Highlight
Brassinosteroids negatively regulate Fe transport and translocation in a strategy II rice plants.
Key words: Brassinosteroids (BRs), d2-1 mutant, Fe deficiency, Fe translocation, Fe uptake, rice (Oryza sativa), strategy II plant.
Abstract
Brassinosteroids (BRs) are steroid hormones that modulate numerous physiological processes in plants. However, few studies have focused on the involvement of BRs in sensing and responding to the stress of mineral nutrient deficiency. In the present study, we evaluated the roles of BRs in the response of rice (Oryza sativa) to iron (Fe) deficiency during Fe uptake, transport, and translocation. Exogenous application of 24-epibrassinolide (EBR) to wild-type (WT) plants exaggerated leaf symptoms of Fe deficiency and suppressed growth. EBR increased and decreased Fe concentrations in roots and shoots, respectively, under both Fe-deficient and Fe-sufficient conditions. Transcripts involved in Fe homeostasis, including OsIRT1, OsYSL15, OsYSL2, OsNAS1, and OsNAS2, were enhanced by EBR under Fe-deficient conditions. EBR depressed expression of OsNAS1, OsNAS2, and OsYSL2 in shoots, and inhibited Fe transport and translocation via the phloem. Rice mutant d2-1, which is defective in BR biosynthesis, was more tolerant to Fe deficiency than the WT, and accumulated greater amounts of Fe in roots than the WT under Fe-sufficient conditions. A greater upregulation of OsIRT1, OsYSL15, OsYSL2, OsNAS1, and OsNAS2 in the d2-1 mutant compared to the WT was found under Fe-sufficient conditions, while expression of these genes in the d2-1 mutant was lower than in the WT under Fe-deficient conditions. The greater tolerance of the d2-1 mutant could be partly mitigated by exogenous application of EBR. These novel findings highlight the important role of BR in mediating the response of strategy II plants to Fe deficiency by regulating Fe uptake and translocation in rice.
Introduction
Iron (Fe) is one of the essential mineral nutrients required for plant growth and development. Plant growth is often limited by low availability of Fe in soil due to its low solubility, especially in calcareous soils (Romera and Alcántara, 2004). Plants have evolved various strategies to cope with Fe deficiency in soil. These strategies have been classified as strategy I and strategy II, and these exist in non-graminaceous monocots and dicots, and in graminaceous monocots, respectively (Römheld and Marschner, 1986; Kobayashi and Nishizawa, 2012). Increased ferric chelate reductase activity, acidification of the rhizosphere, and upregulation of Fe2+ transporters (IRTs) are major mechanisms by which strategy I plants maximize their Fe acquisition (Curie and Briat, 2003; Hell and Stephan, 2003; Romera and Alcántara, 2004). In contrast, strategy II plants commonly secrete phytosiderophores (PSs) belonging to the mugineic acid (MA) family from roots to solubilize Fe3+ (Kobayashi and Nishizawa, 2012). A gene encoding an Fe3+-MA transporter has been isolated from maize mutant yellow trip 1, and an Fe3+-phytosiderophore uptake transporter was named as Yellow Stripe1 (YS1) (von Wiren et al., 1994; Curie et al., 2001). Secretion of MAs from rice roots to the rhizosphere is mediated by OsTOM1 (Nozoye et al., 2011), and the resulting Fe3+-MA complexes are adsorbed into root cells by yellow-stripe like (YSL) transporters in the plasma membrane (Conte and Walker, 2011). In rice, OsYSL15 is the primary transporter responsible for uptake of Fe3+-MA from the rhizosphere (Inoue et al., 2009). Recent studies reveal that, in addition to acquisition of Fe by MA, rice plants also possess the Fe2+-transporter system, and two genes encoding Fe2+ transporters, OsIRT1 and OsIRT2, have been isolated (Bughio et al., 2002; Ishimaru et al., 2006). Expression of OsIRT1 and OsIRT2 is upregulated by Fe deficiency, and mutation in NAAT stimulates the Fe (II) acquisition system in cultures with an abundant Fe2+ source, leading to Fe accumulation in rice plants (Cheng et al., 2007).
Iron is transported in complexed forms once it is loaded into the xylem (Conte and Walker, 2011). In Arabidopsis, FRD3, which belongs to the multidrug and toxic compound extrusion (MATE) family, is involved in loading of citrate into the xylem (Rogers and Guerinot, 2002; Green and Rogers, 2004; Durrett et al., 2007). FRD3 is expressed in the root vasculature, and its expression is enhanced by Fe deficiency (Green and Rogers, 2004). Similar to Arabidopsis, rice also possesses an FRD-like gene (OsFRDL1), which encodes a citrate transporter localized at the rice root pericycle cells and mediates the translocation of Fe to the shoot in the form of Fe-citrate complex (Yokosho et al., 2009). A recent study reported that Fe exists as oxo-bridged tri-Fe3+, tri-citrate (Fe3Cit3) complex in the xylem sap of Fe-deficient tomato (Rellán-Álvarez et al., 2010). In addition to citrate, nicotianamine (NA) is another important agent to complex metals in plants. Nicotianamine exists ubiquitously in roots and shoots as well as in the xylem and phloem sap of higher plants, and it can complex Fe2+, Fe3+, Mg2+, and Zn2+ (Morrissey and Guerinot, 2009; Conte and Walker, 2011). In rice, nicotianamine is synthesized by NA synthase (NAS) from S-adenosylmethionine, and involves long-distance Fe transport (Inoue et al., 2003). OsYSL2 is a rice metal-NA transporter responsible for translocation of Fe and Mn into the grain via the phloem (Koike et al., 2004). Moreover, OsYSL2 can also play roles in the mediation of translocation of Fe from root to shoot (Ishimaru et al., 2010).
A number of phytohormones and messenger molecules such as auxin, ethylene, cytokinins, and nitric oxide (NO) have been reported to be involved in the regulation of Fe deficiency-induced changes in morphological and physiological processes in strategy I plants (Ivanov et al., 2012). In contrast, there is limited information on the role of phytohormones in the response of strategy II plants to Fe deficiency (Wu et al., 2011). As a class of plant polyhydroxysteroids, brassinosteroids (BRs) are ubiquitous in plants (Noguchi et al., 1999). There is emerging evidence demonstrating that BRs play important roles in the response of plants to biotic and abiotic stresses (Sasse, 2003). Our previous studies showed that brassinosteroids are involved in response of cucumber (Cucumis sativus) to Fe deficiency by regulating Fe deficiency-induced FRO and Fe translocation from roots to shoots (Wang et al., 2012). In the present study, we evaluated the role of BRs in the response of strategy II rice plants to Fe deficiency using wild-type rice and the BR-deficient rice mutant d2-1. The d2-1 plants are dwarf mutants resulting from a defect in BR biosynthesis (Hong et al., 2003). The D2 gene encodes a novel cytochrome P450 classified as CYP90D that is highly similar to the reported BR synthesis enzymes. The D2/CYP90D2 enzyme catalyses the steps from 6-deoxoteasterone to 3-dehydro-6-deoxoteasterone and from teasterone to 3-dehydroteasterone in the late BR biosynthesis pathways (Hong et al., 2003). Our results demonstrate that BR also played an important role in the response of strategy II plant to Fe deficiency by regulating long-distance transport and translocation of Fe via the phloem.
Materials and methods
Wild-type (WT) rice (Oryza sativa L. cv. ‘Taichung 65’) and d2-1 mutants derived from Taichung 65 were used. The d2-1 plants were screened from a mutant library produced by N-methyl-N-nitrosourea. Hong et al. (2003) cloned the D2 gene by map-based cloning and showed quantitatively that endogenous BR levels in the mutants were much lower than in their counterpart WT plants under normal growth conditions. The seeds were surface sterilized by incubation for 3min in 75% ethanol followed by 10min in 0.1% HgCl2, and were then washed thoroughly with sterile water. The sterilized seeds were soaked in water for 24h in the dark and then transferred to a nylon net floating on water for 1 week. Thereafter, the 7-d-old seedlings were transferred to nutrient solution containing half-strength Kimura B solution. The nutrient solution contained the macronutrients (mM) (NH4)2SO4 (0.18), MgSO4·7H2O (0.27), KNO3 (0.09), Ca (NO3)2·4H2O (0.18), and KH2PO4 (0.09); and micronutrients (µM) MnCl2·4H2O (0.5), H3BO3 (3), (NH4)6Mo7O24·4H2O (1), ZnSO4·7H2O (0.4), and CuSO4·5H2O (0.2). The solution also contained 50 µM FeEDTA. The pH was adjusted daily to 5.5 and the solution was renewed every 3 d. The hydroponic experiments were carried out in a growth room with a 16-h light (30°C)/8-h dark (22°C) photoperiod, and the relative humidity was kept at ~70%. After 10-d growth, the pre-cultured seedlings were used for the following experiments.
Measurement of chlorophyll
Ten-d-old WT seedlings pre-cultured in solution containing 50 µM Fe-EDTA were transferred to full-strength Kimura B solution supplemented with 0 µM (Fe-deficient medium) or 100 µM Fe-EDTA (Fe-sufficient medium) with varying concentrations of 24-epibrassinolide (EBR) (0, 1, 10, 100, and 500nM) for 2 weeks. The EBR was dissolved in a minimal volume of ethanol, and then made up to volume with nutrient solution (Yu et al., 2004). After treatment, foliar chlorophyll concentrations were measured. Newly formed leaves were weighed and then ground with aqueous acetone (80% v/v) and centrifuged at 10 000g for 5min. Absorbance (A) readings of the supernatant were recorded at 645 and 663nm. Total chlorophyll concentration (mg l–1) was calculated as 8.02A663 + 20.21A645, and expressed as mg chlorophyll g–1 fresh weight. Ten-d-old WT and d2-1 mutant seedlings pre-cultured in solution containing 50 µM Fe-EDTA were transferred to full-strength Kimura B solution supplemented with 0 µM (Fe-deficient medium) or 100 µM Fe-EDTA (Fe-sufficient medium) with or without 100nM EBR for 2 weeks. Foliar chlorophyll concentrations were measured.
Determination of plant biomass and metal analysis in roots and shoots
Ten-d-old WT and d2-1 seedlings pre-cultured in solution containing 50 µM Fe-EDTA were transferred to 0 µM (–Fe) and 100 µM (+Fe) Fe-EDTA full-strength Kimura B solution in the absence and presence of 100nM EBR for 2 weeks. After treatments, seedlings were harvested and separated into roots and shoots, then dried for 2 d at 75ºC. After measurements of biomass, root and shoot samples were ground to fine powder and digested with concentrated nitric acid and hydrogen peroxide. The total Fe concentrations were determined by Inductive Coupled Plasma Emission Spectrometry (ICP-OES).
Expression patterns of genes encoding Fe uptake and translocation proteins
Total RNA was extracted from roots and leaves of rice plants subjected to Fe or EBR treatments for varying periods (1, 3, and 7 d). Total RNA was isolated using RNAiso reagent (Takara) and reverse-transcribed into first-strand cDNA with PrimeScript® RT reagent Kit with gDNA Erager (Takara). Real-time PCR was performed in an optical 96-well plate with an Applied Biosystems SteponeTM Real-Time PCR system. Each reaction contained 7.5 μl of 2 × SYBR Green Master Mix reagent, 0.5 μl of cDNA samples, and 0.6 μl of 10 μM gene-specific primers in a final volume of 15 μl. The thermal cycle used was as follows: 95°C for 10min; and 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. All the primers used for quantitative RT-PCR are listed in Supplementary Table S1. The relative quantification method (Delta-Delta cycle threshold) was used to evaluate quantitative variation between the replicates examined. The PCR signals were normalized to those of actin or rice polyubiquitin (RubQ1).
Analysis of the phloem sap
The protocols described by Corbesier et al. (2003) were used for collection of phloem sap. Briefly, newly formed leaves were detached at their petiole bases, and the petioles were recut in medium containing 20mM EDTA-K2 (pH 7.5). The leaves collected from each individual plant were placed in a 2ml microcentrifuge tube with their petioles immersed in 1.5ml of 15mM EDTA-K2 (pH 7.5). The tubes were placed in airtight transparent plastic containers in an illuminated growth room for 4h to dissolve the phloem sap in EDTA solution (Corbesier et al., 2003); the atmosphere was water saturated (to prevent uptake of EDTA solution by the leaves). Iron concentration in the collected phloem sap was measured by Inductive Coupled Plasma Mass Spectrometry (ICP-MAS). The leaves were dried at 80°C and weighed.
Statistical analysis
Analysis of variance was conducted between different treatments. The significant differences between treatments were evaluated by LSD multiple range tests (P ≤ 0.05) using SAS statistical software.
Results
Exogenous application of EBR enhanced leaf symptoms of Fe deficiency
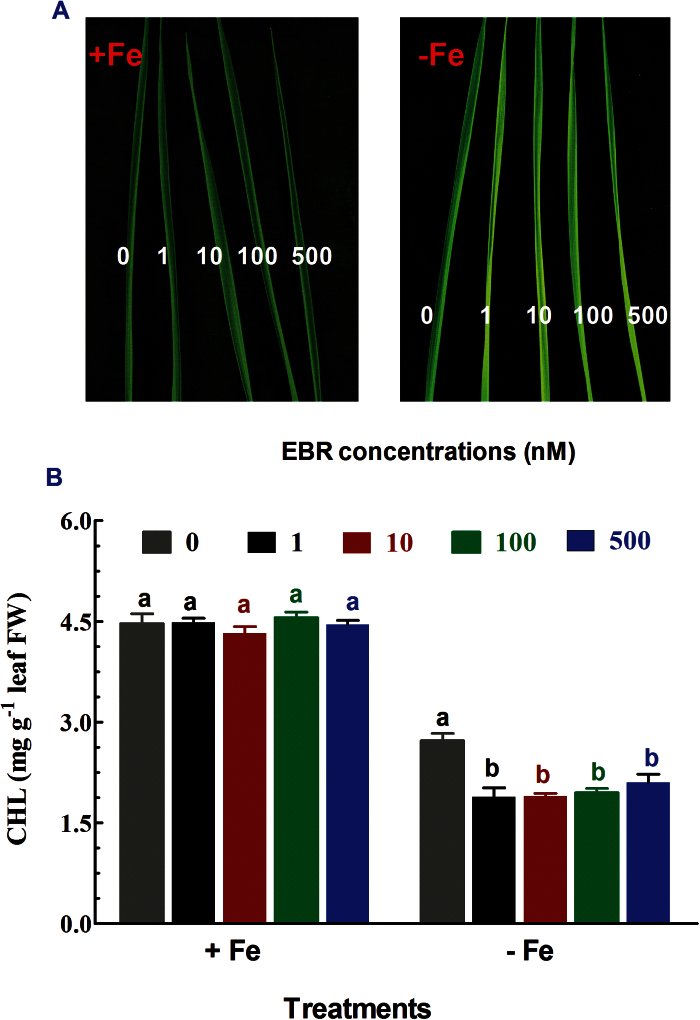
To test whether BR is involved in Fe deficiency-induced changes in physiological processes in rice plants, the effect of exogenous EBR at varying concentrations (0–500nM) on cholorophyll concentrations of rice seedlings grown in Fe-sufficient and Fe-deficient media was studied. Apparent chlorosis was observed in young leaves of rice seedlings grown in Fe-deficient medium for 2 weeks (Fig. 1A), leading to a decrease in cholorophyll concentration (Fig. 1B). Furthermore, the chlorosis became more evident by application of EBR to Fe-deficient seedlings. In contrast, application of EBR had no apparent effect on leaf cholorosis in Fe-sufficient seedlings (Fig. 1A). Accordingly, treatments with EBR led to a significant decrease in chlorophyll concentration of Fe-deficient plants, while the same treatment had no effect on chlorophyll concentration in Fe-sufficient seedlings (Fig. 1B). The reduction in chlorophyll concentration in Fe-deficient seedlings by EBR occurred at a concentration of 1nM, and no further reduction in the chlorophyll concentration was observed when EBR concentration was increased up to 500nM, suggesting that the effect of EBR on the chlorophyll concentration is independent of EBR dose, and that BR may be an important signal during Fe deficiency. Although 1nM EBR can have the negative effect, at >100nM it can partly restore the phenotype of the d2-1 mutant. Therefore, an EBR concentration of 100nM was used throughout this study, which can partly restore growth of d2-1 and has no effect on growth of WT plants. Since rice can take up both Fe2+- and Fe3+-MA, in addition to Fe-EDTA, we also investigated the effects of EBR on plants with different forms of Fe present in the medium (FeSO4, Fe3+EDTA, and FeCl3). Similar to FeEDTA, EBR-induced leaf chlorosis was also detected under conditions of deficiency in FeSO4, Fe3+EDTA, or FeCl3 in the growth medium (Supplementary Figure S1). These results indicate that the effect of BR on Fe nutrition is independent of Fe species in the growth medium. We therefore focused on the effect of BR on rice plants with FeEDTA in the medium throughout our study.
Fig. 1.
Effect of EBR on chlorophyll (CHL). (A) Photographs of 10-d-old WT rice seedlings exposed to Fe-sufficient or Fe-deficient medium with or without different concentrations of EBR (0, 1, 10, 100, and 500nM) for 2 weeks. (B) The chlorophyll concentrations in WT rice leaves were determined following treatment of WT seedlings of –Fe and +Fe plants with different concentrations of EBR (0, 1, 10, 100, and 500nM) for 2 weeks. Data are means ± SE (n = 4). Means with different letters are significantly different (P ≤ 0.05) with regard to treatments.
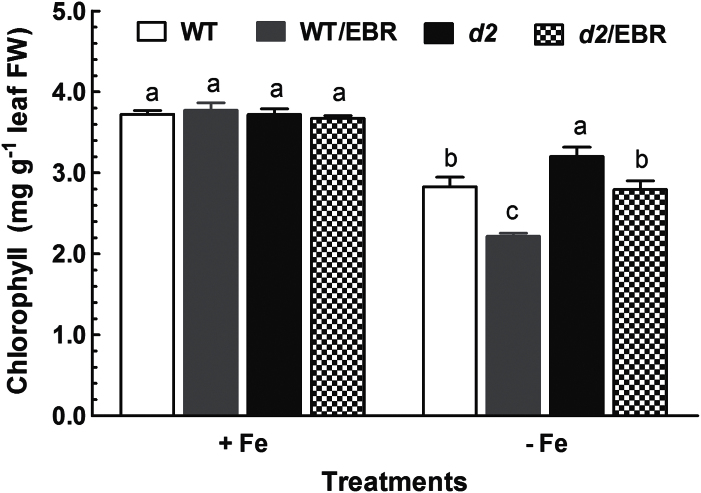
The involvement of BR in the mediation of Fe deficiency-induced changes in physiological processes was further evaluated using a BR-deficient mutant d2-1. Similar to WT plants, Fe deficiency also decreased leaf chlorophyll in the d2-1 mutant, and exogenous application of EBR enhanced Fe-deficiency-induced leaf chlorosis in young leaves of d2-1 seedlings (Fig. 2). However, the d2-1 mutant had a higher chlorophyll concentration than the WT plant, and the magnitude of decrease in chlorophyll concentration by EBR was much greater in WT plants than in d2-1 mutants under Fe- deficient conditions (Fig. 2), indicating that the WT is more sensitive to EBR than d2-1 mutant plants under Fe-deficient conditions. In contrast, application of EBR had no apparent effect on foliar chlorophyll concentration in Fe-sufficient d2-1 mutant seedlings (Fig. 2).
Fig. 2.
Effect of EBR on chlorophyll of the WT and d2-1 mutant. Ten-d-old WT and d2-1mutant seedlings were exposed to Fe-sufficient and Fe-deficient medium with or without 100nM EBR for 2 weeks. After treatments, chlorophyll concentration in leaves were determined. Data are means ± SE (n = 4). Means with different letters are significantly different with regard to treatments (P ≤ 0.05).
BR-deficient mutant d2-1 was less sensitive to Fe deficiency and EBR
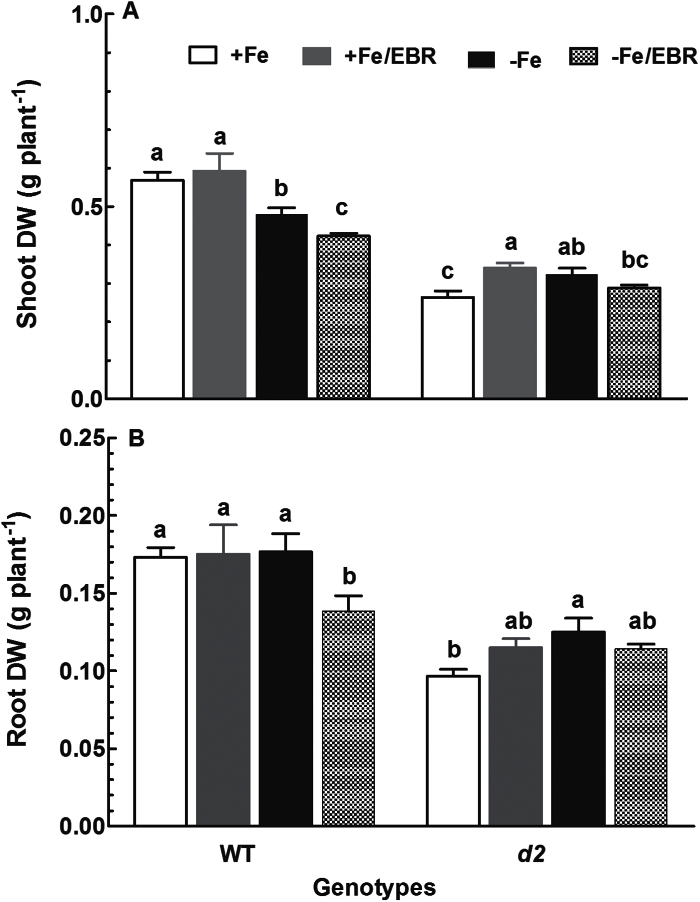
In addition to chlorophyll, dry mass of shoots and roots of WT rice seedlings was significantly reduced when they were grown in Fe-deficient medium. A further reduction in dry weight of shoots and roots of Fe-deficient seedlings was found in the presence of EBR in the growth medium, while no effect of EBR on biomass of Fe-sufficient WT seedlings was observed (Fig. 3A, B). Both shoot and root biomass of d2-1 mutant was significantly lower than that of WT plants when grown under Fe-sufficient conditions (Fig. 3A, B). In contrast to the WT, an increase in dry weight of the shoots and roots of the d2-1 mutant was observed under Fe-deficient conditions (Fig. 3B). There was a significant increase in shoot dry weight and a slight increase in root dry weight in the d2-1 mutant by EBR application under Fe-deficient and Fe-sufficient conditions, respectively (Fig. 3A, B).
Fig. 3.
Effect of EBR on (A) shoot and (B) root dry weight (DW) of WT and d2-1 plants. Ten-d-old WT and d2-1 seedlings were exposed to Fe-sufficient and Fe-deficient medium with or without 100nM EBR for 2 weeks. After treatments, the dry biomass was measured. Data are means ± SE (n = 4). Means with different letters are significantly different (P ≤ 0.05) within the same genotype.
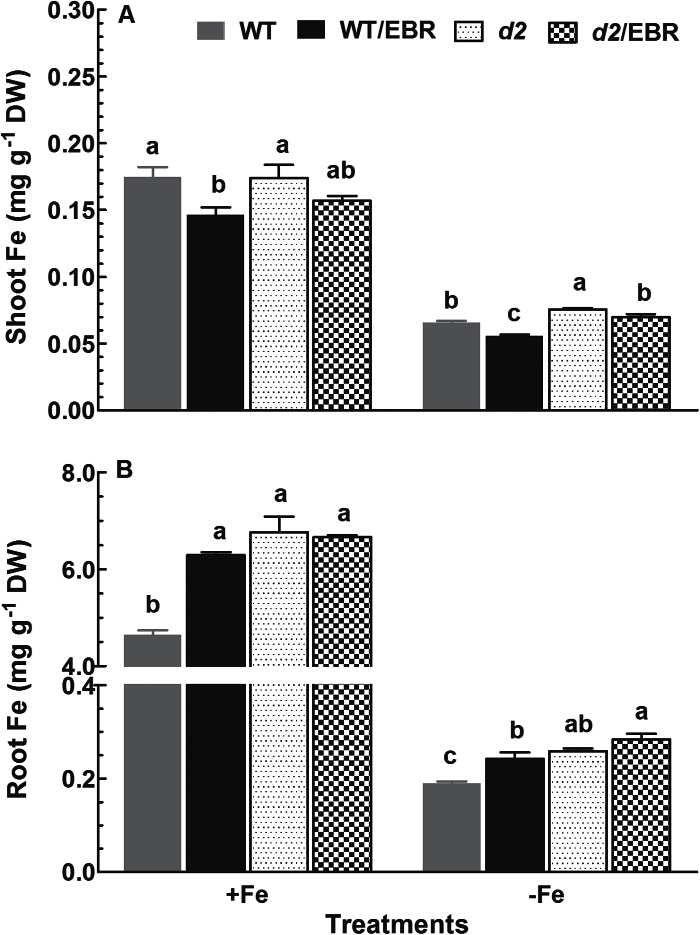
Exposure of rice seedlings to Fe-deficient medium led to a significant decrease of Fe concentrations in roots and shoots in both the WT and the d2-1 mutant (Fig. 4). Also, Fe concentrations in roots of the d2-1 mutant were significantly higher than those of WT plants under both Fe-deficient and Fe-sufficient conditions (Fig. 4B). In contrast to Fe concentrations in roots, concentrations in shoots of d2-1 plants were higher than those of the WT grown in Fe-deficient medium (Fig. 4A), but no differences in shoot Fe concentrations among WT and d2-1 under Fe-sufficient conditions were observed (Fig. 4A). Exogenous application of EBR had contrasting effects on Fe concentrations in roots and shoots of WT rice seedlings regardless of Fe supply, such that treatment with EBR led to decreases in Fe concentrations in shoots by 16 and 15% in Fe-sufficient and Fe-deficient seedlings, respectively (Fig. 4A), while there were 35 and 27% increases in root Fe concentrations of both Fe-sufficient and Fe-deficient seedlings when treated with EBR (Fig. 4B). In contrast to WT plants, EBR had no effect on Fe concentrations of d2-1 in shoots and roots under both Fe-sufficient and Fe-deficient conditions (Fig. 4).
Fig. 4.
Effect of EBR on Fe concentrations in (A) shoots and (B) roots of wild-type and d2-1 mutant plants. Ten-d-old WT seedlings were exposed to Fe-sufficient and Fe-deficient medium with or without 100nM EBR for 2 weeks. After treatments, Fe concentration was measured. Data are means ± SE (n = 4). Means with different letters are significantly different (P ≤ 0.05) within the same Fe treatment.
To test whether the EBR-induced changes in Fe concentrations in shoots and roots were specific to Fe, the effect of EBR on other metals concentrations were studied under Fe-sufficient conditions. In contrast to Fe, Mg and Zn concentrations in shoots and roots were not affected by EBR (Supplementary Figure S2). The concentration of Mn in roots was not affected by EBR, but it was significantly increased by EBR in shoots (Supplementary Figure S2). These results suggest that BR has a specific effect on Fe nutrition in shoots and roots of rice.
Expression patterns of genes related to Fe deficiency in roots
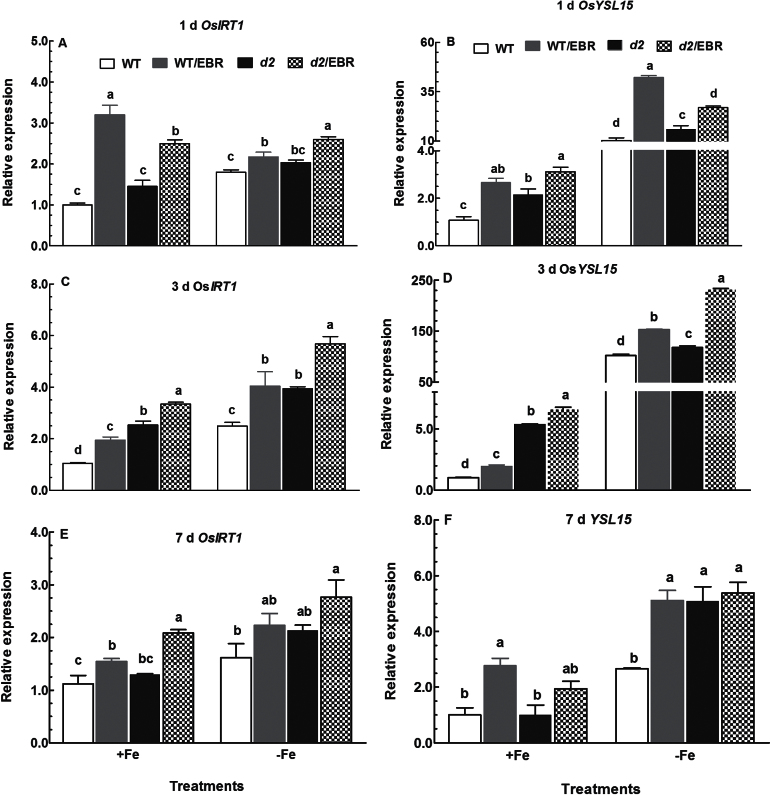
OsIRT1 and OsYSL15 are directly involved in Fe uptake from the rhizosphere (Conte and Walker, 2011). Therefore, we examined the responses of these genes to EBR in WT and d2 mutants under both Fe-deficient and Fe-sufficient conditions. Upregulation of OsIRT1 and OsYSL15 was observed after 1-d treatment with Fe deficiency in both the WT and d2-1 mutants (Fig. 5A, B). The expression of OsIRT1 and OsYSL15 was significantly upregulated by EBR under Fe-sufficient conditions (Fig. 5A, B), and EBR further enhanced the Fe deficiency-induced expression of OsYSL15 and OsIRT1 in WT and d2-1 plants (Fig. 5A, B). Moreover, longer exposure (3 and 7 d) of WT and d2-1 seedlings to Fe-deficient medium and EBR also led to upregulation of OsIRT1 and OsYSL15 (Fig. 5C–F). However, the magnitude of upregulation of OsIRT1 and OsYSL15 by EBR was much greater in WT plants than in d2-1 mutants under both Fe-sufficient and Fe-deficient conditions. In general, similar effects of EBR and Fe deficiency on expression of OsIRT1 and OsYSL15 were observed after treatments with Fe deficiency and EBR for 3 and 7 d (Fig. 5C–F), suggesting that both short- and long-term treatment with Fe deficiency and EBR can alter expression patterns of OsIRT1 and OsYSL15. The expression patterns of OsIRT1 and OsYSL15 in WT and d2-1 mutant plants grown in Fe-sufficient and Fe-deficient media were also compared. Expression levels of OsIRT1 and OsYSL5 in d2-1 mutants were higher than in WT plants grown in Fe-sufficient medium after treatment for 1 and 3 d, and thereafter the mutant and WT plants had comparable expression levels of OsYSL15 (Fig. 5A–F). Exposure of WT and d2-1 mutant plants to Fe-deficient medium led to similar changes in expression patterns of OsIRT1 (Fig. 5 A–F). In contrast to OsIRT1 expression, the Fe deficiency-induced upregulation of OsYSL15 expression in d2-1 mutant plants was less than in WT plants after exposure for 1 and 3 d.
Fig. 5.
Time-course quantitative RT-PCR analysis of Fe uptake-related genes in roots: effect of EBR on OsIRT1 and OsYSL15 expression in roots of WT and d2-1 plants under +Fe and –Fe conditions. Data are means ± SE of three biological replicates. Means with different letters are significantly different within the same gene (P ≤ 0.05).
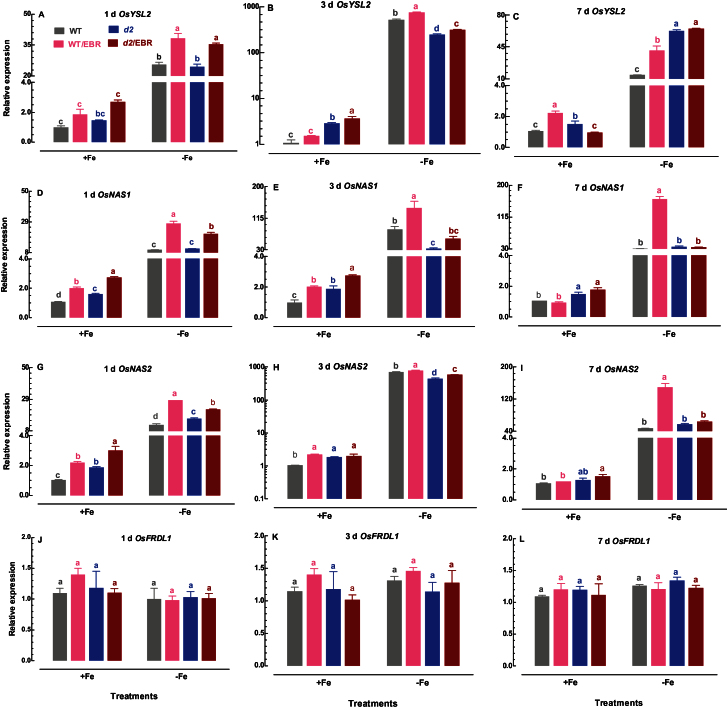
YSL2 and NA have been proposed to mediate long-distance translocation of Fe, and their expression is upregulated under Fe-deficient conditions (Inoue et al., 2003; Ishimaru et al., 2010). OsFRDL1 encodes a citrate transporter that is localized at the pericycle cells, and is essential for translocation of Fe-citrate complex to the shoot (Yokosho et al., 2009). There were increases in expression levels of OsYSL2, OsNAS1, and OsNAS2 in Fe-sufficient WT and d2-1 plants upon addition of EBR to the medium (Fig. 6A–I). In addition to EBR, exposure of WT and d2-1 plants to Fe-deficient medium also led to similar increases in expression levels of OsYSL2, OsNAS1, and OsNAS2 in terms of magnitude and time course (Fig. 6A–I). In addition, the magnitude of upregulation of OsYSL2, OsNAS1 and OsNAS2 expression was much greater in WT and d2-1 mutants challenged by Fe deficiency and EBR together than by treatment with Fe deficiency and EBR alone (Fig. 6A–I), suggesting that Fe deficiency and EBR may have additive effects on the expression of these genes. In contrast to OsYSL2, OsNAS1, and OsNAS2, the expression level of OsFRDL1 was not responsive to treatments with Fe deficiency and EBR alone or Fe deficiency and EBR together (Fig. 6J–L). Transcript levels of OsNAS1 and OsNAS2 in d2-1 plants were generally higher than in the WT under Fe-sufficient conditions (Fig. 6A–I). Treatment with Fe deficiency also induced upregulation of expression of OsYSL2, OsNAS1, and OsNAS2 in d2-1 mutant plants (Fig. 6A–I). However, the magnitude of the upregulation in d2-1 plants was less than the Fe deficiency-induced upregulation of these genes in WT plants (Fig. 6A–I). No apparent differences in expression levels of OsFRDL1 between WT and d2-1 plants under both Fe-sufficient and Fe-deficient conditions were observed (Fig. 6J–L).
Fig. 6.
Time-course quantitative RT-PCR analysis of Fe translocation related genes in roots: effect of EBR on OsYSL2, OsNAS1, OsNAS2, and OsFRDL1 expression in roots of WT and d2-1 plants under +Fe and –Fe conditions. Data are means ± SE of three biological replicates. Means with different letters are significantly different within the same Fe treatment (P ≤ 0.05). (This figure is available in colour at JXB online)
Expression patterns of transcription factor OsIRO2 in roots
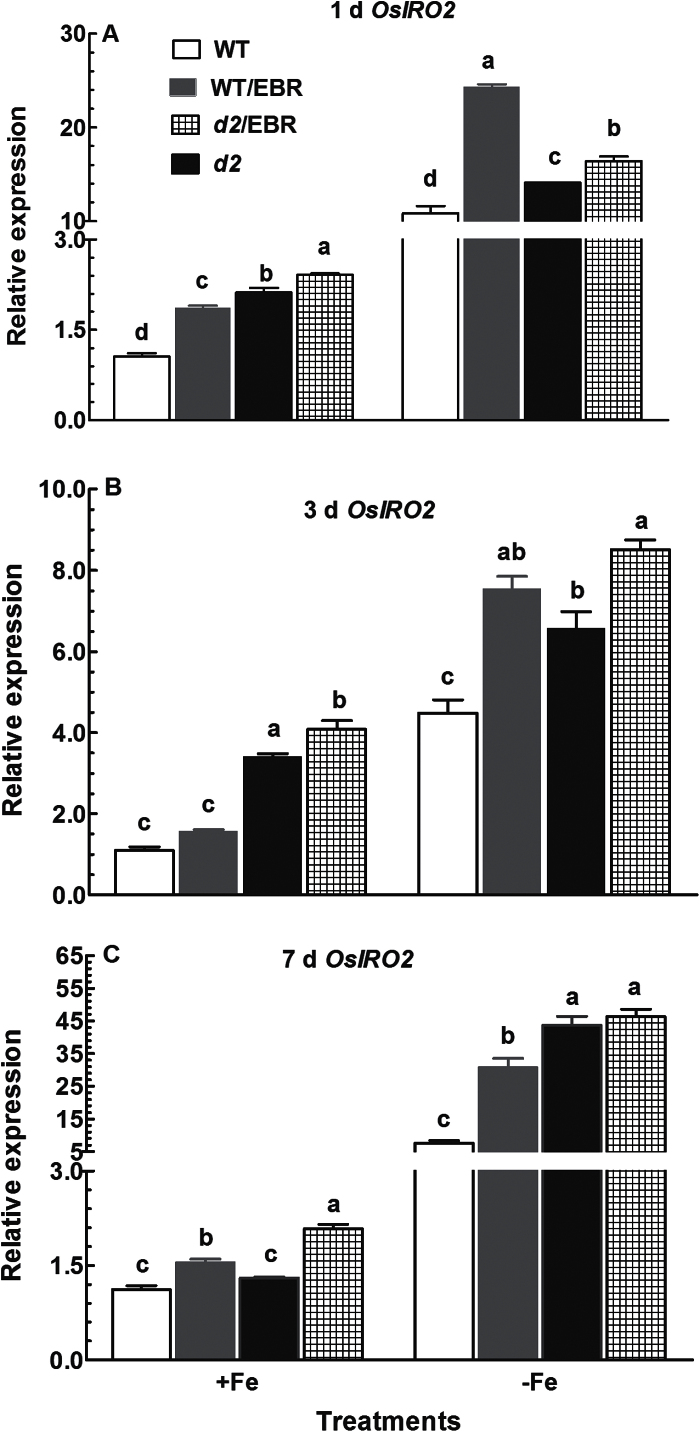
The rice bHLH protein OsIRO2 is an essential regulator involved in mediation of Fe uptake (Ogo et al., 2007). The expression level of OsIRO2 was enhanced after addition of EBR to Fe-sufficient medium in both WT and d2-1 plants (Fig. 7A–C). In addition to EBR, expression of OsIRO2 was also upregulated by exposure to Fe-deficient medium, and the effect of EBR and Fe deficiency on expression of OsIRO2 was additive, such that the expression levels were highest in plants exposed to Fe-deficient medium containing EBR (Fig. 7A–C). However, the magnitude of the upregulation by EBR in d2-1 plants was less than in WT plants under both Fe-sufficient and Fe-deficient conditions. The abundance of OsIRO2 transcript was higher in d2-1 plants than in WT plants under Fe-sufficient conditions after 1 and 3 d of treatment, and became comparable after 7-d exposure to Fe-deficient medium (Fig. 7A–C).
Fig. 7.
Time-course quantitative RT-PCR analysis of OsIRO2 in roots: effect of EBR on OsIRO2 expression in roots of WT and d2-1 plants under +Fe and –Fe conditions. Data are means ± SE of three biological replicates. Means with different letters are significantly different within the same Fe treatments (P ≤ 0.05).
Expression patterns of OsYSL2, OsNAS1, and OsNAS2 in shoots
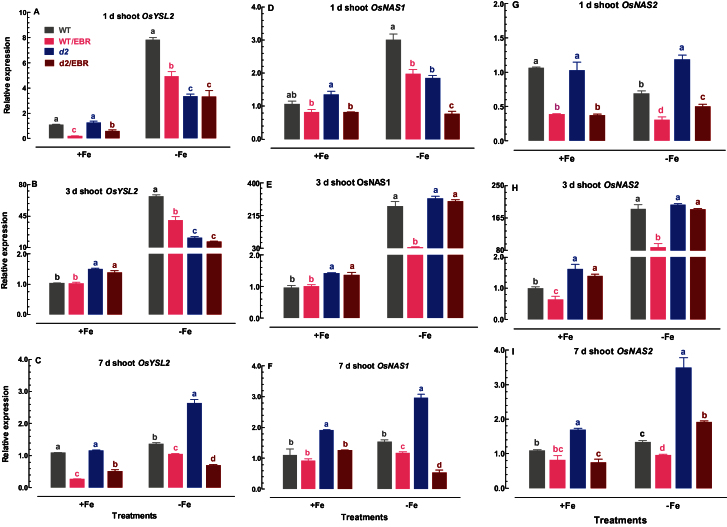
OsYSL2 is a critical Fe-nicotianamine transporter involved in the translocation of Fe, especially in shoots and endosperms (Ishimaru et al., 2010). The responsiveness of OsYSL2 in shoots of WT and d2-1 mutants to EBR under Fe-sufficient and Fe-deficient conditions was monitored. The expression level of OsYSL2 was suppressed by addition of EBR to the Fe-sufficient medium for 1 and 7 d in both WT and d2-1 plants (Fig. 8A–C). In contrast, exposure of WT and d2-1 seedlings to Fe-deficient medium led to a sustained upregulation of OsYSL2 (Fig. 8A–C). Treatment of Fe-deficient seedlings with EBR led to a suppression of OsYSL2 expression at all experimental stages, such that the expression level was lower in plants exposed to Fe-deficient medium with EBR than in plants exposed to Fe-deficient medium without EBR (Fig. 8A–C). The expression level of OsYSL2 in d2-1 plants was slightly higher than in the WT under Fe-sufficient conditions (Fig. 8A–C).
Fig. 8.
Time-course quantitative RT-PCR analysis of OsYSL2, OsNAS1, and OsNAS2 in shoots: Effect of EBR on OsYSL2 expression in shoots of WT and d2-1 plants under +Fe and –Fe conditions. Data are means ± SE of three biological replicates. Means with different letters are significantly different within the same Fe treatment (P ≤ 0.05). (This figure is available in colour at JXB online)
It has been demonstrated that NA is essential for Fe mobilization from the vasculature into the interveinal tissues, and that loss of NA leads to leaf chlorosis (Morrissey and Guerinot, 2009). In the present study, we tested the expression patterns of OsNAS1 and OsNAS2 in shoots under varying conditions. The expression levels OsNAS1 was not affected by EBR from 1 to 7 d in WT plants, but the expression level of OsNAS2 was suppressed by addition of EBR to Fe-sufficient medium for 1 and 3 d in WT plants (Fig. 8D–I). Treatments of EBR inhibited both OsNAS1 and OsNAS2 expression in d2-1 plants at 1 and 7d under Fe-sufficient conditions (Fig. 8D–I). Similar to OsYSL2, treatment of Fe-deficient seedlings of WT plants with EBR led to suppression of OsNAS1 and OsNAS2 at all experimental stages (Fig. 8D–I). The expression level of OsNAS1 and OsNAS2 was depressed by EBR in d2-1 mutants at 1 and 7 d under Fe-deficient conditions (Fig. 8D–I). The expression level of OsNNAS1 and OsNAS2 in d2-1 plants was slightly higher than in WT under Fe-sufficient conditions (Fig. 8D–I).
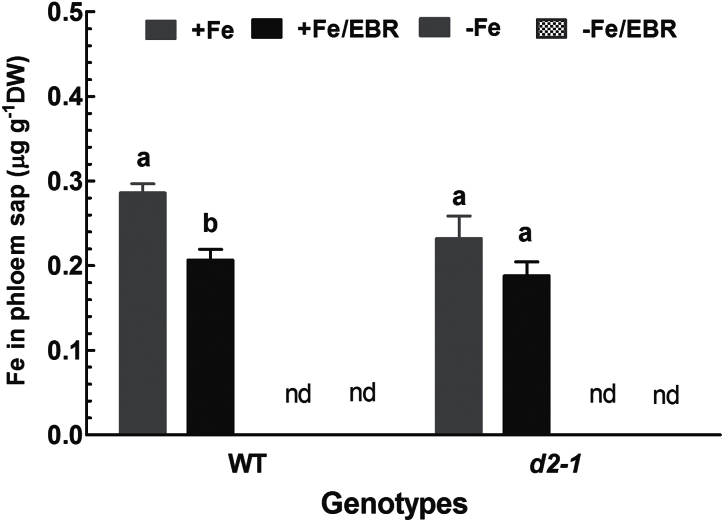
Fe concentrations in the phloem sap
OsYSL2 is a metal-NA transporter responsible for translocation of Fe in the phloem of rice (Koike et al., 2004). To test whether the EBR-induced inhibition of OsYLS2 expression can affect transport or translocation of Fe in the phloem, Fe concentrations in the phloem sap were measured. Fe concentrations in the phloem of the WT were significantly reduced in the presence of EBR under Fe-sufficient conditions (Fig. 9). In contrast, Fe concentrations in the phloem of Fe-sufficient d2-1 plants were not changed by EBR. Little Fe could be detected in the phloem sap when rice plants were grown in Fe-deficient medium in the absence and presence of EBR (Fig. 9). These results indicate that EBR inhibits the translocation of Fe via the phloem.
Fig. 9.
Effect of EBR on Fe concentration in phloem sap of the WT and d2-1 mutant. Ten-d-old WT and d2-1 seedlings were exposed to Fe-sufficient and Fe -deficient medium with or without 100nM EBR for 2 weeks. After treatments, phloem sap was collected and Fe concentration was measured. Data are means ± SE (n = 4). Means with different letters are significantly different within the same genotype (P ≤ 0.05).
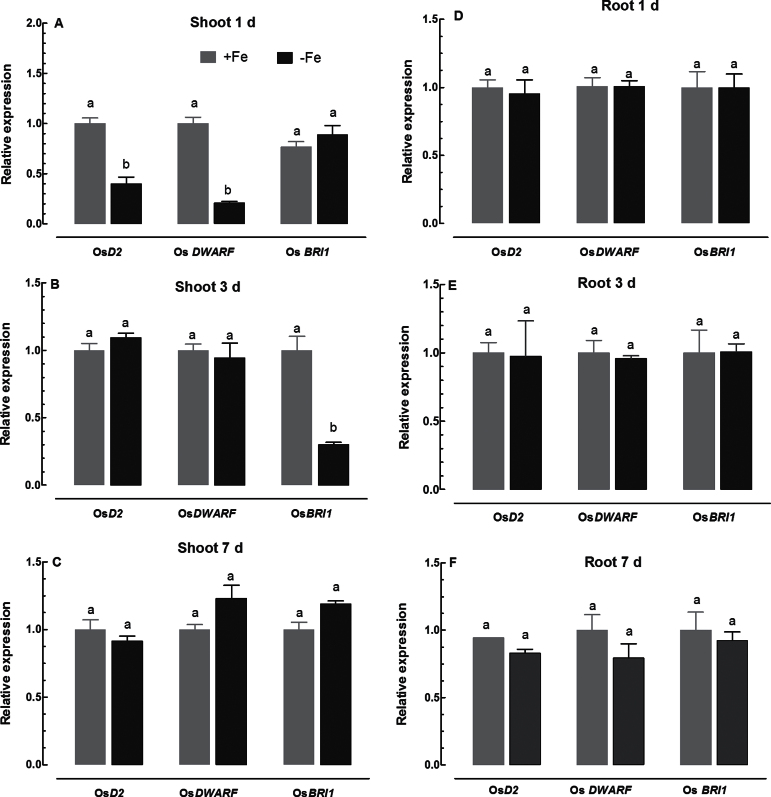
Expression patterns of genes related to BR biosynthesis and signal transduction
To dissect the network associated with BR-dependent Fe uptake and translocation, the response of BR biosynthesis and signalling to Fe deficiency was examined at the transcriptional level in shoots and roots. The expression levels of WRARF and D2, which encode proteins involved in BR biosynthesis (Hong et al., 2002; Hong et al., 2003), and BRI1, which encodes a BR receptor (Morinaka et al., 2006), were substantially suppressed after exposure to Fe-deficient medium for 1 d (Fig. 10A–C). The expression levels of WRARF and D2 in Fe-deficient and Fe-sufficient seedlings became comparable after exposure to Fe-deficient medium for 3 and 7 d (Fig. 10B, C). The Fe deficiency-induced suppression of BRI1 was also observed after 3 d of exposure to Fe-deficient medium, but the expression level became comparable to that in Fe-sufficient plants after exposure to Fe-deficient medium for 7 d (Fig. 10B, C). In contrast, expression of the three genes in roots was relatively constant after exposure to Fe-deficient medium up to 7 d (Fig. 10D–F). These results suggest that both BR biosynthesis and signal transduction in shoots may be closely regulated by Fe status in plants.
Fig. 10.
Time-course quantitative RT-PCR analysis of genes related to BR biosynthesis and signal transduction in shoots and roots: effect of Fe deficiency on OsD2, OsDWARF, and OsBRI1 expression in shoots (A, B, and C) and roots (D, E, and F) of WT plants. Data are means ± s.e. of three biological replicates. Means with different letters are significantly different within the same gene (P ≤ 0.05)
Discussion
There is increasing evidence demonstrating the involvement of BR in the mediation of abiotic stress responses (Sasse, 2003). However, few studies have investigated the role of BR in the response of plants to deficiency in mineral nutrients. Our previous studies revealed that BR is involved in the response of cucumber, a strategy I plant, to Fe deficiency by negatively regulating CsFRO1, CsIRT1, and Fe translocation from roots to shoots (Wang et al., 2012). Since the mechanisms by which Fe uptake and translocation differ between strategy I and II plants, different mechanisms may account for the effect of BR on their Fe acquisition. In the present study, we demonstrate that BR may also play a role in the regulation of the response of rice plants, a strategy II plant, to Fe deficiency using wild-type and BR-deficient mutant d2-1. We further show that an exogenous application of EBR rendered WT plants more sensitive to Fe deficiency by interfering with Fe homeostasis, and that the effect is specific to Fe. Rice mutant d2-1, which is deficient in endogenous BR levels due to disruption of BR biosynthesis, exhibited enhanced tolerance to Fe-deficiency, and the increased tolerance could be partly reversed by EBR complementation. These results suggest that BR is involved in the response of strategy II plants to Fe deficiency. Two mutant lines, d2-1 and d2-2, were originally generated by Hong et al. (2003). The d2-1 mutant exhibits more severe phenotypes than the d2-2 mutant, and the two types of plant are allelic (Hong et al., 2003). Therefore, the results obtained from the d2-1 mutant would not undermine our conclusion.
Exogenous application of EBR to WT rice seedlings enhanced Fe deficiency-induced leaf chlorosis (Fig. 1), but the BR-deficient mutant d2-1 was more tolerant to Fe deficiency than WT plants (Fig. 2). The growth rate was lower in the d2-1 mutant than in WT plants, which may lead to a lower nutrient demand in the d2-1 mutant. To test whether the enhanced tolerance of d2-1 plants to Fe deficiency is not caused by their lower growth rate, we measured other mineral nutrients and compared the response of d2-1 and d61-2 to Fe deficiency. The d61-2 mutation was caused by loss of function of OsBRI1, a putative BR receptor (Hong et al., 2003), and d61-2 mutants exhibit reduced growth compared to WT plants under normal growth conditions (Morinaka et al., 2006). In contrast to increased Fe concentrations in d2-1, there were no differences in Mg and K concentrations in shoots and roots between WT and d2-1 under Fe-sufficient conditions (data not shown). Our data also showed that d2-1 and d61-2 exhibited similar shoot dry weight under Fe-deficient conditions, but d61-2 was more sensitive to Fe deficiency than d2-1 in terms of chlorophyll concentration, shoot dry weight, and shoot Fe concentrations (Supplementary Figure S3). These results suggest that the enhanced tolerance to Fe deficiency of the d2-1 mutant may not simply be accounted for by its reduced growth rate, and that BR may be specifically involved in Fe uptake, transport, and translocation processes in rice. OsIRT1 and OsYSL15 are directly involved in Fe uptake from the rhizosphere in rice (Conte and Walker, 2011). Our results showing that Fe deficiency significantly enhanced expression of OsIRT1 and OsYSL15 (Fig. 5) are in agreement with those reported in the literature. Expression of OsIRT1 and OsYSL15 in the d2-1 mutant was higher than in WT plants under Fe-sufficient conditions (Fig. 5), which may underpin the higher Fe accumulation in roots of the d2-1 mutant than in roots of WT plants. After uptake, Fe is transported in the complexed forms once it is loaded into the xylem (Conte and Walker, 2011). OsFRDL1, a citrate transporter localized at the rice root pericycle cells, is necessary for efficient translocation of Fe-citrate complex to shoots (Yokosho et al., 2009). Expression of OsFRDL1 was relatively insensitive to Fe deficiency and EBR (Fig. 6). A similar insensitivity of OsFRDL1 to Fe deficiency has been reported in the literature (Yokosho et al., 2009). Although citrate plays an important role in long-distance transport of Fe in rice, knockout of OsFRDL1 results in mild defects in Fe homeostasis compared with the severe phenotype of the Arabidopsis frd3 mutant (Kobayashi and Nishizawa, 2012), suggesting that alternative chelators may exist in rice to mediate transport of Fe in the xylem. In addition to citrate, NA levels have a significant effect on metal homeostasis (Morrissey and Guerinot, 2009). Overexpression of NAS in tobacco and Arabidopsis increases NA levels in shoots, resulting in increased Fe in the shoots (Takahashi et al., 2003; Kim et al., 2005). However, it is unclear whether these changes are the result of greater root to shoot translocation facilitated by NA or increased metal uptake in the roots driven by the creation of new Fe sinks in the shoot (Morrissey and Guerinot, 2009). In rice, YSL2 and NA have been proposed to mediate long-distance translocation of Fe and/or are responsible for the phloem transport of Fe in shoots (Inoue et al., 2003; Koike et al., 2004; Ishimaru et al., 2010). In our study, we found that expression of OsYSL2, OsNAS1, and OsNAS2 in shoots was significantly depressed by EBR treatment (Fig. 8), and that Fe concentrations in phloem sap of WT plants were also decreased by EBR application (Fig. 9). EBR treatment may decrease shoot NA and inhibit Fe translocation from roots to shoots or decrease the Fe sink in shoots. The suppression of shoot OsYSL2 may suppress the unloading of Fe from the phloem to mesophyll cells and lead to a low Fe concentration in protoplasts of leaf mesophyll cells and enhanced leaf chlorosis. The lowered shoot Fe by BR feedbacks to roots enhanced the expression of many genes involved in Fe homeostasis, leading to Fe accumulation in roots, but decreased biomass and Fe of shoots.
OsIRO2 is an essential transcription factor modulating Fe uptake and translocation in rice plants (Kobayashi and Nishizawa, 2012). Expression of OsIRO2 is often upregulated upon exposure to Fe-deficient medium, thus activating the expression of genes responsible for Fe homeostasis in roots (Ogo et al., 2007, 2011). Our results are in agreement with those reported in the literature. Moreover, our results showed that EBR significantly enhanced OsIRO2 expression in both Fe-sufficient and Fe-deficient WT rice plants (Fig. 7), which may account for the EBR-induced upregulation of Fe homeostasis-related genes, such as OsYSL15, OsNAS1, and OsNAS2 (Fig. 6). Similar to EBR application, a higher expression of OsIRO2 and genes related to Fe homeostasis was observed by BR deficiency in the d2-1 mutant (Fig. 6). Root Fe and expression levels of genes related to Fe homeostasis in the WT and d2-1 mutant were increased or upregulated by exogenous EBR application and endogenous BR defects, respectively, but the mechanisms may be different. Expression of OsIRT1 and OsYSL15 in d2-1 mutants was higher in d2-1 than in WT plants under Fe-sufficient conditions (Fig. 5), which may account for the higher Fe uptake and accumulation in roots of d2-1 mutants than in roots of WT plants. In addition to efficient Fe-uptake systems of roots in d2-1, the expression of genes related to Fe transport and translocation in shoots of d2-1 was also higher than that in WT plants (Fig. 8). Thus, BR-deficient mutant d2-1 had comparable shoot Fe concentrations with WT plants under Fe-sufficient conditions (Fig. 4A), suggesting that, for the d2-1 mutant, upregulation of genes associated with Fe homeostasis may account for the higher Fe concentrations in shoots. These may provide an explanation for the greater tolerance of d2-1 to Fe deficiency than WT plants. The efficient uptake and translocation of Fe may make the d2-1 mutant more tolerant to Fe deficiency and less responsive to EBR treatment, as evidenced by less suppression of plant biomass and Fe concentrations in the d2-1 mutant than the WT when grown in Fe-deficient medium, and lower expression levels of genes associated with Fe uptake and translocation at early stages of Fe deficiency. Unlike the d2-1 mutant, exogenous EBR suppressed the expression of genes related to Fe transport and translocation in shoots (Fig. 8), leading to reductions in shoot Fe concentrations of WT plants (Fig. 4). Decreased shoot Fe concentration by EBR enhanced leaf chlorosis, which may feed back to the root and activate the expression of Fe homeostasis-related genes, thus leading to Fe accumulation in roots. The symptoms in rice seedlings treated with exogenous BR are comparable to those in Arabidopsis mutant frd3, including leaf chlorosis, constitutive expression of genes associated with Fe uptake, and low Fe level in the plastid, even under Fe-sufficient conditions (Rogers and Guerinot, 2002).
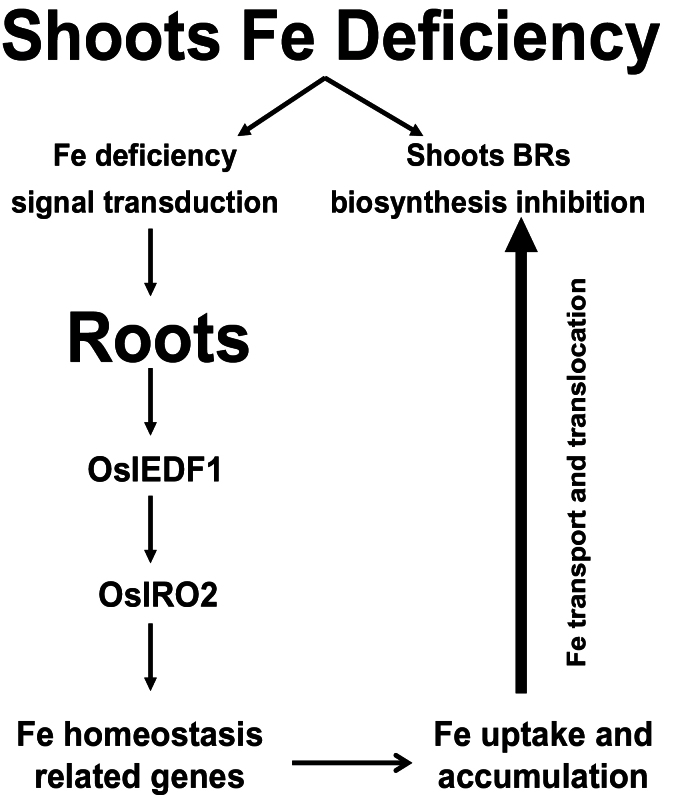
Exogenous application of BRs has been shown to improve stress tolerance by activating the BR signal transduction pathway and BR-regulated expression of stress-related genes (Kagale et al., 2007). In the present study, we found that Fe concentrations in shoots were negatively regulated by BRs, but the signal transduction pathways remain to be dissected. In rice, OsD2 and OsDWARF are responsible for biosynthesis of BRs, and OsBRI1 is a receptor for BR (Yamamuro et al., 2000; Hong et al., 2002, 2003). Under salt and drought stress, expression of salt- and drought-responsive genes is rapidly altered by BR (Kagale et al., 2007). Also, expression of the BR receptor gene in rice can be modified within several hours by treatment with IAA (Sakamoto et al., 2013). These indicate that plants can be quickly responsive to environmental and hormonal cues by modulating BR signalling cascades. Expression of OsD2, OsDWARF, and OsBRI1 in roots of WT plants was not responsive to Fe deficiency (Fig. 10D–F). In contrast, expression of OsD2 and OsDWARF in shoots was markedly suppressed by exposure to Fe-deficient medium for 1 d, while the Fe deficiency-induced inhibition of OsBRI1 expression occurred after 3-d exposure to Fe-deficient medium (cf. Fig. 10). These results imply that BRs may be involved in regulation of Fe transport and translocation from roots to shoots, but the underlying mechanisms remain to be elucidated. In rice, the regulatory network involving OsIDEF1, OsIDEF2, and OsIRO2 in response to Fe deficiency has been elucidated (Kobayashi et al., 2007; Ogo et al., 2008; Kobayashi et al., 2009, 2012; Kobayashi and Nishizawa, 2012). Based on the information and our results, a putative working model is proposed to illustrate the possible role of BR in regulating the response of rice plants to Fe deficiency (Fig. 11). The iron deficiency signal transmits from shoot to root, and activates transcription factors OsIDEF1 and OsIRO2, which in turn modulates the downstream targets involved in Fe homeostasis at the transcriptional level, leading to Fe uptake and accumulation in roots. In contrast, Fe deficiency inhibited BR biosynthesis, and the reduced endogenous BRs may facilitate Fe transport and translocation from roots to shoots. The exogenous application of EBR to rice seedlings would suppress the transport and translocation of Fe from roots to shoots, thus leading to a more severe phenotype of Fe deficiency in shoots and strengthen Fe deficiency signal. The enhanced signal of Fe deficiency will further upregulate downstream genes involved in Fe homeostasis, and lead to Fe uptake and accumulation in roots. BRs negatively regulated Fe transport and translocation from root to shoot in rice seedlings, but the mechanisms are not clear. Therefore, future research focusing on the interaction of BR with Fe transport and translocation in strategy II plants is warranted.
Fig. 11.
A putative working model to illustrate the possible role of BR in regulating the response of rice plants to Fe deficiency. Pathways possibly responsible for Fe uptake or transport and translocation from roots to shoots are separated. Shoot Fe deficiency could induce signal transduction from shoots to roots, and activate Fe mobilization and uptake by roots. On the other hand, Fe deficiency could decrease BR levels in shoots and inhibit Fe transport and translocation from roots to shoots.
In conclusion, our results highlight the importance of BR as a signalling molecule involved in mediating the response of rice plants to Fe deficiency. More specifically, we demonstrate that BR plays a negatively regulatory role in control of transport and translocation from roots to shoots, thus indirectly modulating Fe mobilization and acquisition by possibly regulating OsIRO2.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Primers used for quantitative RT-PCR.
Supplementary Figure S1. Effect of EBR on chlorophyll concentrations in WT rice seedlings in the presence and absence of different forms of Fe (Fe3+, Fe2+).
Supplementary Figure S2. Effect of EBR on Mg, Mn, and Zn concentrations in shoots and roots of WT rice seedlings.
Supplementary Figure S3. Effect of Fe deficiency on chlorophyll concentrations, shoot and root dry weight, and Fe concentrations in shoots of d2-1 and d61-2 mutants.
Supplementary Figure S4. Quantitative RT-PCR analysis of OsD2 in roots and shoots of WT and d2-1 mutant plants under different Fe supply conditions.
Funding
This work was supported by the Natural Science Foundation of China (31101594) and State Key Laboratory of Vegetation and Environmental Change.
Supplementary Material
Acknowledgements
We thank Dr Tomoaki Sakamoto for the gift of d2-1 and d61-2 mutants. We thank the editor and anonymous reviewers for their constructive suggestions on the previous version of the manuscript.
References
- Bughio N, Yamaguchi H, Nishizawa NK, Nakanishi H, Mori S. 2002. Cloning an iron-regulated metal transporter from rice. Journal of Experimental Botany 53, 1677–1682. [DOI] [PubMed] [Google Scholar]
- Cheng L, Wang F, Shou H, et al. 2007. Mutation in nicotianamine aminotransferase stimulated the Fe (II) acquisition system and led to iron accumulation in rice. Plant Physiology 145, 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte SS, Walker EL. 2011. Transporters contributing to iron trafficking in plants. Molecular Plant 4, 464–476. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Prinsen E, Jacqmard A, Lejeune P, Van Onckelen H, Perilleux C, Bernier G. 2003. Cytokinin levels in leaves, leaf exudate and shoot apical meristem of Arabidopsis thaliana during floral transition. Journal of Experimental Botany 54, 2511–2517. [DOI] [PubMed] [Google Scholar]
- Curie C, Briat JF. 2003. Iron transport and signaling in plants. Annual Review of Plant Biology 54, 183–206. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. 2001. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409, 346–349. [DOI] [PubMed] [Google Scholar]
- Durrett TP, Gassmann W, Rogers EE. 2007. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiology 144, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Rogers EE. 2004. FRD3 controls iron localization in Arabidopsis. Plant Physiology 136, 2523–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R, Stephan UW. 2003. Iron uptake, trafficking and homeostasis in plants. Planta 210, 541–551. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, et al. 2002. Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. The Plant Journal 32, 495–508. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M. 2003. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by loss of function of a new member of cytochrome P450. The Plant Cell 15, 2900–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. 2003. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. The Plant Journal 36, 366–381. [DOI] [PubMed] [Google Scholar]
- Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK. 2009. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. The Journal of Biological Chemistry 284, 3470–3479. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa NK. 2010. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. The Plant Journal 62, 379–390. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, et al. 2006. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ . The Plant Journal 45, 335–346. [DOI] [PubMed] [Google Scholar]
- Ivanov R, Brumbarova T, Bauer P. 2012. Fitting into the harsh reality: regulation of iron-deficiency responses in dicotyledonous plants. Molecular Plant 5, 27–42. [DOI] [PubMed] [Google Scholar]
- Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P. 2007. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225, 353–364. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi M, Higuchi K, Tsunoda K, Nakanish H, Yoshimura E, Mori S, Nishizawa NK. 2005. Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants. Plant Cell Physiology 46, 1809–1818. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Itai RN, Aung MS, Senoura T, Nakanishi H, Nishizawa NK. 2012. The rice transcription factor IDEF1 directly binds to iron and other divalent metals for sensing cellular iron status. The Plant Journal 69, 81–91. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Itai RN, Ogo Y, Kakei Y, Nakanishi H, Takahashi M, Nishizawa NK. 2009. The rice transcription factor IDEF1 is essential for the early response to iron deficiency, and induces vegetative expression of late embryogenesis abundant genes. The Plant Journal 60, 948–961. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nishizawa NK. 2012. Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology 63, 132–152. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ogo Y, Itai RN, Nakanishi H, Takahashi M, Mori S, Nishizawa NK. 2007. The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proceedings of the National Academy of Sciences, USA 104, 19150–19155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. 2004. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. The Plant Journal 39, 415–424. [DOI] [PubMed] [Google Scholar]
- Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, Matsuoka M. 2006. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiology 141, 924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J, Guerinot L. 2009. Iron uptake and transport in plants: the good, the bad, and the ionome. Chemical Reviews 109, 4553–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, Yuan H, Feldmann KA, Tax FE. 1999. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiology 121, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. 2011. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. The Journal of Biological Chemistry 286, 5446–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo Y, Itai RN, Kobayashi T, Aung MS, Nakanishi H, Nishizawa NK. 2011. OsIRO2 is responsible for iron utilization in rice and improves growth and yield in calcareous soil. Plant Molecular Biology 75, 593–605. [DOI] [PubMed] [Google Scholar]
- Ogo Y, Itai RN, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK. 2007. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. The Plant Journal 51, 366–377. [DOI] [PubMed] [Google Scholar]
- Ogo Y, Kobayashi T, Itai R N, Nakanishi H, Kakei Y, Takahashi M, Toki S, Mori S, Nishizawa NK. 2008. A novel NAC transcription factor, IDEF2, that recognizes the iron deficiency-responsive element 2 regulates the genes involved in iron homeostasis in plants. The Journal of Biological Chemistry 283, 13407–13417. [DOI] [PubMed] [Google Scholar]
- Rellán-Álvarez R, Giner-Martínez-Sierra J, Orduma J, Orera I, Rodríguez-Castrillón JA, García-Alonso JI, Abadia J, Álvarez-Fernández A. 2010. Identification of a tri-iron (III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: new insights into plant iron long-distance transport. Plant Cell Physiology 51, 91–102. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Guerinot ML. 2002. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. The Plant Cell 14, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E. 2004. Ethylene involvement in the regulation of Fe deficiency stress responses by Strategy I plants. Functional Plant Biology 31, 315–328. [DOI] [PubMed] [Google Scholar]
- Römheld V, Marschner H. 1986. Mobilization of iron in the rhizosphere of different plant species. Advances in Plant Nutrition 2, 155–204. [Google Scholar]
- Sakamoto T, Mornaka Y, Inukai Y, Kitano H, Fujioka S. 2013. Auxin signal transcrip tion factor regulates expression of the brassinosteroid receptor gene in rice. The Plant Journal 73, 676–688. [DOI] [PubMed] [Google Scholar]
- Sasse JM. 2003. Physiological actions of brassinosteroids: an update. Journal of Plant Growth Regulation 22, 276–288. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK. 2003. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. The Plant Cell 15, 1263–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wiren N, Mori S, Marschner H, Romheld V. 1994. Iron inefficiency in maize mutant ys1 (Zea mays L. cv Yellow-Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiology 106, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BL, Li YS, Zhang WH. 2012. Brassinosteroids are involved in response of cucumber (Cucumis sativus) to iron deficiency. Annals of Botany 110, 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang L, Zheng L, Wang L, Chen Y, Whelan J, Shou H. 2011. Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa. Journal of Experimental Botany 62, 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M. 2000. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. The Plant Cell 12, 1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF. 2009. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiology 149, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JQ, Hu WH, Zhou YH, Mao WH, Ye SF, Nogués S. 2004. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. Journal of Experimental Botany 55, 1135–1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.