Abstract
Marine protected area (MPA) designs are likely to require iterative refinement as new knowledge is gained. In particular, there is an increasing need to consider the effects of climate change, especially the ability of ecosystems to resist and/or recover from climate-related disturbances, within the MPA planning process. However, there has been limited research addressing the incorporation of climate change resilience into MPA design. This study used Marxan conservation planning software with fine-scale shallow water (<20 m) bathymetry and habitat maps, models of major benthic communities for deeper water, and comprehensive human use information from Ningaloo Marine Park in Western Australia to identify climate change resilience features to integrate into the incremental refinement of the marine park. The study assessed the representation of benthic habitats within the current marine park zones, identified priority areas of high resilience for inclusion within no-take zones and examined if any iterative refinements to the current no-take zones are necessary. Of the 65 habitat classes, 16 did not meet representation targets within the current no-take zones, most of which were in deeper offshore waters. These deeper areas also demonstrated the highest resilience values and, as such, Marxan outputs suggested minor increases to the current no-take zones in the deeper offshore areas. This work demonstrates that inclusion of fine-scale climate change resilience features within the design process for MPAs is feasible, and can be applied to future marine spatial planning practices globally.
Introduction
Marine protected areas (MPAs), particularly no-take areas, are increasingly considered to be an effective tool to ensure the persistence of healthy marine ecosystems and to increase the resilience of ecological communities [1]. Systematic, quantitative methods [2–4] coupled with robust design criteria which encompass the principles of being comprehensive, adequate, representative, efficient and resilient can facilitate planning of no-take reserves within MPAs to maximise biodiversity conservation outcomes. However, many existing MPAs have been created in an ad hoc or opportunistic manner, resulting in some poorly performing or non-representative protected areas [5,6]. In order to achieve conservation goals, some existing MPAs may require incremental refinement as new ecological or socio-economic information becomes available [7].
Coral reefs are considered to be particularly vulnerable to increased disturbance associated with anthropogenic climate change [8]. Specifically, rising sea temperatures can lead to coral bleaching events, and ocean acidification can disturb the chemical processes essential for reef building [8–11]. Whilst these are both processes for which no-take marine reserves cannot mitigate [9,12], it has been found, in some cases, that the presence of well-enforced, no-take marine reserves may reduce the impacts of these threats, thereby increasing the resilience of marine ecosystems to disturbance caused by climate change [10,13]. More importantly, by including the most resilient areas within no-take marine reserves, particularly areas most likely to resist and/or recover from bleaching events, defined biodiversity conservation outcomes are more likely to be achieved into the future [14].
There is currently limited empirical scientific evidence regarding the features determining coral reef resilience to anthropogenic climate change [15]. There are, however, a vast suite of often contradictory hypotheses about which features may contribute to the resilience of coral reefs (Table 1).
Table 1. Synthesis of current literature identifying key features which may determine coral reef resilience [15–19].
| Resilience Indicator |
|---|
| Temperature variability Corals in areas of high thermal variability, or which have shown quick recovery from a thermal stress event, are more likely to be resilient to future events [10,20–24].Areas least exposed to rising temperatures can also be more resilient [25] |
| Nutrient loads High nutrient load from land-based activities, such as agriculture, can cause macro-algal blooms thereby reducing coral resilience [15,26] |
| Sedimentation High levels of sedimentation result in loss of corals and act as a barrier to settlement of coral larvae [27,28] |
| Substrate availability Successful recruitment following disturbance requires suitable hard substrate upon which larvae can settle [29] |
| Water mixing Mixing through waves, currents and upwelling moderates temperatures and reduces extent of coral exposure to thermal stress events [30–32] |
| Depth Coral reefs in deeper water are more likely to resist and recover from disturbance [19] |
| Light reduction/ shading Factors that cool/ shade from high light levels such as reef aspect, mangroves or cliffs can reduce stress on coral [20,33,34] |
| Structural complexity Reefs which exhibit high rugosity and are more structurally complex recover faster than less complex habitats [19] |
| Resistant coral forms Massive corals tend to be more resistant than branching corals and some species appear to be more resilient than others [20,31,35,36] |
| Coral diversity Increased species diversity gives a higher chance of some species surviving and/or recovering from disturbance [15] |
| Live coral cover Live corals that have survived previous stress events are likely to be more tolerant to disturbance and higher densities improve the ability to recover [21,37–39] |
| Connectivity Coral larvae need to be supplied from upstream reefs following severe disturbance [11,40] |
| Coral disease Coral disease can quickly wipe out colonies making recovery less likely [41] |
| Macro-algal cover Areas with high abundance of macro-algae can prevent coral settlement, dominate benthic space and directly kill corals [42,43] |
| Herbivore biomass Herbivores reduce macro-algal cover and ensure bare substrate is available for settlement by coral larvae [29,39,44] |
| Fishing pressure Reduced fishing pressure lowers biological stress on the ecosystem [45] |
| Proximity to human activities Anchor damage, reef walking, boat strikes etc. damage coral and increase susceptibility to disease and bleaching. Proximity to nodes of human activity leads to increased pollution/run off [17,46,47] |
It is broadly recognised that conservation planning for MPAs should attempt to incorporate resilience features while remaining adaptive to new research findings [7,48]. In particular, Game et al. [14] argue that in order to achieve the best conservation outcomes, MPAs should be placed in areas most likely to be resilient to disturbance induced by climate change. While the growing need to address climate change resilience within marine conservation planning is well documented [1,7], there are many MPAs that were implemented before it became an important consideration. As such, there have been very few spatially explicit attempts to apply resilience theory to the incremental refinement of existing MPAs [7,11,16–18,49].
Ningaloo Marine Park (Fig 1), in Western Australia, is a well-established MPA and UNESCO World Heritage Site with relatively few ecosystem threats in comparison with other coral reefs [50,51]. Following a recent severe ocean warming event which resulted in significant coral bleaching [36,52–54], rising sea surface temperatures (SSTs) associated with climate change are expected to present the greatest threat to this ecosystem. However, the existing management plan does not explicitly consider climate change.
Fig 1. Ningaloo Marine Park indicating management zones: General Use Zone (recreational fishing and limited commercial fishing permitted, IUCN VI), Recreation Zone (recreational fishing permitted, IUCN VI), Special Purpose Zones (recreational shore-based or trolling fishing only, IUCN VI), No-take Sanctuary zones (no fishing permitted, IUCN II).
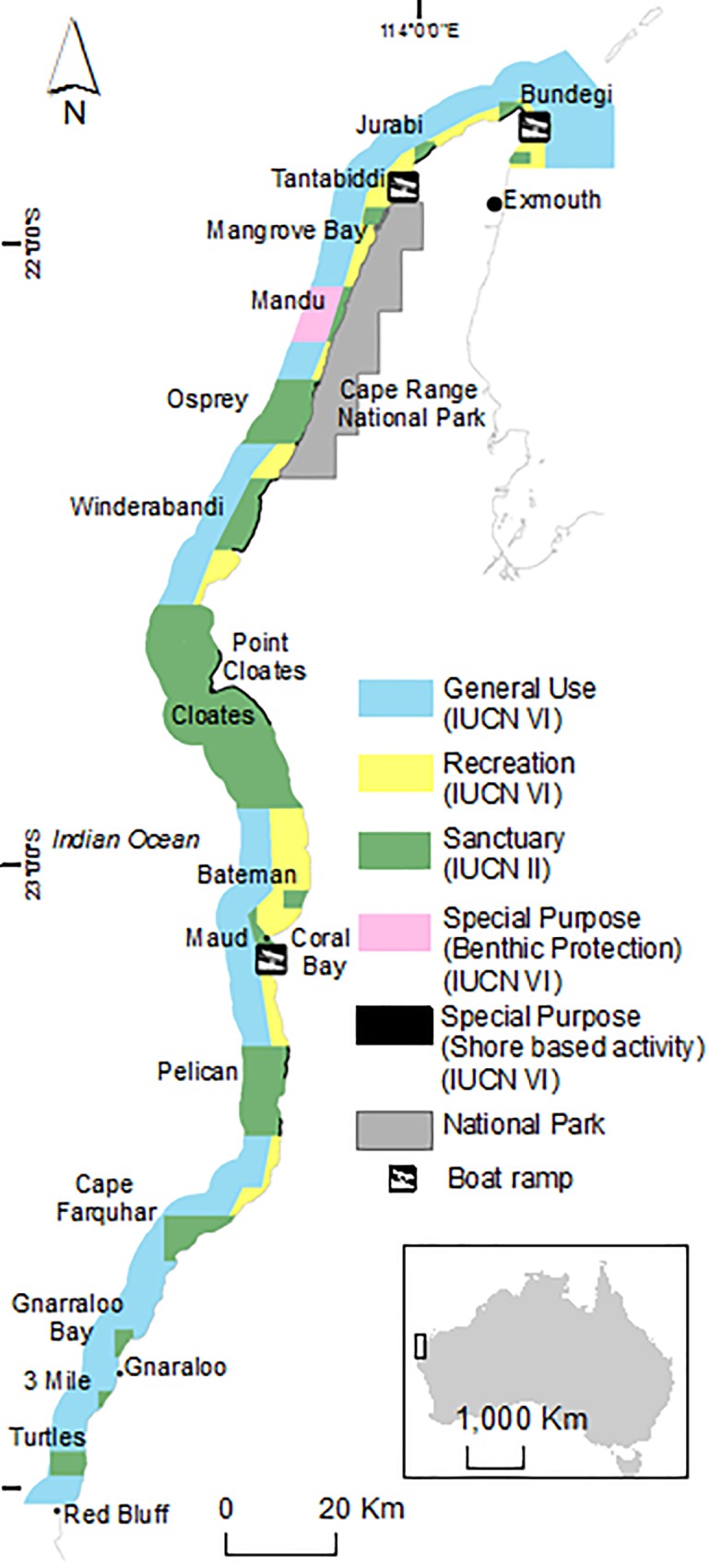
Recent investment in research within the Park has resulted in spatially explicit datasets, which have provided an excellent opportunity to classify climate change resilience features, and to demonstrate one approach for integrating these features into an incremental refinement of the Park’s zonation within the existing adaptive management framework. The aims of this study were to identify any gaps in representation of benthic habitats within existing no-take sanctuary zones; to define and map climate change resilience features and to assess their representation in existing no-take sanctuary zones; and to conduct a spatial analysis using Marxan software to make incremental refinements of existing zones to ensure that the benthic and climate change resilience features are adequately represented within no-take sanctuary zones.
Materials & Methods
Study region
Ningaloo Reef (Fig 1) on Australia’s north-west coast is one of the largest fringing coral reefs in the world [50]. At ~300 km long, it spans three degrees of latitude from 21°S to 24°S, encompasses a diverse range of habitats and has high species richness [55,56]. The reef crest forms a discontinuous barrier along the coast, creating a lagoon with an average width of 2.5 km [57]. At the southern and northern extents of the reef, the lagoons disappear and are replaced by extensive intertidal reef platforms [57]. The reef crest provides a buffer to the prevailing south-westerly winds and waves and is irregularly interspersed by reef passes allowing water circulation in and out of the lagoons [57,58]. The lagoons display high annual sea surface temperature variability of over 10°C in comparison to the relatively more stable temperatures within reef pass systems [59].
In 2011, Ningaloo Reef experienced an extreme marine heat wave event driven by some of the strongest La Niña conditions in the past century [53,60]. High sea level anomalies, increased cyclonic activity and record high water temperatures resulted in mass coral mortality [36,52], with up to 95% loss of coral cover and the complete loss of Acropora and Montipora assemblages in some areas [36]. There has been a discernible increase in the occurrence of SST anomalies, with higher than average ocean temperatures observed in the two austral summers following the 2011 event [54,61], and the most extreme historical anomalies recorded post-1980 [61].
Ningaloo Marine Park is a popular tourism destination with approximately 240,000 visitors annually [62]. There is extensive recreational fishing and some commercial charter fishing activity, but no other commercial fisheries currently operate within the marine park [63].
Datasets
Datasets were acquired from both published and unpublished sources, were all spatially explicit and, when combined, covered the entire extent of the Ningaloo Marine Park (Table 2).
Table 2. Details of datasets used for the incremental refinement of Ningaloo Marine Park no-take sanctuary zones to accommodate resilience features.
| Data type | Description | Source | |
|---|---|---|---|
| BiodiversityFor classes see [S1 Table] | Shallow water habitats (<20 m) | HyMap airborne hyperspectral imagery at 3.5 × 3.5 m resolution. Habitats separated into 46 biotic and abiotic classes | [64] |
| Deeper water benthic communities | Deeper water habitat models with 19 biotic classes at 100 × 100 m resolution | [65] | |
| Physical | Bathymetry (depth, rugosity) | HyMap airborne hyperspectral imagery (125 bands) at 3.5 × 3.5 m resolution | [64] |
| Geomorphic features | Digitized polygons of reef passes, lagoon areas and reef crests | This study | |
| Coastline | Line file depicting mean high water line of Ningaloo coast (1:100,000) | [66] | |
| Human use/ Cost | Boat-based activities | Distribution and density of boat- based activities over year in 3 × 3 km grid | [67] |
| Shore-based activities | Distribution and density of shore-based recreational activities over year in 3 × 3 km grid | [67] | |
| Camp sites and access points | Distribution and density of camp sites and boat launch sites over year in 3 km coastal segments | [68] | |
| Commercial fisheries | Catch and effort of charter fishing from 2009–2013 in 10 × 10 nautical mile data blocks | WA Department of Fisheries (unpublished data) | |
| Other | Current Ningaloo Marine Park management zones | Polygon file depicting spatial boundaries of each zone type | [69] |
The study area was divided into 1 km2 planning units in a grid format [4] using ArcGIS 10.2 software [70]. This layer was overlaid with each of the defined datasets (Table 2) to determine areas of the features present within each planning unit.
Benthic biodiversity
The shallow water marine habitats of Ningaloo to 20 m depth have been mapped using HyMap airborne hyperspectral imagery at 3.5 m pixel resolution [64]. For the deeper water benthic communities, spatial habitat models were constructed using towed video camera imagery of the benthos in depths from 15–130 m throughout the marine park captured together with single beam echo-sounder transects [65] (Table 2) (For detailed methods see S2).
Resilience features
Six resilience features were selected from the available data (Table 2) on the basis of their relevance to Ningaloo Reef and the importance inferred by relevant literature (Table 3, for rationale see S3 Table).
Table 3. Six climate change resilience features highly relevant for Ningaloo Marine Park, and the definition of resilience values assigned to each feature (Percentage values for macro-algal and live coral cover obtained from Kobryn et al. [64]).
| Resilience value | ||||
|---|---|---|---|---|
| Resilience feature | Priority for Ningaloo Reef | High (1) | Moderate (2) | Low (3) |
| Depth | Areas deeper than 8 m [19,39] | >8 m | 6–8m | <6 m |
| Structural complexity | Structurally complex areas with high rugosity values [19] | Rugosity value 4–5 | Rugosity value 2–3 | Rugosity value 0–1 |
| Water mixing | Reef pass areas with high mixing [15] | Reef pass present | N/A | No reef pass present |
| Macro-algal cover | Areas with low macro-algal cover [13] | Sparse macro-algal cover (<35%) | Patchy macro-algal cover (35–65%) | Dominant macro-algal cover (>65%) |
| Live coral cover | Areas with high live coral cover [16,39] | Continuous coral(>65%) | Patchy coral(35–65%) | Sparse coral(<35%) |
| Proximity to human activities | Areas furthest from human activity nodes [10,39] | Low human activity | Moderate human activity | High human activity |
The 3.5 m pixel HyMap bathymetry data [64] layer was utilised to map the water depth throughout the extent of the study area. It was also utilised to create a rugosity map, as a surrogate for habitat complexity, using the Benthic Terrain Modeller Tool for ArcGIS which creates an output of rugosity from digital elevation models. The rugosity values were reclassified into six structural complexity classes following similar criteria to those used by Graham et al. [19] namely, 0 = no vertical relief, 1 = low and sparse relief, 2 = low but widespread relief, 3 = widespread moderately complex relief, 4 = widespread very complex relief and 5 = exceptionally complex relief.
Reef passes, as an indicator for water mixing, were also determined through use of the 3.5 m pixel HyMap bathymetry data [64] and manually digitized in ArcGIS. Reef passes were defined as an opening between reef crests where depths >5 m extended landward beyond the reef crest into the lagoon. Reef passes are <1 km wide; any gaps >1 km in width were considered to be larger reef breaks and are less likely to experience the strong current flow characteristic of reef passes at Ningaloo Reef [58]. Percentage cover of macro-algae and live coral was determined using the 3.5 m resolution shallow water benthic habitat maps which were limited to <20 m depth [64]. Proximity to human activities [67] was calculated using the cost layer outlined below, and the normalised values were subsequently split into equal terciles of high, moderate and low.
Each feature had to meet specific conditions to be assigned a resilience value of 1 for high resilience, 2 for moderate resilience and 3 for low resilience (Table 3). The planning unit grid file was overlaid with each resilience feature layer to create six GIS layers with a resilience value for each 1 km2 planning unit. Any planning units that fell outside the extent of the resilience feature datasets were assigned a score of 1 (high resilience) as they all occurred in deeper water (>20 m) [19].
A test for correlation between each feature found high structural complexity to be strongly correlated with low macro-algal cover (R2 = 0.98) and high live coral cover (R2 = 0.77). High live coral cover and low macro-algal cover also had a positive correlation (R2 = 0.67). These strong correlations could enable the resilience index to be simplified with high structural complexity acting as a surrogate with minimal reductions in accuracy. However, retaining the correlated features increased the irreplaceability of the planning units which contained all three features. With the ultimate aim of incorporating the planning units with the highest resilience within the reserve network, retaining all six features was considered preferable.
Cost data
Boat-based and shore-based human activity data collected through aerial surveys and presented in 3 × 3 km data blocks [67,68] were utilised to derive a spatially explicit socio-economic cost layer. Only peak visitor period data were used and both data files were clipped to the extent of the Ningaloo Marine Park boundary with the shore-based data aggregated to be included within the coastal planning units.
In addition, catch and effort data of commercial charter fishing activities from 2009 to 2014 in 10 × 10 nautical mile data blocks were provided by the Western Australian Department of Fisheries. Total catch values were summed for all years and clipped to the extent of the planning unit layer.
Data detailing distribution of individual recreational activities such as fishing, diving and relaxing enabled a comprehensive socio-economic cost layer to be developed [67]. Values from each of the cost datasets (Table 2) were normalised and fishing values were then multiplied by 10 because displacement of fishing by no-take zones has the highest opportunity cost [71]. All other values were left on a scale of 0–1 as the activities would not be displaced by the presence of a no-take sanctuary zone (S4 Table). Each of the cost data layers was then overlaid with the planning unit grid layer and summed to assign a single opportunity cost to each 1 km2 planning unit.
Incorporating resilience features into conservation planning
A commonly used systematic conservation planning software, Marxan (version 2.43) [72], was used to incorporate the resilience values into a conservation planning exercise. Marxan is an open access software designed to solve the minimum set reserve design problem [73]. It uses a simulated annealing algorithm with spatially derived planning units to find a set of near optimal reserve solutions which minimise socio-economic cost while meeting user-defined biodiversity targets (i.e. conservation features) [74].
A boundary length file was created using a tool developed by ABPmer Marine Environmental Research [75] in ArcGIS and a boundary length modifier of 0.001 was determined using the methods of Stewart and Possingham [76].
Conservation objectives
Current international guidelines recommend that a target of 30–40% minimum representation of conservation features be included within no-take sanctuary zones [1,77]. The existing management plan for Ningaloo Marine Park did not define any quantitative targets for the inclusion of specific habitat types within no-take sanctuary zones [69] and the final boundaries of these zones were delineated by negotiation with stakeholders, resulting in 34% of the total area of the Park being designated as no-take sanctuary zones. However, these zones are biased towards certain habitats and are unrepresentative of others [4]. In order to quantify this problem and recommend redress, we formulated a desired target of 34% representation within sanctuary zones of each benthic biodiversity feature. The target was based on the international guidelines previously mentioned, as well as the stakeholder negotiation previously undertaken in the Park.
With the same rationale used to define 34% targets for habitat types, a 34% target for features conferring high resilience was also used, assuming that these features represent areas most likely to resist and/or recover from future thermal disturbances and are therefore critical areas that require adequate representation within no-take sanctuary zones. Following examination of the literature, six relevant studies were chosen to identify the relative importance of each resilience feature ([15–19,39] (see S3 Table for rationale). As the perceived importance of individual features varied between authors, we used the same (34%) target for each resilience feature.
Analysis
ArcGIS was used to calculate the percentage of shallow water habitats and deeper water benthic communities currently represented within no-take sanctuary zones in the Marine Park. Marxan analyses, following standard methods [78], were then undertaken to identify additional priority areas for inclusion within no-take sanctuary zones that would enable the 34% representation targets to be met. For each of the analyses, 10,000 repetitions and two million iterations were used and a high penalty value was set for each feature to ensure all objectives were achieved. The six resilience features were incorporated as additional conservation features similar to the method used by Levy and Ban [12]. All analyses included the same socio-economic cost layer.
To explore the influence of existing sanctuary zones, distribution of biodiversity features and areas of high resilience on Marxan outputs, six scenarios with different combinations of variables were analysed. These were; S1. Biodiversity features only; S2. Biodiversity features with existing sanctuary zones; S3. Biodiversity and resilience features; S4. Biodiversity and resilience features with existing sanctuary zones; S5. Resilience features only and S6. Resilience features with existing sanctuary zones.
A complete hierarchical cluster analysis based on a Jaccard resemblance matrix similar to the method of Harris et al. [79] was performed in R version 3.2.5 to compare within, and among, the scenarios using the top 100 solutions. Cohen’s Kappa statistic was also used to make pairwise comparisons of the ‘best’ Marxan solutions from each scenario.
Marxan generates a summed solution output for each scenario which provides the selection frequency of each planning unit across all 10,000 repetitions. With a focus on iterative refinement of the existing no-take zones, these outputs for scenarios S2 and S4, and the difference between the two scenarios, were plotted in ArcGIS to determine which planning units had the highest selection frequency and, therefore, were most likely to be present in a final representative reserve design. In order to compare different design options, Marxan calculates an objective function score, which combines the boundary length, any penalties for not meeting targets, and the total cost for the reserve network [74]. The ‘best’ solution output is the run with the lowest objective function value and can be considered a near optimal solution within a suite of other options [78]. The ‘best’ solution outputs were plotted in ArcGIS and total area of the ‘best’ recommended reserve system calculated.
Limitations
Small gaps existed in the benthic datasets, which meant those areas would have been less likely to be included in the reserve network by Marxan. A number of studies have also incorporated SST variables when determining areas of thermal refugia for inclusion within reserves [10,25]. Although SST data could be useful to delineate thermal refuges in deeper environments, the resolution of remotely sensed SST data is relatively coarse and can be inaccurate in the shallow, nearshore and narrow lagoons found at Ningaloo. As the benthic datasets used in this study are very high resolution, in this instance, including SST data would detract from the high level of spatial resolution provided by the benthic datasets and thus was not included in this analysis.
Results
Habitat representation in no-take zones
Of the 46 shallow water habitat classes identified in the Ningaloo Marine Park [64], 45 had >40% representation within current no-take sanctuary zones. The only habitat missing the target (patchy tabulate coral) accounted for a very small proportion of total habitat coverage (<100 m2) and it is only found within the southern regions of the reef. Of the 16 deeper water benthic community classes, three were represented at >34%, while most classes required small increases (1–6%) in representation within sanctuary zones. However, to attain the 34% target, a >10% increase in area within no-take sanctuary zones is required for dense, sparse and medium filter feeder communities and dense sponge communities.
Resilience
Planning units considered to be highly resilient comprised 1360 km2 of the total Marine Park and, of those, 15% fell within existing no-take zones. There was a trend towards higher resilience values being located in offshore areas with moderate resilience values generally associated with the reef crest region and lowest resilience in the inshore, shallower areas.
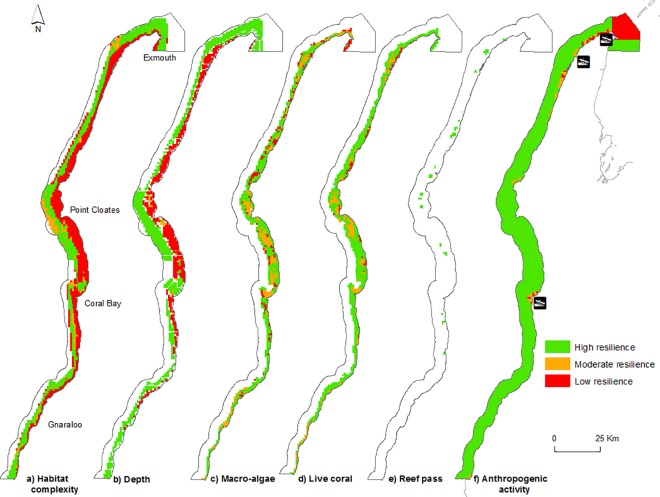
The greatest structural complexity was generally found seaward of the reef crest (Fig 2A). Depth increased with distance from shore although there were some small, deeper areas inside the reef crest (Fig 2B). Areas with high macro-algal cover (low resilience value) were restricted to small areas close to the coast (Fig 2C), whilst continuous live coral cover was evenly distributed throughout the marine park (Fig 2D). Reef passes were distributed throughout most of the marine park, although they diminish in the southern areas where the lagoon systems disappear (Fig 2E). High human impacts were generally located near the two major hubs of Coral Bay and Exmouth with moderate levels throughout the coastal area of Cape Range National Park (Fig 2F).
Fig 2.
Spatial distribution of resilience features at Ningaloo Marine Park; a) Structural complexity, b) Depth, c) Live coral cover, d) Macro-algal cover, e) Reef passes (water mixing), f) Proximity to anthropogenic activity.
Summary of Marxan solutions
When ignoring the existing zones, the target of 34% representation of biodiversity features (S1) could be achieved with 28.3% of the NMP demarcated as sanctuary zones (Table 4).
Table 4. Summary of conditions set and Marxan outputs for 6 different scenarios tested where a target of 34% representation for each feature within each scenario was met.
Note that the total reserve area of existing sanctuary zones is 884 km2 (34%) and total area of Ningaloo Marine Park is 2633 km2.
| Scenario | Variables | Marxan ‘best’solution | |||
|---|---|---|---|---|---|
| Biodiversity features | Resilience features | Existing sanctuaries locked in? | Total sanctuary zone area (% of NMP) | Change in no-take zone area (% change) | |
| S1 Biodiversity only | Yes | No | No | 746.6 km2 (28.3%) | -137.4 km2(-5.2%) |
| S2 Biodiversity & zones | Yes | No | Yes | 1204.6 km2 (45.8%) | +320.6 km2 (+12.1%) |
| S3 Biodiversity & resilience | Yes | Yes | No | 1262.2 km2 (47.9%) | +378.2 km2 (+14.3%) |
| S4 Biodiversity, resilience & zones | Yes | Yes | Yes | 1404.2 km2 (53%) | +518.2 km2(+19.6%) |
| S5 Resilience only | No | Yes | No | 1085.8 km2 (41.2%) | +201.8 km2 (+7.6%) |
| S6 Resilience & zones | No | Yes | Yes | 1087.1 km2 (41.0%) | +197.1 km2 (+7.4%) |
An iterative refinement of the existing zones (S2- Biodiversity & zones) required an increase in the area of no-take sanctuary zones by 12.1% to meet the same objectives. Adding the resilience targets required an increase in no-take sanctuary zones of 14.3% without locking in the existing zones (S3) and an iterative refinement with resilience features (S4) required a total of 53% of the NMP to be demarcated as no-take sanctuary zones (Table 4).
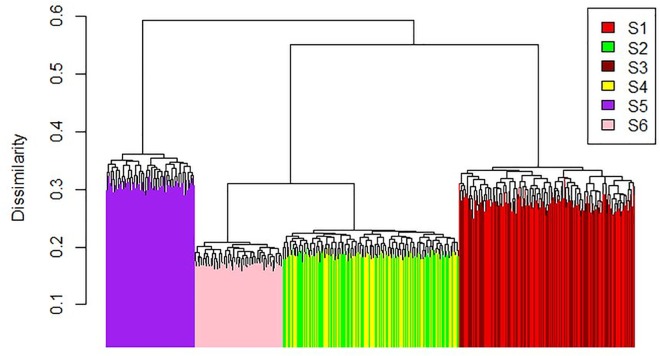
The complete hierarchal cluster analysis of the top 100 solutions per scenario showed S5 (resilience features only without existing zones) was the most dissimilar to the other scenarios (Fig 3). The second split was driven by whether the scenarios had existing sanctuary zones locked in. Finally, within the three scenarios that did have existing sanctuary zones locked in, S6 (resilience only) was separated from the scenarios which included the biodiversity targets (Fig 3). The solutions generated by S1 and S3 and by S2 and S4, respectively showed no dissimilarity thus, for NMP, adding the resilience targets had little bearing on the Marxan solutions.
Fig 3. Dendrogram from a complete hierarchical cluster analysis based on a Jaccard resemblance matrix.
S1–S6 refer to the six scenarios: S1 Biodiversity features only, S2 Biodiversity features with existing sanctuary zones, S3 Biodiversity and resilience features, S4 Biodiversity and resilience features with existing sanctuary zones, S5 Resilience features only and S6 Resilience features with existing sanctuary zones.
With a focus on adaptive management of the existing Marine Park through the incremental refinement of the existing zones, results from scenarios S2 and S4 were examined in more detail.
Incremental refinement of the existing Marine Park
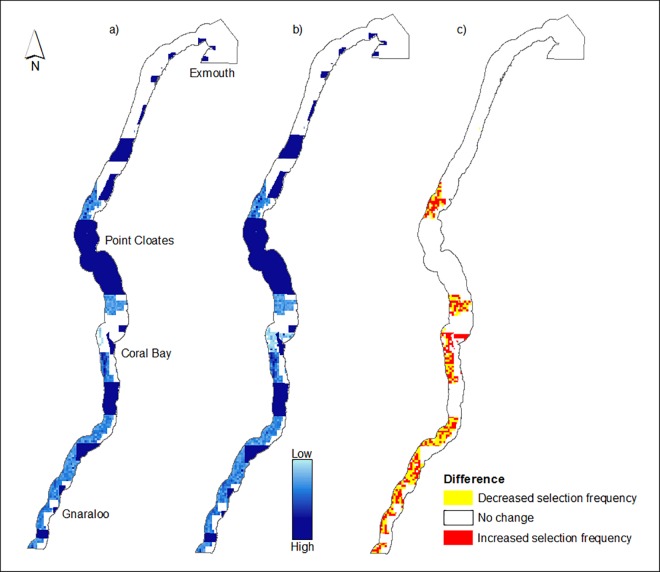
The summed solution output for the S2 scenario identified areas of high selection frequency offshore in the region north of Point Cloates and in the southern reaches offshore from Gnaraloo (Fig 4A). There were a few planning units of high selection frequency in the northern region of the Marine Park corresponding to the area adjacent to Cape Range National Park and Exmouth and relatively evenly distributed areas of high selection frequency outside sanctuary zones throughout the rest of the Marine Park (Fig 4A). When the high resilience features were added as conservation features (Scenario S4), the summed solution output (Fig 4B) was only marginally different to the S2 scenario (Cohens Kappa = 0.798). The greatest increase in selection frequency following the addition of resilience features was off the coast of Coral Bay (Fig 4C).
Fig 4.
a) Selection frequency of Ningaloo Marine Park planning units to achieve 34% target representation for all biological conservation features in an incremental refinement of existing no-take sanctuary zones (Scenario S2), b) Selection frequency of Ningaloo Marine Park planning units to achieve 34% target representation for all biological conservation features and resilience features in an incremental refinement of existing no-take sanctuary zones (Scenario S4), c) Difference in selection frequency of Ningaloo Marine Park planning units between scenario S2 and scenario S4 (S2 subtracted from S4).
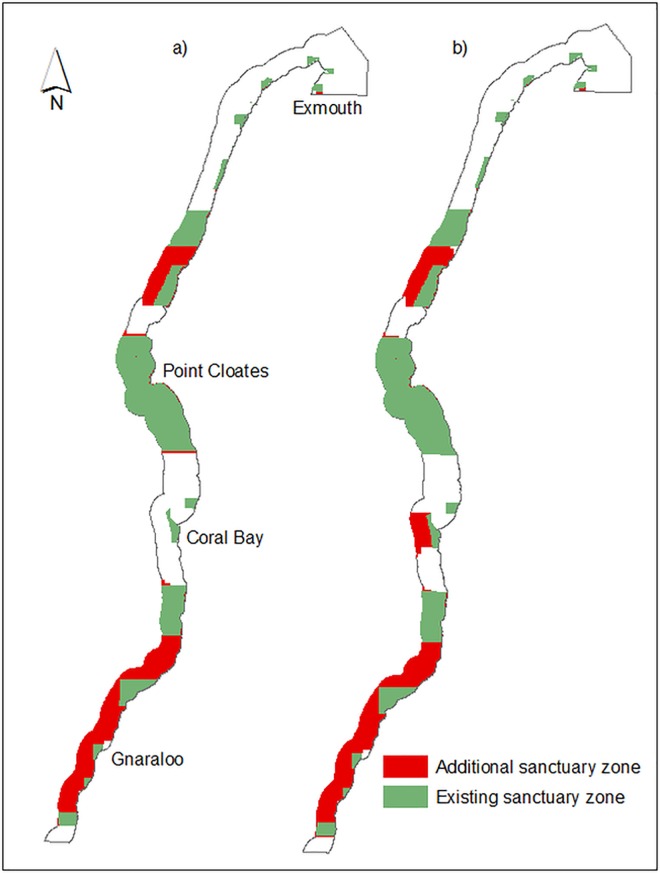
In scenario S2, the southern regions were represented within the ‘best’ reserve design output as two large sanctuary zones with all additional zones increasing the current no-take sanctuary area by 320.2 km2 (12.1%) (Fig 5A). When the resilience features were added (scenario S4), the ‘best’ reserve design was very similar to the output of S2, however, there was one major addition to the sanctuary zone network offshore from Coral Bay which resulted in a further increase in sanctuary zone area of 197.6 km2 (Fig 5B). The Marxan ‘best’ outputs for both scenarios also added the existing shore-based activity special purpose zones (narrow shore-side strips) to the no-take sanctuary network (Fig 5A & 5B).
Fig 5.
a) MARXAN ‘best’ reserve design output meeting 34% target representation for all biological conservation features in an incremental refinement of existing no-take sanctuary zones (Scenario S2), b) MARXAN ‘best’ reserve design output meeting 34% target representation for all biological conservation features and resilience features, in an incremental refinement of existing sanctuary zones (Scenario S4).
Discussion
Method for incorporating climate change resilience features
As many existing marine parks have been established for several decades, there is a growing need for methods which enable an assessment of their management zones against the most recent scientific information, particularly with respect to climate change. This study demonstrates a method by which climate change resilience features can be defined, delineated and then incorporated into the incremental refinement of an existing marine reserve network for coral reefs and contributes to the limited research on the topic [7,80]. The availability of remotely-sensed, hyperspectral data provided a good opportunity to isolate resilience features at a high resolution over a large area. It provided resilience information on a much finer scale than similar research using remote sensing products [12,17], yet without the limited spatial cover associated with fine scale in-water research [18,19]. Further analyses could incorporate fine-scale resilience features with the spatial distribution of past thermal stress [10], models of predicted SSTs into the future [81] or both [12,25]. The method could also be developed to provide insights into marine spatial planning with multiple zones [25], and could consider the level of connectivity between more resilient and less resilient areas [82,83].
This method for developing a spatial distribution of resilience levels from remote sensing products can inform management decisions beyond MPAs through highlighting vulnerable areas that may require other management measures [84]. The depth limitations of hyperspectral imagery (<20 m) were overcome by combining the shallow water dataset with the acoustic single-beam dataset from deeper water. While the single beam dataset has a lower resolution, it enables a comprehensive analysis for the entire extent of the marine park.
Resilience of Ningaloo Reef
Unusually, Ningaloo Reef exhibits a naturally high level of macro-algal cover within the lagoon where both coral and macro-algal habitats exist side by side [85]. This is typically an indicator of a reef in decline, however, high macro-algal cover is considered to be a healthy stable state for Ningaloo Reef [86]. Despite the naturally high occurrence, a recent study indicated that high macro-algal cover still impedes coral larvae settlement at Ningaloo [87] and therefore reduces the ability for corals to recover following a bleaching event. Consequently, naturally high macro-algal cover, coupled with shallower water and less water mixing within the lagoon systems is a likely driver of the lower coral resilience values found in the lagoon areas.
Refinement of Ningaloo Marine Park
The Ningaloo Marine Park management plan is now due for a ten-year review. Investment in research since the establishment of the Marine Park in its current form has resulted in extensive, spatially-explicit, ecological and socio-economic data enabling a robust adaptive management planning process.
The current no-take sanctuary zones have some shortfalls in representation, particularly in deeper, offshore areas. However, all conservation features can be met through incremental increases to existing sanctuary zones in two main regions, namely the seaward extension of the Winderabandi sanctuary zone out to the Ningaloo Marine Park boundary and extending and connecting the very small sanctuary zones from Cape Farquhar to 3 Mile sanctuary in the south, confirming the recommendations of Beckley and Lombard [4].
The suggested increase in sanctuary representation in offshore areas is aligned with global trends where offshore and pelagic waters are often underrepresented within MPAs [88,89]. The biodiversity benefits of increased offshore sanctuary zones could be further enhanced through connectivity with the proposed offshore Commonwealth sanctuary zones within the Exclusive Economic Zone [90]. Furthermore, with the highest densities of human activity occurring within inshore areas, incremental increases to sanctuary zones in offshore regions will enable conservation features to be met with low socio-economic cost [91].
The large reserve area recommended in the south could further be beneficial to the resilience of the Ningaloo Marine Park because of the predicted range shift to higher latitudes of marine species in various climate change scenarios for Western Australia [92]. Furthermore, the regions of the reef at higher latitudes, while still expected to experience a gradual increase in temperature over time, are less likely to experience severe pulse type thermal stress events [60]. With recent research indicating that some coral families can acclimatise to gradually rising temperatures [24], these southern areas may be even more important for persistence than the resilience index implies.
Some highly resilient areas were represented within the current sanctuary zones. However, the shallow water habitat data were collected before the 2011 and subsequent 2012/13 bleaching events and, as such, the benthic composition and coral cover may have changed. For example, within the Bundegi sanctuary zone (Fig 1) 90% of live coral cover was lost [36]. As such, in order to ensure effective adaptive management, a repeat survey, using the same hyperspectral and acoustic methods, could be implemented and included within the resilience framework developed by this study.
Incremental reserve design has been found to be an effective tool for adaptive management [93]. However, this study found similar results to Stewart et al. [94], whereby incremental refinement required a larger area to meet targets than if a systematic conservation planning process was used without considering the existing zoning. While Airamé et al. [95] suggested removal of historic reserves if they were not included within systematic solutions, longevity is important for the success of reserves [96], and increases in the biomass of certain fish species within the current reserves at Ningaloo have already been recorded [97]. Therefore, an iterative refinement of the existing no-take sanctuary zones is the best option for a more representative reserve system that encompasses areas critical for resilience to climate change induced disturbance.
Incorporating resilience features as additional conservation objectives gives managers some assurance that a reserve network might have the best possible chance of achieving biodiversity outcomes in the face of climate change [14,49]. In some cases, achieving this objective might result in a very different reserve system, however, for Ningaloo Marine Park, ensuring that the most resilient areas of the reef were represented within the reserve network could be achieved with only minor additions to the representative no-take sanctuary zones.
Resilience and marine reserves
There is some empirical evidence to suggest that coral reefs within marine reserves may be more resilient to the impacts of climate change [98]. Some areas within reserves have demonstrated an enhanced ability to recover following extreme weather events [99] and Caribbean coral reefs within reserves were found to have significantly better recovery rates after a bleaching event than reefs without reserve protection [13]. The ability for reserves to increase ecosystem resilience to climate change beyond the reserve boundaries themselves is likely to be linked to how well the populations within reserves can enhance the recovery of downstream degraded areas through connectivity [83].
Following the extreme marine heat wave event at Ningaloo Reef in 2011, bleaching was indiscriminate when it came to reserves [36,52], and it will take many years to determine if the areas within the reserves display enhanced recovery. Furthermore, the presence or absence of marine reserves was found to have no bearing on the resistance or recovery of coral reefs in the Seychelles following an extreme bleaching event in 1998 [19]. However, poaching and illegal fishing in these reserves is common, in which case, the benefits from reserve protection would not be apparent [100,101].
Although the role no-take marine reserves play in increasing the resilience of reefs is still uncertain, and likely to be linked to a number of other variables [102], there are many measurable benefits directly linked to no-take reserves [103,104]. The prioritisation of the representation of highly resilient areas, or known areas of refuge to thermal stress, within reserve networks is therefore a sound precautionary principle and fundamental to achieve biodiversity conservation outcomes [81,105].
Conclusions
Consideration of the predicted impacts of climate change in conservation planning is critical, yet, practical application is scarce. This study demonstrates that isolating features likely to confer resilience on a coral reef is feasible and, with the use of hyperspectral remote-sensing and modern acoustics, it can be achieved at high resolution across large study areas. As more empirical evidence regarding the factors that make coral reefs resilient becomes available, conservation planners can further refine resilience features and incorporate areas of high conservation significance within planned MPAs in a relatively straightforward manner. While it is still difficult to predict the impacts of climate change with any degree of certainty, it is imperative that resilience is incorporated into MPAs in order to have the highest probability of long-term persistence.
Supporting Information
(DOCX)
A) Datasets derived from single beam bathymetry that were used as environmental variables for modelling biota, substrate and fish abundance/richness, B) Model accuracy statistic AUC statistic for biotic and abiotic substrate predicted from the presence/absence models (blind validation n = 19872 data points).
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Thanks to Dr Claire Smallwood, Veronique Vanderklift and Dr Brent Wise (WA Department of Fisheries) for providing data and to Matthew Watts for assistance with Marxan. We would like to acknowledge Dr Peter Barnes and Arvid Hogstrom (Department of Parks and Wildlife, Exmouth), Dr Rachel Standish, Dr Rebecca Weeks and Dr Ming Feng for providing valuable input. We would also like to thank the reviewers for helpful comments that assisted in improving the manuscript.
Data Availability
The minimum relevant data used for this study are within the paper and from Figshare at: https://figshare.com/articles/Davies_etal_2016_PLOSdatasets_xlsx/3513953. Deep water data are from the Western Australian Marine Science Study program 1.1.1 study whose authors Ben Radford and Andrew Heyward may be contacted via email at b.radford@aims.gov.au and a.heyward@aims.gov.au. Shallow water data are from a CSIRO Wealth from Oceans project and can be obtained from the author Halina Kobryn at h.kobryn@murdoch.edu.au. Human Use data is from a Murdoch University PhD thesis and can be obtained from the author Claire Smallwood at claire.smallwood@gmail.com.
Funding Statement
H.D. received scholarship funding through Murdoch University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Green A, Fernandes L, Almany G, Abesamis R, McLeod E, Aliño P, et al. Designing marine reserves for fisheries management, biodiversity conservation, and climate change adaptation. Coast Manag. 2014;42: 143–159. 10.1080/08920753.2014.877763 [DOI] [Google Scholar]
- 2.Margules CR, Pressey RL. Systematic Conservation Planning. Nature. 2000;405: 243–253. 10.1038/35012251 [DOI] [PubMed] [Google Scholar]
- 3.Hansen G, Ban N, Jones M, Kaufman L, Panes H, Yasué M, et al. Hindsight in marine protected area selection: A comparison of ecological representation arising from opportunistic and systematic approaches. Biol Conserv. 2011;144: 1866–1875. 10.1016/j.biocon.2011.04.002 [DOI] [Google Scholar]
- 4.Beckley LE, Lombard AT. A systematic evaluation of the incremental protection of broad-scale habitats at Ningaloo Reef, Western Australia. Mar Freshw Res. 2012;63: 17–22. 10.1071/MF11074 [DOI] [Google Scholar]
- 5.Pressey RL, Cowling RM, Rouget M. Formulating conservation targets for biodiversity pattern and process in the Cape Floristic Region, South Africa. Biol Conserv. 2003;112: 99–127. 10.1016/S0006-3207(02)00424-X [DOI] [Google Scholar]
- 6.Stewart RR, Ball IR, Possingham HP. The effect of incremental reserve design and changing reservation goals on the long-term efficiency of reserve systems. Conserv Biol. 2007;21: 346–54. 10.1111/j.1523-1739.2006.00618.x [DOI] [PubMed] [Google Scholar]
- 7.Anthony K, Marshall P, Abdulla A, Beeden R, Bergh C, Black R, et al. Operationalizing resilience for adaptive coral reef management under global environmental change. Glob Chang Biol. 2014;21: 48–61. 10.1111/gcb.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoegh-Guldberg O, Bruno J. The impact of climate change on the world’s marine ecosystems. Science. 2010;323: 1523–1528. [DOI] [PubMed] [Google Scholar]
- 9.Graham N, Ainsworth T, Baird AH, Ban NC, Bay LK. From microbes to people: Tractable benefits of no-take areas for coral reefs In: Gibson R, Atkinson R, Gordon J, editors. Oceanography and Marine Biology, an Annual Review. 49th ed. Boca Raton, Florida: CRC Press; 2011. pp. 117–148. [Google Scholar]
- 10.Mumby P, Elliott I, Eakin C. Reserve design for uncertain responses of coral reefs to climate change. Ecol Lett. 2011;14: 132–140. 10.1111/j.1461-0248.2010.01562.x [DOI] [PubMed] [Google Scholar]
- 11.Magris RA, Pressey RL, Weeks R, Ban NC. Integrating connectivity and climate change into marine conservation planning. Biol Conserv. 2014;170: 207–221. [Google Scholar]
- 12.Levy J, Ban N. A method for incorporating climate change modelling into marine conservation planning: An Indo-west Pacific example. Mar Policy. 2013;38: 16–24. [Google Scholar]
- 13.Mumby PJ, Harborne AR. Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One. 2010;5: 1–7. 10.1371/journal.pone.0008657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Game ET, McDonald-Madden E, Puotinen ML, Possingham HP. Should we protect the strong or the weak? Risk, resilience, and the selection of marine protected areas. Conserv Biol. 2008;22: 1619–1629. 10.1111/j.1523-1739.2008.01037.x [DOI] [PubMed] [Google Scholar]
- 15.McClanahan T, Donner S, Maynard J, MacNeil M, Graham N, Maina J, et al. Prioritizing key resilience indicators to support coral reef management in a changing climate. PLoS One. 2012;7: e42884 10.1371/journal.pone.0042884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maynard J, Marshall P, Johnson J, Harman S. Building resilience into practical conservation: identifying local management responses to global climate change in the southern Great Barrier Reef. Coral Reefs. 2010;29: 381–391. 10.1007/s00338-010-0603-8 [DOI] [Google Scholar]
- 17.Rowlands G, Purkis S, Riegl B, Metsamaa L, Bruckner A, Renaud P. Satellite imaging coral reef resilience at regional scale. A case-study from Saudi Arabia. Mar Pollut Bull. 2012;64: 1222–37. 10.1016/j.marpolbul.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Weeks R, Jupiter S. Adaptive comanagement of a marine protected area network in Fiji. Conserv Biol. 2013;27: 1234–44. 10.1111/cobi.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham NAJ, Jennings S, Macneil MA, Mouillot D, Wilson SK. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature. 2015;518: 94–97. 10.1038/nature14140 [DOI] [PubMed] [Google Scholar]
- 20.Spencer T, Teleki KA, Bradshaw C, Spalding MD. Coral Bleaching in the southern Seychelles during the 1997–1998 Indian Ocean warm event. Mar Pollut Bull. 2000;40: 569–586. 10.1016/S0025-326X(00)00026-6 [DOI] [Google Scholar]
- 21.West JM, Salm RV. Resistance and resilience to coral bleaching: Implications for coral reef conservation and management. Conserv Biol. 2003;17: 956–967. [Google Scholar]
- 22.Obura D, Mangubhai S. Coral mortality associated with thermal fluctuations in the Phoenix Islands, 2002–2005. Coral Reefs. 2011;30: 607–619. 10.1007/s00338-011-0741-7 [DOI] [Google Scholar]
- 23.Selig ER, Casey KS, Bruno JF. New insights into global patterns of ocean temperature anomalies: implications for coral reef health and management. Glob Ecol Biogeogr. 2010;19: 397–411. 10.1111/j.1466-8238.2009.00522.x [DOI] [Google Scholar]
- 24.Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA. Mechanisms of reef coral resistance to future climate change. Science. 2014;344: 895–8. 10.1126/science.1251336 [DOI] [PubMed] [Google Scholar]
- 25.Magris RA, Heron SF, Pressey RL. Conservation planning for coral reefs accounting for climate warming disturbances. PLoS One. 2015;10: e0140828 10.1371/journal.pone.0140828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szmant A. Nutrient enrichment on coral reefs: is it a major cause of coral reef decline? Estuaries. 2002;25: 743–766. [Google Scholar]
- 27.Hughes T, Baird A, Bellwood D, Card M, Connolly S, Folke C, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301: 929 [DOI] [PubMed] [Google Scholar]
- 28.Brown K, Adger W, Tompkins E, Bacon P. Trade-off analysis for marine protected area management. Ecol Econ. 2001;37: 417–434. [Google Scholar]
- 29.Ledlie MH, Graham NAJ, Bythell JC, Wilson SK, Jennings S, Polunin NVC, et al. Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs. 2007;26: 641–653. 10.1007/s00338-007-0230-1 [DOI] [Google Scholar]
- 30.McClanahan T, Polunin N, Done T. Ecological states and the resilience of coral reefs. Conserv Ecol. 2002;6: 18. [Google Scholar]
- 31.Grimsditch GD, Salm RV. Coral Reef Resilience and Resistance to Bleaching. Gland, Switzerland: IUCN; 2006. [Google Scholar]
- 32.McLeod E, Salm R, Green A, Almany J. Designing marine protected area networks to address the impacts of climate change. Front Ecol Environ. 2009;7: 362–370. 10.1890/070211 [DOI] [Google Scholar]
- 33.Fabricius KE, Mieog JC, Colin PL, Idip D, van Oppen MJH. Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol Ecol. 2004;13: 2445–58. 10.1111/j.1365-294X.2004.02230.x [DOI] [PubMed] [Google Scholar]
- 34.Yates KK, Rogers CS, Herlan JJ, Brooks GR, Smiley NA, Larson RA. Diverse coral communities in mangrove habitats suggest a novel refuge from climate change. Biogeosciences. 2014;11: 4321–4337. 10.5194/bg-11-4321-2014 [DOI] [Google Scholar]
- 35.Van Woesik R, Sakai K, Ganase A., Loya Y. Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser. 2011;434: 67–76. 10.3354/meps09203 [DOI] [Google Scholar]
- 36.Depczynski M, Gilmour JP, Ridgway T, Barnes H, Heyward AJ, Holmes TH, et al. Bleaching, coral mortality and subsequent survivorship on a West Australian fringing reef. Coral Reefs. 2013;32: 233–238. 10.1007/s00338-012-0974-0 [DOI] [Google Scholar]
- 37.Aronson R, Precht W, Macintyre I, Murdoch T. Coral bleach-out in Belize. Nature. 2000;405: 36 [DOI] [PubMed] [Google Scholar]
- 38.Williams ID, Polunin NVC. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs. 2001;19: 358–366. 10.1007/s003380000121 [DOI] [Google Scholar]
- 39.Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS. Recovery of an isolated coral reef system following severe disturbance. Science. 2013;340: 69–71. 10.1126/science.1232310 [DOI] [PubMed] [Google Scholar]
- 40.Mumby PJ, Hastings A. The impact of ecosystem connectivity on coral reef resilience. J Appl Ecol. 2008;45: 854–862. 10.1111/j.1365-2664.2008.01459.x [DOI] [Google Scholar]
- 41.Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, et al. Emerging marine diseases-climate links and anthropogenic factors. Science. 1999;285: 1505–1510. 10.1126/science.285.5433.1505 [DOI] [PubMed] [Google Scholar]
- 42.Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, et al. Indirect effects of algae on coral: Algae-mediated, microbe-induced coral mortality. Ecol Lett. 2006;9: 835–845. 10.1111/j.1461-0248.2006.00937.x [DOI] [PubMed] [Google Scholar]
- 43.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318: 1737–1742. 10.1126/science.1152509 [DOI] [PubMed] [Google Scholar]
- 44.Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17: 360–365. 10.1016/j.cub.2006.12.049 [DOI] [PubMed] [Google Scholar]
- 45.Mumby PJ. The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl. 2006;16: 747–769. [DOI] [PubMed] [Google Scholar]
- 46.McManus JW, Reyes RB, Nañola CL. Effects of some destructive fishing methods on coral cover and potential rates of recovery. Environ Manage. 1997;21: 69–78. 10.1007/s002679900006 [DOI] [PubMed] [Google Scholar]
- 47.Edinger EN, Jompa J, Limmon G V., Widjatmoko W, Risk MJ. Reef degradation and coral biodiversity in Indonesia: Effects of land-based pollution, destructive fishing practices and changes over time. Mar Pollut Bull. 1998;36: 617–630. 10.1016/S0025-326X(98)00047-2 [DOI] [Google Scholar]
- 48.Groves CR, Game ET, Anderson MG, Cross M, Enquist C, Ferdaña Z, et al. Incorporating climate change into systematic conservation planning. Biodivers Conserv. 2012;21: 1651–1671. 10.1007/s10531-012-0269-3 [DOI] [Google Scholar]
- 49.Jones KR, Watson JEM, Possingham HP, Klein CJ. Incorporating climate change into spatial conservation prioritisation: A review. Biol Conserv. 2016;194: 121–130. 10.1016/j.biocon.2015.12.008 [DOI] [Google Scholar]
- 50.Wilkinson C. Status of coral reefs of the world:2008 Townsville, Australia: Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre; 2008. [Google Scholar]
- 51.Onton K, Page C, Wilson S, Neale S, Armstrong S. Distribution and drivers of coral disease at Ningaloo reef, Indian Ocean. Mar Ecol Prog Ser. 2011;433: 75–84. [Google Scholar]
- 52.Moore JA, Bellchambers LM, Depczynski MR, Evans RD, Evans SN, Field SN, et al. Unprecedented mass bleaching and loss of coral across 12 degrees of latitude in Western Australia in 2010–11. PLoS One. 2012;7: e51807 10.1371/journal.pone.0051807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng M, McPhaden MJ, Xie S-P, Hafner J. La Niña forces unprecedented Leeuwin Current warming in 2011. Sci Rep. 2013;3: 1277 10.1038/srep01277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng M, Hendon HH, Xie S, Marshall AG, Schiller A, Kosaka Y, et al. Decadal increase in Ningaloo Niño since the late 1990s. Geophys Res Lett. 2015;42: 104–112. [Google Scholar]
- 55.Fox NJ, Beckley LE. Priority areas for conservation of Western Australian coastal fishes: A comparison of hotspot, biogeographical and complementarity approaches. Biol Conserv. 2005;125: 399–410. 10.1016/j.biocon.2005.02.006 [DOI] [Google Scholar]
- 56.Speed CW, Babcock RC, Bancroft KP, Beckley LE, Bellchambers LM, Depczynski M, et al. Dynamic stability of coral reefs on the West Australian coast. PLoS One. 2013;8: e69863 10.1371/journal.pone.0069863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cassata L, Collins L. Coral reef communities, habitats, and substrates in and near sanctuary zones of Ningaloo Marine Park. J Coast Res. 2008;24: 139–151. [Google Scholar]
- 58.Taebi S, Lowe RJ, Pattiaratchi CB, Ivey GN, Symonds G, Brinkman R. Nearshore circulation in a tropical fringing reef system. J Geophys Res. 2011;116: C02016 10.1029/2010JC006439 [DOI] [Google Scholar]
- 59.Falter JL, Zhang Z, Lowe RJ, McGregor F, Keesing J, McCulloch MT. Assessing the drivers of spatial variation in thermal forcing across a nearshore reef system and implications for coral bleaching. Limnol Oceanogr. 2014;59: 1241–1255. 10.4319/lo.2014.59.4.1241 [DOI] [Google Scholar]
- 60.Pearce AF, Feng M. The rise and fall of the “marine heat wave” off Western Australia during the summer of 2010/2011. J Mar Syst. 2013;111–112: 139–156. 10.1016/j.jmarsys.2012.10.009 [DOI] [Google Scholar]
- 61.Zinke J, Rountrey A, Feng M, Xie SP, Dissard D, Rankenburg K, et al. Corals record long-term Leeuwin current variability including Ningaloo Niño/Niña since 1795. Nat Commun. 2014;5: 3607 10.1038/ncomms4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MPRA (Marine Parks and Reserves Authority). Ningaloo Marine Park / Muiron Islands Marine Management Area Management Plan Periodic Audit Report. Perth, Western Australia; 2013.
- 63.Fletcher W, Santoro K. Status report of the fisheries and aquatic resources of Western Australia 2013/14 Western Australian Department of Fisheries, Perth, Western Australia; 2014. [Google Scholar]
- 64.Kobryn HT, Wouters K, Beckley LE, Heege T. Ningaloo Reef: Shallow marine habitats mapped using a hyperspectral sensor. PLoS One. 2013;8: e70105 10.1371/journal.pone.0070105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colquhoun J, Heyward A, Rees M, Twiggs E, Fitzpatrick B, Mcallister F, et al. Ningaloo Reef Marine Park benthic biodiversity survey Western Australian Marine Science Institution, Perth, Western Australia; 2007. [Google Scholar]
- 66.Geoscience Australia. Geodata Coast 100K data file. 2004.
- 67.Smallwood CB, Beckley LE, Moore SA, Kobryn HT. Assessing patterns of recreational use in large marine parks: A case study from Ningaloo Marine Park, Australia. Ocean Coast Manag. 2011;54: 330–340. 10.1016/j.ocecoaman.2010.11.007 [DOI] [Google Scholar]
- 68.Smallwood CB, Beckley LE, Moore SA. An analysis of visitor movement patterns using travel networks in a large marine park, north-western Australia. Tour Manag. 2011;33: 517–528. 10.1016/j.tourman.2011.06.001 [DOI] [Google Scholar]
- 69.CALM, MPRA (Conservation and Land Management, Marine Parks and Reserves Authority). Management plan for the Ningaloo marine park and Muiron Islands marine management area. 2005.
- 70.ESRI. Environmental Systems Research Institute Inc. 2015.
- 71.Weeks R, Russ GR, Bucol AA, Alcala AC. Shortcuts for marine conservation planning: The effectiveness of socioeconomic data surrogates. Biol Conserv. 2010;143: 1236–1244. 10.1016/j.biocon.2010.02.031 [DOI] [Google Scholar]
- 72.Ball I, Possingham H, Watts M. Marxan and Relatives: Software for Spatial Conservation Prioritization In: Moilanen A, Wilson K, Possingham H, editors. Spatial conservation prioritization: quantitative methods and computational tools. Oxford, UK: Oxford University Press; 2009. pp. 185–195. [Google Scholar]
- 73.Ardron J, Possingham H, Klein C. Marxan good practices handbook, version 2 Victoria, Canada: Pacific Marine Analysis and Research Association; 2010. [Google Scholar]
- 74.Ball I, Possingham H. MARXAN (V1. 8.2). Marine reserve design using spatially explicit annealing, a manual Brisbane: University of Queensland; 2000. p. 1:70. [Google Scholar]
- 75.ABPmer (ABP Marine Environmental Research Limited). ArcGIS 10 boundary tool. London UK; 2011.
- 76.Stewart RR, Possingham HP. Efficiency, costs and trade-offs in marine reserve system design. Environ Model Assess. 2005;10: 203–213. 10.1007/s10666-005-9001-y [DOI] [Google Scholar]
- 77.IUCN (International Union for the Conservation of Nature). The promise of Sydney: Recommendations of the 6th IUCN World Parks Congress 2014. IUCN World Parks Congress. Sydney; 2014.
- 78.Game ET, Grantham HS. Marxan user manual: For Marxan version 1.8.10 University of Queensland, St. Lucia, Queensland, Australia, and Pacific Marine Analysis and Research Association, Vancouver, British Columbia, Canada; 2008. [Google Scholar]
- 79.Harris LR, Watts ME, Nel R, Schoeman DS, Possingham HP. Using multivariate statistics to explore trade-offs among spatial planning scenarios. J Appl Ecol. 2014;51: 1504–1514. 10.1111/1365-2664.12345 [DOI] [Google Scholar]
- 80.Maina J, Jones K, Hicks C, McClanahan T, Watson J, Tuda A, et al. Designing climate-resilient marine protected area networks by combining remotely sensed coral reef habitat with coastal multi-use maps. Remote Sens. 2015;7: 16571–16587. 10.3390/rs71215849 [DOI] [Google Scholar]
- 81.Game ET, Watts M, Wooldridge S, Possingham H. Planning for persistence in marine reserves: a question of catastrophic importance. Ecol Appl. 2008;18: 670–680. [DOI] [PubMed] [Google Scholar]
- 82.Keller BD, Gleason DF, McLeod E, Woodley CM, Airame S, Causey BD, et al. Climate change, coral reef ecosystems, and management options for marine protected areas. Environ Manage. 2009;44: 1069–1088. 10.1007/s00267-009-9346-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Selig ER, Casey KS, Bruno JF. Temperature-driven coral decline: The role of marine protected areas. Glob Chang Biol. 2012;18: 1561–1570. 10.1111/j.1365-2486.2012.02658.x [DOI] [Google Scholar]
- 84.McLeod E, Moffitt R, Timmermann A, Salm R, Menviel L, Palmer MJ, et al. Warming seas in the coral triangle: coral reef vulnerability and management implications. Coast Manag. 2010;38: 518–539. 10.1080/08920753.2010.509466 [DOI] [Google Scholar]
- 85.Wilson SK, Depczynski M, Fisher R, Holmes TH, O’Leary R A, Tinkler P. Habitat associations of juvenile fish at Ningaloo Reef, Western Australia: the importance of coral and algae. PLoS One. 2010;5: e15185 10.1371/journal.pone.0015185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johansson CL, Bellwood DR, Depczynski M. Sea urchins, macroalgae and coral reef decline: A functional evaluation of an intact reef system, Ningaloo, Western Australia. Mar Ecol Prog Ser. 2010;414: 65–74. 10.3354/meps08730 [DOI] [Google Scholar]
- 87.Webster FJ, Babcock RC, Van Keulen M, Loneragan NR. Macroalgae inhibits larval settlement and increases recruit mortality at Ningaloo Reef, Western Australia. PLoS One. 2015;10: e0124162 10.1371/journal.pone.0124162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Game E, Grantham H, Hobday A, Pressey R, Lombard A, Beckley L, et al. Pelagic protected areas: the missing dimension in ocean conservation. Trends Ecol Evol. 2009;24: 360–369. 10.1016/j.tree.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 89.Grantham HS, Game ET, Lombard AT, Hobday A, Richardson A, Beckley L, et al. Accommodating dynamic oceanographic processes and pelagic biodiversity in marine conservation planning. PLoS One. 2011;6: e16552 10.1371/journal.pone.0016552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guidetti P, Notarbartolo-Di-Sciara G, Agardy T. Integrating pelagic and coastal MPAs into large-scale ecosystem-wide management. Aquat Conserv Mar Freshw Ecosyst. 2013;23: 179–182. 10.1002/aqc.2314 [DOI] [Google Scholar]
- 91.Klein CJ, Chan A, Kircher L, Cundiff AJ, Gardner N, Hrovat Y, et al. Striking a balance between biodiversity conservation and socioeconomic viability in the design of marine protected areas. Conserv Biol. 2008;22: 691–700. 10.1111/j.1523-1739.2008.00896.x [DOI] [PubMed] [Google Scholar]
- 92.Cheung WWL, Meeuwig JJ, Feng M, Harvey E, Lam VWY, Langlois T, et al. Climate-change induced tropicalisation of marine communities in Western Australia. Mar Freshw Res. 2012;63: 415–427. 10.1071/MF11205 [DOI] [Google Scholar]
- 93.McCook LJ, Ayling T, Cappo M, Choat JH, Evans RD, De Freitas DM, et al. Adaptive management of the Great Barrier Reef: a globally significant demonstration of the benefits of networks of marine reserves. Proc Natl Acad Sci U S A. 2010;107: 18278–85. 10.1073/pnas.0909335107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stewart R, Ball I, Possingham H. The effect of incremental reserve design and changing reservation goals on the long‐term efficiency of reserve systems. Conserv Biol. 2007;21: 346–354. [DOI] [PubMed] [Google Scholar]
- 95.Airamé S, Dugan J, Lafferty K, Leslie H, McArdle D, Warner R. Applying ecological criteria to marine reserve design: a case study from the California Channel Islands. Ecol Appl. 2003;13: 170–184. [Google Scholar]
- 96.Edgar G, Stuart-Smith R, Willis T, Kininmonth S, Baker S, Banks S, et al. Global conservation outcomes depend on marine protected areas with five key features. Nature. 2014;506: 216–221. 10.1038/nature13022 [DOI] [PubMed] [Google Scholar]
- 97.Babcock R, Haywood M, Vanderklift M, Clapin G, Kleczkowski D, Skewes T, et al. Ecosystem impacts of human usage and the effectiveness of zoning for biodiversity conservation: broad-scale fish census Final analysis and recommendations. CSIRO Marine and Atmospheric Research; 2008. [Google Scholar]
- 98.Mellin C, Aaron MacNeil M, Cheal AJ, Emslie MJ, Julian Caley M. Marine protected areas increase resilience among coral reef communities. Ecol Lett. 2016; 19: 629–637. 10.1111/ele.12598 [DOI] [PubMed] [Google Scholar]
- 99.Olds AD, Pitt KA, Maxwell PS, Babcock RC, Rissik D, Connolly RM. Marine reserves help coastal ecosystems cope with extreme weather. Glob Chang Biol. 2014;20: 3050–3058. 10.1111/gcb.12606 [DOI] [PubMed] [Google Scholar]
- 100.Jennings S, Marshall S, Polunin N. Seychelles’ marine protected areas: comparative structure and status of reef fish communities. Biol Conserv. 1996;75: 201–209. [Google Scholar]
- 101.McClanahan TR, Graham NAJ, Wilson SK, Letourneur Y, Fisher R. Effects of fisheries closure size, age, and history of compliance on coral reef fish communities in the western Indian Ocean. Mar Ecol Prog Ser. 2009;396: 99–109. 10.3354/meps08279 [DOI] [Google Scholar]
- 102.Hughes T, Graham N, Jackson J, Mumby P, Steneck R. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol. 2010;25: 633–42. 10.1016/j.tree.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 103.Harrison HB, Williamson DH, Evans RD, Almany GR, Thorrold SR, Russ GR, et al. Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr Biol. 2012;22: 1023–8. 10.1016/j.cub.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 104.Januchowski-Hartley FA, Graham NAJ, Cinner JE, Russ GR. Spillover of fish naïveté from marine reserves. Ecol Lett. 2013;16: 191–7. 10.1111/ele.12028 [DOI] [PubMed] [Google Scholar]
- 105.Chollett I, Mumby PJ. Reefs of last resort: Locating and assessing thermal refugia in the wider Caribbean. Biol Conserv. 2013;167: 179–186. 10.1016/j.biocon.2013.08.010 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
A) Datasets derived from single beam bathymetry that were used as environmental variables for modelling biota, substrate and fish abundance/richness, B) Model accuracy statistic AUC statistic for biotic and abiotic substrate predicted from the presence/absence models (blind validation n = 19872 data points).
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The minimum relevant data used for this study are within the paper and from Figshare at: https://figshare.com/articles/Davies_etal_2016_PLOSdatasets_xlsx/3513953. Deep water data are from the Western Australian Marine Science Study program 1.1.1 study whose authors Ben Radford and Andrew Heyward may be contacted via email at b.radford@aims.gov.au and a.heyward@aims.gov.au. Shallow water data are from a CSIRO Wealth from Oceans project and can be obtained from the author Halina Kobryn at h.kobryn@murdoch.edu.au. Human Use data is from a Murdoch University PhD thesis and can be obtained from the author Claire Smallwood at claire.smallwood@gmail.com.