Xie DX. 2011. The jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis . The Plant Cell 23, 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, et al. 2003. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant Cell 15, 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Huang JF, Rhee Y. 1991. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiology 96, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I. 2004. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis . The Plant Cell 16, 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Browse J. 2000. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proceedings of the National Academy of Sciences, USA 97, 10625–10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Heard JE, Asai T, Ausubel FM. 2000. Simulation of fungal-mediated cell death by Fumonisin B1 and selection of Fumonisin B1-resistant (fbr) Arabidopsis mutants. The Plant Cell 12, 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J. 2005. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis . Plant Physiology 137, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C, Dong H, Shi L, Deng Y, Mu J, Zhang J, Yang X, Zuo J. 2008. Serine palmitoyltransferase, a key enzyme for de novo synthesis of sphingolipids, is essential for male gametophyte development in Arabidopsis . Plant Physiology 146, 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu GH, Nomura K, He SY, Howe GA, Browse J. 2007. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448, 661–665. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, Broekaert WF. 1998. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences, USA 95, 15107–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A. 2002. The jasmonate signal pathway. The Plant Cell 14 Suppl, S153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does D, Leon-Reyes A, Koornneef A, et al. 2013. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. The Plant Cell 25, 744–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Beers EP, Dangl JL, et al. 2011. Morphological classification of plant cell deaths. Cell Death and Differentiation 18, 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees SC, Glazebrook J. 2003. Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. The Plant Journal 33, 733–742. [DOI] [PubMed] [Google Scholar]
- Vierstra RD. 2009. The ubiquitin-26S proteasome system at the nexus of plant biology. Nature Reviews Molecular Cell Biology 10, 385–397. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565. [DOI] [PubMed] [Google Scholar]
- Wolpert TJ, Dunkle LD, Ciuffetti LM. 2002. Host-selective toxins and avirulence determinants: what’s in a name? Annual Review of Phytopathology 40, 251–285. [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. 1998. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280, 1091–1094. [DOI] [PubMed] [Google Scholar]
- Xu LH, Liu FQ, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang DF, Xie DX. 2002. The SCFCOl1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis . The Plant Cell 14, 1919–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XJ, Volk S, Ljung K, et al. 2007. Ubiquitin lysine 63 chain-forming ligases regulate apical dominance in Arabidopsis . The Plant Cell 19, 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Chung WI, Choi D. 2009. NbHB1, Nicotiana benthamiana homeobox 1, is a jasmonic acid-dependent positive regulator of pathogen-induced plant cell death. New Phytologist 184, 71–84. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu Q, Ren J, Qian W, He S, Huang K, Yu X, Gao Y, Huang P, An C. 2012. Two novel RING-type ubiquitin ligases, RGLG3 and RGLG4, are essential for jasmonate-mediated responses in Arabidopsis . Plant Physiology 160, 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.