Abstract
Background
Fecal microbiota transplantation (FMT) is a microbiota-based therapy that shows therapeutic potential in recurrent or refractory Clostridium difficile infections and other intestinal or extra-intestinal disorders. Nonetheless, adverse events (AEs) remain a major challenge in the application of FMT.
Aim
To review the AEs of FMT and to address the concerns of safety during the procedure.
Methods
Publications were retrieved in the databases of Medline, Embase and Cochrane Library. AEs were classified according to their causality with FMT or their severity.
Results
A total of 7562 original articles about FMT were identified in this study, 50 of them fulfilled the inclusion criteria. Totally 78 kinds of AEs were revealed enrolled in these 50 selected publications. The total incidence rate of AEs was 28.5%. Among the 42 publications, 5 kinds were definitely and 38 kinds were probably related to FMT. The commonest FMT-attributable AE was abdominal discomfort, which was reported in 19 publications. For upper gastrointestinal routes of FMT, 43.6% (89/204) patients were compromised by FMT-attributable AE, while the incidence dropped to 17.7% (76/430) for lower gastrointestinal routes. In contrast, the incidences of serious adverse events (SAEs) were 2.0% (4/196) and 6.1% (40/659) for upper and lower gastrointestinal routes, respectively. A total of 44 kinds of SAEs occurred in 9.2% patients, including death (3.5%, 38/1089), infection (2.5%, 27/1089), relapse of inflammatory bowel diseases (0.6%, 7/1089) and Clostridium difficile infection (0.9%, 10/1089).
Conclusion
Consequently, both AEs and SAEs are not rare and should be carefully monitored throughout FMT. However, high quality randomized controlled trials are still needed for the more definite incidence of AEs of FMT.
Introduction
The gut microbiota is one of the most complex systems in human body, which comprises about 1014 microbes, outnumbering human cells by 10-fold [1–3]. The majority of microbes have an extensive influence on human, including digestion, immunity, energy homeostasis, vitamin synthesis, etc. [4–7]. Alteration of the gut microbiota has been associated with both digestive and extra-digestive disorders [8–12]. Novel strategy for treatment of bacteria-associated diseases, via modulating the gut microbiota, is underway to establish its pivotal role.
Fecal microbiota transplantation (FMT), also known as fecal bacteriotherapy or intestinal microbiota transplantation, is defined as the perfusion of treated feces from a healthy donor via the upper or lower gastrointestinal route [13]. About 1700 years ago, Ge Hong, a well-known traditional Chinese medicine doctor, firstly described the use of human fecal suspension by mouth for patients with food poisoning or severe diarrhea [14]. In 1958, Eiseman et al applied FMT to treat antibiotic-associated diarrhea [15]. Since Schwan et al reported the first FMT therapy for CDI in 1983 [16], the application of FMT in CDI has been practiced extensively [17–19]. The effective rate of FMT for recurrent or refractory CDI was over 90% [20, 21]. Although FMT is still regarded as an investigational agent and requires an investigational new drug (IND) application, the US FDA has already recommended FMT as an alternative therapy for recurrence of CDI after a pulsed vancomycin regimen [22]. Moreover, FMT shows remarkable therapeutic potential in diverse conditions [13, 23] including inflammatory bowel diseases (IBD) [24, 25], irritable bowel syndrome (IBS) [26–28], metabolic diseases [4, 29, 30], neuropsychiatric conditions [31], autoimmune diseases [32, 33], allergic disorders [34, 35], and chronic fatigue syndrome [36].
Although patients benefit from FMT, concerns about this emerging strategy remain to be addressed, including long-term outcomes of FMT and the AEs. So far, the exact roles of the gut microbiota in FMT are not yet fully understood. And the AEs that happen during or after FMT still perplex clinicians and fundamental researchers. Hence, we systematically reviewed the AEs of FMT in all related publications aiming to elucidate the causality between FMT and the AEs. Furthermore, the AEs of FMT were divided into different degrees according to the severity and SAEs were emphatically introduced to arouse attention in FMT.
Methods
Information Sources and Search Strategy
Electronic databases for literature search included the Medline, Embase, and Cochrane Library. The last search was run on July 2015. The complete string used for the electronic search is shown in Table 1. All the deriving terms were combined by the Boolean operator “OR” to assure the identification of studies regarding FMT.
Table 1. Complete String Used for the Electronic Search.
| (fecal microbiota transplantation) OR (fecal transplantation) OR (feces transplantation) OR (stool transplantation) OR (microflora transplantation) OR (fecal flora transplantation) OR (fecal transplant) OR (fecal microbiota transplant) OR (feces transplant) OR (stool transplant) OR (microflora transplant) OR (fecal flora transplant) OR (fecal bacteriotherapy) OR (fecal microbiota bacteriotherapy) OR (feces bacteriotherapy) OR (stool bacteriotherapy) OR (microflora bacteriotherapy) OR (fecal flora bacteriotherapy) OR (fecal suspension) OR (fecal microbiota suspension) OR (feces suspension) OR (stool suspension) OR (microflora suspension) OR (fecal flora suspension) OR (fecal donation) OR (fecal microbiota donation) OR (feces donation) OR (stool donation) OR (microflora donation) OR (fecal flora donation) OR (fecal donor) OR (fecal microbiota donor) OR (feces donor) OR (stool donor) OR (microflora donor) OR (fecal flora donor) OR (fecal transfer) OR (fecal microbiota transfer) OR (feces transfer) OR (stool transfer) OR (microflora transfer) OR (fecal flora transfer) OR (fecal infusion) OR (fecal microbiota infusion) OR (feces infusion) OR (stool infusion) OR (microflora infusion) OR (fecal flora infusion) OR (fecal implantation) OR (fecal microbiota implantation) OR (feces implantation) OR (stool implantation) OR (microflora implantation) OR (fecal flora implantation) OR (fecal implant) OR (fecal microbiota implant) OR (feces implant) OR (stool implant) OR (microflora implant) OR (fecal flora implant) OR (fecal instillation) OR (fecal microbiota instillation) OR (feces instillation) OR (stool instillation) OR (microflora instillation) OR (fecal flora instillation) OR (fecal microbiota reconstitution) OR (fecal reconstitution) OR (feces reconstitution) OR (stool reconstitution) OR (microflora reconstitution) OR (fecal flora reconstitution) |
Study selection
Titles, abstracts, and keywords were independently assessed by two investigators (WSN and XMQ) to determine the appropriateness of the publications. Both investigators checked all the articles in accordance with the inclusion criteria and exclusion criteria. Disagreement was resolved by a third investigator (CHL). Original full-text articles, letters to the editor, abstracts of scientific conferences, case reports and case series which were published between 1913 and 2015 were reviewed. Studies involving the AEs of FMT for human sujects of any age were included into this study. Studies evaluating treatments with cultured bacteria other than human feces, animal studies and non-original reports (reviews, systematic reviews, meta-analyses, editorials, etc) were excluded.
Data Collection and List of Items
Data extraction was conducted according to the above mentioned inclusion and exclusion criteria and cross-checked by the two independent investigators (WSN and XMQ). When publications included patients from a previous study and newly enrolled ones, only the latter were brought into the study. Items of this study were listed as follows: (i) the study characteristics (the first author, the year of publication, the length of follow-up); (ii) the patients (the number, the reason for FMT, the prior therapy); (iii) the relationship between donors and recipients; (iv) the FMT procedure (the patient preparation for FMT, the weight of infused stools, the route of infusion, the number of infusion); (v) the detailed descriptions of AEs (the onset time, the causality between AEs and FMT, how the AEs relieve and the outcomes).
Definition of causality between the AEs and FMT
The relationship between the AEs and FMT were categorized into four types as previously dsecribed with minor modifications: definitely related, probably related, possibly related, and unrelated to FMT [37].
Definitely related
AEs caused by endoscopic procedure during FMT; an event that follows a reasonable temporal sequence from FMT exposure; that follows a known or expected response pattern to the FMT; that is confirmed by stopping the FMT procedure; and that is not explained by any other reasonable hypothesis.
Probably related
An event that follows a reasonable temporal sequence from FMT procedure; that follows a known or expected response pattern to the FMT; that is confirmed by stopping the FMT procedure; and that is unlikely to be explained by the known characteristics of the subject’s clinical state or by other interventions.
Possibly related
An event that follows a reasonable temporal sequence from FMT procedure; that follows a known or expected response pattern to FMT; but that could readily have been caused by a number of other factors.
Unrelated
An event that can be determined with certainty to have no relationship to FMT.
Definition of severity of AEs
Adverse events (AEs)
AE is defined as any untoward medical occurrence in a patient after administration of FMT that does not necessarily have a causal relationship with this treatment. Therefore, an AE can be any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with FMT, whether or not related to the FMT [37].
Serious adverse events (SAEs)
A SAE is any adverse experience occurring during or after FMT that results in any of the following outcomes: death, life-threatening experience, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability or incapacity, congenital anomaly or birth defect, or an important medical event [37].
Results
Included studies
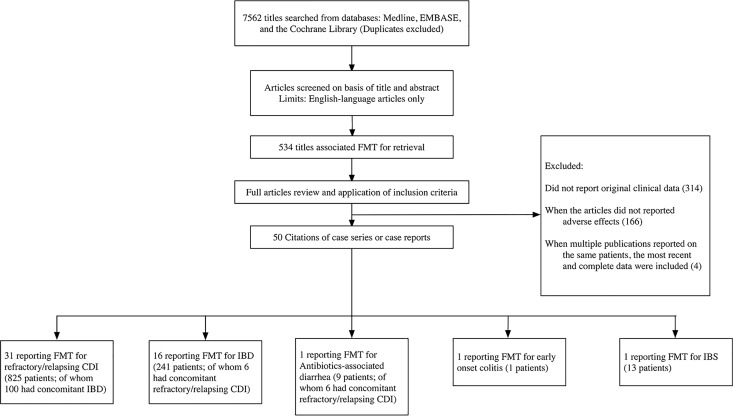
A total of 7562 original articles about FMT were identified. Among them, 534 were selected for further assessment according to the titles. After reviewing the full text articles, 50 of them fulfilled the inclusion criteria among which 16 were case series, 9 were case reports and 4 were randomized controlled trials (Fig 1). The excluded articles were presented in S1 Appendix. Besides, conference abstracts and letters to the editor were included. The included studies were published during the period from 1998 to 2015, with a span of 18 years. The follow-up time after FMT ranged between 2 weeks to 68 months.
Fig 1. Flow chart of studies of adverse events of fecal microbiota transplantation.
FMT: fecal microbiota transplantation, CDI: Clostridium difficile infection, IBD: inflammatory bowel disease, IBS: irritable bowel syndrome.
Patient characteristics
In the selected 50 publications, a total of 1089 patients were treated with FMT (age range: 1–95 years). Among them, 831 patients were affected by refractory or recurrent CDI, of whom 106 had concomitant IBD; 235 were affected by independent IBD; 1 affected by early onset colitis; 9 affected by antibiotic-associated diarrhea (AAD), of whom 6 had concomitant CDI; and 13 affected by IBS. Overall, 78 kinds of AEs were reported to happen on 310 patients during or after FMT. The majority of AEs were presented as mild symptoms such as abdominal disomfort, diarrhea, transient fever, nausea, vomiting and constipation. Each AE was cited once for one patient since the AE always recorded only once during a multiple FMT in one course of treatment.
Since AEs usually overlapped, we could not obtain the exact total number of patients with AEs in a publication. Hence, we took the number of patients with the most frequent kind of AEs from each publication for further calculating the overall incidence of AEs. Based on the above mentioned statistical principles, the overall incidence of AEs was 28.5% (310/1089). The incidences of AEs in CDI and non-CDI (IBD, AAD, IBS and early onset colitis) were 28.0% (233/831) and 29.8% (77/258) respectively.
Causality between AEs and FMT
Many factors could be involved in the development of AEs, including the individual difference of recipients, donors, methods of administration and regimen of FMT. The causality between AEs (including SAEs) and FMT was analyzed according to the description as above [37]. As a result, AEs were described to be attributable to FMT in 42 publications (Tables 2 and 3). Five kinds of AEs were reported to be definitely related to FMT in 5 publications. Thirty-eight kinds of AEs, probably related to FMT, were reported in 35 articles and were considered as results of temporary systemic immune response to the applied bacteria. In addition, 25 kinds of AEs were reported to be possibly related to FMT in 13 articles. Finally, 38 kinds of AEs were reported to be unrelated to FMT in 22 articles (Table 4).
Table 2. Attributable adverse events are grouped by route of administration (Upper gastrointestinal routes; Lower gastrointestinal routes).
| First Author, Year (Ref.) | AEs | The number of patients with AEs | Sample size | Causality between AEs and FMT | Routes of infusion |
|---|---|---|---|---|---|
| Upper gastrointestinal routes | |||||
| Vermeire, 2012 [60] | Fever; Abdominal tenderness | 3 | 4 | Probably | Nasojejunal tube |
| Cui, 2014 [41] | Fever; Increased diarrhea | 7 | 30 | Probably | Gastroscopy (mid-gut) |
| Van Nood, 2013 [61] | Belching; Nausea; Abdominal cramps; Diarrhea; Abdominal pain; Infection; Dizziness combined with diarrhea; Constipation | 27 | 29 | Probably | Nasoduodenal tube |
| Aas, 2003 [62] | Death from peritonitis | 1 | 18 | Possibly | Nasogastric tube |
| MacConnachie, 2009 [42] | Upper gastrointestinal hemorrhage | 1 | 15 | Possibly | Nasogastric tube |
| Kronman, 2015 [63] | Vomiting; Mucoid stools | 1 | 10 | Probably | Nasogastric, nasoduodenal or nasojejunal tube |
| Pinn, 2014 [26] | Flatulence | 1 | 13 | Probably | Esophagogastroduodenoscopy |
| Wang, 2013 [64] | Diarrhea | 5 | 16 | Probably | Gastroscopy |
| Suskind, 2015 [65] | Rhinorrhea; Sore throat | 5 | 9 | Definitely | Nasogastric tube |
| Abdominal pain; Bloating; Diarrhea; Flatulence | Probably | ||||
| Suskind, 2015 [66] | Nasal stuffiness; Flatulence | 1 | 4 | Probably | Nasogastric tube |
| Bloating | Possibly | ||||
| Rossen, 2015 [50] | Discomfort tube placement; Fever; Nausea; Malaise; Increase of stool frequency/diarrhea; Headache; Vomited fecal infusion; Vomited bowel prep; Vomiting; Abdominal cramps; Abdominal pain; Abdominal murmurs; Dizziness; Mild constipation | 34 | 48 | Probably | Nasoduodenal tube |
| Borody, 2003 [67] | Sore throat | 3 | 8 | Definitely | Nasojejunal tube |
| Lower gastrointestinal routes | |||||
| Kump, 2013 [68] | Fever; Temporary increase of CRP and IL-6; Increase in stool frequency | 1 | 6 | Probably | Colonoscopy |
| Zhang, 2013 [69] | Severe cold | 1 | 1 | Possibly | Colonoscopy |
| Quera, 2013 [44] | Fever; Bacteremia | 1 | 1 | Probably | Colonoscopy |
| Kunde, 2013 [70] | Fever; Chills; Abdominal fullness | 2 | 10 | Probably | Enema |
| UC flare | Possibly | ||||
| Gustafsson, 1998 [71] | Diarrhea | 3 | 9 | Probably | Enema |
| Lee, 2014 [72] | Transient constipation; Excess flatulence | 9 | 94 | Probably | Enema |
| Hamilton, 2012 [73] | Irregularity of bowel movements; Excessive flatulence | 14 | 43 | Probably | Colonoscopy |
| Khoruts, 2010 [74] | Constipation; Irregularity of bowel movements | 1 | 1 | Probably | Colonoscopy |
| Pierog, 2014 [75] | Appendicitis | 1 | 6 | Possibly | Colonoscopy |
| Silverman, 2010 [76] | Urinary tract infections | 2 | 7 | Possibly | Enema |
| Hohmann, 2014 [39] | Cytomegalovirus colitis | 1 | 1 | Probably | Enema |
| De Leon, 2013 [40] | Transient relapse of UC | 1 | 1 | Probably | Colonoscopy |
| Schwartz, 2013 [43] | Norovirus Gastroenteritis | 1 | 13 | Probably | Colonoscopy |
| Brandt, 2012 [20] | Peripheral neuropathy; Sjogren’s disease; Idiopathic Thrombocytopenic purpura; Rheumatoid arthritis | 4 | 77 | Possibly | Colonoscopy |
| Mellow, 2011 [77] | Relapse of CDI | 1 | 13 | Possibly | Colonoscopy |
| Mandalia, 2014[78] | Diverticulitis; Fever | 1 | 1 | Probably | Colonoscopy |
| Dutta, 2014 [79] | Fever; Bloating | 5 | 27 | Probably | Enteroscopy and colonoscopy |
| Ray, 2014 [80] | Pain/nausea; Bloating/cramps; Gas/nausea | 4 | 20 | Probably | Colonoscopy |
| Continuing diarrhea | Possibly | ||||
| Satokari, 2015 [53] | Mild transient fever | 2 | 49 | Probably | Colonoscopy |
| Sun, 2014 [45] | Multi-organism bacteremia | 1 | 1 | Probably | Colonoscopy |
| Mandalia, 2014 [81] | Abdominal pain | 1 | 29 | Probably | Colonoscopy |
| Cammarota, 2015 [49] | Diarrhea; Bloating and abdominal cramping | 19 | 20 | Probably | Colonoscopy |
Table 3. Attributable adverse events are grouped by route of administration (Upper and lower gastrointestinal routes; Not mention of the routes; Capsule).
| First Author, Year (Ref.) | AEs | The number of patients with AEs | Sample size | Causality between AEs and FMT | Routes of infusion |
|---|---|---|---|---|---|
| Upper and lower gastrointestinal routes | |||||
| Angelberger, 2013 [52] | Sore throat | 5 | 5 | Definitely | Nasojejunal tube and enema |
| Fever; Temporary increase in CRP; Worsening of diarrhea; Flatulence; Vomiting | Probably | ||||
| Vandelplas, 2014 [38] | Vomiting; Profuse sweating; Paleness; Tachycardia; Fever | 1 | 1 | Probably | Colonoscopy and nasoduodenal tube |
| Russell, 2014 [82] | Mucoid stools; Bloating; Cramping; Loose stools; Abdominal pain; Gassiness; Diarrhea; Blood in stool | 3 | 10 | Probably | Colonoscopy and nasogastric tube |
| Greenberg, 2013 [83] | Transient worsening of abdominal distension | 3 | 16 | Probably | Colonoscopy and nasojejunal infusion |
| Not mention of the routes | |||||
| Kelly, 2014 [84] | Death from aspiration; Minor mucosal tear | 12 | 80 | Definitely | NR |
| Fever; Bloating and abdominal discomfort; Abdominal pain | Probably | ||||
| IBD flare; Self-limited diarrheal illness; Hip pain; Pertussis; Nausea; Death from pneumonia; Diarrhea, encephalopathy and pancytopenia; Colectomy | Possibly | ||||
| Brandt, 2013 [85] | Transient abdominal distension with bloating | 2 | 12 | Probably | NR |
| Wilson, 2014 [86] | Diarrhea or nullloose stoolnull; Bloating; Flatus; Constipation; Abdominal pain; GERD (gastroesophageal reflux disease) | 12 | 45 | Probably | NR |
| Obi, 2014 [87] | Bowel perforation | 1 | 20 | Definitely | NR |
| Borody, 2003 [67] | Flatulence; Rectal discomfort; Nausea; Abdominal cramping; Bloating; Headache; Abdominal pain | 7 | 24 | Probably | Combination of colonoscopy and/ or rectal enema and/or nasojejunal tube |
| Capsule | |||||
| Youngster, 2014 [59] | Abdominal cramping and bloating | 6 | 20 | Possibly | Capsule |
Table 4. Adverse events are grouped by their causality with fecal microbiota transplantation.
| First Author, Year (Ref.) | Definitely related to FMT | Probably related to FMT | Possibly related to FMT | Unrelated to FMT |
|---|---|---|---|---|
| Vermeire, 2012 [60] | Fever; Abdominal tenderness; Transient increase of CRP | |||
| Kump, 2013 [68] | Fever; Temporary increase of CRP and IL-6; Increase in stool frequency | |||
| Angelberger, 2013 [52] | Sore throat | Fever; Temporary increase in CRP; Worsening of diarrhea; Flatulence; Vomiting | Itchiness; Erythema; Paresthesia of the hip; Blisters on the tongue | Common cold; Unexplained pancreatitis; Collapse due to orthostatic disorder |
| Zhang, 2013 [69] | Severe cold | |||
| Cui, 2014 [41] | Fever; Increased diarrhea | |||
| Quera, 2013 [44] | Fever; Bacteremia | |||
| Kunde, 2013 [70] | Fever; Chills; Abdominal fullness; Lower back pain; Nausea; Vomiting | Headache, UC flare | Cervical lymphadenopathy | |
| Vandelplas, 2014 [38] | Vomiting; Profuse sweating; Paleness; Tachycardia; Fever | |||
| Russell, 2014 [82] | Mucoid stools; Bloating; Cramping, Loose stools; Abdominal pain; Gassiness; Diarrhea; Blood in stool | |||
| Van Nood, 2013 [61] | Belching; Nausea; Abdominal cramps; Diarrhea; Abdominal pain; Infection; Dizziness combined with diarrhea; Constipation | Symptomatic choledocholithiasis | ||
| Gustafsson, 1998 [71] | Diarrhea | |||
| Lee CH, 2014 [72] | Transient constipation; Excess flatulence | |||
| Hamilton, 2012 [73] | Irregularity of bowel movements; Excessive flatulence | |||
| Khoruts, 2010 [74] | Constipation; Irregularity of bowel movements | |||
| Kelly, 2014 [84] | Death from aspiration; Minor mucosal tear | Fever; Bloating; Abdominal discomfort;Abdominal pain post FMT colonoscopy | IBD flare; Self-limited diarrheal illness; Hip pain; Pertussis; Nausea; Death from pneumonia; Diarrhea; Encephalopathy and pancytopenia; Colectomy | Cerebrovascular accident, nausea and vomiting; Fall and sustained hip fracture; Influenza and diarrhea (non-CDI); Catheter infection |
| Pierog, 2014 [75] | Appendicitis | |||
| Youngster, 2014 [59] | Infectious irritable bowel symptoms | Relapse of severe CDI | ||
| Silverman, 2010 [76] | Urinary tract infections; | |||
| Hohmann, 2014 [39] | Irregularity of bowel movements; Weakness; Fatigue; Decreased appetite; Abdominal pain; Night sweats; Fever; Cytomegalovirus colitis | Minor joint pains; Weight loss | ||
| De Leon, 2013 [40] | Transient relapse of UC | |||
| Schwartz, 2013 [43] | Norovirus Gastroenteritis | |||
| Brandt, 2012 [20] | Peripheral neuropathy; Sjogren ‘ s disease; Idiopathic Thrombocytopenic purpura; Rheumatoid arthritis | Death for unknown causes, metastatic colon cancer, metastatic ovarian cancer, pneumonia, myocardial infarction, cerebral vascular accident and sepsis | ||
| Mellow, 2011 [77] | CDI relapse | Death from pneumonia; Death from superior mesenteric vein thrombosis; Death from ovarian cancer | ||
| Aas, 2003 [62] | Death from peritonitis | Death from pneumonia | ||
| Mandalia, 2014 [78] | Diverticulitis; Fever | |||
| MacConnachie, 2009 [42] | Upper gastrointestinal hemorrhage | |||
| Kronman, 2015 [63] | Vomiting; Mucoid stools | |||
| Dutta, 2014 [79] | Low-grade fever; Bloating | |||
| Friedman-Moraco, 2014 [88] | Cerebral vascular event; Bronchiolitis obliterans | |||
| Ray, 2014 [80] | Pain/nausea; Bloating/cramps; Vomiting; Abdominal pain | Continuing diarrhea | Cerebrovascular accident | |
| Pinn, 2014 [26] | A transient increase in flatus | |||
| Mattila, 2012 [19] | Died of unrelated illnesses | |||
| Satokari, 2015 [53] | Mild transient fever | |||
| Trubiano, 2013 [89] | Renal failure, episodes of VAP (ventilator-associated pneumonia) and death | |||
| Garborg, 2010 [90] | Died of serious co-morbid conditions | |||
| Borody, 2003 [67] | Sore throat | Flatulence, rectal discomfort, nausea, abdominal cramping, bloating, headache, abdominal pain | ||
| Sun, 2014 [45] | Multi-organism bacteremia | |||
| Mandalia, 2014 [81] | Abdominal pain | |||
| Greenberg, 2013 [83] | Transient worsening of abdominal distension | |||
| Brandt, 2013 [85] | Transient abdominal distension with bloating | |||
| Fischer, 2014 [91] | Multi-organ failure | |||
| Wilson, 2014 [86] | Diarrhea; Bloating; Flatus; Constipation; Abdominal pain; GERD (gastroesophageal reflux disease) | Infections; recurrent CDI; Death of lung cancer | ||
| Wang, 2013 [64] | Diarrhea | |||
| Fischer, 2014 [92] | Refractory CD, refractory CDI, UC flare, non-infectious severe diarrhea, recurrent CDI and worsening CD | |||
| Obi, 2014 [87] | Bowel perforation | Diarrhea | ||
| Suskind, 2015 [65] | Rhinorrhea, sore throat | Abdominal pain, bloating, diarrhea, flatulence | Mild stuffy nose, | |
| Suskind, 2015 [66] | Nasal stuffiness, flatulence | Bloating | Vomiting, developed C difficile diarrhea | |
| Cammarota, 2015 [49] | Diarrhea, bloating and abdominal cramping | |||
| Rossen, 2015 [50] | Transient borborygmia, increase of stool frequency, vomiting, transient fever, | Suspicion of a small bowel perforation, cytomegalovirus infection, abdominal pain, cervix carcinoma | ||
| Moayyedi, 2015 [56] | Crohn’s colitis, active ulcerative colitis, Clostridium difficile toxin positive |
Donors and AEs
So far, there is no unified standard to screen the stool samples from donors. The following donor screening tests were applied to the donors in the selected 50 publications: viral screenings (hepatitis A virus, hepatitis B virus, hepatitis C virus, Epstein–Barr virus, human immunodeficiency virus, treponema pallidum, and cytomegalovirus), stool tests for Clostridium difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), parasites and ova. However, the donors who were in the latent period of infection could not be excluded by the above screening tests and thus these donors might contribute to the development of infectious AEs.
In addition, individual differences of donors may also lead to AEs. For example, one patient (a 1-year-old girl) developed fever, vomiting and tachycardia after receiving the fecal transplant from her brother, while she well tolerated the transplant from her niece [38]. For the infrequent infection of cytomegalovirus in FMT receipt, it may be caused by the donors with young age [39]. Finally unrecognized pathogens that were carried by donors might induce AEs [40].
Related donors (family members) for FMT were reported in 11 publications. Unrelated donors were reported in 9 publications.
Preparation and route of administration and AEs
For FMT via upper gastrointestinal routes, the recipients were prepared in fasting condition. For FMT via lower gastrointestinal routes, bowel lavage and/or antibiotics were given to the recipients before FMT. However, no association of AEs with the preparation of FMT was found in the 50 publications. Of note, in Bota Cui’s report, the recipients who took metoclopramide before FMT manifested fewer AEs, suggesting metoclopramide might potentially help avoid the AEs [41].
The routes of administration are listed as follows according to the frequency that they were used: colonoscopy (26 publications), retention enema (8 publications), nasogastric tube (6 publications), nasojejunal tube (5 publications), gastroscopy (2 publications), sigmoidoscopy (1 publication), nasoduodenal tube (4 publications), enteroscopy (1 publication), esophagogastroduodenoscopy (1 publication) and capsule (1 publication). Among the above routes of administration, lower gastrointestinal routes include colonoscopy, sigmoidoscopy and retention enema. Upper gastrointestinal routes include the remaining means. Compared with upper gastrointestinal routes, lower gastrointestinal routes were more widely used. After exclusion of the publications in which the routes of administration were not clearly stated, the proportion of patients affected by FMT-attributable AE is 43.6% (89/204) for upper gastrointestinal routes of FMT administration, while the incidence dropped to 17.7% (76/430) for lower gastrointestinal routes. The FMT-attributable AEs were grouped by routes of administration (Tables 2 and 3).
Among the 78 kinds of AEs, 5 kinds were definitely related to endoscopic manipulation. Of these, nasal stuffiness, sore throat, rhinorrhea and upper gastrointestinal hemorrhage happened on a total of 8 patients in 4 publications, which were attributable to upper gastrointestinal routes administration. It seems that patients are likely to be injured by invasive endoscope procedures for upper gastrointestinal routes of FMT administration.
The commonest attributable AE was abdominal discomfort for both upper and lower gastrointestinal routes, including abdominal pain, increased stool frequency, flatulence, bloating, cramps and other nonspecific symptoms. For upper gastrointestinal routes of administration in 12 publications, 29.9% (61/204) patients (in 9 publications) were reported to suffer abdominal discomfort after FMT. For lower gastrointestinal routes in 22 publications, 13.0% (56/430) patients (in 10 publications) developed abdominal discomfort after FMT. The upper gastrointestinal routes were therefore more likely to develop abdominal discomfort compared with lower gastrointestinal. The second commonest attributable AE was transient fever which was happened on 3.4% (7/204) and 2.8% (12/430) patients for upper and lower gastrointestinal routes of FMT administration, respectively (Table 2).
Classification of AEs based on severity
Mild to moderate AEs such as abdominal pain, abdominal cramping, flatulence, increased stool frequency, constipation, vomiting, belching, fever and transient increase of C-reactive protein (CRP) were reported in most of the selected 50 publications and ususally did not cause critical clinical outcome. Hence, we paid emphatic attention to SAEs and listed 44 kinds of SAEs that were reported in 27 publications (Table 5), of which 18 kinds were associated with FMT procedure. Totally 9.2% (100/1089) patients developed SAEs. The incidences of SAEs were 2.0% (4/196) and 6.1% (40/659) for upper and lower gastrointestinal routes respectively, which suggest that lower gastrointestinal routes of FMT administration induce more SAEs compared with upper routes.
Table 5. Serious adverse events (SAEs) of fecal microbiota transplantation.
| First Author, Year (Ref.) | The total number of patients | Patient Preparation to FMT | Infused Stools | Route of Infusion | Donor Relation-ship | Number of Infusion | SAE | Causality between AEs and FMT | Day post-FMT event occurred | How to relieve the AE | Follow-Up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Leon, 2013 [40] | 1 UC/CDI | Antibiotics | 600ml infusion | Colonoscopy | Related | 1 | Transient relapse of UC | Probably | 9 days | Prednisone, mesalamine | 2 weeks | |
| Hohmann, 2014 [39] | 1 UC | NR | NR | Home FMT | Related | 4 | Cytomegalovirus colitis | Probably | Several weeks | Anti- cytomegalovirus therapy | NR | |
| Van Nood, 2013 [61] | 16CDI | Bowel lavage | 500ml infusion | Nasoduodenal tube | Unrelated | 1 or 2 | Symptomatic choledocholithiasis | Unrelated | During follow-up | Stone extraction | 15 weeks | |
| Schwartz, 2013 [43] | 13 CDI | Antibiotics, Bowel preparation | NR | Colonoscopy | Related | NR | Norovirus Gastroenteritis; | Probably | 2 days | Self-limited | NR | |
| Norovirus Gastroenteritis; Relapse of CDI | Unrelated | 12 days | ||||||||||
| Brandt, 2012 [20] | 77 CDI | Antibiotics, Bowel preparation | 300–700ml infusion | Colonoscopy | Related/Unrelated | 1 or 2 | Peripheral neuropathy | Possibly | NR | NR | 3–68 months | |
| Sjogren ‘ s disease | ||||||||||||
| Idiopathic thrombocytopenic purpura | ||||||||||||
| Rheumatoid arthritis | ||||||||||||
| Died of unrelated diseases | ||||||||||||
| Mellow, 2011 [77] | 13 CDI | NR | 300–600 ml infusion | Colonoscopy | NR | 1 | Death | B strep pneumonia | Unrelated | 1 month | Died | 1–10 months |
| Superior mesenteric vein thrombosis | Unrelated | 5 months | ||||||||||
| Ovarian cancer | Unrelated | 7 months | ||||||||||
| Relapse of CDI | Unrelated | 7 months | Relapse of CDI | |||||||||
| Youngster, 2014 [59] | 20 CDI | NR | 650μl*15 | Capsule | Unrelated | 1 or 2 | Relapse of severe CDI | Unrelated | NR | Receiving the remaining 15 capsules | 8 weeks | |
| Aas, 2003 [62] | 18 recurrent Clostridium difficile Colitis | NR | ≤30 g | Nasogastric tube | NR | 1 | Death | Peritonitis | Possibly | 3 days | Died | 90 days |
| Pneumonia | Unrelated | 14 days | ||||||||||
| Kunde, 2013 [70] | 10 UC | NR | 70–113 g | Enema | Related and unrelated | 5 | UC flare | Possibly | Third week | Corticosteroid enema | 1 month | |
| Kelly, 2014 [84] | 80 CDI in Immunoco-mpromised Patients | NR | NR | Colonoscopy or others | NR | 1 or more | Death | Pneumonia | Possibly | 13 days | Died | 3–46 months |
| Aspiration | Definitely | 1 day | ||||||||||
| Hospitalizations | Fever, diarrhea, encephalopathy and pancytopenia | Possibly | 4 days | NR | ||||||||
| Abdominal pain post FMT colonoscopy | Probably | 0 day | Self-limited | |||||||||
| IBD flare | Possibly | < 84 days | NR | |||||||||
| Cerebrovascular accident; nausea and vomiting | Unrelated | 21 days | ||||||||||
| Colectomy | Possibly | < 28 days | ||||||||||
| Fall and sustained hip fracture | Unrelated | 84 days | ||||||||||
| Influenza B and diarrhea (non-CDI) | Unrelated | 3 days | ||||||||||
| Catheter infection | Unrelated | 14 days | ||||||||||
| Mandalia, 2014 [78] | 1 CDI/CD | NR | 100g | Colonoscopy | NR | 1 | Diverticulitis, fever | Probably | 2–3 hours | Antibiotics | 3 months | |
| Quera, 2013 [44] | 1 CD/CDI | NR | NR | Colonoscopy | NR | 1 | Bacteriemia | Probably | 24 hours | Aztreonam | 5 months | |
| Pierog, 2014 [75] | 6 CDI | Bowel lavage | 250–500 mL infusion | Colonoscopy | Related | 1 | Appendicitis | Possibly | 2 weeks | Appendectomy | 12 weeks | |
| Silverman, 2010 [76] | 7 CDI | Stop anti-CDI antimicrobial | 50 mL infusion | Enema | Related | 1 or 2 | Post infectious irritable bowel symptoms | Unrelated | NR | Cotrimoxazole | 14 months | |
| Urinary tract infections | Ampicillin/ gentamicin and ciprofloxacin | |||||||||||
| Friedman-Moraco, 2014 [88] | 2 CDI | NR | 80mL | Nasojejunal tube | Related | 2 | Cerebral vascular event | Unrelated | NR | NR | 1 year | |
| 250mL | Colonoscopy | |||||||||||
| 325mL | Colonoscopy | Unrelated | 2 | Bronchiolitis obliterans and death | 5 days | NR | ||||||
| 100mL | Nasojejunal tube | |||||||||||
| Ray, 2014 [80] | 20 CDI | Stop all antibiotics | NR | Colonoscopy | Related and unrelated | 1 | Cerebrovascular accident | Unrelated | > 1 month | NR | 3 months | |
| Mattila, 2012 [19] | 70 CDI | Antibiotics were stopped Colonic lavage | 100mL suspension | Colonoscopy | Related and unrelated | 1 or more | Died of unrelated illnesses | Unrelated | Within 1 year | NR | 1 year | |
| No response and death | Unrelated | Within 3 months | ||||||||||
| Trubiano, 2013 [89] | 1 CDI | NR | 30mL suspension | Nasogastric tube | Related | 1 | Renal failure, episodes of ventilator-associated pneumonia and death | Unrelated | NR | Continuous renal replacement therapy | NR | |
| Garborg, 2010 [90] | 40 recurrent Clostridiumdifficile-associated diarrhea | Fast | 50–100 g | Gastroscopy or colonoscopy | Related and unrelated | 1 or 2 | Wegener ‘ s granulomatosis, acute myelogenous leukaemia, advanced cardiovascular disease developed fulminant colitis and underwent subtotal colectomy | Unrelated | 3 weeks– 2 months | Died | 2 months | |
| Sun, 2014 [45] | 1 CDI | NR | NR | Colonoscopy | NR | NR | Multi-organism bacteremia | Probably | NR | Ampicillin/sulbactam; vancomycin | NR | |
| Fischer, 2014 [91] | 17 CDI | Bowel preparation | NR | Colonoscopy | Related and unrelated | 1, 2 or 3 | Multi-organ failure and death | Immunosuppression | Unrelated | NR | NR | NR |
| Septic shock | ||||||||||||
| Wilson, 2014 [86] | 45 CDI | NR | NR | NR | NR | NR | Infections; Recurrent CDI; Death of lung cancer | Unrelated | NR | NR | 6 months | |
| Fischer, 2014 [92] | 41 CDI/IBD (21 CD, 19UC, 1 indeterminate colitis) | NR | NR | Colonoscopy or Sigmoidoscopy | NR | 1 or 2 | Refractory CD, refractory CDI, UC flare, non-infectious severe diarrhea, recurrent CDI and worsening CD | Unrelated | NR | NR | NR | |
| Obi, 2014 [87] | 20 CDI | NR | NR | NR | NR | NR | Bowel perforation | Definitely | NR | Colectomy | 4 months | |
| Suskind, 2015 [66] | 4 UC | Omeprazole, rifaximin, MiraLAX and bowel preparation | Infusion of 30 mL | Nasogastric tube | NR | 1 | Developed C difficile diarrhea | Unrelated | 3 months | Vancomycin | 6 months | |
| 4 months | NR | |||||||||||
| Rossen, 2015 [50] | 50 UC | Bowel lavage | 120 g | Nasoduodenal tube | Unrelated | 2 | Suspicion of a small bowel perforation | Unrelated | 5 weeks | Antibiotics | 12 weeks | |
| Cytomegalovirus infection | 7 weeks | Ganciclovir | ||||||||||
| Abdominal pain | 11 weeks | Spontaneous recovery | ||||||||||
| Cervix carcinoma | 6 weeks | Operation | ||||||||||
| Moayyedi, 2015 [56] | 75 UC | NR | 50 mL | Retention enema. | Unrelated | 6 | Three patients had their diagnoses changed to Crohn’s colitis from ulcerative colitis. | Unrelated | NR | NR | 12 months | |
| Active ulcerative colitis | Three weeks | Urgent colectomy | ||||||||||
| Clostridium difficile toxin positive | After study exit | NR | ||||||||||
The FMT-attributable (definitely, probably and possibly related) SAEs included death, pathogen infections, IBD flare, auto-immune diseases, and FMT procedure related injury, etc, while the FMT unrelated SAEs covered death or hospitalization caused by underlying conditions. The commonest SAEs were death, severe infections and relapse of CDI and IBD.
As the most devastating SAEs, death happened on 38 patients in 10 publications (Table 6) and the mortality rate was 3.5% (38/1089). Of these deaths, 1 was definitely related, 2 were possibly related, and 35 were unrelated to FMT. The death that was definitely related to FMT was caused by aspiration during sedation of colonoscopy [42]. The other two deaths were associated with infections which might be outcomes of either FMT procedures or underlying immunocompromised status. Except for the above 3 patients, no evidence supported the notion that the remaining deaths could have been caused or facilitated by preparation, route of infusion, donor, number of infusion or the FMT procedure.
Table 6. Summary of death after fecal microbiota transplantation.
| First Author, Year (Ref.) | The total number of patients | Patient Preparation to FMT | Infused Stools | Route of Infusion | Donor Relationship | Number of Infusion | Cause of death | Causality between AEs and FMT | Day post-FMT event occurred | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Garborg, 2010 [90] | 40 recurrent Clostridiumdifficile-associated diarrhea | Fast | 50–100 g | Gastroscopy or colonoscopy | Related and unrelated | 1 or 2 | Wegener ‘ s granulomatosis, acute myelogenous leukaemia, advanced cardiovascular disease developed fulminant colitis and underwent subtotal colectomy | Unrelated | 3 weeks– 2 months | 2 months |
| Mellow, 2011 [77] | 13 CDI | NR | 300–600 ml infusion | Colonoscopy | NR | 1 | B strep pneumonia | Unrelated | 1 month | 1–10 months |
| Superior mesenteric vein thrombosis | Unrelated | 5 months | ||||||||
| Ovarian cancer | Unrelated | 7 months | ||||||||
| Aas, 2003 [62] | 18 recurrent Clostridium difficile Colitis | NR | ≤30 g | Nasogastric tube | N46R | 1 | Peritonitis | Possibly | 3 days | 90 days |
| Pneumonia | Unrelated | 14 days | ||||||||
| Kelly, 2014 [84] | 80 CDI in Immunocom-promised Patients | NR | NR | Colonoscopy or others | NR | 1 or more | Pneumonia | Possibly | 13 days | 3–46 months |
| Aspiration | Definitely | 1 day | ||||||||
| Friedman-Moraco, 2014 [88] | 2 CDI | NR | 325mL | Colonoscopy | Unrelated | 2 | Bronchiolitis obliterans | Unrelated | 5 days | NR |
| 100mL | Nasojejunal tube | |||||||||
| Mattila, 2012 [19] | 70 CDI | Antibiotics were stopped, colonic lavage | 100mL suspension | Colonoscopy | Related and unrelated | 1 or more | Unrelated illnesses | Unrelated | Within 1 year | 1 year |
| No response and died | Unrelated | Within 3 months | ||||||||
| Brandt, 2012 [20] | 77 CDI | Antibiotics, bowel preparation | 300–700ml infusion | Colonoscopy | Related/Unrelated | 1 or 2 | Death for unknown causes, metastatic colon cancer, metastatic ovarian cancer, pneumonia, myocardial infarction, cerebral vascular accident and sepsis | Unrelated | NR | 3–68 months |
| Trubiano, 2013 [89] | 1 CDI | NR | 30 mL | Nasojejunal tube | Related | 1 | Renal failure, episodes of ventilator-associated pneumonia | Unrelated | NR | NR |
| Fischer, 2014 [92] | 17 CDI | Bowel preparation | NR | Colonoscopy | Related and unrelated | 1 or 2 or 3 | Immunosuppression | Unrelated | NR | NR |
| Septic shock | ||||||||||
| Wilson, 2014 [86] | 45 CDI | NR | NR | NR | NR | NR | Lung cancer | Unrelated | NR | 6 months |
Twenty-seven patients were reported to be hospitalized or die for infection in 12 publications (CDI was not included) (Table 7). The incidence of severe infection was 2.5% (27/1089). Among the 27 cases of severe infection, 8 cases were probably or possibly related to FMT and the remaining 19 cases were unrelated to FMT.Out of the 8 cases of severe infection, 2 were viral infection, 2 were bacteriemia infection, and the remaining 4 were infection of unknown pathogens. The pathogens that caused the 2 cases of viral infection were cytomegalovirus [39] and norovirus [43] respectively and the pathogens that caused the 2 cases of bacteriemia infection were Escherichia coli, Proteus mirabilis, Citrobacter koseri, and Enterococcus faecium [44, 45]. The cytomegalovirus infection happened after home FMT and was suspected to be probably related to a child donor without strict donor screening. The noroviurs infection was speculated to be probably related to environmental contamination by an endoscopy suite employee. IBD flare happened on 7 patients (4 UC and 3 CD) post-FMT in the 50 selected publications (Table 8) and its incidence reached 0.6% (7/1089). Most patients who suffered from IBD flare were those with low immunity, such as kids, aged people and immunocompromised ones. Of note, patients with IBD flare were administered FMT via lower gastrointestinal routes, such as colonoscopy, sigmoidoscopy or enema. Therefore, IBD flare should arouse attention when FMT was administered via lower gastrointestinal routes. So far, the association between donors and IBD flare has not been defined, for some unrecognized pathogens from donors’ stool might also lead to IBD flare.
Table 7. Summary of infections after fecal microbiota transplantation.
| First Author, Year (Ref.) | The total number of patients | Patient Preparation to FMT | Infused Stools | Route of Infusion | Donor Relationship | Number of Infusion | SAE (infections) | Causality between AEs and FMT | Day post-FMT event occurred | How to relieve the AE | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hohmann, 2014 [39] | 1 UC | NR | NR | Home FMT | Related | 4 | Cytomegalovirus colitis | Probably | Several weeks | Anti- cytomegalovirus therapy | NR |
| Schwartz, 2013 [43] | 13 CDI | Antibiotics, Bowel preparation | NR | Colonoscopy | Related | NR | Norovirus Gastroenteritis | Probably | 2 days | Self-limited | NR |
| Norovirus Gastroenteritis | Unrelated | 12 days | |||||||||
| Mellow, 2011 [77] | 13 CDI | NR | 300–600 ml infusion | Colonoscopy | NR | 1 | B strep pneumonia | Unrelated | 1 month | Died | 1–10 months |
| Aas, 2003 [62] | 18 recurrent Clostridium difficile Colitis | NR | ≤30 g | Nasogastric tube | NR | 1 | Peritonitis | Possibly | 3 days | Died | 90 days |
| Pneumonia | Unrelated | 14 days | |||||||||
| Kelly, 2014 [84] | 80 CDI in Immunocom-promised Patients | NR | NR | Colonoscopy or others | NR | 1 or more | Died of pneumonia | Possibly | 13 days | Died | 3–46 months |
| Influenza B and diarrhea (non-CDI) | Unrelated | 3 days | NR | ||||||||
| Catheter infection | Unrelated | 14 days | |||||||||
| Mandalia, 2014 [78] | 1 CDI/CD | NR | 100g | Colonoscopy | NR | 1 | Diverticulitis, fever | Probably | 2–3 hours | Antibiotics | 3 months |
| Quera, 2013 [44] | 1 CD/CDI | NR | NR | Colonoscopy | NR | 1 | Bacteriemia | Probably | 24 hours | Aztreonam | 5 months |
| Pierog, 2014 [75] | 6 CDI | Bowel lavage | 250–500 mL infusion | Colonoscopy | Related | 1 | Appendicitis | Possibly | 2 weeks | Appendectomy | 12 weeks |
| Silverman, 2010 [76] | 7 CDI | Stop anti-CDI antimicrobial | 50 mL infusion | Enema | Related | 1 or 2 | Post infectious irritable bowel symptoms | Unrelated | NR | Cotrimoxazole | 14 months |
| Urinary tract infections | Ampicillin/ gentamicin and ciprofloxacin | ||||||||||
| Sun, 2014 [45] | 1 CDI | NR | NR | Colonoscopy | NR | NR | Multi-organism bacteremia | Probably | NR | Ampicillin/sulbactam; vancomycin | NR |
| Wilson, 2014 [86] | 45 CDI | NR | NR | NR | NR | NR | HCV seroconversion, urinary tract infection, viral upper respiratory infection, foot infection, eye infection, and shingles | Unrelated | NR | NR | 6 months |
| Rossen, 2015 [50] | 50 UC | Bowel lavage | 120 g | Nasoduodenal tube | Unrelated | 2 | Cytomegalovirus infection | Unrelated | 7 weeks | Ganciclovir | 12 weeks |
Table 8. Summary of relapse of inflammatory bowel disease or Clostridium difficile infection after fecal microbiota transplantation.
| SAE | First Author, Year (Ref.) | The total number of patients | Patient Preparation to FMT | Infused Stools | Route of Infusion | Donor Relationship | Number of Infusion | Causality between SAEs and FMT | Day post-FMT event occurred | How to relieve the AE | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Relapse of CDI | Schwartz, 2013 [43] | 13 CDI | Antibiotics, Bowel preparation | NR | Colonoscopy | Related | NR | Unrelated | 12 days | NR | NR |
| Mellow, 2011 [77] | 13 CDI | NR | 300–600 ml infusion | Colonoscopy | NR | 1 | Unrelated | 7 months | NR | 1–10 months | |
| Youngster, 2014 [59] | 20 CDI | NR | 650μl*15 | Capsule | Unrelated | 1 or 2 | Unrelated | NR | Receiving the remaining 15 capsules | 8 weeks | |
| Wilson, 2014 [86] | 45 CDI | NR | NR | NR | NR | NR | Unrelated | NR | NR | 6 months | |
| Suskind, 2015 [66] | 4 UC | Omeprazole, rifaximin, MiraLAX and bowel preparation | Infusion of 30 mL | Nasogastric tube | NR | 1 | Unrelated | 3 months | Vancomycin | 6 months | |
| 4 months | NR | ||||||||||
| Relapse of IBD | De Leon, 2013 [40] | 1 UC/CDI | Antibiotics | 600ml infusion | Colonoscopy | Related | 1 | Probably | 9 days | Prednisone, mesalamine | 2 weeks |
| Kunde, 2013 [70] | 10 UC | NR | 70–113 g | Enema | Related and unrelated | 5 | Possibly | Third week | Corticosteroid enema | 1 month | |
| Kelly, 2014 [84] | 80 CDI in Immunocomprom-ised Patients | NR | NR | Colonoscopy or others | NR | 1 or more | Possibly | < 84 days | NR | 3–46 months | |
| Relapse of UC, relapse of CDI | Fischer, 2014 [92] | 41 CDI/IBD (21 CD, 19UC, 1 indeterminate colitis) | NR | NR | Colonoscopy or Sigmoidoscopy | NR | 1 or 2 | Unrelated | NR | NR | NR |
Although unrelated to FMT, CDI relapse was still another unignorable SAE, the incidence of which reached 0.9% (10/1089) in the selected 50 publications (Table 7).
Discussion
The human gastrointestinal tract harbors the largest number of microbes in the human body, which is referred to as the gut microbiota. Perturbations in the gut microbiota have been associated with conditions as diverse as gastrointestinal diseases and even systemic disorders [46]. As a microbiota-targeted therapy, FMT shows promise in controlling bacteria-associated disorders, especially recurrent or refractory CDI. Nevertheless, with the growing application of FMT, safety evaluation for FMT is increasingly urgent and potential risks of FMT must be paid attention to. Previous studies focus on the effectiveness of FMT treatment on CDI [47], IBD [25] and other digestive and nondigestive disorders [23, 48], lacking of emphasizing AEs of FMT. Landy et al [48] reviewed publications about FMT therapy for gastrointestinal diseases that were published before 2011 and did not find any reports regarding FMT related AEs. A more recent systematic review about FMT for IBD treatment (2012) summarized AEs that were reported in just three citations [25]. Lately, two randomized controlled trials of FMT for CDI [49] and UC [50] reported a high incidence of AEs, suggesting that under-reporting AEs of FMT may exist in many other cases. Therefore, there is an urgent need to systematically review and analyze the characteristics of AEs of FMT to evaluate the safety of the procedure.
Here, we selected 50 publications in which AEs of FMT were reported. Totally 78 kinds of AEs happened on 310 patients following FMT treatment. The AEs of FMT were divided into two major categories, namely related and unrelated to FMT. Our analytical results showed that the AEs related to FMT (including definitely, probably and possibly related) accounted for a larger proportion than the AEs unrelated to FMT. Moreover, we found that SAEs related to FMT such as death, viral and bacterial infections, transient relapse of IBD, were not rare and therefore deserved attention and consideration in the procedure of FMT.
Human Microbiome Project (HMP) has sampled the microbiome of many people to get a better idea of variability, and how microbes work together in complex communities. HMP implied that because the microbiome is more varied than the genome, and easier to modify, it gives a more logical starting point for individual treatments [51]. As observed by Angelberger et al, most AEs may be caused by the applied bacteria into the gut [52], which, in our opinion, could be further supported by the notion that most patients receiving FMT were under the conditions of impaired intestinal mucosal barrier and severe inflammation. In a recent observational cohort study of FMT for treating recurrent CDI, mild transient fever happened on two patients receiving FMT [53]. The authors speculated that FMT itself rather than glycerol used in the frozen preparations caused the AE, which was in agreement with Angelberger’s opinion. Though all donors underwent blood and stool tests before FMT as recommended by FDA, some unrecognized infective agents might cause AEs of FMT. Since variability in donor microbiotas exists, it is necessary to establish a better donor screening methodologies. Moreover, the inclusion criteria of FMT donors for recurrent or refractory CDI have been established, but an agreement of the inclusion criteria of donors for IBD, IBS, metabolic diseases, and other extra-CDI have not been reached, which might engender potential risks for AEs of FMT [54, 55]. A recent placebo-controlled randomized trial demonstrated that FMT with the donation of two donors were more effective but with milder AEs than the other donors. Sequencing analysis of the microbiota was conducted for the two donors and they turned out to have similar taxonomic profiles [56]. Previous study also demonstrated that genetic variation in immune genes could result in variability in susceptibility to enteric infection in germfree mice [57]. Thus, genetic variation may paly a key role in variability in microbiota composition, susceptibility to enteric infection, response to FMT treatments, and even AEs. The emerging metagenomics, genetic and microbiota screening methodologies could be useful for identifying better donor sources for FMT therapies in the future [58].
Route of fecal infusion is another concern in FMT that may lead to AEs. Lower gastrointestinal routes, including colonoscopy, sigmoidoscopy, and retention enema, were more widely used than upper gastrointestinal routes. We found that the patients who received FMT treatment via upper gastrointestinal routes were more likely to develop AEs than those who received FMT treatment via lower gastrointestinal routes (43.9% vs. 20.6%). To avoid injury associated AEs during endoscopic process, noninvasive and patient-acceptable routes can be chosen for FMT treatment. Actually, a recent pilot study in which frozen capsules FMT was administered orally for patients with recurrent CDI demonstrated a high incidence of diarrhea resolution (overall 90%) but few mild AEs such as abdominal cramping and bloating [59]. Therefore, capsules would potentially make FMT procedure safer by avoiding procedure-related complications as well as availability for long-term usage. Furthermore, the encapsulated FMT can be accessible to a wider range of patients, especially to those who cannot withstand the endoscopic procedure. Hence, large randomized controlled studies for the safety and therapeutic efficacy of encapsulated FMT are warranted.
Though this systematic review provides a handful of valuable messages for clinical application of FMT, some limitations need to be addressed. First, the incidences of AEs might be underestimated. On one hand, since AEs usually overlapped, we took the number of patients with the most frequent kind of AE from a publication for further calculating the overall incidence of AEs. On the other hand, transient or mild AEs were sometimes ignored by researchers, resulting in the missing data of AE occurrence. Secondly, some potential confounding factors such as the health conditions of the donors, the time span from FMT exposure to the onset of the AEs and the outcomes of AEs could have substantial impacts on the classification of AEs. Thirdly, there was subjective nature in the classification of AEs. In most instances, it is impossible for us to obtain the original data of the publications we selected. Therefore we categorized AEs according to the authors’ subjective description or FDA general definitions of AEs for drugs or therapy [37].
Conclusion
Though FMT was validated to be a beneficial therapeutic strategy, we should pay enough attention to AEs of FMT. In order to prevent or treat AEs during or after FMT, more clinical trials and fundamental research are urged to elucidate the exact mechanism of how FMT causes AEs and set up a guideline on how to handle FMT-related AEs in different situations.
Supporting Information
(DOCX)
(DOC)
(DOC)
Acknowledgments
We thank all of the authors of the primary studies included in this systematic review.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study is supported by the grants (81570478 to B.W., 81300272 to H.C. and 81470796 to F.Y.) from the National Natural Science Foundation of China, and the grants (13JCQNJC10600 to H.C. and 15JCZDJC36600 to F.Y.) from Tianjin Research Program of Application Foundation and Advanced Technology of China.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–48. 10.1016/j.cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 2.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33. 10.1146/annurev.mi.31.100177.000543 [DOI] [PubMed] [Google Scholar]
- 3.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 5.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–69. 10.1038/nri2710 [DOI] [PubMed] [Google Scholar]
- 6.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23. 10.1038/nri2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20. 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- 8.Lepage P, Hasler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141(1):227–36. 10.1053/j.gastro.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):179–84. 10.1002/ibd.21339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondot S, Kang S, Furet JP, Aguirre de Carcer D, McSweeney C, Morrison M, et al. Highlighting new phylogenetic specificities of Crohn's disease microbiota. Inflamm Bowel Dis. 2011;17(1):185–92. 10.1002/ibd.21436 [DOI] [PubMed] [Google Scholar]
- 11.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 13.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–9. 10.1016/j.cgh.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755; author reply p -6. 10.1038/ajg.2012.251 [DOI] [PubMed] [Google Scholar]
- 15.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–9. . [PubMed] [Google Scholar]
- 16.Schwan A, Sjolin S, Trottestam U, Aronsson B. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2(8354):845 . [DOI] [PubMed] [Google Scholar]
- 17.Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11):1591–7. 10.1086/430315 [DOI] [PubMed] [Google Scholar]
- 18.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359(18):1932–40. 10.1056/NEJMra0707500 [DOI] [PubMed] [Google Scholar]
- 19.Mattila E, Uusitalo-Seppala R, Wuorela M, Lehtola L, Nurmi H, Ristikankare M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142(3):490–6. 10.1053/j.gastro.2011.11.037 [DOI] [PubMed] [Google Scholar]
- 20.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–87. 10.1038/ajg.2012.60 [DOI] [PubMed] [Google Scholar]
- 21.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–8. 10.1038/ajg.2013.59 [DOI] [PubMed] [Google Scholar]
- 22.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98; quiz 99. 10.1038/ajg.2013.4 [DOI] [PubMed] [Google Scholar]
- 23.Sha S, Liang J, Chen M, Xu B, Liang C, Wei N, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10):1003–32. 10.1111/apt.12699 [DOI] [PubMed] [Google Scholar]
- 24.Ianiro G, Bibbo S, Scaldaferri F, Gasbarrini A, Cammarota G. Fecal microbiota transplantation in inflammatory bowel disease: beyond the excitement. Medicine (Baltimore). 2014;93(19):e97 10.1097/md.0000000000000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36(6):503–16. 10.1111/j.1365-2036.2012.05220.x [DOI] [PubMed] [Google Scholar]
- 26.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am J Gastroenterol. 2014;109(11):1831–2. 10.1038/ajg.2014.295 [DOI] [PubMed] [Google Scholar]
- 27.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150(10):604 . [DOI] [PubMed] [Google Scholar]
- 28.Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38(6):475–83. . [DOI] [PubMed] [Google Scholar]
- 29.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121(6):2126–32. 10.1172/jci58109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6 e7. 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 31.Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. 2013;25(4):488–795. 10.1097/BOR.0b013e32836208de [DOI] [PubMed] [Google Scholar]
- 32.Luckey D, Gomez A, Murray J, White B, Taneja V. Bugs & us: the role of the gut in autoimmunity. Indian J Med Res. 2013;138(5):732–43. . [PMC free article] [PubMed] [Google Scholar]
- 33.Borody T, Leis S, Campbell J, Torres M, Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am J Gastroenterol. 2011;106:S352. [Google Scholar]
- 34.Borody T CJ, Rorers M. Reversal of idiopathic thrombocytopenic purpura with fecal microbiota transplantation (FMT). Am J Gastroenterol. 2011;106:941. [Google Scholar]
- 35.Russell SL, Finlay BB. The impact of gut microbes in allergic diseases. Curr Opin Gastroenterol. 2012;28(6):563–9. 10.1097/MOG.0b013e3283573017 [DOI] [PubMed] [Google Scholar]
- 36.Borody T NA, Torres M. Bacteriotherapy in chronic fatique syndrome (CFS): a retrospective review. Am J Gastroenterol. 2012;107:S591. [Google Scholar]
- 37.Kelly CR, Kunde SS, Khoruts A. Guidance on preparing an investigational new drug application for fecal microbiota transplantation studies. Clin Gastroenterol Hepatol. 2014;12(2):283–8. 10.1016/j.cgh.2013.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenplas Y, Veereman G, van der Werff Ten Bosch J, Goossens A, Pierard D, Samsom JN, et al. Fecal Microbial Transplantation in Early-Onset Colitis: Caution Advised. J Pediatr Gastroenterol Nutr. 2015;61(3):e12–4. 10.1097/mpg.0000000000000281 [DOI] [PubMed] [Google Scholar]
- 39.Hohmann EL, Ananthakrishnan AN, Deshpande V. Case Records of the Massachusetts General Hospital. Case 25–2014. A 37-year-old man with ulcerative colitis and bloody diarrhea. N Engl J Med. 2014;371(7):668–75. 10.1056/NEJMcpc1400842 [DOI] [PubMed] [Google Scholar]
- 40.De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11(8):1036–8. 10.1016/j.cgh.2013.04.045 [DOI] [PubMed] [Google Scholar]
- 41.Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: Safety, feasibility and efficacy trial results. J Gastroenterol Hepatol. 2014. 10.1111/jgh.12727 [DOI] [PubMed] [Google Scholar]
- 42.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM. 2009;102(11):781–4. 10.1093/qjmed/hcp118 [DOI] [PubMed] [Google Scholar]
- 43.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108(8):1367 10.1038/ajg.2013.164 [DOI] [PubMed] [Google Scholar]
- 44.Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn's disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014;8(3):252–3. 10.1016/j.crohns.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 45.Sun W, Arunachalam A, Siddique S, Zandman D. Multi-organism bacteremia after fecal microbiota transplantation for relapsing clostridium difficile infection. Am J Gastroenterol. 2014;109:S420. [Google Scholar]
- 46.Xu MQ, Cao HL, Wang WQ, Wang S, Cao XC, Yan F, et al. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol. 2015;21(1):102–11. 10.3748/wjg.v21.i1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo B, Harstall C, Louie T, Veldhuyzen van Zanten S, Dieleman LA. Systematic review: faecal transplantation for the treatment of Clostridium difficile-associated disease. Aliment Pharmacol Ther. 2012;35(8):865–75. 10.1111/j.1365-2036.2012.05033.x [DOI] [PubMed] [Google Scholar]
- 48.Landy J, Al-Hassi HO, McLaughlin SD, Walker AW, Ciclitira PJ, Nicholls RJ, et al. Review article: faecal transplantation therapy for gastrointestinal disease. Aliment Pharmacol Ther. 2011;34(4):409–15. 10.1111/j.1365-2036.2011.04737.x [DOI] [PubMed] [Google Scholar]
- 49.Cammarota G, Masucci L, Ianiro G, Bibbo S, Dinoi G, Costamagna G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–43. 10.1111/apt.13144 [DOI] [PubMed] [Google Scholar]
- 50.Rossen NG, Fuentes S, van der Spek MJ, Tijssen J, Hartman JH, Duflou A, et al. Findings from a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology. 2015. 10.1053/j.gastro.2015.03.045 [DOI] [PubMed] [Google Scholar]
- 51.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108(10):1620–30. 10.1038/ajg.2013.257 [DOI] [PubMed] [Google Scholar]
- 53.Satokari R, Mattila E, Kainulainen V, Arkkila PE. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection—an observational cohort study. Aliment Pharmacol Ther. 2015;41(1):46–53. 10.1111/apt.13009 [DOI] [PubMed] [Google Scholar]
- 54.Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60(5):631–7. 10.1136/gut.2010.223263 [DOI] [PubMed] [Google Scholar]
- 55.Hedin CR, Stagg AJ, Whelan K, Lindsay JO. Family studies in Crohn's disease: new horizons in understanding disease pathogenesis, risk and prevention. Gut. 2012;61(2):311–8. 10.1136/gut.2011.238568 [DOI] [PubMed] [Google Scholar]
- 56.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized, Controlled Trial. Gastroenterology. 2015. 25857665 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 57.Kubinak JL, Stephens WZ, Soto R, Petersen C, Chiaro T, Gogokhia L, et al. MHC variation sculpts individualized microbial communities that control susceptibility to enteric infection. Nat Commun. 2015;6:8642 10.1038/ncomms9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–80. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–8. 10.1001/jama.2014.13875 [DOI] [PubMed] [Google Scholar]
- 60.Vermeire S, Joossens M, Verbeke K, Hildebrand F, Machiels K, Van Den Broeck K, et al. Pilot study on the safety and efficacy of faecal microbiota transplantation in refractory crohn's disease. Gastroenterology. 2012;142 (5 Suppl 1):S360. [Google Scholar]
- 61.Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15. 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 62.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–5. 10.1086/367657 [DOI] [PubMed] [Google Scholar]
- 63.Kronman MP, Nielson HJ, Adler AL, Giefer MJ, Wahbeh G, Singh N, et al. Fecal microbiota transplantation via nasogastric tube for recurrent clostridium difficile infection in pediatric patients. J Pediatr Gastroenterol Nutr. 2015;60(1):23–6. 10.1097/mpg.0000000000000545 [DOI] [PubMed] [Google Scholar]
- 64.Wang M, Wang H, Zhang F. Standard fecal microbiota transplantation through mid-gut is effective therapy for refractory ulcerative colitis. J Gastroenterol Hepatol. 2013;28(Suppl. 3):590.23527756 [Google Scholar]
- 65.Suskind DL, Brittnacher MJ, Wahbeh G, Shaffer ML, Hayden HS, Qin X, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn's disease. Inflamm Bowel Dis. 2015;21(3):556–63. 10.1097/mib.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suskind DL, Singh N, Nielson H, Wahbeh G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2015;60(1):27–9. 10.1097/mpg.0000000000000544 [DOI] [PubMed] [Google Scholar]
- 67.Borody TJ, Leis SM, Chongnan J, Wettstein A, Herdman K, Llorente RA, et al. Faecal bacteriotherapy (FB) for chronic C. difficile (Cd) syndromes. J Gastroenterol Hepatol. 2003;18(Suppl. 18):B8. [Google Scholar]
- 68.Kump PK, Grochenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2155–65. 10.1097/MIB.0b013e31829ea325 [DOI] [PubMed] [Google Scholar]
- 69.Zhang FM, Wang HG, Wang M, Cui BT, Fan ZN, Ji GZ. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn's disease. World J Gastroenterol. 2013;19(41):7213–6. 10.3748/wjg.v19.i41.7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr., et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56(6):597–601. 10.1097/MPG.0b013e318292fa0d [DOI] [PubMed] [Google Scholar]
- 71.Gustafsson A, Lund-Tonnesen S, Berstad A, Midtvedt T, Norin E. Faecal short-chain fatty acids in patients with antibiotic-associated diarrhoea, before and after faecal enema treatment. Scand J Gastroenterol. 1998;33(7):721–7. . [DOI] [PubMed] [Google Scholar]
- 72.Lee CH, Belanger JE, Kassam Z, Smieja M, Higgins D, Broukhanski G, et al. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014;33(8):1425–8. 10.1007/s10096-014-2088-9 [DOI] [PubMed] [Google Scholar]
- 73.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761–7. 10.1038/ajg.2011.482 [DOI] [PubMed] [Google Scholar]
- 74.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44(5):354–60. 10.1097/MCG.0b013e3181c87e02 [DOI] [PubMed] [Google Scholar]
- 75.Pierog A, Mencin A, Reilly NR. Fecal Microbiota Transplantation in Children With Recurrent Clostridium difficile Infection. Pediatr Infect Dis J. 2014;33(11):1198–200. 10.1097/inf.0000000000000419 [DOI] [PubMed] [Google Scholar]
- 76.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8(5):471–3. 10.1016/j.cgh.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 77.Mellow M, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent clostridium difficile infection-results and follow-up. Am J Gastroenterol. 2010;105(Suppl. 1):S135. [PubMed] [Google Scholar]
- 78.Mandalia A, Kraft CS, Dhere T. Diverticulitis after Fecal Microbiota Transplant for C. difficile Infection. Am J Gastroenterol. 2014;109(12):1956–7. 10.1038/ajg.2014.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dutta SK, Girotra M, Garg S, Dutta A, von Rosenvinge EC, Maddox C, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol. 2014;12(9):1572–6. 10.1016/j.cgh.2013.12.032 [DOI] [PubMed] [Google Scholar]
- 80.Ray A, Smith R, Breaux J. Fecal Microbiota Transplantation for Clostridium difficile Infection: The Ochsner Experience. Ochsner J. 2014;14(4):538–44. . [PMC free article] [PubMed] [Google Scholar]
- 81.Mandalia A, Ward A, Kraft CS, Dhere TA. Outcomes for route and immunocompromised status do not significantly differ in fecal microbiota transplant for recurrent clostridium difficile. Gastroenterology. 2014;146(5):S252–S3. [Google Scholar]
- 82.Russell GH, Kaplan JL, Youngster I, Baril-Dore M, Schindelar L, Hohmann E, et al. Fecal transplant for recurrent Clostridium difficile infection in children with and without inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58(5):588–92. 10.1097/mpg.0000000000000283 [DOI] [PubMed] [Google Scholar]
- 83.Greenberg A, Aroniadis O, Shelton C, Brandt L. Long-term follow-up study of fecal microbiota transplantation (FMT) for inflammatory bowel disease (IBD). American Journal of Gastroenterology. 2013;108(Suppl. 1):S540. [Google Scholar]
- 84.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–71. 10.1038/ajg.2014.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brandt L, Aroniadis O, Greenberg A, Borody T, Finlayson S, Furnari V, et al. Safety of fecal microbiota transplantation (FMT) in immunocompromised (IC) patients with inflammatory bowel disease (IBD). Am J Gastroenterol. 2013;108(Suppl. 1):S556. [Google Scholar]
- 86.Wilson D, Rahni D, Kelly C. Safety outcomes after fecal microbiota transplantation (FMT) For C. Difficile Infection (CDI). Am J Gastroenterol. 2014;109(Suppl. 2):S207. [Google Scholar]
- 87.Obi O, Hampton D, Anderson T, Leung P, Abdul MKM, Chandra G, et al. Fecal microbiota transplant for treatment of resistant C. Difficile infection using a standardized protocol: A community hospital experience. Am J Gastroenterol. 2014;109(Suppl. 2):S629.25289912 [Google Scholar]
- 88.Friedman-Moraco RJ, Mehta AK, Lyon GM, Kraft CS. Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. Am J Transplant. 2014;14(2):477–80. 10.1111/ajt.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trubiano JA, Gardiner B, Kwong JC, Ward P, Testro AG, Charles PG. Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol. 2013;25(2):255–7. 10.1097/MEG.0b013e32835b2da9 [DOI] [PubMed] [Google Scholar]
- 90.Garborg K, Waagsbo B, Stallemo A, Matre J, Sundoy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis. 2010;42(11–12):857–61. 10.3109/00365548.2010.499541 [DOI] [PubMed] [Google Scholar]
- 91.Fischer M, Cook G, Rogers N, Sipe B, Vuppalanchi R. Rescue therapy with fecal microbiota transplantation in hospitalized patients with severe and severe-complicated clostridium difficile infection. Am J Gastroenterol. 2014;109(Suppl. 2):S195. [Google Scholar]
- 92.Fischer M, Kelly C, Kao D, Kuchipudi A, Jafri SM, Blumenkehl M, et al. Outcomes of fecal microbiota transplantation for C. Difficile infection in patients with inflammatory bowel disease. Am J Gastroenterol. 2014;109(Suppl. 2):S487. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.