Abstract
Background
Pituitary carcinomas (PC) and atypical pituitary adenomas (APA) are rare variants of pituitary tumors for which no evidence-based treatment currently exists. We sought to determine whether temozolomide represents an effective chemotherapeutic option for patients with PC and APA.
Methods
A systematic review was performed using all published cases of PC and APA treated with temozolomide, and for which information on treatment regimen, clinical response, and survival could be identified. The primary goal of this analysis was to describe overall survival and progression-free survival among PC and APA patients after temozolomide treatment. Secondary goals included assessment of response rate and biomarkers of response.
Results
We identified 57 cases and obtained follow-up data on 54 patients (31 APA and 23 PC) for analysis. Estimates of 5-year progression-free survival and overall survival were 21.9% and 57.4% for patients with APA and 36.1% and 56.2% for patients with PC. Among those who responded to temozolomide, overall survival was marginally statistically significantly greater for patients on long-term temozolomide therapy compared with those who were not (5-year overall survival 91.7% vs 54.1%, P = .08); Progression-free survival results were similar but not statistically significant. The objective response rate was 48.4% for patients with APA and 65.2% for patients with PC. Stable disease occurred in 29% of APA and 17.4% of PC patients. Neither histology nor expression of Ki-67 correlated with response; however, negative O6-methylguanine-DNA methyltransferase staining was strongly related to response to temozolomide in patients with APA (P < .001).
Conclusions
Temozolomide is an effective treatment of both PC and APA, and long-term treatment can be considered for particularly aggressive cases.
Keywords: atypical pituitary adenoma, MGMT, pituitary carcinoma, pituitary tumors, temozolomide
Tumors of the pituitary gland are a relatively common form of central nervous system (CNS) neoplasm in the general population.1 A recent systematic review analyzed data from 3577 patients and reported the overall estimated prevalence rate of pituitary adenoma demonstrated by either autopsy or brain images to be 16.7% (autopsy alone, 14.4% and imaging alone, 22.5%).2 Based on the Central Brain Tumor Registry of the United States 2007–2011, the pituitary tumor incidence rate is 3.29 per 100 000 and accounts for 15.1% of all CNS tumors.3 It is the most common CNS tumor in adolescents and young adults (age 15-34 years). Most pituitary tumors are benign adenomas. About 10% to 15% of patients have atypical pituitary adenomas (APA), which display a distinct growth pattern and clinical course. According to World Health Organization criteria, both APA and pituitary carcinomas (PC) have invasive growth, elevated mitotic index, Ki-67 staining greater than 3%, and positive p53 nuclear staining; however, only PC demonstrates metastasis, either systemically or within the craniospinal space.4 Staining for p53 may thus be a potential useful marker for differentiating benign from more invasive forms of pituitary tumors. However, despite evidence suggesting utility of immunostaining for p53 is limited to invasive pituitary tumors and correlates with positive Ki-67 nuclear staining, it has not been widely used in the clinical setting.5 As a result, most published cases have not reported this information.
Both APA and PC respond poorly to standard treatment. Many patients die from disease progression despite multimodal therapy that includes surgery, conformal radiotherapy, radiosurgery, use of dopamine agonists, or other pharmacological treatments. Because of the rarity and heterogeneous nature of the disease, information regarding optimal treatment is fairly limited.6,7
Temozolomide (TMZ) is a prodrug that is quickly converted to its active compound 3-methyl-(triazen-1-yl) imidazole-4-carboxamide (MTIC) in physiological pH. The activated form readily crosses the blood-brain barrier; thus, this drug has been investigated and used commonly for treatment of primary and secondary intracranial neoplasms. It exerts its cytotoxic activity by alkylating DNA at the O6 position of guanine. As a result, TMZ resistance can result through a mechanism that reverts this alkylation, such as high expression of O6-methylguanine-DNA methyltransferase (MGMT). The U.S. Food and Drug Administration (FDA) approved TMZ in 1999 for the treatment of glioblastoma multiforme and anaplastic astrocytoma. Clinicians started to use TMZ to treat APA and PC in 2006,8,9 likely because Stupp et al demonstrated that concurrent TMZ with radiation led to significant survival benefit for patients with newly diagnosed glioblastoma in 2005.10 However, treatment outcomes have only been published in case reports or in small case series. It has been difficult to draw conclusions from case reports or series regarding the benefit of TMZ because of variability in treatment initiation, treatment regimens, and different patterns of disease evolution.8,9,11–36 Moreover, many reported cases had relatively short follow-up times at the time of their publication. In this systematic review of reported cases, we summarize the long-term outcomes of 57 patients with APA or PC treated with TMZ to determine 5-year overall survival (OS), progression-free survival (PFS), and objective response rate. We conducted subgroup analyses to assess the benefit of long-term TMZ therapy beyond 12 months, and whether biological markers, including expression of Ki-67 and MGMT, were associated with TMZ response.
Methods
Identification of eligible cases
We searched PubMed and Medline for articles published from January 2006 to October 2014 using the following key words: pituitary, tumor, atypical adenoma, carcinoma, neoplasm, malignant, and temozolomide. We did not find any published cases in which TMZ was used to treat PC or APA prior to 2006. A total of 88 articles were identified. Articles were included in the analysis if they reported cases of PC or APA treated with TMZ at any point during the course of treatment (ie, first-line or beyond). Articles were excluded if they were published in a language other than English or if information regarding treatment regimen, clinical response, or survival was not available or obtainable by contacting the corresponding authors. After reviewing the abstracts and references of each article, 29 case and case-series reports were included in the study based on our eligibility criteria.
Data collection
Corresponding authors of articles in which the patient was alive at the time of publication were contacted for updated follow-up data (including progression and death). Data abstracted from the published reports included gender, age, time of diagnosis, histology, status of Ki-67, MGMT methylation or expression (positive was defined as positive nuclei staining of more than or equal to 10%; otherwise, negative), and treatment modality including surgeries, radiosurgeries, radiation therapy, and medical treatment (dopamine agonist, somatostatin analog, growth hormone antagonist, hormonal replacement therapy, chemotherapy, and TMZ). Details regarding TMZ treatment were also collected, including time of initiation, duration, regimen, and any concurrent therapy.
Study definitions
According to World Health Organization criteria, both APA and PC have invasive growth, elevated mitotic index, positive Ki-67 staining, and positive p53 nuclear staining. In addition, PC can demonstrate metastatic lesions.4 Because immunostaining of p53 has not been widely used in the clinical setting, APA was defined as an atypical adenoma that rapidly proliferated (high Ki-67 staining and mitotic index), invaded adjacent tissue, and had frequent recurrences. PC was defined as a tumor that developed from the anterior pituitary gland and had developed one or more cerebral, meningeal, or systemic metastases. According to the World Health Organization Response Evaluation Criteria In Solid Tumors (RECIST) criteria, we defined complete response as complete disappearance of all known disease. Partial response was defined as a decrease of 50% or more in tumor load. Stable disease was defined as a decrease of less than 50% or increase less than 25% in one or more measurable lesions. Progressive disease was defined as more than 25% increase in total tumor load or appearance of new lesion(s). Short-term therapy was defined as 1 to 12 months of treatment with adjuvant TMZ, and long-term therapy as more than 12 months of treatment.
Statistical analysis
The primary goal of this analysis was to describe the OS and PFS in eligible patients after TMZ administration. Secondary goals included assessment of response rate and biomarkers of TMZ response. OS was calculated from the date of initial TMZ treatment to date of death or censored at the date of last follow-up if still alive. PFS was calculated from date of initial TMZ treatment to date of first known progression, recurrence, or death or censored at date of last follow-up if disease-free and alive. For patients with reports indicating progression but not the timing of progression, we assigned their PFS as 2 months based on the fact that the first restaging image is commonly done after 2 cycles of treatment. Both OS and PFS were censored at 5 years. Median time to event and 95% confidence intervals (CI) were calculated as available using Kaplan-Meier methods. Comparisons of OS and PFS by pathology (PC vs APA), timing of TMZ initiation, use of additional chemotherapy, and use of TMZ as a maintenance therapy among those who responded to treatment were conducted using log-rank tests. Association between response to TMZ and histology or expression of Ki-67 and MGMT was assessed using Fisher's exact test. Due to the sample size, we did not account for correlations between patients included in the same publication or treated at the same clinic; all patients were treated as independent individuals in the analysis.
Results
We identified 29 case or case-series reports published between 2006 and 2014 that met all inclusion criteria.8,9,11–37 A total of 55 cases were collected from the literature. Among them, 31 cases were APA and 24 cases were PC (Table 1). Two additional eligible cases (1 APA and 1 PC) were included in the analysis from our institution, the University of Minnesota Cancer Center for a total sample size of 57. Multiple attempts were made to reach all corresponding authors for updated clinical information. Of the 18 articles in which patients were alive at the time of publication, 9 authors responded with updated information on 22 cases; these authors are noted in Table 1 with an asterisk.
Table 1.
Summary of collected studies
| First Author | Year | Country | Case # | Reference |
|---|---|---|---|---|
| Lim* | 2006 | US | 1 PC | 9 |
| Fadul | 2006 | US | 2 PC | 8 |
| Neff | 2007 | US | 1 APA | 12 |
| Kovacs | 2007 | Canada | 1 APA | 11 |
| Hagen* | 2009 | Denmark | 2 APA, 1 PC | 14 |
| McCormack* | 2009 | Australia | 1 APA, 1 PC | 15 |
| Byrne | 2009 | Australia | 1 PC | 13 |
| Syro | 2009 | Colombia | 1 APA | 18 |
| Moyes | 2009 | Canada | 1 APA | 17 |
| Takeshita* | 2009 | Japan | 1 PC | 19 |
| Mohammed | 2009 | Canada | 1 APA, 1 PC | 16 |
| Losa | 2010 | Australia | 5 APA, 1 PC | 23 |
| Bush* | 2010 | US | 5 APA, 2PC | 21 |
| Raverot* | 2010 | France | 3 APA, 4 PC | 24 |
| Curtò | 2010 | Italy | 1 PC | 22 |
| Bode | 2010 | Germany | 1 PC | 20 |
| Moshkin | 2011 | Canada | 1 PC | 27 |
| Murakami | 2011 | Japan | 1 PC | 26 |
| Dillard* | 2011 | US | 1 APA | 25 |
| Thearle | 2011 | US | 1 PC | 28 |
| Morin | 2012 | Canada | 1 APA | 30 |
| Whitelaw* | 2012 | UK | 3 APA | 33 |
| Ersen | 2012 | Colombia | 1 APA | 29 |
| Phillips | 2012 | US | 1 PC | 32 |
| Morokuma* | 2012 | Japan | 1 PC | 31 |
| Zemmoura | 2013 | France | 1 PC | 35 |
| Vieira Neto | 2013 | Brazil | 1 PC | 34 |
| Batisse | 2013 | France | 1 APA | 37 |
| Zacharia | 2014 | US | 3 APA | 36 |
*authors responded with updated patient information.
Patients' baseline characteristics are outlined in Table 2. The majority of patients were men (59.3% APA and 72.0% PC). The mean age at diagnosis was 46.1 ± 13.1 years (45.5 ± 13.6 in APA and 46.7 ± 12.6 in PC). The median diagnosis time was 7 years (range, 0-25 years) from the initial diagnosis of adenoma. The most common history included tumors that secreted adrenocorticotropic hormone (43.8% APA and 40.0% PC) and prolactin (31.3% APA and 32.0% PC). Ki-67 and MGMT immunostaining was performed in 82.4% and 78.9% of APA and PC cases, respectively. The mean positive staining percentages for Ki-67 were 10.2 ± 6.4 and 12.9 ± 11.1 in APA and PC, respectively. Negative MGMT expression was found in 43.8% of APA cases and 36.0% of PC cases.
Table 2.
Patients' baseline characteristics (N = 57)
| APA (N = 32) | PC (N = 25) | Total | |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 19 (59.3) | 18 (72.0) | 37 (64.9) |
| Female, n (%) | 13 (40.7) | 7 (28.0) | 20 (35.1) |
| Age, yr, mean (SD) | 45.5 (13.6) | 46.7 (12.6) | 46.1 (13.1) |
| Histology | |||
| ACTH, n (%) | 14 (43.8) | 10 (40.0) | 24 (42.1) |
| PRL, n (%) | 10 (31.3) | 8 (32.0) | 18 (31.6) |
| LH, n (%) | 2 (6.2) | 1 (4.0) | 3 (5.3) |
| GH, n (%) | 2 (6.2) | 2 (8.0) | 4 (7.0) |
| Null, n (%) | 4 (12.5) | 4 (16.0) | 8 (14.0) |
| Ki-67, mean (SD) | 10.2 (6.4) | 12.9 (11.1) | 11.1 (9.3) |
| MGMT expression | |||
| Positive, n (%) | 9 (28.1) | 6 (24.0) | 15 (26.3) |
| Negative, n (%) | 14 (43.8) | 9 (36.0) | 23 (40.3) |
| Mixed, n (%) | 1 (3.1) | 0 (0.0) | 1 (1.8) |
| N/A, n (%) | 8 (25.0) | 10 (40.0) | 18 (31.6) |
ACTH, Adrenocorticotropic hormone; APA, atypical pituitary adenoma; GH, growth hormone; LH, luteinizing hormone; MGMT, O6-methylguanine-DNA methyltransferase; N/A, not available; Null, null cell; PC, pituitary carcinoma; PRL, prolactin; SD, standard deviation.
Prior to TMZ treatment, all patients had been initially treated with surgical resections, and 86% of patients had multiple resections; 95% of patients received radiation therapy, and 43% of patients received at least 2 cycles of radiation; 75% of patients were treated with a variety of agents including dopamine agonists, somatostatin analogs, ketoconazole, metyrapone, and steroids at different stages. Among all patients, TMZ was initiated at a median time of 10.1 years after initial diagnosis (range, 9 months-25 years) and was similar for patients with APA (9.6 years) and PC (10.7 years). The majority of patients (87.8%) received TMZ at 150–200 mg/m2 for 5 days every 28 days, while 7 patients (12.2%) received 75 mg/m2 for 21 days every 28 days. Three patients received concurrent chemoradiation therapy with 75 mg/m2 TMZ daily while getting brain irradiation. On average, patients received 13 cycles of TMZ treatment (range, 1-45 cycles). Among the patients who received short-term TMZ treatment, the median number of cycles was 8 (range, 1-12 cycles), while it was 26 (range, 14-45 cycles) among those who received long-term TMZ therapy. The majority of patients (77.2%) did not receive additional chemotherapy. Among patients who received chemotherapy or other therapy, the most common regimens were capecitabine (5 cases), bevacizumab (3 cases), platinum plus etoposide (3 cases), and cisplatin plus adriblastin (a form of doxorubicin, 1 case).
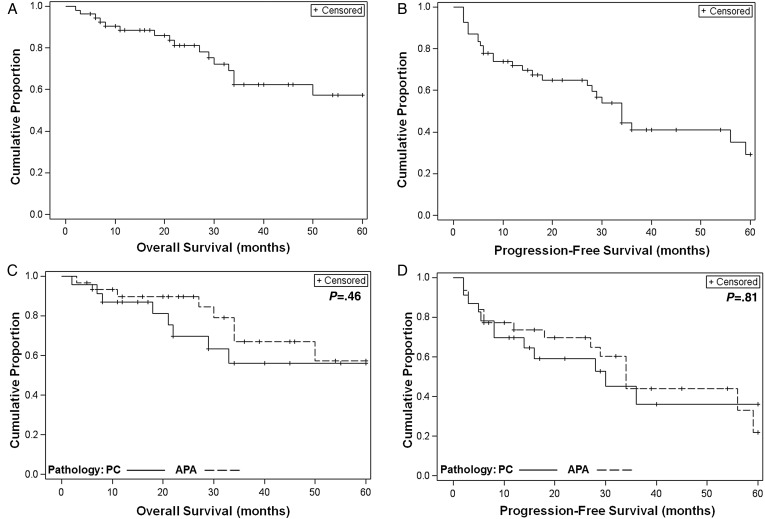
Follow-up data were available for 54 (31 APA, 23 PC) of the 57 cases. The estimate of OS at 5 years was 57.2% (95% CI, 38.1-72.4%; Fig. 1A); median OS could not be estimated due to too few deaths reported. PFS at 5 years was 29.3% (95% CI, 13.4-47.3%; Fig. 1B); median PFS was 34 months (95% CI, 27-59 months). No statistically significant differences in OS and PFS were seen between patients with APA and patients with PC. The 5-year OS was 57.4% (95% CI, 29.6-77.7%) for patients with APA and 56.2% (95% CI, 30.2-75.8%) for patients with PC; P = .46, Fig. 1C. The 5-year PFS was 21.9% (95% CI, 4.5-47.8%) for patients with APA and 36.1% (95% CI, 13.8-59.2%) for patients with PC; P = .81, Fig. 1D).
Fig. 1.
Overall survival (OS) and progression-free survival (PFS) from the start of temozolomide among all patients. (A) Kaplan-Meier curve of OS of all cases with follow-up information (N = 54). (B) Kaplan-Meier curve of PFS of all cases with follow-up information (N = 54). (C) Kaplan-Meier curves of OS by pathology (N = 54; P = .46). (D) Kaplan-Meier curves of PFS by pathology (N = 54; P = .81). PC, pituitary carcinoma; APA, atypical pituitary adenoma.
The objective response rate (complete response plus partial response) was 48.4% for APA and 65.2% for PC. Stable disease occurred in 29% of APA and 17.4% of PC patients. Approximately one-fifth of patients did not respond to TMZ (22.5% APA and 17.4% PC) (Table 3). The median duration of response was 30 months (range, 5.5-120 months).
Table 3.
Summary of response rate to TMZ (N = 54)
| CR | PR | SD | PD | |
|---|---|---|---|---|
| APA (N = 31), n (%) | 2 (6.5) | 14 (45.2) | 9 (29.0) | 6 (19.3) |
| PC (N = 23), n (%) | 2 (8.7) | 14 (60.9) | 3 (13) | 4 (17.4) |
APA, atypical pituitary adenoma; CR, complete response; PC, pituitary carcinoma; PD, progression of disease; PR, partial response; SD, stable disease; TMZ, temozolomide.
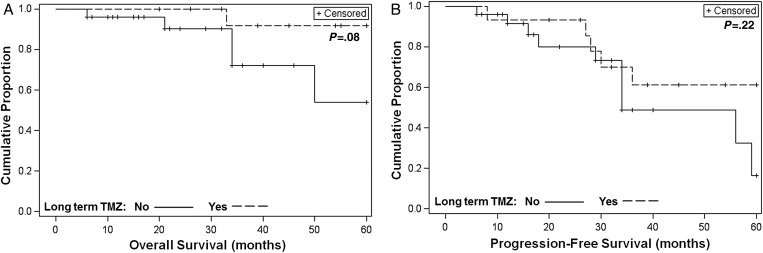
Among those who responded to TMZ, 35.7% of patients received long-term TMZ treatment. The 5-year PFS rate was 61.3% (95% CI, 30.0-81.9% among patients who responded and received long-term TMZ therapy vs 16.3% (95% CI, 1.0-48.9%; P = .22) among patients not receiving long-term therapy. The 5-year OS rate was 91.7% (95% CI, 53.9-98.8%) among patients receiving long-term TMZ treatment vs 54.1% (95% CI, 16.5-81.1%; P = .08) among patients not receiving long-term treatment. Although the differences for OS and PFS did not reach statistical significance, there was a trend toward benefit from long-term TMZ therapy (Fig 2A and B). To further investigate the duration of TMZ treatment for patients responding to the therapy, we studied the clinical outcomes including response, PFS, OS, and overall clinical status in all 15 patients who received long-term TMZ treatment (Table 4). Among the 15 patients who received long-term treatment, the majority stayed free of disease progression for relatively long durations (14-120 months). Most of the patients remain under surveillance. Four patients had died; but all of them died from causes unrelated to their pituitary tumors. Among 11 patients still living, 9 of them remained free of disease progression after stopping TMZ (2-97 months). Two out of the 9 patients had extended the interval of TMZ therapy to every 2 or 4 months to reduce the toxicity.
Fig. 2.
Overall survival (OS) and progression-free survival (PFS) from the start of temozolomide (TMZ) among patients who responded, by use of long-term TMZ. (A) Kaplan-Meier curves of OS by use of long-term TMZ (N = 41; P = .08). (B) Kaplan-Meier curves of PFS by use of long-term TMZ (N = 41; P = .22).
Table 4.
Summary of clinical outcomes of patients who received long-term TMZ therapy (N = 15)
| Patient | APA/PC | No. of Rx cycles | Response | PFS (mo) | OS (mo) | Clinical status |
|---|---|---|---|---|---|---|
| Long-term TMZ 12–15 mo: | ||||||
| 1 | PC | 14 | PR | 14 | 22 | Died of multiorgan failure with sepsis |
| 2 | APA | 15 | SD | 27 | 75 | Alive, underwent radiation and surgery after 15 cycles when tumor regrew |
| 3 | APA | 15 | PR | 84 | 84 | Died of unrelated disease, stable pituitary tumor at time of death |
| Long-term TMZ 16–18 mo: | ||||||
| 4 | PC | 18 | PR | 30 | 66 | Died, improvement of PRL level and clinical symptoms for 2.5 years |
| 5 | APA | 18 | SD | 20 | 20 | Alive, finished TMZ in July 2014, currently under surveillance |
| Long-term TMZ 22–24 mo: | ||||||
| 6 | PC | 23 | PR | 120 | 120 | Alive, normal IGF1 & PRL levels and stable clinical symptoms |
| 7 | APA | 24 | PR | 26 | 26 | Alive, still on TMZ, stable tumor size and PRL level |
| 8 | PC | 24 | CR | 84 | 84 | Alive, no radiological or biochemical recurrence 5 years after stopping TMZ |
| Long-term TMZ 28–32 mo: | ||||||
| 9 | PC | 28 | PR | 28 | 33 | Died of bowel carcinoma |
| 10 | APA | 30 | PR | 54 | 54 | Alive, stable ACTH level and tumor size, was on TMZ every 2 months for 28 cycles |
| 11 | PC | 32 | PR | 8 | 55 | Alive, disease progression after 8 mo, currently stable disease on bevacizumab |
| 12 | APA | 32 | CR | 32 | 32 | Alive |
| Long-term TMZ ≥ 36 mo: | ||||||
| 13 | PC | 36 | PR | 36 | 45 | Alive, slight increase in size of vertebral metastases first noticed in December 2013, but stable brain lesion |
| 14 | PC | 42 | PR | 84 | 84 | Alive, currently on TMZ every 4 months |
| 15 | APA | 45 | CR | 45 | 45 | Alive |
ACTH, adrenocorticotropic hormone; APA, atypical pituitary adenoma; CR, complete response; IGF1, insulin-like growth factor 1; OS: overall survival; PC, pituitary carcinoma; PFS: progression free survival; PR, partial response; PRL, prolactin; Rx, treatment; SD, stable disease; TMZ, temozolomide.
Among those with available data regarding histology and biomarkers, we further investigated whether TMZ responses were associated with histology or expression of Ki-67 and MGMT (Table 5). There was no statistically significant correlation between histology or Ki-67 and response to TMZ (P = .05, .47, .38, .28 for histology/APA, histology/PC, Ki-67/APA, and Ki-67/PC, respectively); however, it appeared that the negative expression of MGMT was clearly associated with TMZ response among patients with APA (P < .001). There was a similar trend among patients with PC, although the association was not statistically significant (P = .15).
Table 5.
Summary of histology, expression of MGMT and Ki-67 according to the response to TMZ (N = 54)
| PC, No. of patients (%) |
APA, No. of patients (%) |
||||||
|---|---|---|---|---|---|---|---|
| CR + PR | SD | PD | CR + PR | SD | PD | ||
| Histology | ACTH | 7 (30.4) | 1 (4.3) | 1 (4.3) | 8 (25.8) | 2 (6.5) | 3 (9.7) |
| PRL | 6 (26.1) | 0 | 1 (4.3) | 7 (22.6) | 2 (6.5) | 1 (3.2) | |
| LH | 1 (4.3) | 0 | 0 | 0 | 2 (6.5) | 0 | |
| GH | 1 (4.3) | 1 (4.3) | 0 | 0 | 0 | 2 (6.5) | |
| Null | 1 (4.3) | 1 (4.3) | 2 (8.7) | 1 (3.2) | 3 (9.7) | 0 | |
| MGMT | Pos | 2 (8.7) | 1 (4.3) | 1 (4.3) | 0 | 6 (19.3) | 4 (12.9) |
| Neg | 8 (34.8) | 0 | 2 (8.7) | 11 (35.5) | 2 (6.5) | 0 | |
| N/A | 6 (26.2) | 2 (8.7) | 1 (4.3) | 5 (16.1) | 1 (3.2) | 2 (6.5) | |
| Ki-67 | ≤ 1% | 3 (13.1) | 0 | 0 | 1 (3.2) | 0 | 0 |
| 1–5% | 2 (8.7) | 0 | 1 (4.3) | 6 (19.3) | 6 (19.3) | 3 (9.7) | |
| 5–10% | 3 (13.1) | 1 (4.3) | 0 | 2 (6.5) | 2 (6.5) | 1 (3.2) | |
| >10% | 3 (13.1) | 1 (4.3) | 3 (13.1) | 5 (16.1) | 0 | 0 | |
| N/A | 5 (21.7) | 1 (4.3) | 0 | 2 (6.5) | 1 (3.2) | 2 (6.5) | |
ACTH, adrenocorticotropic hormone; APA, atypical pituitary adenoma; CR, complete response; GH, growth hormone; LH, luteinizing hormone; MGMT, O6-methylguanine-DNA methyltransferase; N/A, not available; Neg, negative; Null, null cell; PC, pituitary carcinoma; PD, progression of disease; Pos, positive; PR, partial response; PRL, prolactin; SD, stable disease.
Discussion
APA and PC are relatively rare tumors that respond poorly to current treatment modalities. In the present study, we examined whether TMZ was effective as a treatment because it has been widely used to treat these tumors since 2006; however, data regarding its efficacy are limited and its clinical utility remains controversial. We analyzed all available cases with clinical outcome data available, including 32 APA and 25 PC cases. Our study is the most comprehensive analysis to date that addresses clinical outcomes of patients with APA and PC treated with TMZ. We collected a broad range of data, including patients' demographic data and details of diagnosis and treatment. According to the World Health Organization's classification of pituitary tumors, APA and PC are characterized for their invasive growth, elevated mitotic index, high Ki-67 labeling index, and extensive nuclear reactivity for p53; however, the mitotic index and p53 staining were not reported in the majority of cases. Centralized review of all published cases used in our analysis could not be practically performed.
On the basis of historical data, we speculated that patients with APA would have a survival advantage compared with patients with PC.38 However, there was no statistically significant difference in either OS or PFS between APA and PC at 5 years after TMZ therapy. The improvement in survival among patients with PC could be confounded by different patient samples or advancement of surgical and radiation therapies; however, it also could be due to treatment with TMZ. We noted a high response rate to TMZ. The clinical benefit rate (objective response plus stable disease rates) was about 80% in both APA and PC patients. Among those who responded to TMZ, there was a clear trend toward benefit from long-term therapy. Although these data strongly argue for use of long-term TMZ in treatment of aggressive pituitary tumors, the duration of TMZ therapy remains unknown. Due to the limited number of cases, we were not able to perform any statistical analysis to compare the different treatment intervals or durations. Nonetheless, we believe our study provides information that will be helpful to clinicians for meaningful decision making regarding the duration of TMZ treatment of pituitary tumors.
Approximately one-fifth of patients did not respond to TMZ. Hence, in this era of molecular targeting and genomic profiling, it is important to identify biological marker(s) that can predict positive TMZ response for pituitary tumors. Historically, MGMT expression or promoter methylation and tumor proliferation capacity (Ki-67) as well as histology have been studied and/or used for this purpose.39–42 In subgroup analyses, we identified an association between negative expression of MGMT and response of APA to TMZ, and a similar, but not statistically significant, trend was seen with PC. These findings emphasize the value of checking MGMT expression before starting TMZ, especially for patients with APA, in order to identify patients most likely to benefit from this medication. This conclusion is consistent with the findings of a large case series recently published by Bengtsson et al.43 The authors concluded that response to TMZ was associated with MGMT expression based on clinical and histology data from 21 patients with aggressive pituitary tumors (13 APA and 8 PC), who were treated with TMZ in 4 European countries.
The present study has several strengths and limitations. Strengths include a large patient cohort representative of cases treated worldwide, an analysis with information updated since publication of the original case reports/series, 5-year outcome data on patients with invasive pituitary tumors treated with TMZ, and a subgroup analysis on the benefit of maintenance TMZ treatment. The retrospective study design reliant on data reported in published case reports is a limitation of this study. This design led to some missing data, particularly relating to MGMT methylation. However, we predicted a positive relationship between MGMT methylation and TMZ response based on convincing evidence from previous studies showing that low MGMT expression inversely correlated with its promoter methylation status.15,40,42 Finally, there are possible confounding factors that may affect study outcomes that we have not accounted for in the analysis.
In conclusion, the high response rates and duration of responses observed in this study provide evidence that TMZ is an effective option for treating both APA and PC. Many aspects of optimal TMZ therapy remain unknown, and future studies should examine the timing of TMZ treatment (immediate vs delayed) and combination with other treatment modalities (chemotherapy or radiotherapy, concurrent vs sequential).
Funding
This research was supported by NIH Clinical and Translational Science KL2 Scholar Award 8UL1TR000114 (to E.L.); the University of Minnesota Deborah E. Powell for Women′s Health Interdisciplinary Seed Grant support (Grant #PCWH-2013–002); Minnesota Medical Foundation/University of Minnesota Foundation; the Masonic Cancer Center and Department of Medicine, Division of Hematology, Oncology and Transplantation, University of Minnesota; Institutional Research Grant #118198-IRG-58-001-52-IRG94 from the American Cancer Society; the National Pancreas Foundation; Mezin-Koats Colon Cancer Research Award; The Randy Shaver Cancer Research and Community Fund; and NIH grant P30 CA77598 and the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (supporting R.I.V.).
Acknowledgments
We thank Michael Franklin, M.S. (University of Minnesota) for excellent editorial assistance and critical review of the manuscript. We thank Dr. Weihua Guan (University of Minnesota), for providing us outstanding statistical analysis of the correlation between response and biomarkers. We appreciate all the authors who responded to our emails and provided the updated patient information. The authors are listed here in alphabetical order: Drs. Anthony P Harney (University of California- Los Angeles), Casper Hagen (Odense University Hospital, Denmark), Marianne S Andersen (Odense University Hospital, Denmark), Ann I McCormack (Royal North Shore Hospital, Australia), Akira Takeshita (Toranomon Hospital, Japan), Beatriz S Lopes (University of Virginia), Gérald Raverot (University of Lyon, France), Maria Fleseriu (Oregon Health & Science University), Ben Whitelaw (King's College Hospital, England), Takao Ando (Nagasaki University Graduates School of Biomedical Sciences, Japan).
Conflict of interest statement. none declared.
References
- 1. Kopczak A, Renner U, Karl Stalla G. Advances in understanding pituitary tumors. F1000 Prime Reports. 2014;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ezzat S, Asa SL, Couldwell WT et al. The prevalence of pituitary adenomas. Cancer. 2004;101(3):613–619. [DOI] [PubMed] [Google Scholar]
- 3. Ostrom QT, Gittleman H, Liao P et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology. 2014;16(suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeLellis RA LR, Heitz PU, Eng C. World Health Organization classification of tumours: tumours of endocrine organs. IARC, Lyons. 2004;36–39. [Google Scholar]
- 5. Saeger WLD, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156(2):203–216. [DOI] [PubMed] [Google Scholar]
- 6. Heaney A. Clinical review: Pituitary carcinoma: difficult diagnosis and treatment. J Clin Endocrinol Metab. 2011;96(12):3649–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaltsas GA NP, Kontogeorgos G, Buchfelder M, Grossman AB. Clinical review: Diagnosis and management of pituitary carcinomas. J Clin Endocrinol Metab. 2005;90(5):3089–3099. [DOI] [PubMed] [Google Scholar]
- 8. Fadul CE, Kominsky AL, Meyer LP et al. Long-term response of pituitary carcinoma to temozolomide. Journal of Neurosurgery. 2006;105(4):621–626. [DOI] [PubMed] [Google Scholar]
- 9. Lim S, Shahinian H, Maya MM, Yong W, Heaney AP. Temozolomide: a novel treatment for pituitary carcinoma. The Lancet Oncology. 2006;7(6):518–520. [DOI] [PubMed] [Google Scholar]
- 10. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 11. Kovacs K, Horvath E, Syro LV et al. Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm: morphological findings. Human Pathology. 2007;38(1):185–189. [DOI] [PubMed] [Google Scholar]
- 12. Neff L, Weil M, Cole A et al. Temozolomide in the treatment of an invasive prolactinoma resistant to dopamine agonists. Pituitary. 2007;10(1):81–86. [DOI] [PubMed] [Google Scholar]
- 13. Byrne S, Karapetis C, Vrodos N. A novel use of temozolomide in a patient with malignant prolactinoma. Journal of Clinical Neuroscience. 2009;16(12):1694–1696. [DOI] [PubMed] [Google Scholar]
- 14. Hagen C, Schroeder HD, Hansen S, Hagen C, Andersen M. Temozolomide treatment of a pituitary carcinoma and two pituitary macroadenomas resistant to conventional therapy. European Journal of Endocrinology. 2009;161(4):631–637. [DOI] [PubMed] [Google Scholar]
- 15. McCormack AI, McDonald KL, Gill AJ et al. Low O6-methylguanine-DNA methyltransferase (MGMT) expression and response to temozolomide in aggressive pituitary tumours. Clinical Endocrinology. 2009;71(2):226–233. [DOI] [PubMed] [Google Scholar]
- 16. Mohammed S, Kovacs K, Mason W, Smyth H, Cusimano MD. Use of temozolomide in aggressive pituitary tumors: case report. Neurosurgery. 2009;64(4):E773–E774. [DOI] [PubMed] [Google Scholar]
- 17. Moyes VJ, Alusi G, Sabin HI et al. Treatment of Nelson's syndrome with temozolomide. European Journal of Endocrinology. 2009;160(1):115–119. [DOI] [PubMed] [Google Scholar]
- 18. Syro LV, Scheithauer BW, Ortiz LD et al. Effect of temozolomide in a patient with recurring oncocytic gonadotrophic pituitary adenoma. Hormones (Athens). 2009;8(4):303–306. [DOI] [PubMed] [Google Scholar]
- 19. Takeshita A, Inoshita N, Taguchi M et al. High incidence of low O6-methylguanine DNA methyltransferase expression in invasive macroadenomas of Cushing's disease. European Journal of Endocrinology. 2009;161(4):553–559. [DOI] [PubMed] [Google Scholar]
- 20. Bode H, Seiz A, Lammert A et al. SOM230 (pasireotide) and temozolomide achieve sustained control of tumour progression and ACTH secretion in pituitary carcinoma with widespread metastases. Exp Clin Endocrinol Diabetes. 2010;118(10):760–763. [DOI] [PubMed] [Google Scholar]
- 21. Bush ZM, Longtine JA, Cunningham T et al. Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcome with O6-methylguanine methyltransferase (MGMT) promoter methylation and expression. The Journal of Clinical Endocrinology and Metabolism. 2010;95(11):E280–E290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curto L, Torre ML, Ferrau F et al. Temozolomide-induced shrinkage of a pituitary carcinoma causing Cushing's disease—report of a case and literature review. ScientificWorldJournal. 2010;10:2132–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Losa M, Mazza E, Terreni MR et al. Salvage therapy with temozolomide in patients with aggressive or metastatic pituitary adenomas: experience in six cases. European Journal of Endocrinology. 2010;163(6):843–851. [DOI] [PubMed] [Google Scholar]
- 24. Raverot G, Sturm N, de Fraipont F et al. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. The Journal of Clinical Endocrinology and Metabolism. 2010;95(10):4592–4599. [DOI] [PubMed] [Google Scholar]
- 25. Dillard T, Gultekin SH, Delashaw J Jr., Yedinak C, Neuwelt E, Fleseriu M. Temozolomide for corticotroph pituitary adenomas refractory to standard therapy. Pituitary. 2011;14(1):80–91. [DOI] [PubMed] [Google Scholar]
- 26. Murakami M, Mizutani A, Asano S et al. A mechanism of acquiring temozolomide resistance during transformation of atypical prolactinoma into prolactin-producing pituitary carcinoma: case report. Neurosurgery. 2011;68(6):E1761–E1767. [DOI] [PubMed] [Google Scholar]
- 27. Moshkin O, Syro LV, Scheithauer BW et al. Aggressive silent corticotroph adenoma progressing to pituitary carcinoma. The Role of Temozolomide Therapy. 2011;10(2):162–167. [DOI] [PubMed] [Google Scholar]
- 28. Thearle MS, Freda PU, Bruce JN, Isaacson SR, Lee Y, Fine RL. Temozolomide (Temodar®) and capecitabine (Xeloda®) treatment of an aggressive corticotroph pituitary tumor. Pituitary. 2011;14(4):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ersen A, Syro LV, Penagos LC et al. Non-uniform response to temozolomide therapy in a pituitary gonadotroph adenoma. Canadian Journal of Neurological Sciences/Journal Canadien des Sciences Neurologiques. 2012;39(5):683–685. [DOI] [PubMed] [Google Scholar]
- 30. Morin E, Berthelet F, Weisnagel J, Bidlingmaier M, Serri O. Failure of temozolomide and conventional doses of pegvisomant to attain biochemical control in a severe case of acromegaly. Pituitary. 2012;15(1):97–100. [DOI] [PubMed] [Google Scholar]
- 31. Morokuma H, Ando T, Hayashida T et al. A case of nonfunctioning pituitary carcinoma that responded to temozolomide treatment. Case Reports in Endocrinology. 2012;2012:645914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phillips J, East HE, French SE et al. What causes a prolactinoma to be aggressive or to become a pituitary carcinoma? Hormones. 2012;11(4):477–482. [DOI] [PubMed] [Google Scholar]
- 33. Whitelaw BC, Dworakowska D, Thomas NW et al. Temozolomide in the management of dopamine agonist–resistant prolactinomas. Clinical Endocrinology. 2012;76(6):877–886. [DOI] [PubMed] [Google Scholar]
- 34. Vieira Neto L, Chimelli L, Pereira PJdM et al. The role of temozolomide in the treatment of a patient with a pure silent pituitary somatotroph carcinoma. Endocrine Practice. 2013;19(6):E145–E149. [DOI] [PubMed] [Google Scholar]
- 35. Zemmoura I, Wierinckx A, Vasiljevic A, Jan M, Trouillas J, François P. Aggressive and malignant prolactin pituitary tumors: pathological diagnosis and patient management. Pituitary. 2013;16(4):515–522. [DOI] [PubMed] [Google Scholar]
- 36. Zacharia BE, Gulati AP, Bruce JN et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery. 2014;74(4):E447–E455. [DOI] [PubMed] [Google Scholar]
- 37. Batisse M1 RG, Maqdasy S, Durando X et al. Aggressive silent GH pituitary tumor resistant to multiple treatments, including temozolomide. Cancer Invest. 2013;31(3):190–196. [DOI] [PubMed] [Google Scholar]
- 38. Hansen T, Batra S, Lim M et al. Invasive adenoma and pituitary carcinoma: a SEER database analysis. Neurosurg Rev. 2014;37(2):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kovacs K, Scheithauer B, Lombardero M et al. MGMT immunoexpression predicts responsiveness of pituitary tumors to temozolomide therapy. Acta Neuropathol. 2008;115(2):261–262. [DOI] [PubMed] [Google Scholar]
- 40. McCormack AI, Wass JAH, Grossman AB. Aggressive pituitary tumours: the role of temozolomide and the assessment of MGMT status. European Journal of Clinical Investigation. 2011;41(10):1133–1148. [DOI] [PubMed] [Google Scholar]
- 41. Zuhur S, Tanik C, Karaman Ö et al. MGMT immunoexpression in growth hormone-secreting pituitary adenomas and its correlation with Ki-67 labeling index and cytokeratin distribution pattern. Endocrine. 2011;40(2):222–227. [DOI] [PubMed] [Google Scholar]
- 42. McCormack A, Kaplan W, Gill A et al. MGMT expression and pituitary tumours: relationship to tumour biology. Pituitary. 2013;16(2):208–219. [DOI] [PubMed] [Google Scholar]
- 43. Bengtsson DSH, Andersen M, Maiter D et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689–1698. [DOI] [PubMed] [Google Scholar]