Abstract
Cigarette smoking is associated with elevated risk of anxiety and mood disorder. Using the 7.5% carbon dioxide (CO2) inhalation model of anxiety induction, we examined the effects of smoking status and abstinence from smoking on anxiety responses.
Physiological and subjective responses to CO2 and medical air were compared in smokers and non-smokers (Experiment One) and in overnight abstinent and non-abstinent smokers (Experiment Two).
CO2 induced greater increases in blood pressure in non-smokers compared with smokers (ps < 0.043), and greater increases in anxiety (p = 0.005) and negative affect (p = 0.054) in non-abstinent compared with abstinent smokers.
CO2 increased physiological and subjective indices of anxiety. There were differences across smoking groups indicating that the CO2 inhalation model is a useful tool for examining the relationship between smoking and anxiety. The findings suggested that both acute smoking and acute abstinence may protect against anxious responding. Further investigation is needed in long-term heavy smokers.
Introduction
Cigarette smoking is strongly associated with elevated risk of anxiety and depression, and there is growing evidence that the relationship is bidirectional [Paperwalla et al., 2004, Munafo et al., 2008, Cosci et al., 2010, Munafo et al., 2010, Lewis et al., 2011]. Smokers often report smoking in times of low mood and anxiety, and identify anxiety as a trigger of cigarette seeking and relapse. The belief that smoking reduces anxiety is strong among smokers, but there is little empirical evidence of anxiolytic effects in non-deprived smokers. In contrast, acute withdrawal from smoking increases feelings of anxiety [Hughes, 2007]. Therefore, while cigarette smokers report smoking to alleviate anxiety, they may in fact simply be smoking to alleviate abstinence-induced anxiety, in particular given the fast action and clearance of nicotine.
While the link between cigarette smoking and anxiety is well described, the causal nature of the relationship remains unclear. Epidemiological studies that prospectively examine this relationship have identified anxiety as a risk factor for smoking initiation, nicotine dependence and relapse [Patton et al., 1998, Park et al., 2009, Mckenzie et al., 2010]. The proposed mechanisms for this include increased peer influence (particularly in relation to initiation of smoking) [Patton et al., 1998] and smoking as anxiolytic self-medication [Park et al., 2009]. However, smoking has also been shown to predict the incidence of depression [Johnson et al., 2000, Mojtabai et al., 2013, Moylan et al., 2013] generalised anxiety and panic disorder [Zvolensky et al., 2005, Moylan et al., 2012].
A number of explanations have been offered to account for the elevated risk of anxiety and anxiety disorders in smokers, and these vary depending on the specific anxiety disorder considered. The relationship between panic and smoking has been attributed to smoking-induced respiratory abnormalities [Caldirola et al., 2004], which may increase sensitivity to respiratory signals that induce or maintain panic symptoms [Klein, 1993]. Zvolensky [2003] proposes a pathoplasty model in which smoking modifies panic disorder by inducing acute physical changes akin to fearful bodily sensations (such as autonomic effects, noradrenaline release). Alongside these changes, the act of smoking also negatively impacts on perception of general health and can produce actual medical disturbance that further contribute to panic symptomology [Zvolensky et al., 2003].
There is also general consensus that repeated tobacco exposure can directly alter the stress systems of the body [Bruijnzeel, 2012], which may explain more generalised changes in anxiety and anxiety sensitivity. Nicotine, the main psychoactive constituent of tobacco, acts primarily at the nicotinic acetylcholine receptors (nAChRs), which are widespread throughout the brain [Picciotto et al., 2002]. Their activation affects many neurotransmitter systems and neural regions involved in the stress response, including the hippocampus which is a key output centre regulating hypothalamic-pituitary-adrenal axis (HPA) function [Jacobson et al., 1991]. Acutely, cigarette smoking activates the HPA axis and increases adrenocorticotropic hormone (ACTH) and cortisol levels [Kirschbaum et al., 1992, Steptoe et al., 2006], and repeated smoking results in desensitisation and upregulation of nAChRs [Dani et al., 1996] and changes basal activity of the HPA [Rohleder et al., 2006].
These changes provide a biological explanation for the smoking-related vulnerability towards anxiety, and the differences in reported anxiety between smokers and non-smokers. Despite smokers reporting anxiolytic effects of smoking [Nichter et al., 1997, Fidler et al., 2009], they often show higher levels of anxiety and anxious arousal than non-smokers [Parrott, 1999, Collins et al., 2009, Ameringer et al., 2010]. Acute smoking abstinence is associated with a negative affective comprising low mood, irritability, anxiety and craving [Hughes et al., 1986, Perkins et al., 1992, Benowitz, 2008], and smoking relieves these symptoms. Therefore, smokers may come to believe that smoking reduces anxiety, when in fact it simply alleviates withdrawal-related anxiety [Hughes et al., 1984]. In contrast, long-term abstinence from smoking is associated with marked reductions in anxiety [Mcdermott et al., 2013]. Understanding the true nature of the smoking-anxiety interaction is important as a misperception of anxiolytic effects of smoking and anxiogenic effects of smoking cessation may promote smoking maintenance and negate quit attempts. In order to investigate these relationships, it is logical to stimulate anxiety in a controlled research setting, and a variety of anxiogenic models have been used in laboratory settings. Childs and de Wit [2010] exposed non-deprived smokers to a psychosocial stressor (Trier Social Stress Test) and found that cigarette craving and pleasure from first cigarette, but not smoking, were increased in response to the stressor compared to a non-stressful control task. Increases in actual smoking behaviour have been observed in response to other “stressful” manipulations including loud noise exposure [Cherek, 1985] and stage performances [Rose et al., 1983]. These findings indicate that anxiety-provoking situations incite craving and increased smoking in some instances, but subjective anxiety levels were not measured directly, which limits the interpretation of these findings. Furthermore, psychological models of anxiety induction are often limited in terms of their external validity, practicality and the degree of individual variation in responding.
To circumvent some of these issues, respiratory challenges that involve breathing air with increased levels of carbon dioxide have been used [Zvolensky et al., 2001]. This can be achieved in a number of ways, one of which is by individuals rebreathing air in a closed system. Using this method Abrams [2008] found decreased respiratory rate but increased report of panic symptoms in a group of nicotine withdrawn heavy smokers compared with non-smokers. In a follow up study comparing abstinent and non-abstinent smokers, Abrams [2011] did not find an effect of smoking status on response to a 5-minute rebreathing procedure, although pre-challenge withdrawal symptoms were associated with stronger subjective responses to the challenge. A limitation of this technique is that the level of CO2 inhaled gradually increases over time and therefore inhalation techniques that deliver fixed levels of CO2-enriched air afford the researcher greater control of the anxiety response.
Another respiratory challenge involves controlled inhalation of air mixtures that have higher CO2 concentrations than normal air. Like other CO2 challenges, these inhalations induce physiological and psychological symptoms of anxious arousal. The effects of CO2 appear to be dose dependent with higher doses producing panic-like symptoms and lower doses producing less intense generalised anxiety [Bailey et al., 2007]. In support, single breath of 35% CO2 concentration, but not lower doses, reliably stimulates ACTH and cortisol release [Kaye et al., 2004]. However, some individuals experience panic attacks and panic-like symptoms at lower doses [Zvolensky et al., 2001]. Due to the high prevalence of smoking among panic disorder patients [Mccabe et al., 2004], several studies have examined the effects of higher CO2 concentrations (around 20% – 35%). Cosci et al. [2006] investigated the effect of transdermal nicotine (via skin patch) on reactivity to 35% CO2 challenge in non-smokers. Although nicotine patch increased baseline autonomic activation compared to placebo patch, there was no effect of patch type on CO2 reactivity. Using 20% challenge, Zvolensky et al. [2001] were able to differentiate between smokers who able and unable to maintain quit attempts for seven days, with evidence of increase reactivity in those who reported being unable do so. The effects of lower CO2 concentration (i.e., 5% – 10%) have been largely unexplored in smoking research. The effects of withdrawal on stress reactivity have been explored using 10% CO2 challenge and these studies have shown that higher levels of withdrawal are associated with greater stress reactivity in individuals with panic disorder [Leyro et al., 2013] and high anxiety sensitivity [Vujanovic et al., 2009].
The current study extends previous research by exploring the relationship between smoking and anxiety using a lower 7.5% CO2 concentration. The effects of this dose in healthy volunteers are now well described [Bailey et al., 2005]; compared with the inhalation of air, this produces marked increases in heart rate and blood pressure, and corresponding increases in subjective ratings of anxiety, fear and tension, with decreased subjective ratings of feeling relaxed and happy. These effects are sustained throughout the 20 minute inhalation, with rapid recovery when inhalation of normal air is resumed. A single dose of the benzodiazepines lorazepam, alprazolam and zolpidem reduces the subjective effects of 7.5% CO2 [Bailey et al., 2007, Bailey et al., 2009], as does 3-week treatment with the serotonin reuptake inhibitor paroxetine [Bailey et al., 2007]. Furthermore, this challenge induces cognitive biases characteristic of anxiety [Garner et al., 2011, Cooper et al., 2012].
Based on previous findings, we hypothesised that smokers would show greater stress reactivity than non-smokers (Experiment One) and abstinent smokers would show greater anxiogenic response and higher craving compared to non-abstinent smokers (Experiment Two). These studies also informed the validity of the 7.5% CO2 model as a tool for future smoking research.
Experiment One
Methods
Design and overview
The experiment consisted of a repeated-measures design, with gas (air, 7.5% CO2) as the within-subjects factor and smoking status (smoker, non-smoker) as a between-subjects factor. The order of gas inhalation was counterbalanced across participants.
Participants
Twelve smokers (smoke at least 5 cigarettes per day and within one hour of waking) and twelve non-smokers (current non-smokers and have smoked no more than 100 cigarettes in lifetime) were recruited from the staff and students of the University of Bristol and from a participant database. Exclusion criteria were current or a history of psychiatric disorder or drug or alcohol dependence (excluding caffeine), assessed using a structured interview by a trained researcher, based on the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Blood pressure (<140/90 mmHg), heart rate (50–90 bpm) and body mass index (BMI; 18–30 kg/m2) were required to be within the normal range. Prior to the study, participants were asked to refrain from alcohol for 36 hours, and from caffeine after midnight. Participants were reimbursed £20 for their time at the end of testing. The study was reviewed and approved by the University of Bristol Faculty of Science Research Ethics Committee.
Gas mixtures
The gas mixtures used were CO2 7.5%/O2 21%/N 71.5% and medical air (O2 21%; BOC Ltd.). These were administered to participants using a oro-nasal mask (Hans Rudolph, Kansas City, MO, USA), which was attached to a 500 L bag with tubing. For safety reasons, the gas was administered single-blind.
Physiological assessment
Participants were tested for alcohol and carbon monoxide levels in exhaled breath using the Alcohawk PT400 (Q3 Innovations, Independence, IA, USA) and the Bedfont Micro Smokerlyser (Bedfont Scientific Ltd, Maidstone, UK), respectively. Blood pressure was recorded using the OMRON M6 Comfort Digital Blood Pressure Monitor (OMRON Healthcare Ltd, Milton Keynes, UK). Fresh urine samples were provided to test for the presence of barbiturates, benzodiazepines, opiates, tetrahydrocannabinol (THC), methamphetamine, amphetamine and cocaine, and to test for pregnancy in female participants (Surescreen Diagnostics Ltd, Derby, UK).
Psychological assessment
Participants completed the Spielberger State-Trait Anxiety Inventory State (STAI-S) and Trait (STAI-T) subscales (Spielberger, 1983), Anxiety Sensitivity Inventory (ASI) [Reiss et al., 1986], and the Positive and Negative Affect Schedule positive (PANAS-P) and negative (PANAS-N) subscales [Watson et al., 1988]. Cigarette craving was measures using the Questionnaire on Smoking Urges (QSU) [Tiffany et al., 1991].
Procedure
Participants completed an initial telephone screening before attending the testing session, in order to ascertain likely eligibility for the study. Smokers were required to be abstinent from smoking overnight (i.e., at least 12 hours), but were asked to smoke one of their own cigarettes before entering the laboratory in order to keep recent smoking levels consistent within the smoker group. On the day of testing, participants provided signed informed consent, and then completed further screening procedures, including exhaled breath and urine testing, assessment of blood pressure, heart rate, height and weight, and completion of the psychiatric screening interview. Eligible participants then completed the STAI-S, STAI-T, ASI, PANAS-P, and PANAS-N measures prior to the first inhalation. Inhalations of air and CO2 each lasted for twenty minutes. Inhalations periods were separated by 40 minutes of rest, and the order of air and CO2 was counterbalanced across participants. Throughout the study, an experimenter remained within close proximity of the gas cylinders to ensure the bag was adequately full at all times. Participants sat with their back to the gas cylinders. Immediately after each inhalation, blood pressure and heart rate were recorded while the participant remained seated, and the STAI-S, PANAS and QSU (smokers only) were then completed. While anxiety induced by the inhalation is sustained during inhalation, it declines very rapidly on inhalation of normal air. Participants were therefore asked to indicate how they felt during the inhalation when the effects of the gas were “at their strongest” when completing the questionnaire measures at the end of the inhalation. Participants were then required to stay in the laboratory with the researcher for a further 30 minutes (post end of last inhalation) to allow for recovery (as assessed through heart rate and blood pressure response and participant self-report).
Statistical analysis
Data were analysed by repeated measures ANOVA, with gas (air, CO2) as a within-subjects factor, and smoking status (smoker, non-smoker) as a between-subjects factor. For effects of gas on craving, t-tests were run on craving scores after CO2 and air in smoking group only. To explore the relationship between subjective anxiety and craving Pearson’s correlations were conducted on post-CO2 SSAI and QSU scores. All analyses were performed using PASW Statistics version 18 (SPSS Inc., Chicago IL, USA). Precise p-values are reported throughout.
Previous research has indicated that inhalation of 7.5% CO2 increases state anxiety by approximately 19 points (SD 8.5 points) compared to inhalation of air. Our sample size therefore provided 99% power to detect an effect of inhalation of 7.5% CO2 versus inhalation of air on subjective anxiety, and 80% power to detect a difference of 10 points between smokers and non-smokers in the increase in anxiety induced by inhalation of 7.5% CO2, assuming an alpha level of 5%. This effect is comparable to that observed for the effects of anxiolytic medications on response to inhalation of 7.5% CO2 (Bailey et al., 2007).
Results
Characteristics of participants
Participant characteristics (n = 24; 50% female) at baseline are shown in Table 1, and did not differ between smokers and non-smokers (ps > 0.48).
Table 1.
Characteristics of Participants: Means and standard deviations for entire sample of Experiment One and Two, and p-value of independent group t-tests (smoker vs. non-smoker in Experiment One and abstinent vs. non-abstinent in Experiment Two)
| Experiment One (Smoking Status) | Experiment Two (Abstinence) | |||
|---|---|---|---|---|
| Mean (SD) | p-value | Mean (SD) | p-value | |
| Age | 23.71 (3.26) | 0.50 | 24.88 (7.40) | 0.69 |
| BMI | 22.90 (2.80) | 0.56 | 23.25 (3.89) | 0.66 |
| Systolic BP | 109.96 (13.14) | 0.87 | 113.88 (16.21) | 0.10 |
| Diastolic BP | 72.33 (7.51) | 0.67 | 69.67 (10.48) | 0.22 |
| Pulse rate | 69.96 (9.48) | 0.76 | 74.21 (11.18) | 0.01 |
| ASI | 12.83 (7.25) | 0.48 | 19.92 (10.19) | 0.31 |
| STAI Trait | 32.92 (6.43) | 0.54 | 37.42 (7.62) | 0.21 |
| STAI State | 30.83 (7.94) | 0.84 | 33.00 (8.70) | 0.23 |
| PANAS Positive | 27.75 (6.12) | 0.95 | 25.33 (5.92) | 0.17 |
| PANAS Negative | 11.17 (2.62) | 0.88 | 12.04 (2.97) | 0.64 |
Cardiovascular measures
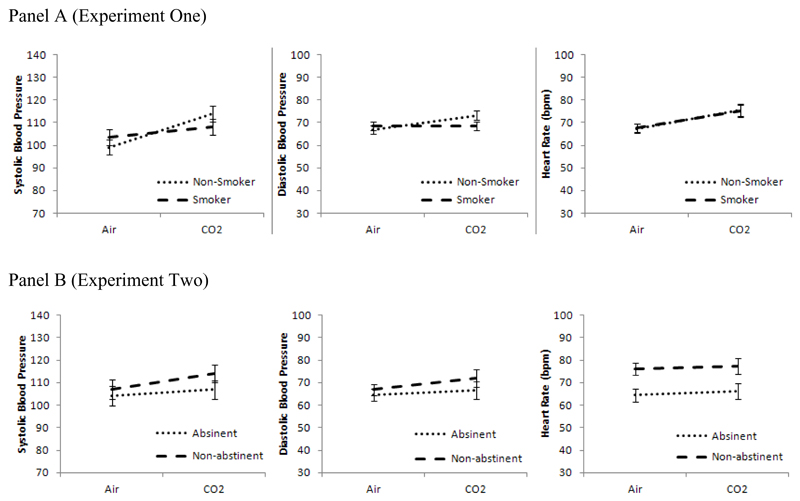
CO2 inhalation increased systolic blood pressure (F [1, 22] = 28.48, p < 0.001, η2 = 0.56), diastolic blood pressure (F [1, 22] = 4.89, p = 0.038, η2 = 0.18), and heart rate (F [1, 22] = 13.31, p = 0.001, η2 = 0.38) compared with air inhalation (Table 2). For systolic and diastolic blood pressure, the gas × smoking status interaction terms indicated that these effects were stronger in non-smokers than smokers (ps < 0.043). This was not observed for heart rate (p = 0.89).
Table 2.
Cardiovascular responses, anxiety and mood after CO2 and air inhalation in Experiments One and Two.
| Experiment One | Experiment Two | |||||
|---|---|---|---|---|---|---|
| CO2 | Air | p-value | CO2 | Air | p-value | |
| Systolic BP | 111.0 (12.6) | 101.3 (12.0) | <.001 | 110.5 (14.1) | 105.6 (14.7) | .038 |
| Diastolic BP | 70.8 (7.3) | 67.7 (6.0) | .038 | 69.3 (13.3) | 65.8 (9.0) | .13 |
| Heart rate | 75.3 (9.6) | 67.5 (6.8) | .001 | 71.8 (13.5) | 70.2 (11.1) | .65 |
| QSU craving | 32.9 (9.76) | 30.3 (12.0) | .38 | 35.3 (16.5) | 34.7 (14.1) | .82 |
| STAI state | 47.0 (11.9) | 33.1 (8.0) | <.001 | 48.4 (10.6) | 35.8 (7.1) | <.001 |
| PANAS positive | 20.3 (5.0) | 22.0 (5.2) | .12 | 19.7 (4.5) | 21.8 (5.9) | .032 |
| PANAS negative | 15.6 (4.8) | 11.3 (2.9) | .002 | 17.1 (6.2) | 11.9 (2.4) | <.001 |
Values represent mean (SD).
Anxiety and mood
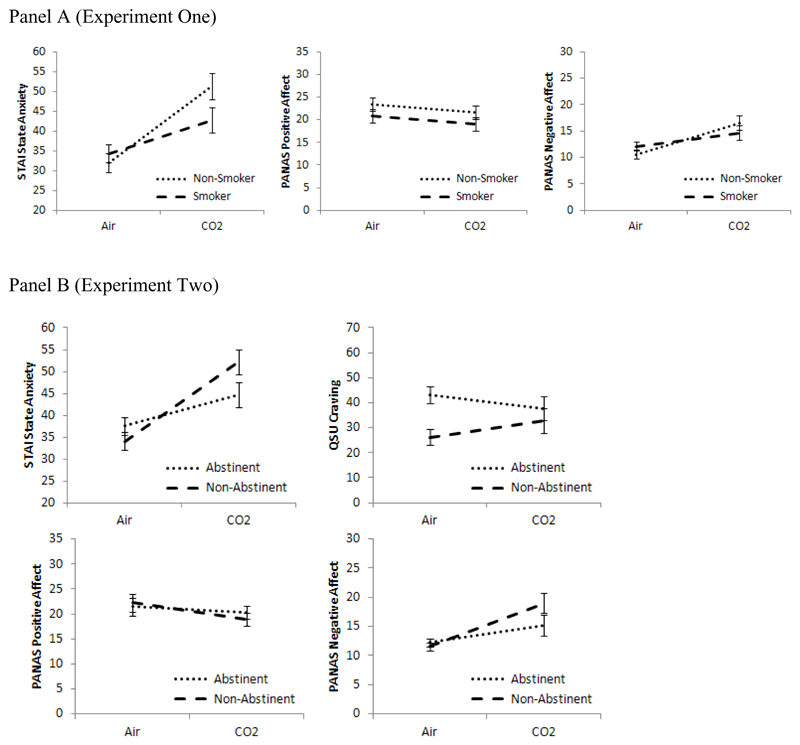
CO2 inhalation increased state anxiety (F [1, 22] = 18.72, p < 0.001, η2 = 0.46) and negative affect (F [1, 22] = 11.98, p = 0.002, η2 = 0.35), compared with air inhalation (Table 2). There was no main effect on positive affect (p = 0.12), and the gas × smoking status interaction terms indicated that there was no evidence that these effects differed by smoking status (ps > 0.10). These results are shown in Figure 1 (panel a).
Figure 1.
Effects of CO2 vs. air on anxiety (SSAI), positive and negative affect (PANAS) and craving (QSU) (Experiment Two only) in smokers vs. non-smokers (Experiment One) and abstinent vs. non-abstinent smokers (Experiment Two)
Cigarette craving
There was no difference in craving among smokers after CO2 (M = 32.9, SD = 12.0) compared to air (M = 30.3, SD = 9.8) (t = .91, df = 11, p = .38). Post-CO2 craving did not correlate with post-CO2 anxiety (p = .67).
Discussion
CO2 inhalation consistently increased cardiovascular parameters and self-reported anxiety and negative affect compared with air. Smokers showed reduced physiological responses to CO2 inhalation than non-smokers, with lesser increases in blood pressure. There was no interaction between gas and smoking status for self-reported anxiety and negative affect. The lesser physiological response in the smokers may be due to an anxiolytic effect of smoking, which may be a conditioned response (i.e., learned association between smoking and anxiolysis that may be withdrawal related) or due to a pharmacological anxiolytic effect of nicotine. The smokers in this study smoked before entering the laboratory. While these findings suggest no exacerbated anxiety responses to this challenge in non-abstinent smokers, it remains unclear how abstinence may explain these responses. In regular smokers, abstinence from smoking results in a range of physiological and subjective symptoms, including increased anxiety, and previous research has reported increased panic symptoms in withdrawn smokers following a rebreathing procedure [Abrams et al., 2008]. Furthermore, it is widely accepted that anxiety is a risk factor for smoking and smoking relapse [Parrott, 1998, Albertsen et al., 2006]. Therefore, smokers may be particularly vulnerable to anxiety when in a withdrawn state, and this was investigated in Experiment Two, which compared reactivity to the CO2 challenge in a group of abstinent and non-abstinent daily smokers.
Experiment Two
Methods
Design and overview
The experimental design and study procedures were identical to those described in Experiment One, with the exception that only smokers were recruited. Participants were randomised to abstain from smoking for 12 hours prior to testing, or smoke as usual (with their last cigarette being smoked just prior to entering the laboratory). A balanced randomisation schedule (i.e., equal numbers per group) was produced using random number generation software. Gas order was not counterbalanced (the air inhalation was administered first). Participants were told that they would complete two inhalations, one of (normal) air and one of CO2–enriched air, but they were not told the order in which they would receive these mixtures. The fixed order schedule was utilised due to increases in anxiety during the first inhalation (regardless of gas content), which may have inflated the anxiolytic effect of the gas by interacting with the CO2-induced anxiety. To create a more conservative test, medical air was administered first throughout.
Participants
Twenty-four smokers (smoke at least 5 cigarettes per day and within one hour of waking) were recruited from the staff and students of the University of Bristol and from a participant database, and randomised to either abstinent or non-abstinent conditions (twelve participants per group). All inclusion and exclusion criteria were identical to those described in Experiment One. None of the participants recruited had taken part in Experiment One. Abstinence status on the day of testing was confirmed by exhaled CO monitoring (< 7 ppm).
Statistical analysis
Data were analysed by repeated measures ANOVA, with gas (air, CO2) as a within-subjects factor, and smoking abstinence (abstinent, non-abstinent) as a between-subjects factor. Analyses were performed with and without adjustment for changes in craving between gas conditions. To explore the relationship between subjective anxiety and craving Pearson’s correlations were conducted on post-CO2 SSAI and QSU scores. All analyses were performed using PASW Statistics version 18 (SPSS Inc., Chicago IL, USA). Precise p-values are reported throughout.
Results
Characteristics of participants
Participant characteristics (n = 24; 46% female) at baseline are shown in Table 1. There were baseline differences in craving (t = 4.31, df = 22, p <0.001) and heart rate (t = 2.81, df = 22, p = 0.010) with higher craving (M = 42.9, SD = 12.8 versus M = 22.3, SD = 10.5) and lower heart rate (M = 68.6, SD = 9.8 versus M = 79.8, SD = 9.8) in abstinent versus non-abstinent smokers. There were no other differences between abstinence groups (ps > 0.10).
Cardiovascular measures
CO2 inhalation increased systolic blood pressure (F [1, 22] = 4.89, p = 0.038, η2 = 0.18), compared with air inhalation (Table 2). There were no main effects on diastolic blood pressure (p = 0.13) or heart rate (p = 0.65), and the gas × abstinence interaction terms indicated that there was no evidence that these effects differed by abstinence status (ps > 0.36). These results were not altered when change in craving was included as a covariate.
Anxiety and mood
CO2 inhalation increased state anxiety as measured by SSAI (F [1, 22] = 52.13, p < 0.001, η2 = 0.70) and negative affect (F [1, 22] = 21.76, p < 0.001, η2 = 0.50), and decreased positive affect (F [1, 22] = 5.24, p = 0.032, η2 = 0.19) compared with air inhalation (Table 2). For state anxiety, the gas × abstinence interaction term indicated that this effect was stronger in non-abstinent smokers than abstinent smokers (p = 0.005). A similar pattern was observed more weakly for negative affect (p = 0.054), but not positive affect (p = 0.27). These results are shown in Figure 1 (panel b), and were not altered when change in craving was included as a covariate.
Craving
There was no effect of gas on craving (p = .82), but craving was higher in the abstinent group (M = 40.4, SD = 12.5) than non-abstinent group (M = 29.5, SD = 13.1) (F [1, 22] = 4.37, p = 0.048, η2 = 0.17). There was a gas × abstinence interaction (F [1, 22] = 5.67, p = 0.026, η2 = 0.21), with non-abstinent smokers showing higher craving after CO2 compared to air (t = 2.24, df = 11, p = 0.046), but no difference between CO2 and air in abstinent smokers (p = 0.21) (Figure 1, panel b). Post-CO2 craving did not correlate with post-CO2 anxiety across the collective data set (p = .998), but based on the gas × abstinence interaction correlations were conducted separately for each abstinent group. There was evidence of a correlation in the non-abstinent group (r = .53, n = 12, p = 0.075) but not the abstinent group (r = -.35, n = 12, p = 0.26).
Discussion
CO2 inhalation increased subjective anxiety and systolic blood pressure, and induced negative changes in mood. Overnight abstinent smokers showed lesser response to CO2 than did non-abstinent smokers. Furthermore, abstinent smokers did not show increases in craving after CO2 compared to air as was observed in non-abstinent smokers. These findings suggest that abstinence from smoking may have beneficial effects on anxious responding in smokers [Parrott, 1995, West et al., 1997]. The relationship between smoking and anxiety is undoubtedly complex. Paradoxically, smokers often assert that smoking is anxiolytic, yet at a population level, smokers report higher levels of anxiety than do non-smokers [Parrott, 1998]. Anxiolytic effects reported by smokers may actually be due to reversal of an anxiogenic smoking withdrawal state that develops with chronic smoking, and as a result smokers may come to associate the act of smoking with reductions in anxiety and smoke in times of stress. Although our findings did not indicate heightened reactivity to CO2 in abstinent smokers, it is noteworthy that this group did not differ from the non-abstinent group on state measures of anxiety or positive or negative affect, as would be expected in individuals experiencing acute nicotine withdrawal. This lack of withdrawal symptoms suggests that our sample were not strongly tobacco dependent. We recruited individuals who smoked at least five cigarettes per day and who smoked within one hour of waking in the morning (which are considered reliable indicators or smoking dependence). There were group differences in baseline levels of craving with higher craving in abstinent smokers as would be expected for smokers who usually smoke upon waking, but as noted above this craving difference was independent of other withdrawal symptoms such as negative affect. We did not record the number of years smoked. Given the young age of many of our participants, it is unlikely that many have sustained the regular pattern of smoking for very long, and therefore may not have experienced the physiological changes associated with long-term regular smoking. These data indicate a dissociation between craving and negative affect in our sample of abstinent smokers. Future research should replicate this study in a sample of heavier smokers with a longer smoking history, who would be expected to show withdrawal syndrome upon acute abstinence from smoking.
General Discussion
Inhalation of CO2-enriched air increased physiological and subjective indices of anxiety and negative affect in both experiments, providing validation of its use as a model of anxiety induction in experimental studies. Furthermore, gas by smoking group interactions were observed in both experiments, indicating that responses to the gas are influenced by smoking-related variables. Lower reactivity to CO2 was observed in smokers compared with non-smokers (Experiment One) and in abstinent compared with non-abstinent smokers (Experiment Two).
These findings suggest that during periods of smoking, smokers do not show substantial increases in anxious responding, as demonstrated by lesser reactivity to CO2 than non-smokers (Experiment One). Despite this, there was also evidence that acute abstinence from smoking may be beneficial, reducing anxious responding beyond that of non-abstinent smokers (Experiment Two). It is important to note that there was little evidence of negative withdrawal symptoms in our overnight abstinent sample, and therefore these findings may only generalise to abstinence in the absence of marked withdrawal symptoms.
Interactions between gas and smoking group were observed in both experiments, yet the dependent variable for which the interaction was obtained differed across studies. These were systolic and diastolic blood pressure in Experiment One and state anxiety and negative affect in Experiment Two. While it is possible that different smoking variables have differential effects across anxiety parameters, it seems that there were more general patterns of reactivity change that simply did not reach statistical significance on all dependent measures. For example, in Experiment One, in addition to reduced blood pressure response to the gas, smokers also showed a pattern consistent with reduced subjective anxiety and negative (see Figures 1 and two, panels A). However, the smoking groups in Experiments One and Two differed in relation to gas-related craving. The non-abstinent group in Experiment Two reported higher craving after CO2 compared to air but this effect was not observed in Experiment One (where the smokers had the same abstinence profile). This suggests possible differences in the smokers between the two studies, despite use of the same inclusion criteria. This variation again may be partly due to the inclusion of some smokers with short smoking histories and low dependence. Both groups reported increases in anxiety after CO2 suggesting a partial dissociation between anxiety and craving.
Figure 2.
Effects of CO2 vs. air on systolic blood pressure, diastolic blood pressure and heart rate in smokers vs. non-smokers (Experiment One) and abstinent vs. non-abstinent smokers (Experiment Two)
Taken together, these findings support an acute anxiolytic effect of smoking as has been reported elsewhere [Jarvik et al., 1989, Cohen et al., 2009]. However, it has been suggested that this effect is relatively short-lived [Szyndler et al., 2001], and that chronic smoking induces physiological changes [Rohleder et al., 2006] that may increase basal levels anxiety in smokers compared to non-smokers [Sheahan et al., 2006]. The absence of withdrawal symptoms (i.e., negative affect) in the abstinent group suggests low levels of dependency. Instead of the expected higher reactivity to the gas in abstinent smokers, we observed lower anxiety and craving in this group. These findings imply that abstinence may be beneficial when not accompanied by a pronounced withdrawal syndrome which supports the contention that long term abstinence (i.e., post-withdrawal) from smoking has beneficial effects on anxiety and anxious responding [Mcdermott et al., 2013]. However, Leyro et al. [2013] investigated panic responses to 10% CO2 challenge in heavier smokers, and found that high nicotine withdrawal was only associated with greater panic attack symptoms in individuals with panic disorder. In contrast, healthy controls had lower symptomology when heavily withdrawn from smoking, suggesting that the withdrawal may not be strongly associated with stress reactivity. However, this study examined more extreme (panic-related) outcomes and future research should examine withdrawal effects in heavy long-term smokers on subjective anxiety.
The relationship between smoking and anxiety is complex and may vary depending on the smoking variable under investigation [Leventhal et al., 2013] and also as a function of individual differences. There are also reports of gender differences with females showing greater increases in negative affect (including anxiety) during smoking abstinence [Pang et al., 2013]. In a qualitative review of the smoking-affect literature, Ameringer and Leventhal [2010] identified associations with negative affect and emotional arousal with smoking status but not smoking heaviness. The current studies assessed changes in phasic craving as a function of anxiety-induction but future research should consider a wider range of smoking variables and individual differences. Furthermore, due to the nature of the manipulation and sample, it is difficult to directly extrapolate from the effect sizes we observed to clinical significance. However, the study was powered to detect effects comparable in magnitude to studies of the effects of anxiolytic medications on response to inhalation of 7.5% CO2 [Bailey et al., 2007].
There are some limitations of the current studies which should be acknowledged. First, the sample was predominately students from the University of Bristol. This may limit the generalisability of findings to the wider population. Second, while the studies were sufficiently powered to demonstrate effects of the CO2 inhalation on anxious outcomes, the small sample size may underlie the relatively weak statistical evidence for the key interaction effects. Therefore, these findings should be replicated using a larger sample. Third, a number of personal and situational variables may affect smoking and anxiety outcomes, including fitness, sleep levels and menstrual cycle, which were not controlled for in the current studies.
These experiments are the first to use the 7.5% CO2 anxiety induction model to investigate the relationship between smoking and anxiety. The model consistently increased physiological and subjective indices of anxiety across all experiments. Furthermore, we observed differences between smoking groups in anxious responding in both experiments, thereby validating the use of this model as a tool to be used in future smoking research. Given the important role that anxiety is believed to play particularly in the maintenance and relapse to smoking, developing novel and valid tools is an important advancement in the field. As well as investigating the effects of smoking variables, such as smoking status and abstinence, future research should strive to further elucidate the mechanisms underlying the smoking-anxiety relationship. Habitual smoking induces changes in a number of biological systems; however, other cognitive and psychological factors, including emotional dysregulation, outcome expectancies and smoking motives all warrant future investigation. The relationship between smoking and anxiety in undoubtedly complex, and while further research is required, these studies provide an important first step in the use of physiological anxiety manipulation techniques to understand smoking behaviour.
Acknowledgements
This work was supported by a National Alliance for Research in Schizophrenia and Affective Disorders Young Investigator Grant to MRM. ASA, AFA and MRM are members of the UK Centre for Tobacco Control Studies, a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
Footnotes
Conflict of Interest Statement
ASA and MRM have received research funding from Pfizer Inc. The authors declare that there are no other conflicts of interest.
References
- Abrams K, Leger K, Schlosser L, Merrill A, Bresslour M, Jalan A. Nicotine Withdrawal Exacerbates Fear Reactivity to Co(2)-Induced Bodily Sensations among Smokers. Nicotine Tob Res. 2011;13:1052–1058. doi: 10.1093/ntr/ntr113. [DOI] [PubMed] [Google Scholar]
- Abrams K, Zvolensky MJ, Dorflinger L, Galatis A, Blank M, Eissenberg T. Fear Reactivity to Bodily Sensations among Heavy Smokers and Nonsmokers. Exp Clin Psychopharmacol. 2008;16:230–239. doi: 10.1037/1064-1297.16.3.230. [DOI] [PubMed] [Google Scholar]
- Albertsen K, Borg V, Oldenburg B. A Systematic Review of the Impact of Work Environment on Smoking Cessation, Relapse and Amount Smoked. Prev Med. 2006;43:291–305. doi: 10.1016/j.ypmed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Ameringer KJ, Leventhal AM. Applying the Tripartite Model of Anxiety and Depression to Cigarette Smoking: An Integrative Review. Nicotine Tob Res. 2010;12:1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Kendrick AH, Nutt DJ. Behavioral and Cardiovascular Effects of 7.5% Co2 in Human Volunteers. Depress Anxiety. 2005;21:18–25. doi: 10.1002/da.20048. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Kendrick A, Diaper A, Potokar JP, Nutt DJ. A Validation of the 7.5% Co2 Model of Gad Using Paroxetine and Lorazepam in Healthy Volunteers. J Psychopharmacol. 2007;21:42–49. doi: 10.1177/0269881106063889. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Papadopoulos A, Seddon K, Nutt DJ. A Comparison of the Effects of a Subtype Selective and Non-Selective Benzodiazepine Receptor Agonist in Two Co(2) Models of Experimental Human Anxiety. J Psychopharmacol. 2009;23:117–122. doi: 10.1177/0269881108089603. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Neurobiology of Nicotine Addiction: Implications for Smoking Cessation Treatment. Am J Med. 2008;121:S3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW. Tobacco Addiction and the Dysregulation of Brain Stress Systems. Neurosci Biobehav Rev. 2012;36:1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldirola D, Bellodi L, Cammino S, Perna G. Smoking and Respiratory Irregularity in Panic Disorder. Biol Psychiatry. 2004;56:393–398. doi: 10.1016/j.biopsych.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Cherek DR. Effects of Acute Exposure to Increased Levels of Background Industrial Noise on Cigarette Smoking Behavior. Int Arch Occup Environ Health. 1985;56:23–30. doi: 10.1007/BF00380697. [DOI] [PubMed] [Google Scholar]
- Childs E, De Wit H. Effects of Acute Psychosocial Stress on Cigarette Craving and Smoking. Nicotine Tob Res. 2010;12:449–453. doi: 10.1093/ntr/ntp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Young RW, Velazquez MA, Groysman M, Noorbehesht K, Ben-Shahar OM, et al. Anxiolytic Effects of Nicotine in a Rodent Test of Approach-Avoidance Conflict. Psychopharmacology (Berl) 2009;204:541–549. doi: 10.1007/s00213-009-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BN, Lepore SJ. Association between Anxiety and Smoking in a Sample of Urban Black Men. J Immigr Minor Health. 2009;11:29–34. doi: 10.1007/s10903-008-9164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Howard CJ, Attwood AS, Stirland R, Rostant V, Renton L, et al. Acutely Induced Anxiety Increases Negative Interpretations of Events in a Closed-Circuit Television Monitoring Task. Cogn Emot. 2012 doi: 10.1080/02699931.2012.704352. [DOI] [PubMed] [Google Scholar]
- Cosci F, Abrams K, Schruers KR, Rickelt J, Griez EJ. Effect of Nicotine on 35% Co2-Induced Anxiety: A Study in Healthy Volunteers. Nicotine Tob Res. 2006;8:511–517. doi: 10.1080/14622200600789643. [DOI] [PubMed] [Google Scholar]
- Cosci F, Knuts IJ, Abrams K, Griez EJ, Schruers KR. Cigarette Smoking and Panic: A Critical Review of the Literature. J Clin Psychiatry. 2010;71:606–615. doi: 10.4088/JCP.08r04523blu. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and Cellular Aspects of Nicotine Abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Fidler JA, West R. Self-Perceived Smoking Motives and Their Correlates in a General Population Sample. Nicotine Tob Res. 2009;11:1182–1188. doi: 10.1093/ntr/ntp120. [DOI] [PubMed] [Google Scholar]
- Garner M, Attwood A, Baldwin DS, James A, Munafo MR. Inhalation of 7.5% Carbon Dioxide Increases Threat Processing in Humans. Neuropsychopharmacology. 2011;36:1557–1562. doi: 10.1038/npp.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of Abstinence from Tobacco: Valid Symptoms and Time Course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and Symptoms of Tobacco Withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of Nicotine on the Tobacco Withdrawal Syndrome. Psychopharmacology (Berl) 1984;83:82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The Role of the Hippocampus in Feedback Regulation of the Hypothalamic-Pituitary-Adrenocortical Axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Caskey NH, Rose JE, Herskovic JE, Sadeghpour M. Anxiolytic Effects of Smoking Associated with Four Stressors. Addict Behav. 1989;14:379–386. doi: 10.1016/0306-4603(89)90025-7. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between Cigarette Smoking and Anxiety Disorders During Adolescence and Early Adulthood. JAMA. 2000;284:2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Kaye J, Buchanan F, Kendrick A, Johnson P, Lowry C, Bailey J, et al. Acute Carbon Dioxide Exposure in Healthy Adults: Evaluation of a Novel Means of Investigating the Stress Response. J Neuroendocrinol. 2004;16:256–264. doi: 10.1111/j.0953-8194.2004.01158.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Strasburger CJ. 'Normal' Cigarette Smoking Increases Free Cortisol in Habitual Smokers. Life Sci. 1992;50:435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- Klein DF. False Suffocation Alarms, Spontaneous Panics, and Related Conditions. An Integrative Hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and Depressive Symptoms and Affective Patterns of Tobacco Withdrawal. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Araya R, Smith GD, Freathy R, Gunnell D, Palmer T, et al. Smoking Is Associated with, but Does Not Cause, Depressed Mood in Pregnancy--a Mendelian Randomization Study. PLoS One. 2011;6:e21689. doi: 10.1371/journal.pone.0021689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ. The Interaction of Nicotine Withdrawal and Panic Disorder in the Prediction of Panic-Relevant Responding to a Biological Challenge. Psychol Addict Behav. 2013;27:90–101. doi: 10.1037/a0029423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccabe RE, Chudzik SM, Antony MM, Young L, Swinson RP, Zolvensky MJ. Smoking Behaviors across Anxiety Disorders. J Anxiety Disord. 2004;18:7–18. doi: 10.1016/j.janxdis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Mcdermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P. Change in Anxiety Following Successful and Unsuccessful Attempts at Smoking Cessation: Cohort Study. Br J Psychiatry. 2013;202:62–67. doi: 10.1192/bjp.bp.112.114389. [DOI] [PubMed] [Google Scholar]
- Mckenzie M, Olsson CA, Jorm AF, Romaniuk H, Patton GC. Association of Adolescent Symptoms of Depression and Anxiety with Daily Smoking and Nicotine Dependence in Young Adulthood: Findings from a 10-Year Longitudinal Study. Addiction. 2010;105:1652–1659. doi: 10.1111/j.1360-0443.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Crum RM. Cigarette Smoking and Onset of Mood and Anxiety Disorders. Am J Public Health. 2013;103:1656–1665. doi: 10.2105/AJPH.2012.300911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan S, Gustavson K, Karevold E, Overland S, Jacka FN, Pasco JA, et al. The Impact of Smoking in Adolescence on Early Adult Anxiety Symptoms and the Relationship between Infant Vulnerability Factors for Anxiety and Early Adult Anxiety Symptoms: The Topp Study. PLoS One. 2013;8:e63252. doi: 10.1371/journal.pone.0063252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan S, Jacka FN, Pasco JA, Berk M. Cigarette Smoking, Nicotine Dependence and Anxiety Disorders: A Systematic Review of Population-Based, Epidemiological Studies. BMC Med. 2012;10:123. doi: 10.1186/1741-7015-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Araya R. Cigarette Smoking and Depression: A Question of Causation. Br J Psychiatry. 2010;196:425–426. doi: 10.1192/bjp.bp.109.074880. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Heron J, Araya R. Smoking Patterns During Pregnancy and Postnatal Period and Depressive Symptoms. Nicotine Tob Res. 2008;10:1609–1620. doi: 10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- Nichter M, Vuckovic N, Quintero G, Ritenbaugh C. Smoking Experimentation and Initiation among Adolescent Girls: Qualitative and Quantitative Findings. Tob Control. 1997;6:285–295. doi: 10.1136/tc.6.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RD, Leventhal AM. Sex Differences in Negative Affect and Lapse Behavior During Acute Tobacco Abstinence: A Laboratory Study. Exp Clin Psychopharmacol. 2013;21:269–276. doi: 10.1037/a0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paperwalla KN, Levin TT, Weiner J, Saravay SM. Smoking and Depression. Med Clin North Am. 2004;88:1483–1494. x–xi. doi: 10.1016/j.mcna.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Park ER, Chang Y, Quinn V, Regan S, Cohen L, Viguera A, et al. The Association of Depressive, Anxiety, and Stress Symptoms and Postpartum Relapse to Smoking: A Longitudinal Study. Nicotine Tob Res. 2009;11:707–714. doi: 10.1093/ntr/ntp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. Smoking Cessation Leads to Reduced Stress, but Why? Int J Addict. 1995;30:1509–1516. doi: 10.3109/10826089509055846. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Nesbitt's Paradox Resolved? Stress and Arousal Modulation During Cigarette Smoking. Addiction. 1998;93:27–39. doi: 10.1046/j.1360-0443.1998.931274.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does Cigarette Smoking Cause Stress? Am Psychol. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, Anxiety, and Smoking Initiation: A Prospective Study over 3 Years. Am J Public Health. 1998;88:1518–1522. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased Desire to Smoke During Acute Stress. Br J Addict. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of Nicotine and Nicotinic Receptors on Anxiety and Depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, Mcnally RJ. Anxiety Sensitivity, Anxiety Frequency and the Prediction of Fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The Hypothalamic-Pituitary-Adrenal (Hpa) Axis in Habitual Smokers. Int J Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette Smoking During Anxiety-Provoking and Monotonous Tasks. Addict Behav. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Sheahan SL, Rayens MK, An K, Riegel B, Mckinley S, Doering L, et al. Comparison of Anxiety between Smokers and Nonsmokers with Acute Myocardial Infarction. Am J Crit Care. 2006;15:617–625. [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, Cortisol and Nicotine. Int J Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Szyndler J, Sienkiewicz-Jarosz H, Maciejak P, Siemiatkowski M, Rokicki D, Czlonkowska AI, et al. The Anxiolytic-Like Effect of Nicotine Undergoes Rapid Tolerance in a Model of Contextual Fear Conditioning in Rats. Pharmacol Biochem Behav. 2001;69:511–518. doi: 10.1016/s0091-3057(01)00548-2. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The Development and Initial Validation of a Questionnaire on Smoking Urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Zvolensky MJ. Anxiety Sensitivity, Acute Nicotine Withdrawal Symptoms, and Anxious and Fearful Responding to Bodily Sensations: A Laboratory Test. Exp Clin Psychopharmacol. 2009;17:181–190. doi: 10.1037/a0016266. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and Validation of Brief Measures of Positive and Negative Affect: The Panas Scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. What Happens to Anxiety Levels on Giving up Smoking? Am J Psychiatry. 1997;154:1589–1592. doi: 10.1176/ajp.154.11.1589. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH. A Review of Psychological Factors/Processes Affecting Anxious Responding During Voluntary Hyperventilation and Inhalations of Carbon Dioxide-Enriched Air. Clin Psychol Rev. 2001;21:375–400. doi: 10.1016/s0272-7358(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Eifert GH, Brown RA. Affective Style among Smokers: Understanding Anxiety Sensitivity, Emotional Reactivity, and Distress Tolerance Using Biological Challenge. Addict Behav. 2001;26:901–915. doi: 10.1016/s0306-4603(01)00242-8. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner EW, Mcleish AC. Smoking and Panic Attacks, Panic Disorder, and Agoraphobia: A Review of the Empirical Literature. Clin Psychol Rev. 2005;25:761–789. doi: 10.1016/j.cpr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, Mccreary BT. The Impact of Smoking on Panic Disorder: An Initial Investigation of a Pathoplastic Relationship. J Anxiety Disord. 2003;17:447–460. doi: 10.1016/s0887-6185(02)00222-0. [DOI] [PubMed] [Google Scholar]