Abstract
Purpose
To study the effects of masked auditory feedback (MAF) on speech fluency in adults with aphasia and/or apraxia of speech (APH/AOS). We hypothesized that adults with AOS would increase speech fluency when speaking with noise. Altered auditory feedback (AAF; i.e., delayed/frequency-shifted feedback) was included as a control condition not expected to improve speech fluency.
Method
Ten participants with APH/AOS and 10 neurologically healthy (NH) participants were studied under both feedback conditions. To allow examination of individual responses, we used an ABACA design. Effects were examined on syllable rate, disfluency duration, and vocal intensity.
Results
Seven of 10 APH/AOS participants increased fluency with masking by increasing rate, decreasing disfluency duration, or both. In contrast, none of the NH participants increased speaking rate with MAF. In the AAF condition, only 1 APH/AOS participant increased fluency. Four APH/AOS participants and 8 NH participants slowed their rate with AAF.
Conclusions
Speaking with MAF appears to increase fluency in a subset of individuals with APH/AOS, indicating that overreliance on auditory feedback monitoring may contribute to their disorder presentation. The distinction between responders and nonresponders was not linked to AOS diagnosis, so additional work is needed to develop hypotheses for candidacy and underlying control mechanisms.
Acquired apraxia of speech (AOS) is a neurologic disorder of speech production that, in the setting of stroke, usually results from lesions that include left inferior-posterior frontal cortex. The disorder is manifested through substituted and distorted articulation of speech sounds, slow rate of speech, and abnormal prolongation of pauses or sound segments (Wambaugh, Duffy, McNeil, Robin, & Rogers, 2006). 1 Speech fluency is often impaired in additional ways, with frequent attempts to self-correct errors, audible and visible groping of the articulators, and repetition of speech sounds and syllables (Duffy, 2013). These self-corrections often do not improve articulation accuracy (Marshall & Tompkins, 1982) and may exacerbate the communication problem due to misleading prosodic marking of revisions (Liss, 1998).
Few treatment studies have focused on reducing disfluencies in people with AOS (although see Brendel & Ziegler, 2008; Goldberg, Haley, & Jacks, 2012; Mauszycki & Wambaugh, 2008). Instead the literature has been largely focused on articulatory-kinematic treatment approaches for AOS, with most outcomes including accuracy of speech sound production (Ballard et al., 2015; Wambaugh et al., 2006). The focus of the present research is to determine if masked auditory feedback (MAF) can increase speech fluency in adults with AOS.
The study was motivated by a theoretical account of how MAF may affect speech fluency in AOS. Effects on left-hemisphere stroke survivors with other impairment profiles were also examined as a point of comparison and because the majority of the extant literature on the effects of auditory feedback modification was based on study participants from a broad population of people with aphasia.
Disfluency in Aphasia and AOS
Fluency in aphasia is linked to diverse speech and language qualities (Feyereisen, Pillon, & de Partz, 1991; Gordon, 1998). Although early work attributed much of the fluency distinction to phrase length (Goodglass, Quadfasel, & Timberlake, 1964), other factors, including impaired grammatical form, articulatory struggle, effortful initiation, reduced speech rate, abnormal prosody, and awkward articulation, are routinely included in the concept (Benson, 1967; Goodglass et al., 1964; Park et al., 2011).
Disfluent speech production is a prominent feature of AOS as well. When Darley (1968) described the disorder, he noted that it was characterized by repetitious output that “sounds much like stuttering” (p. 9). Repetition of speech sounds is a common disfluency in AOS, comprising 18% of speech errors in one early study (Johns & Darley, 1970) and 6% of all productions in another (Trost, 1970). More complex disfluencies also occur, reflecting apparent attempts to repair inaccurate productions. These are described variously in the literature as false starts (Johns & Darley, 1970), re-approach behavior (Trost, 1970), and revisions (Liss, 1998). They have in common the production of a final utterance preceded by audible vocal output, including one or more speech sounds differing from the target (e.g., filler words er, um), and they are quite common, occurring in 12% of all productions in one study (Trost, 1970) and 18% in another (McNeil, Odell, Miller, & Hunter, 1995).
Self-corrections are also common, and possibly more prominent, in conduction aphasia. Odell, McNeil, Rosenbek, and Hunter (1991) noted a high rate of apparent “struggle” behavior in study participants with AOS, but an even higher rate in participants with conduction aphasia. Likewise, McNeil et al. (1995) found that attempts (i.e., unsuccessful production attempts resulting in revisions), although common in speakers with AOS, were even more common in speakers with conduction aphasia. Disfluency may also be observed during word-finding problems and is therefore linked to a variety of aphasia profiles.
In addition to repetitions and revisions, prosodic and articulatory features of AOS contribute to the perception of disfluent output. Overall slow rate of speech (Kent & Rosenbek, 1983), prolongation of sound segments, and silent intervals between segments (McNeil, Liss, Tseng, & Kent, 1990; Odell et al., 1991; Strand & McNeil, 1996) also represent deviations from the normal time course of speech production, thus affecting the forward flow of speech.
Regardless of the cause, speech disfluency can be problematic for the listener, requiring greater effort to process and resulting in poorer recall of messages (Panico & Healey, 2009) and making speakers vulnerable to losing their conversational turn (Perkins, 1995). Self-corrections may be justified if they improve the eventual communicative message. However, several studies unfortunately have found that self-corrections for speakers with nonfluent aphasia or AOS are successful less than half of the time (Farmer, 1978; Marshall & Tompkins, 1982; McNeil et al., 1995).
The Impaired Feedforward, Intact Feedback Hypothesis for AOS
The speech impairment in AOS is thought to reflect damage to learned motor programs for speech production (McNeil, Robin, & Schmidt, 2008). In the directions into velocities of articulators (DIVA) model of speech processing (Guenther, Ghosh, & Tourville, 2006), speech motor programs are conceptualized as “feedforward” control processes that represent mappings between phonemic representations and spatial and temporal movement parameters. These mappings are learned during development and allow the mature speaker to produce rapid, accurate, and consistent speech movements in a variety of contexts. The feedforward system relies on feedback from both somatosensory and auditory processing systems to evaluate speech output. The feedback processing system for speech is slower than the feedforward system, but allows the speaker to make adjustments for altered speaking conditions (e.g., speaking with a pencil between the teeth or with a dental appliance) or to repair errors.
In previous work, we have suggested that speech characteristics of AOS reflect impaired feedforward control as well as spared or overactive somatosensory and auditory feedback (Jacks, 2008; see also Maas, Mailend, & Guenther, 2015; Robin, Jacks, Hageman, Clark, & Woodworth, 2008; Rogers, Eyraud, Strand, & Storkel, 1996). Therefore, imprecise articulation and difficulty producing complex articulatory combinations may be explained by impairment of the feedforward system, whereas the slow rate of speech and prolongation of vowels and consonants may be accounted for by compensatory use of the slower operating feedback processing for speech. In addition, overreliance on feedback control may cause a speaker to be hypervigilant to the speech errors, such that he or she repeatedly interrupts and repairs speech production, resulting in unproductive disfluencies such as those described in the previous section.
Predictions of MAF on Speech Output
The feedforward-impaired, feedback-spared account of AOS leads to some logical predictions in running speech, particularly when considering what happens when access to the feedback system is diminished—for example, by masking the auditory signal with noise. On the one hand, if people with AOS use auditory information to monitor articulatory performance and provide corrective feedback, then masking this signal may result in less precise speech output. For example, neurologically healthy (NH) speakers may produce “blurred” or less intelligible speech when talking in noise (Pickett, 1958; Pitman, 1943; but see Van Summers et al., 1988).
On the other hand, if attending to speech errors results in speech disfluencies in people with AOS, then speaking while listening to noise may allow them to reduce these disfluencies by reducing access to the auditory signal. MAF has often been shown to temporarily reduce disfluencies in persons who stutter (e.g., Andrews, Howie, Dozsa, & Guitar, 1982; Cherry, Sayers, & Marland, 1955; Ingham et al., 2009; Sternberg, 1946). Although AOS and stuttering are not the same disorder, auditory masking is generally thought to affect persons who stutter by suppressing a hypervigilant auditory perception system (e.g., Cherry et al., 1955; Civier, Tasko, & Guenther, 2010; but see Postma & Kolk, 1993; Wingate, 1970). Because this is similar to the mechanism we have hypothesized for AOS, we reasoned that auditory masking might have a fluency-inducing effect in this population.
Effects of MAF on Speech Output in Aphasia and AOS
Early research suggested that auditory masking improves verbal output in people with aphasia (e.g., Birch & Lee, 1955). However, this literature is marked by discrepant findings and is limited by sparse and inconsistent characterization of the disorder presentation and the affected speech behavior. Birch and colleagues (Birch, 1956; Birch & Lee, 1955) found that a majority of speakers with motor aphasia increased the quantity and “enunciation” of verbal output and decreased the response latency when reading or naming words while hearing a 60–80 dB pure tone presented over headphones. Later studies were unable to replicate Birch's strong findings in people diagnosed with expressive aphasia (Weinstein, 1959; Wertz & Porch, 1970) or in people diagnosed with AOS (Deal & Darley, 1972). These early researchers examined diverse outcome measures, including naming accuracy (Weinstein, 1959), speech errors (Deal & Darley, 1972), and a multidimensional rating of speech quality (Wertz & Porch, 1970), but did not consider potential changes to speech fluency. More recently, two studies (Manasco, Dagenais, Holdsworth, & Brown, 2010; Manasco, 2008) examined the effects of auditory masking on fluency in 13 adults with aphasia. As in earlier studies, the results were mostly negative, with only one participant decreasing disfluencies with masking noise and one other reducing speech errors. Although these findings are not promising for potential effects of masking on speech fluency, the speech task used—primarily single-word repetition—did not provide realistic opportunities for speech disfluency.
Two studies have examined the effects of auditory masking on acoustic measures of speech production in adults with AOS and aphasia. Rogers et al. (1996) tested the effects of auditory masking noise on vowel durations in monosyllabic words for three speakers with AOS. They reasoned that if people with AOS lengthen vowels due to a compensatory feedback processing strategy, then blocking access to auditory feedback would reduce vowel duration. Contrary to this prediction, vowel productions for two of the three participants were actually longer in noise than in quiet. The result is in keeping with reports that speaking in noise leads to longer vowel durations, both for NH speakers and for people with AOS or aphasia (Maas et al., 2015).
Maas et al. (2015) hypothesized that inability to compensate via auditory feedback mechanisms for impaired feedforward control would result in reduced vowel space. A group of six speakers with AOS showed significantly reduced vowel contrast when speaking in noise, whereas older controls and adults with aphasia who did not have AOS did not show this effect. Although these results appear to support the feedforward-impaired, feedback-spared account of AOS, individual results showed that this group difference was driven by only three of the six participants with AOS, reflecting similar individual variability as seen in previous studies.
In summary, the literature on masking studies in APH/AOS remains sparse, and the studies available are mixed with respect to the predictions we advanced from the feedforward-impaired, feedback-spared account of AOS. With respect to effects on articulatory precision, Birch (1956) reported that 75% of her participants improved verbal performance with masking noise; however, details were limited to subjective description of speech. Later studies resulted in mixed results (Deal & Darley, 1972; Manasco et al., 2010; Manasco, 2008; Rogers et al., 1996; Weinstein, 1959; Wertz & Porch, 1970), whereas the most recent study (Maas et al., 2015) supported the notion that speakers with AOS are less precise when speaking in noise. The effect of masking noise on disfluency was only studied by Manasco, also showing mixed results (Manasco, 2008; Manasco et al., 2010).
Effects of Altered Auditory Feedback on Speech Output in Aphasia and AOS
We began this line of research with the prediction that MAF would benefit speech fluency in adults with AOS. However, to determine if effects were specific to auditory masking, we included a second feedback condition that has enhanced fluency in other populations. We chose to alter the speaker's auditory perception of his or her own speech with a combination of delayed auditory feedback (DAF) and frequency shifted feedback—together considered altered auditory feedback (AAF). This was a natural choice, because delayed and frequency shifted feedback have been documented to reduce disfluencies in persons who stutter (Howell, El-Yaniv, & Powell, 1987; Ryan & Van Kirk, 1974; Stuart, Kalinowski, Rastatter, Saltuklaroglu, & Dayalu, 2004). However, because delayed feedback often results in slowed speech rate—the opposite of our prediction for auditory masking—AAF provides an opportunity to observe a distinct response pattern.
Although many studies have demonstrated fluency-inducing effects of AAF in persons who stutter, it is unclear by what mechanism this occurs (Alm, 2004; Kalinowski & Saltuklaroglu, 2003; Stuart et al., 2004). Recent evidence suggests that stuttering may be caused by slowed integration of auditory feedback with corrective motor commands for ongoing movements (Cai, Beal, Ghosh, Guenther, & Perkell, 2014; Loucks, Chon, & Han, 2012). It is possible that by artificially delaying the presentation of feedback, the auditory system is able to more effectively integrate the information with ongoing speech processes. Notably, delayed feedback usually results in a slowed speech production rate (Lee, 1950), likely an adaptation to allow feedback to be received more synchronously with speech commands. This slow speech rate with DAF led early researchers to attribute increased fluency to the speed itself (Wingate, 1970). However, Kalinowski and colleagues (Kalinowski, Armson, & Stuart, 1995; Macleod, Kalinowski, Stuart, & Armson, 1995; Stuart, Kalinowski, Armson, Stenstrom, & Jones, 1996) have repeatedly shown that stuttering can be reduced with delayed and frequency-shifted feedback while maintaining a normal or even fast rate. Nevertheless, Howell and colleagues (Howell, Au-Yeung, & Pilgrim, 1999; Howell & Sackin, 2000) attributed the fluency effect with AAF to slowed articulation at the syllable level (i.e., increased syllable durations), suggesting that the overall normal speech rates found by Kalinowski et al. (1995) were achieved by reducing longer pauses in the utterances.
If AAF operates on stuttering disfluencies by allowing additional time for a slowed auditory processing system to integrate feedback with ongoing movements, then we would not expect increased fluency for persons with AOS, for whom auditory feedback is generally considered to be unimpaired (e.g., Square-Storer, Darley, & Sommers, 1988). In fact, we might expect people with AOS to reduce their already slow speech rate with AAF, assuming their auditory feedback processing is normal. In contrast, persons with aphasia characterized by some level of auditory processing impairment and faster speech rate might benefit from fluency inducing effects of AAF, by slowing the speech rate and allowing more time for processing auditory input.
Frequency-shifted feedback has not yet been explored in adults with aphasia or AOS, though effects of delayed feedback have been reported. In the earliest study of DAF in aphasia, Stanton (1958) found that most participants with expressive-only aphasia responded to a 180-ms delay in a manner similar to NH controls—with prolonged vowels (i.e., slowed rate), flattened intonation, sound repetitions, and substitution errors. In other words, DAF worsened, rather than improved, speech accuracy and fluency in participants with expressive aphasia. In contrast, the majority of participants with some receptive impairment improved speech production with the delay. Later studies also suggest a difference in response according to aphasia type. For example, Boller and colleagues (Boller, Vrtunski, Kim, & Mack, 1978; Chapin, Blumstein, Meissner, & Boller, 1981) found that speakers with Broca's aphasia responded to DAF in a similar manner as NH speakers—with longer vowel durations, greater intensity, and increased errors, whereas people with fluent aphasia types (e.g., Wernicke's, conduction, posterior) showed little change in vowel duration and intensity. Consistent with Stanton's report, Chapin et al. (1981) also found that speakers with conduction aphasia reduced repetitions and sound substitutions with DAF, whereas those with Broca's aphasia increased repetitions and substitution errors. Although these reports do not provide a definitive explanation for the improvement with DAF in adults with conduction aphasia or receptive involvement, the results indicate that speakers may benefit from a delayed signal by allowing them additional time to process auditory feedback information.
One study examined the effect of DAF in people with dyspraxia of speech (i.e., AOS). Lozano and Dreyer (1978) studied the effect of 180-ms DAF on word production in five adults with AOS and minimal aphasia. 2 Although there was no group effect on disfluencies or speech errors, individual speakers showed different response patterns. Two participants produced fewer repetitions, self-corrections, and pauses under DAF conditions, whereas one participant clearly increased repetitions and pauses.
Individual Variability
The question of whether MAF has a beneficial or detrimental effect on speech in adults with aphasia or AOS has been revisited several times in the past 60 years (Birch, 1956; Manasco et al., 2010; Weinstein, 1959; Wertz & Porch, 1970). In each study except for Birch, the conclusion was that masking was not generally beneficial to the participants studied, and this was the message passed forward to future researchers. Despite the overall negative reports, however, there were participants in each study who benefited, at least in a subset of tasks. To us, this suggests that MAF may yet provide clinical benefit to some—though not all—participants with aphasia or AOS. The observation of individual differences in previous work strongly informed our approach in this study. In particular, although our predictions are based on a theoretical concept of AOS, the variable results from prior studies led us to consider each participant individually to determine whether they responded to the masking condition or not. In this report we will consider the characteristics of responders and nonresponders to seek potential commonalities—related to diagnosis type or other factors—that may predict a potential response in other participants not yet observed.
Purpose
The purpose of this study was to investigate the effect of MAF on speech fluency in adults with aphasia and AOS (APH/AOS), with comparison to delayed and frequency-shifted (i.e., altered) auditory feedback (AAF). We hypothesized that participants with AOS would increase speech rate or decrease duration of disfluencies under MAF due to decreased distraction from auditorily perceived errors. In contrast, we predicted a slower speech rate with AAF in people with AOS, as found previously for many speaker groups. Performance of NH speakers also was examined to serve as a reference point for typical response to these conditions.
Method
Participants
The participants were 10 adults (five women, five men) with speech or language impairments due to left-hemisphere damage to the brain (see Table 1). 3 In all cases but one, the injury was due to a left-hemisphere stroke; P2 experienced focal damage due to blunt force trauma to the head. Nine participants were native speakers of English; one individual was a native speaker of an African creole language (P9). Five participants were excluded from the study because they were unable to attempt sentence production, two because they had no perceptible speech impairment, and one due to a moderate cognitive impairment. Ten neurologically healthy participants were also recruited as controls (five women; five men, average age 63;10 [years;months]; range = 35;4–75;10). All participants were native speakers of English, passed a pure-tone hearing screening, and scored 0 on the Questionnaire for Verifying Stroke-Free Status (Jones, Williams, & Meschia, 2001). The study was approved by the University of North Carolina at Chapel Hill Institutional Review Board, and all participants provided signed consent.
Table 1.
Participant information.
| Participant ID | Age | Sex | Time postonset | Single word intell. | Speech diagnosis | Harsh vocal rating | Hypernasal rating | Hearing screen level | TONI (%) | WAB-R Results |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQ | Type | Fluency | Spont. speech | Aud. Comp. | Repetition | Naming | ||||||||||
| P1 | 66;6 | F | 2;3 | 21 | AOS | Mod. | Mild | 25 | 37 | 76 | Broca | 4 | 13 | 9.6 | 6.8 | 8.4 |
| P2 a | 30;3 | F | 0;3 | 87 | AOS | Equiv. | Equiv. | 25 | 32 | 96 | Anomic | 9 | 19 | 10 | 9.2 | 9.8 |
| P3 | 39;10 | M | 1;1 | 93 | MIN | Norm. | Norm. | 25 | 58 | 85 | Anomic | 9 | 18 | 8.4 | 7.5 | 8.8 |
| P4 | 52;6 | M | 0;6 | 44 | AOS b | Mild-Mod | Equiv. | 40 | 39 | 30 | Broca | 3 | 5 | 5 | 4 | 1 |
| P5 | 46;2 | F | 6;2 | 92 | BORD c | Norm. | Norm. | 40 | 21 | 94 | Anomic | 9 | 19 | 10 | 8.8 | 9 |
| P6 | 66;8 | F | 0;5 | 55 | AOS | Equiv. | Equiv. | 40 | 37 | 60 | Broca | 2 | 11 | 7.8 | 4.8 | 6.7 |
| P7 | 47;2 | M | 0;5 | 95 | APP | Equiv. | Equiv. | 25 | 19 | 93 | Anomic | 9 | 19 | 9.4 | 9.7 | 8.5 |
| P8 | 80;8 | M | 13;2 | 70 | AOS | Mild | Norm. | 40 | 17 | 69 | Cond. | 6 | 14 | 9.3 | 6 | 5.1 |
| P9 | 66;7 | M | 2;7 | 58 | DYS d | Mild-Mod | Mild | 25 | 24 | 76 | Anomic | 5 | 14 | 7.6 | 8.2 | 8.4 |
| P10 | 39;3 | F | 0;1 | 35 | BORD e | Mild | Equiv. | 25 | 17 | 70 | Transcortical Motor | 4 | 12 | 8.4 | 8 | 6.8 |
Note. TONI = Test of Nonverbal Intelligence (Brown et al., 2010); WAB-R = Western Aphasia Battery- Revised (Kertesz, 2006); AQ = aphasia quotient; AOS = apraxia of speech; MIN = minimal speech impairment; BORD = borderline AOS; APP = aphasia with phonemic paraphasia; DYS = dysarthria; Aud. Comp. = auditory comprehension. Age and Time post-onset are represented in years and months (YY;MM). Hearing screening was completed at 25 dB at 0.5, 1, 2, 4, 6, and 8 kHz or at 40 dB at 1, 2, and 4 kHz. Ratings for harsh vocal quality and hypernasality were completed on a rating scale with the following levels: Normal (Norm.); Mild, Equivocal (Equiv.); Mild, Mild-Moderate (Mild-Mod.), Moderate, Severe, and Profound.
P2 experienced a focal traumatic injury, whereas all others had survived stroke.
P4 had all signs of AOS in multisyllabic words (articulatory distortions, slow rate, and prolongations); however, in sentences slow rate and prolongations occurred rarely.
Borderline AOS classification was identified in P5 due to articulatory distortions in approximately half of multisyllabic words and less than half of sentences, and abnormal prosody at the sentence level only.
P9 was a nonnative speaker of English, with voice characteristics consistent with a unilateral upper motor neuron dysarthria.
Borderline AOS classification was identified in P10 because of articulatory distortions, but she had no evidence of dysprosody.
Evaluation
Testing included administration of the Western Aphasia Battery–Revised (WAB-R; Kertesz, 2006), the Test of Nonverbal Intelligence, Fourth Edition (TONI-IV; Brown, Sherbenou, & Johnsen, 2010), an oral mechanism examination, a 38-item Motor Speech Examination, single-word intelligibility testing (Haley, Roth, Grindstaff, & Jacks, 2011), and hearing screening (see Table 1 for individual results). Aphasia Quotients (AQs) from the WAB-R ranged from 30 to 96, with a median of 76. Three participants profiled with Broca's, five with anomic, one with transcortical motor, and one with conduction aphasia. On the TONI-IV, percentile scores ranged from 17 to 58, with a median of 28. Single-word intelligibility ranged from 21% to 95%, with a median of 64%. Six participants passed pure-tone hearing screening at 25 dB bilaterally (0.5, 1, 2, 4, 6, and 8 kHz), whereas three passed at a more lenient 40 dB criterion for 0.5, 1, and 2 kHz in at least one ear (Weinstein & Ventry, 1983).
Differential Diagnosis of AOS
Diagnoses of AOS (n = 5), aphasia with phonemic paraphasia (APP; n = 1), minimal speech impairment (MIN; n = 1), or borderline AOS (n = 2) were determined on the basis of consensus ratings of segmental errors and temporal prosody by the authors and one additional rater—all certified speech-language pathologists (see Table 1 for participant diagnoses and the online supplemental material for detail on the diagnostic process, including Supplemental Table 1 for consensus ratings for each participant). In one case (P4), an atypical presentation of AOS was noted. Although pervasive speech sound errors were noted in both multisyllabic words and sentences, the prosodic features of AOS (slow rate and prolongations) were present only in multisyllabic words and not in sentences. In another case (P9), the presence of dysarthria and second-language speech features predominated, and none of the above diagnoses was deemed appropriate. When dysarthria was present in other speakers, the features of the diagnosed disorder (e.g., AOS, APP) were considered more salient than those due to dysarthria alone.
Dysarthria Ratings
To determine the presence of dysarthria, ratings of vocal quality and nasal resonance were completed by the authors (see the online supplemental material). Four participants had evidence of unilateral upper-motor neuron dysarthria based on mild (P8) or moderate (P4) harsh vocal quality, moderate strained-strangled quality (P1), or moderate hoarse quality (P9). Imprecise consonants and intermittent hypernasality were also noted, but were not used for dysarthria diagnosis, due to the confounding presence of AOS with segmental distortion and distorted substitution/addition errors. Confirmatory nonspeech signs of dysarthria (asymmetric smile, weak lip seal, and asymmetric velar elevation) were present in the four participants with phonatory involvement.
Procedures
A treatment introduction and withdrawal design were used to test the effects of modified feedback on speech fluency. Participants produced sentences in an ABACA paradigm, with normal auditory feedback (NAF) in the A conditions and modified feedback in the B and C conditions. For eight participants, AAF (i.e., delayed and pitch-shifted) was the first modified condition; in the remaining two (P8, P9), auditory masking was the first modified condition. Each experimental phase included 20 sentence trials, with stimuli drawn from the Harvard sentences corpus (IEEE Audio and Electroacoustics Group, 1969), which includes 72 phonetically balanced lists of 10 sentences. A different set of 20 sentences was used for each phase, and they were presented in random order.
Each trial began by presenting the written sentence on the screen, accompanied by a recording of the sentence. When the participant was ready to produce the sentence, the investigator advanced the computer program. The written cue disappeared for 3 s while the condition was introduced (i.e., silence for NAF and AAF; masking noise for MAF). Following the 3-s delay, the written sentence reappeared, cueing the participant to begin speaking. During normal feedback conditions, participants were able to hear their voice as normal, as the audio channel for the microphone was routed to their earphones using the sound card control panel. Throughout the masked and altered conditions, the microphone channel was muted so that participants could only hear the masking noise or the delayed and shifted version of their voice via air conduction. In the masking condition, the noise was turned off immediately following each trial to allow the auditory cue for the following trial to be heard. Prior to the experimental trials, participants were introduced to the listening conditions and the sequence of trials during short practice sessions, including two sentences per condition. Participants were instructed to speak at a natural rate and loudness, regardless of the auditory condition.
Experimental tasks were presented with Alvin2 software (Hillenbrand & Gayvert, 2005) on a PC running 64-bit Windows 7. Speech was recorded with a headset microphone via an external USB sound card (M-Audio Fast Track Ultra; Avid Technologies, Inc., Burlington, MA). Audio was recorded using a headset microphone (C555L; AKG Acoustics GmbH, Vienna, Austria), and audio stimuli were delivered via foam-tipped earphones (ER-3A; Etymotic Research, Inc., Elk Grove Village, IL).
In the masking condition, pink noise was delivered binaurally at 85 dB SPL, calibrated using a Larson-Davis System 824 sound level meter (Depew, NY) with a 2-cc coupler (GRAS RA0038, Holte, Denmark). Pink noise, a random signal similar to white noise, was used as a masker due to its characteristics of having greater intensity at lower frequencies and lower intensity at higher frequencies, similar to the spectrum of human speech. In the AAF condition, our goal was to induce a delay of 100 ms and a one-octave frequency shift, consistent with AAF parameters used in persons who stutter (Lincoln, Packman, Onslow, & Jones, 2010). For the two participants tested first (P1, P6), the altered feedback was generated with Live Lite 8 software(Ableton, Inc., Pasadena, CA) with a plugin to shift frequencies down by one octave. The time delay was 116 ms for P1 but only 25 ms for P6. In order to improve the consistency of the temporal delay and to allow us to use an upward frequency shift, a custom command-line utility was developed to alter feedback for the remaining participants. This command-line utility was based on the Synthesis ToolKit in C++ (Cook & Scavone, 2012) and consistently yielded a one-octave upward shift and 106-ms delay. Accuracy of the auditory manipulation was confirmed by creating a two-channel recording with the microphone input in the first channel and the altered auditory signal in the second channel. This allowed us to measure both the temporal delay and the frequency shift.
Speech Coding
The effect of masking on speech production was assessed through measurement of speech rate (syllables/second), disfluency duration (seconds), and vocal intensity (dB). Prior to manual coding, a series of processing steps was completed using Praat (Boersma & Weenink, 2014) with custom scripts. First, vocal intensity in decibels was obtained from each speech sample for later analysis by condition. The samples then were intensity-normalized to a common value to reduce potential listener bias in manual coding of disfluencies. An automated syllable identification routine was used to locate syllable peaks, based on a script by De Jong and Wempe (2009). The script was modified to identify syllable boundaries, using an iterative algorithm using intensity-based thresholds (Haley, Jacks, de Riesthal, Abou-Khalil, & Roth, 2012; Rosen, Kent, Delaney, & Duffy, 2006). Syllable peaks and boundaries were saved to Praat TextGrids for later editing by research assistants.
Manual Correction of Syllables
Praat TextGrids were corrected by inserting syllable peaks and boundaries that were missed by the automated script and by deleting aberrant peaks and syllable boundaries detected by the script. Editing judgments were based on a combination of auditory perception via headphones and visual analysis of wideband spectrograms and intensity contours.
Coding of Disfluency Duration and Syllable Rate Calculation
Disfluency duration was defined as the total duration of intervals in each sentence that was coded as disfluent. Although six specific disfluency categories were coded (see below), disfluency duration depended on the placement of temporal boundaries of disfluent intervals rather than the differentiation among disfluency types. Coders were instructed to mark disfluency boundaries such that if these intervals were deleted from the sound file, the remaining signal would be maximally fluent. Six disfluency types coded in Praat TextGrids were included: (a) repetitions of speech sounds, words, or phrases; (b) self-corrections/revisions; (c) filler words; (d) pauses greater than 0.5 s; (e) filled pauses (i.e., pauses interrupted with filler words); and (f) vowel or consonant prolongations. Coders were instructed to use their own perceptual judgment to determine whether a vowel or consonant was abnormally prolonged; when such segments occurred, the entire segment was coded as prolonged. After syllable peaks and boundaries had been verified manually, syllable rate was calculated automatically as the total number of syllables produced divided by the total time of each utterance.
Reliability
Two graduate students in speech-language pathology independently coded 100% of the samples from seven of the participants. Reliability between listeners for syllable rate and disfluency duration measures was assessed using a Pearson correlation, with median coefficients of 0.93 for syllable rate (minimum = 0.87, maximum = 0.97) and 0.91 for disfluency duration (minimum = 0.73, maximum = 0.96). Three other participants were coded by only one student, who had demonstrated high interrater reliability (mean Pearson correlation = 0.91 across five participants). Two primary coders, with primary coder defined as the first person to begin coding for each speaker, provided data for the study.
Data Analysis
Visual Inspection
To establish experimental control according to the logic of the single-case research design and demonstrate that fluency measures are related to the listening condition, it is necessary to observe change in the dependent measure both on introduction and withdrawal of a given condition (e.g., increased rate with masking noise, decreased rate on removal). To this end, median values for adjacent conditions, illustrated in Figures 1 and 2, were compared.
Figure 1.
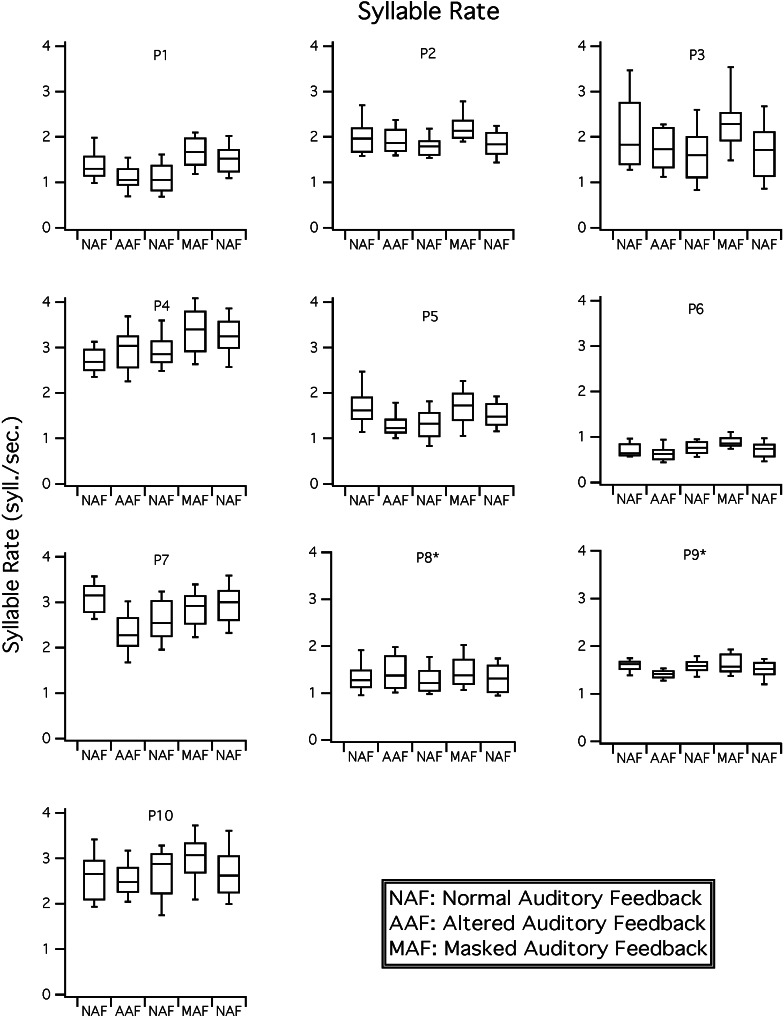
Syllable rate results. Boxplots for rate (syllables per second) are shown for each participant in NAF, delayed and frequency shifted feedback (i.e., AAF), and MAF conditions. Participants 8 and 9 received the conditions with MAF in the second position and AAF in the fourth position; these conditions were presented in the reverse order for all other participants.
Figure 2.
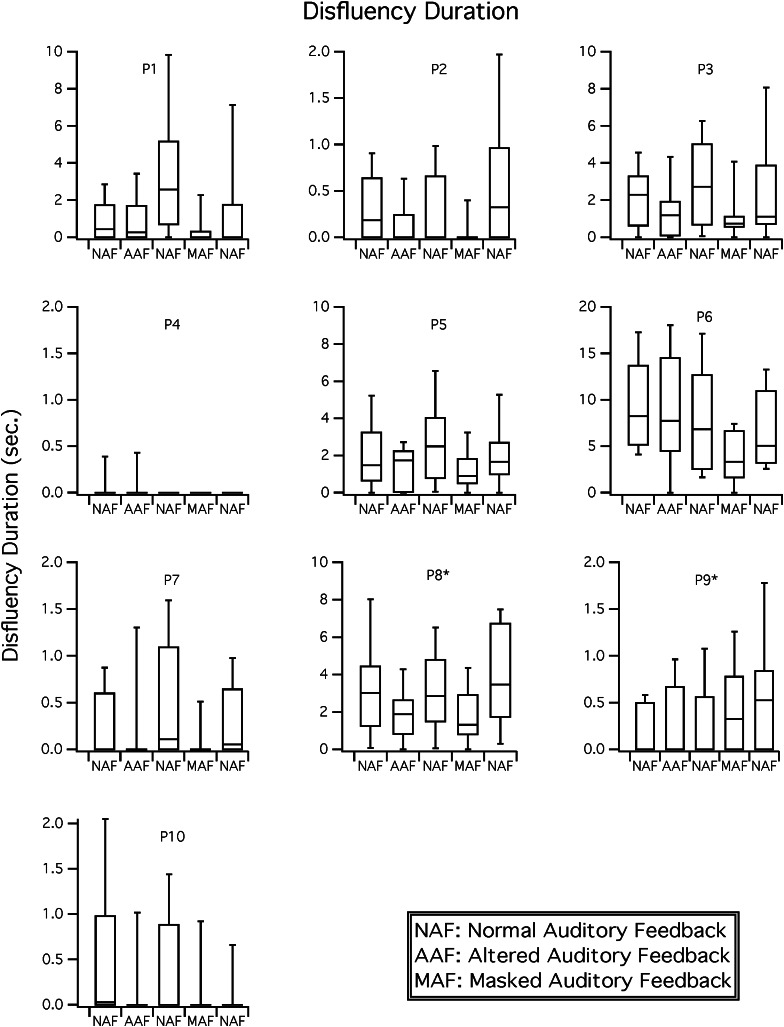
Disfluency duration results. Boxplots for disfluency duration (in seconds) are shown for each participant in NAF, delayed and frequency shifted feedback (i.e., AAF), and MAF conditions. Participants 8 and 9 received the conditions with MAF in the second position and AAF in the fourth position; these conditions were presented in the reverse order for all other participants.
Nonparametric Tests
Due to skewed distributions and unequal variances between conditions for some measures, nonparametric tests were used to compare performance across phases within each participant. First, Kruskal–Wallis tests were run for each of the dependent measures in all participants to determine whether there was an overall effect of condition. Wilcoxon rank-sum tests were used to compare dependent measures for each significant Kruskal–Wallis test, with separate comparisons between each consecutive phase (B vs. A, A′ vs. B, etc.). Tests were completed using JMP statistical software (SAS Institute, Inc., Cary, NC), and results reported for the two-sample normal approximation test, using the Z statistic. Significance values for the Wilcoxon analyses were adjusted to control false discovery rate for multiple comparisons using the Benjamini–Hochberg procedure (Benjamini & Yekutieli, 2001). Statistical comparisons of the medians are presented in Tables 2 and 3 for effects of MAF and AAF, respectively.
Table 2.
Masked auditory feedback (MAF) effects on syllable rate, disfluency duration, and vocal intensity.
| Participant ID | Speech diagnosis | Syllable rate |
Disfluency duration |
Vocal intensity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level change |
Variability change |
Level change |
Variability change |
Level change |
|||||||
| MAF effect | MAF release | MAF effect | MAF release | MAF effect | MAF release | MAF effect | MAF release | MAF effect | MAF release | ||
| P1 | AOS | 4.21*** | −1.53 | — | — | −3.34* | 0.71 | −11.30** | 0.53 | 5.37*** | −5.33*** |
| P2 | AOS | 4.04*** | −3.18* | — | — | −1.64 | 2.79* | −3.34 | 17.27*** | 5.40*** | −5.40*** |
| P3 | MIN | 2.80* | −2.31* | — | — | −2.64* | 1.7 | −8.22* | 3.34 | 5.40*** | −5.40*** |
| P4 | AOS | 2.69* | −0.74 | — | — | — | — | — | — | 5.40*** | −5.40*** |
| P5 | BORD | 2.58* | −1.37 | — | — | — | — | — | — | 5.40*** | −5.40*** |
| P6 | AOS | 2.50* | −2.85* | — | — | −2.33 | 2.39 | — | — | 4.58*** | −5.13*** |
| P7 | APP | 1.64 | 0.61 | — | — | −2.49* | 2.26 | −15.95*** | 13.75** | 5.40*** | −5.40*** |
| P8 a | AOS | — | — | — | — | −1.83 | 1.83 | −2.64 | 2.3 | 5.40*** | −5.40*** |
| P9 a | DYS | 0.5 | −0.01 | 2.59 | −5.08 | — | — | — | — | 5.40*** | −5.40*** |
| P10 | BORD | — | — | — | — | — | — | −0.64 | 0.06 | 5.40*** | −5.40*** |
Note. AOS = apraxia of speech; MIN =minimal speech impairment; BORD = borderline AOS; APP = aphasia with phonemic paraphasia; DYS = dysarthria. The effect of MAF on changed level and variability of syllable rate and disfluency is represented by nonparametric analyses (Wilcoxon [Z score] for level changes; Fligner-Killeen [X 2] for variability change). The effect of MAF represents comparison to the preceding baseline phase. Release from MAF indicates comparison of MAF measures to the following baseline condition. Negative values indicate that level or variability was reduced in comparison to the prior phase. Significance figures were adjusted for multiple comparisons using the Benjamini-Hochberg method (Benjamini & Yekutieli, 2001). Comparisons were only made for participants for whom a Kruskal–Wallis test indicated an overall effect of condition on a given factor. Em dashes indicate that tests among conditions were not completed because an overall effect was not found for that participant using the Kruskal-Wallis test.
P8 and P9 received the ACABA order (i.e., masking before altered feedback; A= NAF; B = AAF; C = MAF); all others received AAF before masking (ABACA order).
p < .05,
p < .01,
p < .001.
Table 3.
Altered auditory feedback (AAF) effects on syllable rate, disfluency duration, and vocal intensity.
| Participant ID | Speech diagnosis | Syllable rate |
Disfluency duration |
Vocal intensity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level change |
Variability change |
Level change |
Variability change |
Level change |
|||||||
| AAF effect | AAF release | AAF effect | AAF release | AAF effect | AAF release | AAF effect | AAF release | AAF effect | AAF release | ||
| P1 | AOS | −2.91* | −0.2 | — | — | 0.00 | 2.35 | −1.75 | 8.15* | 5.40*** | −4.45*** |
| P2 | AOS | −0.20 | −1.28 | — | — | −1.76 | 0.83 | −5.49 | 0.93 | 4.91*** | −5.37*** |
| P3 | MIN | −0.72 | −0.91 | — | — | −1.3 | 2.21 | −0.48 | 4.51 | 3.34*** | −5.32*** |
| P4 | AOS | 1.95 | −0.59 | — | — | — | — | — | — | −3.49*** | −1.87 |
| P5 | BORD | −2.85* | 0.53 | — | — | — | — | — | — | 5.15*** | −5.40*** |
| P6 | AOS | −1.25 | 2.21 | — | — | −0.21 | −0.60 | — | — | 5.18*** | −4.43*** |
| P7 | APP | −4.42*** | 1.85 | — | — | −2.11 | 2.39 | −2.82 | 13.38** | 4.61*** | −3.64*** |
| P8 a | AOS | — | — | — | — | −1.56 | 2.30 | −3.36 | 7.60* | 5.40*** | −5.40*** |
| P9 a | DYS | −3.77** | 2.12 | −0.13 | 3.34 | — | — | — | — | 4.29*** | −5.21*** |
| P10 | BORD | — | — | — | — | — | — | −12.92** | 1.03 | 2.77 ** | −4.87*** |
Note. AOS = apraxia of speech; MIN = minimal speech impairment; BORD = borderline AOS; APP = aphasia with phonemic paraphasia; DYS = dysarthria. The effect of AAF on changed level and variability of syllable rate and disfluency is represented by nonparametric analyses (Wilcoxon [Z score] for level changes; Fligner-Killeen [X 2] for variability change). The effect of AAF represents comparison to the preceding baseline phase. Release from AAF indicates comparison of AAF measures to the following baseline condition. Negative values indicate that level or variability was reduced in comparison to the prior phase. Significance figures were adjusted for multiple comparisons using the Benjamini-Hochberg method (Benjamini & Yekutieli, 2001). Comparisons were only made for participants for whom a Kruskal–Wallis test indicated an overall effect of condition on a given factor. Em dashes indicate that tests among conditions were not completed because an overall effect was not found for that participant using the Kruskal-Wallis test.
P8 and P9 received the ACABA order (i.e., masking before altered feedback; A = NAF; B = AAF; C = MAF); all others received AAF before masking.
p < .05,
p < .01,
p < .001.
Variability Analysis
In addition to changes in the level of syllable rate or disfluency duration, marked change in variability was noted between conditions in some participants. Therefore, additional tests were completed to determine the significance of this change. The Fligner–Killeen median test (Fligner & Killeeen, 1976) was used as an omnibus test to determine whether variability of disfluency duration or syllable rate differed across conditions for a given participant, using an uncorrected alpha of 0.05. For significant tests, follow-up Fligner-Killeen tests were completed to compare the variance of adjacent conditions. Significance values were adjusted for multiple comparisons using the Benjamini-Hochberg procedure. Results are reported in Tables 2 and 3 for effects of MAF and AAF, respectively.
Results
The two feedback conditions had different effects on speech fluency. For most speakers with aphasia or AOS, speech rate increased under masking noise (MAF) but decreased with delayed and frequency-shifted feedback (AAF; see Figure 1). For several participants with aphasia or AOS, MAF resulted in both decreased level and variability of disfluency duration, whereas AAF decreased the variability but not the overall level of disfluency duration (see Figure 2). In contrast to the APH/AOS speakers, none of the NH speakers increased speech rate with masking, whereas AAF resulted in a slowed rate as predicted. NH speakers had negligible disfluencies in any condition, and no effect of either condition was seen for this measure.
In the following, results are presented first for the effects of MAF on speech in participants with APH/AOS (see Table 2), followed by AAF in APH/AOS (see Table 3). Within each section, we examine syllable rate, disfluency duration, and vocal intensity measures using visual inspection of Figures 1 and 2 and nonparametric statistics (see Tables 2 and 3). Note that the organization of Figures 1 and 2 differ from Tables 2 and 3. In particular, each Figure focuses on a single dependent measure (e.g., syllable rate, disfluency duration), displaying individual results for all feedback conditions. In contrast, the Tables focus on the feedback condition, showing the effect for each dependent measure. Results of the NH participants are presented following the APH/AOS findings.
Effects of MAF
Syllable Rate
As shown in Figure 1, nine of 10 participants (all but P9) increased syllable rate numerically with masking noise. When masking noise was removed, speech rate decreased in eight participants (all but P7). Wilcoxon comparisons between conditions indicated that syllable rate increased significantly with MAF for participants 1–6, though the return to baseline resulted in a significant decrease for only three speakers (P2, P3, P6; see Table 2). Syllable rate variability was not systematically affected by speaking condition, including masking noise. Although the omnibus Fligner-Killeen test for unequal variances was significant for P9 (X 2 = 10.32, p < .05), follow-up comparisons revealed no significant differences in variability of syllable rate for masking noise (see Table 2).
Relating the syllable rate results to the speech diagnoses, four of the six participants who significantly increased rate with masking noise had profiles consistent with AOS, one with borderline AOS, and one with minimally impaired speech. Of the four participants who did not increase rate with masking, one had AOS and one borderline AOS. These results provide partial support for our hypothesis that participants with AOS increase speech rate with masking noise, as most, but not all, participants with AOS showed the response and some participants without AOS profiles did increase rate.
Disfluency Duration
As shown in Figure 2, effects of masking on median disfluency duration were small or absent. Disfluency duration decreased somewhat with MAF and increased somewhat upon return to baseline conditions for seven participants (all but P4, P9, and P10). Wilcoxon tests revealed that only three participants significantly reduced disfluency duration from baseline to MAF (P1, P3, and P7), whereas another (P2) significantly increased disfluency following removal of masking noise (see Table 2). However, for several participants, variability of disfluency duration decreased with MAF conditions and increased on the return to baseline. The effect is illustrated in Figure 2, by the large differences in interquartile ranges depicted in the boxplots (noted with masking noise for all but P4 and P9). The Fligner-Killeen analysis indicated that decreased variability with MAF was significant for three participants (P1, P3, and P7; see Table 2). On removal of MAF, variability significantly increased for two participants (P2 and P7),
Our hypotheses for the effects of masking noise on disfluency duration in participants with AOS received little support. To be specific, although we predicted that speakers with AOS would reduce the duration of disfluencies, only one of the three participants with this response had a profile of AOS.
Vocal Intensity
As expected from previous masking studies in aphasia (e.g., Birch & Lee, 1955), MAF had a robust effect on vocal intensity, resulting in increased intensity when noise was introduced and decreased intensity when it was removed. Wilcoxon contrasts between conditions showed that these effects were significant for all participants (see Table 2).
Effects of AAF
Syllable Rate
As shown in Figure 1, delayed and frequency-shifted feedback had the opposite effect as masking noise, as eight speakers (all but P4 and P8) slowed their median syllable rate during the AAF condition. Five of these speakers then increased syllable rate when AAF was removed (P5, P6, P7, P9, and P10). Wilcoxon comparisons between conditions showed that the syllable rate decline was significant for four participants (P1, P5, P7, and P9), but the return to normal feedback was not significant for any participant (see Table 3). As noted in the masking results, only P9 showed any effect of condition on syllable rate variability (omnibus Fligner-Killeen Χ 2 = 10.32, p < .05); however, pairwise comparisons revealed no significant effect of AAF.
In comparing the AAF results to the participant diagnoses, we note that only one of the four participants who significantly reduced rate with AAF had an AOS diagnosis, whereas others with AOS had nonsignificantly reduced rate or, in one person (P4), increased rate. These results did not support our hypothesis that speech rate would be reduced with AAF in participants with AOS.
Disfluency Duration
Visual inspection of Figure 2 shows that seven participants reduced disfluency duration with AAF (all participants except P4, P5, and P9). Upon return to the baseline condition, disfluencies increased somewhat in four of them (P1, P3, P7, and P8). Although disfluencies declined numerically for most participants, Wilcoxon tests showed that the change was not significant for any participant, either on the introduction or removal of AAF. Although there were no significant changes in level of disfluency duration, Fligner-Killeen tests showed decreased variability in the AAF condition for one participant (P10). On removal of AAF, variability significantly increased for three speakers (P1, P7, and P8).
Vocal Intensity
Similar to previous studies of DAF in aphasia (e.g., Stanton, 1958), delayed and frequency-shifted feedback resulted in increased vocal intensity for all participants but one (P4), with corresponding decreased intensity when normal feedback was resumed. Wilcoxon tests between conditions show that the increase with AAF and decrease on removal of AAF was significant for the nine participants (see Table 3). The results of P4 warrant further consideration, as he actually reduced intensity in the AAF condition in comparison to the prior normal feedback trials, whereas all others increased intensity with AAF. A point of interest is that AAF also affected P4's syllable rate differently than the other speakers, in that he increased rather than decreased rate. This participant's results suggest that he was relatively unresponsive to AAF.
Summary of Results for NH Control Participants
NH control participants responded to the auditory conditions in a predictable way on the basis of the extant literature. Most control participants slowed their speech rate slightly, but not significantly, when speaking with MAF (mean decrease of 0.06 syllables per second; see the online supplemental material, Supplemental Table 2). Three participants (C1, C8, and C10) increased their rate nonsignificantly with masking noise (p > .10), and one significantly decreased rate with masking (0.41 syllables per second reduction; p < .05). On the release from masking, two participants had significantly increased syllable rate (p < .05), whereas the remainder had nonsignificant increases (n = 5) or decreases (n = 3) in rate.
All control participants slowed their speech rate in the AAF condition relative to the preceding baseline with normal feedback, and the reduction was substantial and significant for eight of the 10 participants (p < .01; see the online supplemental material, Supplemental Table 2). With an overall average rate of 3.05 syllables per second (minimum = 2.63, maximum = 3.68), participants reduced their rate by an average of 0.68 syllables per second in the AAF condition compared to the preceding normal condition. There was a corresponding average increase of 0.78 syllables per second when AAF was removed, which was significant for all participants.
Disfluencies occurred very infrequently in the NH control participants, with an overall mean of 0.026 s of disfluencies coded per sentence. No participant demonstrated a significant change in disfluency time either on the introduction or removal of either auditory condition.
Discussion
The purpose of this study was to test the effects of MAF in a broad sample of adults with speech sound production disorders, including those with and without characteristics of AOS, with comparison to NH adults. Our overall prediction was that participants with AOS would increase fluency when speaking with masking noise, with faster syllable rate, and with reduced disfluency duration. AAF was also considered as an experimental control condition. Although AAF has previously been shown to increase fluency in persons who stutter, we thought it most likely that speech rate would decrease in participants with AOS, as shown previously in NH adults and those with aphasia. Each of these conditions will be considered in turn and the results considered in light of theoretical models of speech processing for AOS. Last, alternative interpretations of the results will be discussed, as well as potential limitations.
Effects of MAF on Fluency
Auditory masking had a beneficial effect on speech fluency in several participants, most consistently resulting in increased syllable rate. This result contrasted with our NH participants, for whom one participant experienced a significantly reduced rate and none a significantly increased rate. This result also differs substantially from previous studies in aphasia and AOS; only Birch and colleagues (Birch, 1956; Birch & Lee, 1955) had found a beneficial effect of masking noise on speech. In part, the results may reflect our focus on speech fluency, whereas other studies focused on measures less likely to improve with masking, such as speech fluency in single-word repetition (Manasco et al., 2010; Manasco, 2008), naming accuracy (Weinstein, 1959), or articulatory quality (Wertz & Porch, 1970). In the present report, we did not examine the question of whether articulatory precision was affected by masking noise, though our theoretical framework and recent results (Maas et al., 2015) suggest that reduced access to the auditory signal may reduce precision. Analysis of articulatory precision and intelligibility for the present sample is currently under way.
The relatively high level of response to masking in our study may also relate to our clinical sample. We predicted that speakers with AOS would more likely benefit from masking than those without AOS on the basis of the posited mechanism of impaired motor programming ability with spared auditory feedback. Four of the seven participants with a strong or possible response to masking noise presented with clear evidence of AOS (e.g., articulatory distortions and segmental prolongations), and one additional participant had borderline characteristics of the disorder. Although six participants significantly increased their speech rate with masking noise, only three rebounded significantly to baseline performance following removal of the MAF stimulus. A complete return to baseline following removal of masking would have demonstrated the effect of masking more definitively. Nevertheless, the incomplete return to baseline is consistent with previous reports in adults with expressive aphasia (Birch, 1956; Birch & Lee, 1955) and in adults who stutter (Dewar, Dewar, Austin, & Brash, 1979), in which effects of masking persist following the removal of masking. Although such temporary aftereffects may be inconvenient in the context of the single-case design used here, they may be beneficial in the context of a treatment paradigm. For example, if the duration of a positive carryover effect of auditory masking can be extended for longer periods, it may be possible to use the condition to gradually increase fluency behavior in more generalized contexts.
We note that one person classified with AOS and one with borderline AOS showed no response to masking, whereas one person with APP was a responder. Furthermore, because our sample included only three people without characteristics of AOS, recruitment is ongoing to help determine whether masking noise improves fluency in other speaker groups (e.g., people with predominance of phonemic paraphasias).
Mechanisms for Feedback Effects
Our prediction that speakers with AOS would improve fluency measures with masking noise was based on the hypothesis that auditory feedback control is intact, whereas feedforward control is impaired (Jacks, 2008). According to this account, disfluencies (e.g., repetitions, revisions, and extended pausing) are caused by unproductive attention to speech errors heard in the auditory signal. We reasoned that auditory masking might be beneficial for fluency by preventing the speaker from attending auditorily to speech errors, thus reducing the likelihood of disfluency-generating correction.
The basic mechanism of overactive feedback processes with impaired feedforward control has been advanced to explain speech characteristics in two other disorders: childhood AOS (Terband, Maassen, Guenther, & Brumberg, 2009) and developmental stuttering (Civier et al., 2010). These studies used computational simulations in the DIVA speech processing model (Guenther et al., 2006) to generate sound errors and disfluencies. It is crucial that the simulation models were altered to rely primarily on feedback control processes (e.g., auditory feedback, somatosensory feedback). This resulted in stuttering-like disfluencies, which were reduced when masking noise was introduced in the model (Civier et al., 2010.)
The simulation studies suggest that a feedback-intensive processing strategy is a plausible mechanism to explain the symptoms of AOS, CAS, or developmental stuttering, including disfluencies. Furthermore, Civier et al.'s (2010) study provided further evidence to suggest that auditory masking might increase fluency in diverse speaker populations. However, a word of caution is warranted in discussing this posited theoretical mechanism—although these disorders share some features (e.g., presence of disfluencies), they are not identical in their etiology or behavioral presentation (e.g., different types of disfluencies), and therefore in their underlying mechanism. The discrepancy in response to AAF and MAF in our sample, given that persons who stutter often respond favorably to both, further suggests that the two conditions operate on different mechanisms. In fact, recent experimental work by Cai and colleagues (Cai et al., 2014; Cai et al., 2012) casts doubt on the feedback overreliance hypothesis for stuttering, suggesting instead that auditory feedback processing itself may be impaired in persons who stutter. For these various reasons, we think it fitting to consider alternative explanations for the fluency improvements seen in this study.
External Influences on Fluency During MAF
Baseline speech rate or fluency may determine responsiveness to altered feedback. Relatively faster speech rate and fluent speech production may show less positive effects of potentially fluency-inducing conditions. Indeed, five of the six participants who increased rate significantly under masking noise had a slow baseline syllable rate (i.e., <2.5 syllables per second; P1, P2, P3, P5, and P6), whereas only one had a normal speech rate (P4). 4 Nevertheless, a normal baseline rate did not preclude a positive rate change with masking, nor did presence of a slow baseline rate guarantee the effect. Several speakers with substantial disfluencies were not clearly affected by either AAF or MAF. Of the three participants who significantly reduced disfluency under MAF (P1, P3, and P7), one had minimal disfluencies at baseline (P7) and the other two had moderate disfluencies, with a median duration of 3 s during (the second) baseline.
Speaking while listening to loud noise is known to increase vocal intensity, a phenomenon known as the Lombard effect (Lane & Tranel, 1971). Because masking resulted in changes in both syllable rate and in vocal intensity, it is conceivable that the rate increase might be related to the change in vocal loudness. However, vocal intensity increased for both MAF (in which speech rate was generally faster) and for AAF (in which speech rate was generally slower). Therefore, the changes in fluency measures are not likely a direct effect of the increased intensity experienced with masking noise.
Another potential explanation for the effect of masking noise is that the unpleasant stimulus creates a time pressure, causing the participant to speak faster because the noise will be turned off when he or she finishes the sentence. An alternative experimental condition that creates a time pressure might be tested to determine if similar effects can be achieved without the use of auditory noise.
Active speaker strategies may also have influenced the results. For example, when listening to loud noise, individuals may use different strategies, consciously or subconsciously, for completing a sentence production task. One speaker may try to focus on the feeling of her speech articulators in the absence of auditory feedback, another might attempt to ignore the masking noise or altered feedback, and a third may be distracted by the unusual speaking conditions. Even as NH adults have varied strategies for accomplishing difficult or tedious tasks, so may speakers with aphasia or AOS. It would be worthwhile to record participants' impressions of the different conditions and any intentional strategies they used as they completed the experiment. In so doing, we might adapt existing procedures to be more effective or develop new conditions on the basis of the strategies that individuals independently use successfully during intervention and practice.
Effects of AAF on Fluency
We found no significant evidence that delayed and frequency-shifted feedback improved speech fluency measures in the 10 participants studied. Instead, AAF significantly reduced syllable rate in four participants with varying speech diagnoses (viz. AOS, borderline AOS, dysarthria, APP). This result is consistent with the slow rate found in our NH participants and others (Black, 1951) as well as persons who stutter (Wingate, 1970) and people with aphasia (Stanton, 1958). Although DAF reduces disfluencies in persons who stutter (Andrews et al., 1982), it often results in increased disfluencies in NH speakers and many people with aphasia (Boller et al., 1978).
However, not all people respond the same way to DAF, and speakers with conduction aphasia in particular have been found to produce fewer substitution errors, repetitions, and vowel prolongations with DAF (Chapin et al., 1981). Our one speaker with APP (P7) had lower disfluencies when speaking with AAF than in the baseline condition. Although this result was not statistically significant, it is nevertheless possible that AAF may benefit some speakers with aphasia. In particular, it may help people whose baseline speech rate is overly rapid, slowing them down and thereby resulting in fewer errors and consequent revisions. However, it may benefit people with impaired auditory processing by providing additional time to process incoming signals. On the basis of our results, AAF is not likely to be a speech facilitator for people with AOS. However, previous literature and the nonsignificant fluency reduction observed in one participant with APP suggest that it may be helpful for some people with aphasia.
Conclusion
The results of this study show that some people with AOS or aphasia can be influenced to alter their speech fluency by speaking in the presence of an intense auditory masking noise, though more research is needed to determine whether the changes are sufficiently robust to affect communicative effectiveness. Although delayed and frequency-shifted feedback did not significantly improve fluency for any participant, it also did not have a particularly deleterious effect. Ongoing recruitment and study of participants with diverse speech and language profiles may yield further insights into the factors that cause one person but not another to respond with increased fluency to masking noise.
Supplementary Material
Acknowledgments
Research reported in this article was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award R03DC011881. A limited report of data included in this article was given as a poster session at the Motor Speech Conference in Sarasota, FL in February 2014. We gratefully acknowledge research assistants Lúcia Fischer and Peter Schultz for syllable and disfluency coding, Tyson Harmon for analysis of the NH participants, and colleagues Emily Buss and Lori Leibold for comments on a draft of this article. Last, we appreciate the participants who gave their time to be in this study.
Funding Statement
Research reported in this article was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award R03DC011881.
Footnotes
Variability and consistency of speech sound production have been omitted from the discussion of diagnostic characteristics of AOS, due to recent research casting doubt on the usefulness of this feature for diagnosis (Haley, Jacks, & Cunningham, 2013; Staiger, Finger-Berg, Aichert, & Ziegler, 2012).
Diagnosis of dyspraxia of speech was based on characteristics used for diagnosis of AOS at that time (Johns & Darley, 1970).
For ease of interpretation, the participants are ordered according to the mean difference in syllable rate between the masking condition and the preceding baseline condition, with participants having a greater response to masking presented first.
As noted in the Method and in coding results (see the online supplemental material, Supplemental Table 1), P4's presentation of AOS appears idiosyncractic; his rate was slowed in multisyllabic words but not in sentences.
References
- Alm P. A. (2004). Stuttering and the basal ganglia circuits: A critical review of possible relations. Journal of Communication Disorders, 37, 325–369. [DOI] [PubMed] [Google Scholar]
- Andrews G., Howie P. M., Dozsa M., & Guitar B. E. (1982). Stuttering: Speech pattern characteristics under fluency-inducing conditions. Journal of Speech and Hearing Disorders, 25, 208–216. [PubMed] [Google Scholar]
- Ballard K. J., Wambaugh J. L., Duffy J. R., Layfield C., Maas E., Mauszycki S., & McNeil M. R. (2015). Treatment for acquired apraxia of speech: A systematic review of intervention research between 2004 and 2012. American Journal of Speech-Language Pathology, 24(2), 316–337. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., & Yekutieli D. (2001). The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics, 29, 1165–1188. [Google Scholar]
- Benson D. F. (1967). Fluency in aphasia: Correlation with radioactive scan localization. Cortex, 3, 373–394. [Google Scholar]
- Birch H. G. (1956). Experimental investigations in expressive aphasia. New York State Journal of Medicine, 56, 3849–3852. [PubMed] [Google Scholar]
- Birch H. G., & Lee J. (1955). Cortical inhibition in expressive aphasia. Archives of Neurology & Psychiatry, 74(5), 514. [DOI] [PubMed] [Google Scholar]
- Black J. W. (1951). The effect of delayed side-tone upon vocal rate and intensity. Journal of Speech and Hearing Disorders, 16(1), 56–60. [DOI] [PubMed] [Google Scholar]
- Boersma P., & Weenink D. (2014). Praat: Doing phonetics by computer. Retrieved from http://www.praat.org/
- Boller F., Vrtunski P. B., Kim Y., & Mack J. L. (1978). Delayed auditory feedback and aphasia. Cortex, 14, 212–226. [DOI] [PubMed] [Google Scholar]
- Brendel B., & Ziegler W. (2008). Effectiveness of metrical pacing in the treatment of apraxia of speech. Aphasiology, 22(1), 77–102. http://doi.org/10.1080/02687030600965464 [Google Scholar]
- Brown L., Sherbenou R. J., & Johnsen S. K. (2010). Test of Nonverbal Intelligence, Fourth Edition. San Antonio, TX: Pearson. [Google Scholar]
- Cai S., Beal D. S., Ghosh S. S., Guenther F. H., & Perkell J. S. (2014). Impaired timing adjustments in response to time-varying auditory perturbation during connected speech production in persons who stutter. Brain and Language, 129, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Beal D. S., Ghosh S. S., Tiede M. K., Guenther F. H., & Perkell J. S. (2012). Weak responses to auditory feedback perturbation during articulation in persons who stutter: Evidence for abnormal auditory-motor transformation. PloS One, 7(7): e41830 doi:10.1371/journal.pone.0041830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin C., Blumstein S. E., Meissner B., & Boller F. (1981). Speech production mechanisms in aphasia: A delayed auditory feedback study. Brain and Language, 14, 106–113. [DOI] [PubMed] [Google Scholar]
- Cherry E. C., Sayers B. M., & Marland P. M. (1955). Experiments on the complete suppression of stammering. Nature, 176, 874–875. [DOI] [PubMed] [Google Scholar]
- Civier O., Tasko S. M., & Guenther F. H. (2010). Overreliance on auditory feedback may lead to sound/syllable repetitions: Simulations of stuttering and fluency-inducing conditions with a neural model of speech production. Journal of Fluency Disorders, 35, 246–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R., & Scavone G. P. (2012). The Synthesis ToolKit in C++ (STK) (v. 4.4.4). Retrieved from ccrma.stanford.edu/software/stk/
- Darley F. L. (1968, November). Apraxia of speech: 107 years of terminological confusion. Paper presented at the convention of the American Speech and Hearing Association, Denver, CO. [Google Scholar]
- Deal J. L., & Darley F. L. (1972). The influence of linguistic and situational variables on phonemic accuracy in apraxia of speech. Journal of Speech and Hearing Disorders, 15, 639–653. [DOI] [PubMed] [Google Scholar]
- De Jong N. H., & Wempe T. (2009). Praat script to detect syllable nuclei and measure speech rate automatically. Behavior Research Methods, 41, 385–390. [DOI] [PubMed] [Google Scholar]
- Dewar A., Dewar A. D., Austin W. T. S., & Brash H. M. (1979). The long term use of an automatically triggered auditory feedback masking device in the treatment of stammering. International Journal of Language & Communication Disorders, 14, 219–229. [Google Scholar]
- Duffy J. R. (2013). Motor speech disorders: Substrates, differential diagnosis, and management (3rd ed.). St. Louis, MO: Elsevier Mosby. [Google Scholar]
- Farmer A. (1978). Sound error self-correction in the conversational speech of nonfluent and fluent aphasics. Folia Phoniatrica, 30, 293–302. [DOI] [PubMed] [Google Scholar]
- Feyereisen P., Pillon A., & de Partz M. P. (1991). On the measures of fluency in the assessment of spontaneous speech production by aphasic subjects. Aphasiology, 5, 1–21. [Google Scholar]
- Fligner M. A., & Killeen T. J. (1976). Distribution-free two-sample tests for scale. Journal of the American Statistical Association, 71, 210–213. [Google Scholar]
- Goldberg S., Haley K. L., & Jacks A. (2012). Script training and generalization for people with aphasia. American Journal of Speech-Language Pathology, 21, 222–238. [DOI] [PubMed] [Google Scholar]
- Goodglass H., Quadfasel F. A., & Timberlake W. H. (1964). Phrase length and the type and severity of aphasia. Cortex, 1, 133–153. [Google Scholar]
- Gordon J. K. (1998). The fluency dimension in aphasia. Aphasiology, 12, 673–688. doi:10.1080/02687039808249565 [Google Scholar]
- Guenther F. H., Ghosh S. S., & Tourville J. A. (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language, 96, 280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K. L., Jacks A., & Cunningham K. T. (2013). Error variability and the differentiation between apraxia of speech and aphasia with phonemic paraphasia. Journal of Speech, Language, and Hearing Research, 56, 891–905. http://doi.org/10.1044/1092-4388(2012/12-0161) [DOI] [PubMed] [Google Scholar]
- Haley K. L., Jacks A., de Riesthal M., Abou-Khalil R., & Roth H. L. (2012). Toward a quantitative basis for assessment and diagnosis of apraxia of speech. Journal of Speech, Language, and Hearing Research, 55, S1502–S1517. [DOI] [PubMed] [Google Scholar]
- Haley K. L., Roth H., Grindstaff E., & Jacks A. (2011). Computer-mediated assessment of intelligibility in aphasia and apraxia of speech. Aphasiology, 25, 1600–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenbrand J. M., & Gayvert R. T. (2005). Open source software for experiment design and control. Journal of Speech, Language, and Hearing Research, 48, 45–60. [DOI] [PubMed] [Google Scholar]
- Howell P., Au-Yeung J., & Pilgrim L. (1999). Utterance rate and linguistic properties as determinants of lexical dysfluencies in children who stutter. The Journal of the Acoustical Society of America, 105, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell P., El-Yaniv N., & Powell D. J. (1987). Factors affecting fluency in stutterers when speaking under altered auditory feedback. In Peters H. F. M. & Hulstijn W. (Eds.), Speech motor dynamics in stuttering (pp. 361–369). Vienna, Austria: Springer-Verlag. [Google Scholar]
- Howell P., & Sackin S. (2000). Speech rate modification and its effects on fluency reversal in fluent speakers and people who stutter. Journal of Developmental and Physical Disabilities, 12, 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IEEE Audio and Electroacoustics Group. (1969). IEEE recommended practice for speech quality measurements. IEEE Transactions on Audio and Electroacoustics, AU-17, 225–246. [Google Scholar]
- Ingham R. J., Bothe A. K., Jang E., Yates L., Cotton J., & Seybold I. (2009). Measurement of speech effort during fluency-inducing conditions in adults who do and do not stutter. Journal of Speech, Language, and Hearing Research, 52(5), 1286–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks A. (2008). Bite block vowel production in apraxia of speech. Journal of Speech, Language and Hearing Research, 51, 898–913. [DOI] [PubMed] [Google Scholar]
- Johns D. F., & Darley F. L. (1970). Phonemic variability in apraxia of speech. Journal of Speech and Hearing Disorders, 13, 556–583. [DOI] [PubMed] [Google Scholar]
- Jones W. J., Williams L. S., & Meschia J. F. (2001). Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke, 32(10), 2232–2236. [DOI] [PubMed] [Google Scholar]
- Kalinowski J., Armson J., & Stuart A. (1995). Effect of normal and fast articulatory rates on stuttering frequency. Journal of Fluency Disorders, 20, 293–302. [Google Scholar]
- Kalinowski J., & Saltuklaroglu T. (2003). Choral speech: The amelioration of stuttering via imitation and the mirror neuronal system. Neuroscience & Biobehavioral Reviews, 27, 339–347. [DOI] [PubMed] [Google Scholar]
- Kent R. D., & Rosenbek J. C. (1983). Acoustic patterns of apraxia of speech. Journal of Speech & Hearing Disorders, 26, 231–249. [DOI] [PubMed] [Google Scholar]
- Kertesz A. (2006). Western Aphasia Battery–Revised. San Antonio, TX: Pearson. [Google Scholar]
- Lane H., & Tranel D. (1971). The Lombard sign and the role of hearing in speech. Journal of Speech and Hearing Disorders, 14, 677–709. [Google Scholar]
- Lee B. S. (1950). Effects of delayed speech feedback. The Journal of the Acoustical Society of America, 22, 824–826. [Google Scholar]
- Lincoln M., Packman A., Onslow M., & Jones M. (2010). An experimental investigation of the effect of altered auditory feedback on the conversational speech of adults who stutter. Journal of Speech, Language, and Hearing Research, 53, 1122–1131. [DOI] [PubMed] [Google Scholar]
- Liss J. M. (1998). Error-revision in the spontaneous speech of apraxic speakers. Brain and Language, 62, 342–360. [DOI] [PubMed] [Google Scholar]
- Loucks T., Chon H., & Han W. (2012). Audiovocal integration in adults who stutter. International Journal of Language & Communication Disorders, 47, 451–456. [DOI] [PubMed] [Google Scholar]
- Lozano R. A., & Dreyer D. E. (1978). Some effects of delayed auditory feedback on dyspraxia of speech. Journal of Communication Disorders, 11, 407–415. [DOI] [PubMed] [Google Scholar]
- Maas E., Mailend M. L., & Guenther F. H. (2015). Feedforward and feedback control in apraxia of speech (AOS): Effects of noise masking on vowel production. Journal of Speech, Language, and Hearing Research, 58, 185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod J., Kalinowski J., Stuart A., & Armson J. (1995). Effect of single and combined altered auditory feedback on stuttering frequency at two speech rates. Journal of Communication Disorders, 28, 217–228. [DOI] [PubMed] [Google Scholar]
- Manasco H., Dagenais P., Holdsworth L., & Brown A. (2010, December). Effects of binaural masking on self-repairs and disfluencies in aphasia and apraxia of speech. PSHA Journal, 19–29. [Google Scholar]
- Manasco M. H. (2008). Effects of auditory masking on speech errors, disfluencies, response latency, and accuracy in aphasia (Doctoral dissertation). University of South Alabama, Mobile. [Google Scholar]
- Marshall R. C., & Tompkins C. A. (1982). Verbal self-correction behaviors of fluent and nonfluent aphasic subjects. Brain and Language, 15, 292–306. [DOI] [PubMed] [Google Scholar]
- Mauszycki S. C., & Wambaugh J. L. (2008). The effects of rate control treatment on consonant production accuracy in mild apraxia of speech. Aphasiology, 22(7), 906–920. [Google Scholar]
- McNeil M. R., Liss J. M., Tseng C. H., & Kent R. D. (1990). Effects of speech rate on the absolute and relative timing of apraxic and conduction aphasic sentence production. Brain and Language, 38, 135–158. [DOI] [PubMed] [Google Scholar]
- McNeil M. R., Odell K. H., Miller S. B., & Hunter L. (1995). Consistency, variability, and target approximation for successive speech repetitions among apraxic, conduction aphasic, and ataxic dysarthric speakers. Clinical Aphasiology, 23, 39–55. [Google Scholar]
- McNeil M. R., Robin D. A., & Schmidt R. A. (2008). Apraxia of speech: Definition, differentiation, and treatment. In McNeil M. R. (Ed.), Clinical management of sensorimotor speech disorders (2nd ed., pp. 311–344). New York, NY: Thieme. [Google Scholar]
- Odell K., McNeil M. R., Rosenbek J. C., & Hunter L. (1991). Perceptual characteristics of vowel and prosody production in apraxic, aphasic, and dysarthric speakers. Journal of Speech and Hearing Research, 34, 67–80. [DOI] [PubMed] [Google Scholar]
- Panico J., & Healey E. C. (2009). Influence of text type, topic familiarity, and stuttering frequency on listener recall, comprehension, and mental effort. Journal of Speech, Language, and Hearing Research, 52, 534–546. [DOI] [PubMed] [Google Scholar]
- Park H., Rogalski Y., Rodriguez A. D., Zlatar Z., Benjamin M., Harnish S., … Reilly J. (2011). Perceptual cues used by listeners to discriminate fluent from nonfluent narrative discourse. Aphasiology, 25, 998–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. (1995). Applying conversation analysis to aphasia: Clinical implications and analytic issues. International Journal of Disorders of Communication, 30, 372–383. [DOI] [PubMed] [Google Scholar]
- Pickett J. M. (1958). Limits of direct communication in noise. The Journal of the Acoustical Society of America, 30(4), 278–281. [Google Scholar]
- Pitman L. K. (1943). A device for detecting simulated unilateral deafness. Journal of the American Medical Association, 121(10), 752–753. [Google Scholar]
- Postma A., & Kolk H. (1993). The covert repair hypothesis: Prearticulatory repair processes in normal and stuttered disfluencies. Journal of Speech and Hearing Research, 36, 472–487. [PubMed] [Google Scholar]
- Robin D. A., Jacks A., Hageman C., Clark H. M., & Woodworth G. (2008). Visuomotor tracking abilities of speakers with apraxia of speech or conduction aphasia. Brain and Language, 106(2), 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. A., Eyraud R., Strand E. A., & Storkel H. (1996). The effects of noise masking on vowel duration in three patients with apraxia of speech and a concomitant aphasia. Clinical Aphasiology, 24, 83–96. [Google Scholar]
- Rosen K. M., Kent R. D., Delaney A. L., & Duffy J. R. (2006). Parametric quantitative acoustic analysis of conversation produced by speakers with dysarthria and healthy speakers. Journal of Speech, Language, and Hearing Research, 49, 395–411. [DOI] [PubMed] [Google Scholar]
- Ryan B. P., & Van Kirk B. (1974). The establishment, transfer, and maintenance of fluent speech in 50 stutterers using delayed auditory feedback and operant procedures. Journal of Speech and Hearing Disorders, 39, 3–10. [DOI] [PubMed] [Google Scholar]
- Square-Storer P., Darley F. L., & Sommers R. K. (1988). Nonspeech and speech processing skills in patients with aphasia and apraxia of speech. Brain and Language, 33, 65–85. [DOI] [PubMed] [Google Scholar]
- Staiger A., Finger-Berg W., Aichert I., & Ziegler W. (2012). Error variability in apraxia of speech: A matter of controversy. Journal of Speech, Language, and Hearing Research, 55(5), S1544–S1561. [DOI] [PubMed] [Google Scholar]
- Stanton J. B. (1958). The effects of delayed auditory feedback on the speech of aphasic patients. Scottish Medical Journal, 3, 378–384. [DOI] [PubMed] [Google Scholar]
- Sternberg M. L. (1946). Auditory factors in stuttering (Unpublished doctoral dissertation). State University of Iowa, Iowa City. [Google Scholar]
- Strand E. A., & McNeil M. R. (1996). Effects of length and linguistic complexity on temporal acoustic measures in apraxia of speech. Journal of Speech and Hearing Research, 39, 1018–1033. [DOI] [PubMed] [Google Scholar]
- Stuart A., Kalinowski J., Armson J., Stenstrom R., & Jones K. (1996). Fluency effect of frequency alterations of plus/minus one-half and one-quarter octave shifts in auditory feedback of people who stutter. Journal of Speech and Hearing Research, 39, 396–401. [DOI] [PubMed] [Google Scholar]
- Stuart A., Kalinowski J., Rastatter M., Saltuklaroglu T., & Dayalu V. (2004). Investigations of the impact of altered auditory feedback in-the-ear devices on the speech of people who stutter: Initial fitting and 4-month follow-up. International Journal of Language & Communication Disorders, 39, 93–113. [DOI] [PubMed] [Google Scholar]
- Terband H., Maassen B., Guenther F. H., & Brumberg J. (2009). Computational neural modeling of speech motor control in childhood apraxia of speech (CAS). Journal of Speech, Language, and Hearing Research, 52, 1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost J. E. (1970). A descriptive study of verbal apraxia in patients with Broca's aphasia (Doctoral dissertation). Northwestern University, IL. [Google Scholar]
- Van Summers W., Pisoni D. B., Bernacki R. H., Pedlow R. I., & Stokes M. A. (1988). Effects of noise on speech production: Acoustic and perceptual analyses. The Journal of the Acoustical Society of America, 84(3), 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh J. L., Duffy J. R., McNeil M. R., Robin D. A., & Rogers M. A. (2006). Treatment guidelines for acquired apraxia of speech: A synthesis and evaluation of the evidence. Journal of Medical Speech-Language Pathology, 14, 15–34. [Google Scholar]
- Weinstein B. E., & Ventry I. M. (1983). Audiometric correlates of the hearing handicap inventory for the elderly. Journal of Speech and Hearing Disorders, 48(4), 379–384. [DOI] [PubMed] [Google Scholar]
- Weinstein S. (1959). Experimental analysis of an attempt to improve speech in cases of expressive aphasia. Neurology, 9, 632–635. [DOI] [PubMed] [Google Scholar]
- Wertz R. T., & Porch B. E. (1970). Effects of masking noise on the verbal performance of adult aphasics. Cortex, 6, 399–409. [DOI] [PubMed] [Google Scholar]
- Wingate M. E. (1970). Effect on stuttering of changes in audition. Journal of Speech & Hearing Research, 13, 861–873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.