Abstract
Objective:
Limited prospective data indicate that premorbid sleep disturbances elevate the risk for subsequent alcohol and other drug problems, yet the implications for subsequent substance involvement trajectories remain unclear. In the present analyses, we examined risk associations between sleep characteristics during late childhood and the onset of substance use and substance use disorders into adulthood.
Method:
A sample of 707 children was recruited at ages 9–13 years and followed over seven additional visits through age 30 years. In 304 participants, fathers had a history of substance use disorder involving illicit drugs. Self-reported baseline sleep characteristics (restless sleep and variable sleep timing) were assessed at approximately ages 9–13 years. Assessment of alcohol, cannabis, and cocaine involvement occurred at follow-up visits. Cox proportional hazard models tested sleep characteristics as predictors of two substance-related outcomes (age at first use or diagnosis of disorder), as well as the onset of major depressive disorder.
Results:
Restless sleep at baseline significantly predicted an earlier onset age for trying alcohol and cannabis and showed a trend toward predicting early onset of cannabis use disorder. Restless sleep also predicted an earlier onset of depression. Irregular sleep timing at baseline significantly predicted an earlier onset age for alcohol use disorder and showed trends toward predicting early onsets of disorders of cannabis and cocaine use.
Conclusions:
Disturbed sleep during late childhood appears to accelerate the onset of not only initial substance use but also the development of clinically defined substance use disorder. Sleep-focused preventative efforts during late childhood may reduce the incidence of mood and substance use disorders.
Abundant cross-sectional data and clinical lore recognize that sleep disturbances are highly prevalent among individuals with alcohol and other drug problems (Blank et al., 2015; Conroy & Arnedt, 2014; Johnson & Breslau, 2001). A few prospective studies indicate that premorbid sleep disturbances elevate the risk for subsequent alcohol and drug problems (Breslau et al., 1996; Hasler et al., 2014; Wong et al., 2004, 2010, 2015). Nevertheless, the implications of sleep disturbances for subsequent substance involvement remain unclear. Adolescence is a particularly important period to study sleep and substance use relationships, given that problems in both realms spike during this time (e.g., Johnson et al., 2006; Kessler et al., 2005). Indeed, approximately 9% of 13- to 16-year-olds meet the criteria for current insomnia, with much higher proportions reporting subclinical sleep problems (Johnson et al., 2006).
A few prospective studies have suggested that sleep complaints precipitate alcohol and drug problems throughout the life span. Early childhood sleep problems may predict the onset of substance use during adolescence as well as continued sleep problems that, in turn, predict substance problems in young adulthood (Wong et al., 2004, 2010). Sleep complaints among young adults increase the risk for new-onset alcohol use disorders (AUDs) or other substance use disorders (SUDs) (Breslau et al., 1996). Furthermore, once an AUD is established, sleep disturbance can predict relapse (Brower et al., 2001; Drummond et al., 1998).
Among the handful of adolescent studies examining prospective sleep–substance use associations, two studies used data from the National Longitudinal Study of Adolescent Health (Add Health). Roane and Taylor (2008) reported cross-sectional associations between the presence or absence of insomnia and substance use among 4,494 participants at baseline (Mage = 15.83 years) but no significant prospective associations between baseline insomnia and substance use 6–7 years later. In contrast, Wong and colleagues used continuous measures of insomnia (“trouble falling asleep”) and sleep duration in the Add Health sample and reported that baseline sleep predicted a range of alcohol-related problems (e.g., interpersonal problems, heavy episodic [“binge”] drinking, and drunk driving), illicit drug use, and drug-related problems at a 1-year follow-up and, to a lesser degree, at the 6- to 7-year follow-up (Wong et al., 2015). Finally, a longitudinal study of 696 adolescents (ages 12–19 years) from the Pittsburgh Adolescent Alcohol Research Center (PAARC) reported that adolescents with AUD at baseline reported more overall sleep disturbance—including insomnia and hypersomnia complaints—relative to adolescents with no baseline AUD, which persisted at 3- and 5-year follow-up visits (Hasler et al., 2014). Furthermore, among the adolescents without baseline AUDs, baseline insomnia (but not hypersomnia) predicted an increase in alcohol symptoms at the 1-year follow-up. The mixed findings among the adolescent studies underscore the need for further clarifying the associations among sleep disturbances and subsequent alcohol and drug outcomes.
These prior studies suggest that the degree of risk for various substance-related outcomes may vary according to the nature of the sleep problem. Studies have primarily focused on the consequence of two categories of sleep problems: insomnia and/or insufficient sleep duration. A third category of sleep problem—variability in sleep timing and/ or duration—has received less attention in prospective studies. In adolescents, sleep variability may be operationalized as the difference between weekday and weekend sleep and is thought to reflect the degree of circadian misalignment (or “social jet lag”) suffered because of imposed academic schedules (Hasler & Clark, 2013; Wittmann et al., 2006). Given cross-sectional evidence that sleep variability is associated with increased substance involvement (Hasler & Clark, 2013), it is important to examine prospective associations between sleep variability and substance-related outcomes. Of note, among adolescents without baseline AUDs in the aforementioned PAARC study, larger weekday–weekend differences in sleep duration at baseline predicted more alcohol symptoms 3 and 5 years later.
The present study examines sleep characteristics and subsequent substance use outcomes in a sample enriched for high-risk characteristics followed from late childhood (ages 9–13 years) over eight visits through age 30 years at the Center for Education and Drug Abuse Research (CEDAR) at the University of Pittsburgh. The CEDAR protocol includes an extensive substance use assessment, including direct assessment of both parents via interviews. Prior CEDAR analyses have identified a variety of important contributors to adolescent and adult SUDs (Clark et al., 1999; Moss et al., 1999; Vanyukov et al., 2003) but have not yet focused on sleep. The present study assessed two distinct sleep parameters relevant to adolescence: restless sleep, as an indicator of insomnia, and variability in sleep timing, as an indicator of sleep/circadian misalignment. Substance-related outcomes focused on first use and first diagnosis of a disorder for alcohol, cannabis, and cocaine. We predicted that more restless and variable sleep at baseline would predict the earlier onset of substance use and earlier diagnosis of a disorder. We also examined whether restless and variable sleep would show differential associations with the various substance-related outcomes. Last, we tested whether the two sleep parameters predicted the onset of depressive disorders, given abundant prior evidence of sleep disturbance as a risk factor for depression (Baglioni et al., 2011).
Method
Participants
Participants were 707 children who were identified, recruited, and initially assessed in late childhood (ages 9-13 years) by contacting their biological fathers. Recruitment occurred through multiple sources, including treatment programs, advertisements, and random-digit dialing. All parents provided written informed consent; children provided assent. The study was approved by the Institutional Review Board of the University of Pittsburgh.
Recruitment used a high-risk design based on paternal SUD history, including (a) children of fathers with SUDs (n = 304), (b) children of fathers with other psychiatric disorders but no SUDs (n = 76), and (c) children of fathers without SUDs or other major psychiatric disorders (n = 327) (Clark et al., 2001). Fathers were considered to have SUDs if structured clinical interviews (as described in Clark et al., 2001) determined that they ever met Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R; American Psychiatric Association, 1987), criteria for abuse or dependence involving illicit substances (i.e., substances other than nicotine, caffeine, or alcohol). Other psychiatric disorders were not exclusionary for the fathers with SUDs. Maternal SUDs were similarly diagnosed, although they did not affect inclusion/exclusion decisions. This approach was intended to lead to the acquisition of a sample including a substantial proportion of children likely to develop accelerated substance use trajectories during adolescence and subsequently to meet diagnostic criteria for SUDs.
In addition to the baseline assessment (Visit 1: approximately ages 10-12 years), the study included information from seven follow-up visits that assessed participants throughout adolescence and early-middle adulthood. The visits were scheduled at ages 12-14 years (Visit 2), 16 years (Visit 3), 19 years (Visit 4), 22 years (Visit 5), 25 years (Visit 6), 28 years (Visit 7), and 30 years (Visit 8); participants were assessed as close to these ages as possible.
Although the original sample included 732 participants, we excluded 18 participants who did not report either European American or African American ancestry in order to improve interpretability of differences between those groups. (Among those 18 “other” participants, no specific race/ethnicity group had sufficient numbers to be interpretable.) Of the remaining 714 participants, 7 (1%) were missing data on one or both parents’ SUD history. Thus, the initial sample of 707 participants for the present analyses included 204 females and 503 males (28.9% and 71.1% of the sample, respectively), with 562 participants with a European American background and 145 with an African American background (79.4% and 20.5% of the sample, respectively). The mean age at baseline (Visit 1) was 11.39 years (SD = 0.91) (range: 9.42-13.34), and the mean age at the last visit completed was 22.66 years (SD = 5.02) (range: 10.00-31.34).
Measures
Sleep variables were based on two subscales, originally derived via factor analysis from the Dimensions of Temperament Survey-Revised (DOTS-R; Windle & Lerner, 1986):
Activity-Sleep and Rhythmicity-Sleep. In the original validation study, these subscales had Cronbach’s alphas of .81 and .69, respectively, in a sample of sixth graders. In the current sample, Cronbach’s alphas were .78 and .61, respectively. Each of these scales was based on four or six 4-point response scales ranging from 1 (usually false) to 4 (usually true). Activity-Sleep (score = 4-16) assessed restless sleep, with representative items including, “I move a great deal in my sleep” and “In the morning, I am still in the same place as I was when I fell asleep” (reverse scored). Rhythmicity- Sleep (score = 6-24) assessed variability in sleep timing, with representative items including, “I wake up at different times” (reversed scored) and “I seem to get sleepy just about the same time every night.”
The assessment of alcohol, cannabis, and cocaine involvement determined the age at first use utilizing an adaptation of the Lifetime Alcohol Use History (Clark et al., 2001; Skinner & Allen, 1982; Skinner & Horn, 1984). Determination of AUD and SUD diagnoses occurred via a modified version of the Structured Clinical Interview for DSM-IV (First et al., 2002; Martin et al., 1995, 2000). At each assessment, subjects were asked about the period since the prior assessment or, for the first assessment, their lifetime. The onset dates were estimated to the nearest month. Prior research has shown that onset dates for SUD symptoms and diagnoses can be reliably assessed in adolescent samples (Martin et al., 2000).
Given abundant evidence that sleep problems are a risk factor for depression (e.g., Baglioni et al., 2011), a diagnosis of major depression was also considered in the analyses. Major depression was assessed by the CEDAR version of the Schedule for Affective Disorders and Schizophrenia (see Clark et al., 1997).
Covariates of interest included sex (i.e., male and female), age at baseline (Visit 1), race (dichotomized between European American and African American), and the presence of parental SUD. Diagnoses of parental SUDs were made by DSM-III-R (American Psychiatric Association, 1987), the latest DSM edition when the study was initiated. Sex was coded as 1 (male) and 2 (female). Race was coded as 1 (European American) and 3 (African American). The presence of parental SUDs was coded as 0 (neither parent; n = 377), 1 (one parental SUD; n = 220), or 2 (both parents with SUDs; n = 110).
Data analyses
Cox proportional hazard models in IBM SPSS Statistics for Windows, Version 21 (IBM Corp., Armonk, NY), tested sleep characteristics as predictors of the onset of alcohol and drug outcomes. The independent variables were restless sleep and variability of sleep timing (sleep rhythmicity) at Visit 1 (ages 10-12 years). These variables were converted to z scores before inclusion in the hazard models to facilitate interpretation. The dependent variables were the first age at which participants tried alcohol, cannabis, or cocaine and the first age at which participants were diagnosed with an alcohol, cannabis, or cocaine use disorder. We also ran a parallel model using the age at which participants were first diagnosed with a depressive disorder. All models included sex, age at Visit 1, race, and the presence of parental SUD as covariates. In the present sample of 707 participants, no data were missing for the sleep variables, covariates, or baseline-dependent variables. With regard to missing data in the dependent variables after baseline, Cox proportional hazard models use all available data; thus, all individuals are included in the analysis. The test statistic was Wald’s chi-square test.
Results
Baseline sleep characteristics
At baseline, the mean DOTS-R Activity-Sleep (restless sleep) was 10.43 (SD = 3.45, range: 4-16), and mean DOTS-R Rhythmicity-Sleep (variability of sleep timing) was 15.45 (SD = 3.65, range: 6-24). There were no significant sex differences on either variable (restless sleep: t = 0.53, p = .60; variability of sleep timing: t = -0.17, p = .86). African American participants reported more variable sleep timing (t = 2.48, p = .01) relative to European American participants. There were no ethnic differences in restless sleep (t = 0.99, p = .32).
Ages at onset for alcohol and drug involvement
The number of participants with (and mean ages at onset for) substance use, from youngest to oldest, were as follows: alcohol (n = 575; Mage = 15.15 [SD = 2.92]), cannabis (n = 415; Mage = 15.86 [SD = 2.26]), and cocaine (n = 67; Mage = 19.91 [SD = 3.63]). The mean ages at diagnosis for the substance use and depressive disorders, from youngest to oldest, were as follows: cannabis use disorder (n = 181; Mage = 16.98 [SD = 2.41]), AUD (n = 195; Mage = 17.77 [SD = 2.67]), cocaine use disorder (n = 37; Mage = 20.08 [SD = 3.15]), and depressive disorder (n = 206; Mage = 26.78 [SD = 3.62]).
Sleep-related risk of alcohol and drug involvement
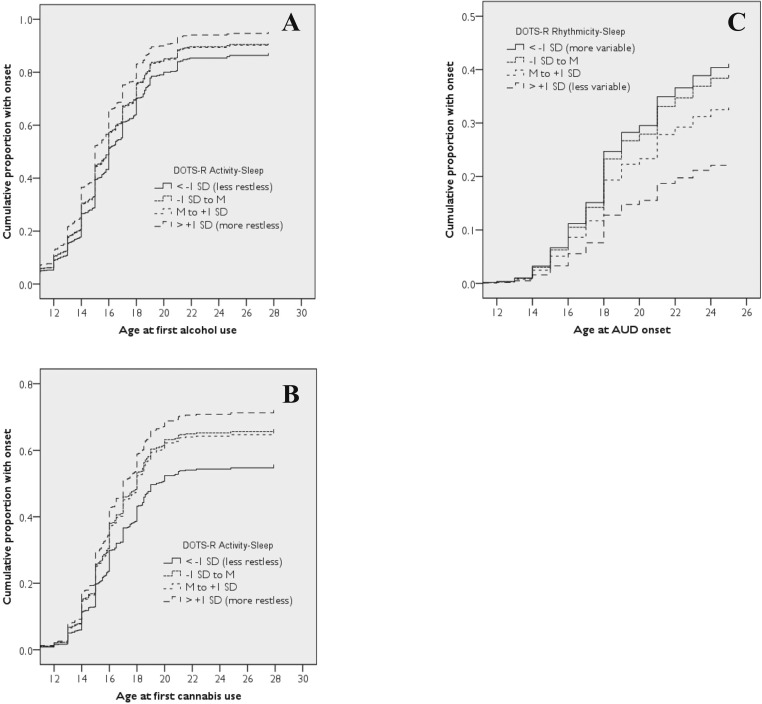
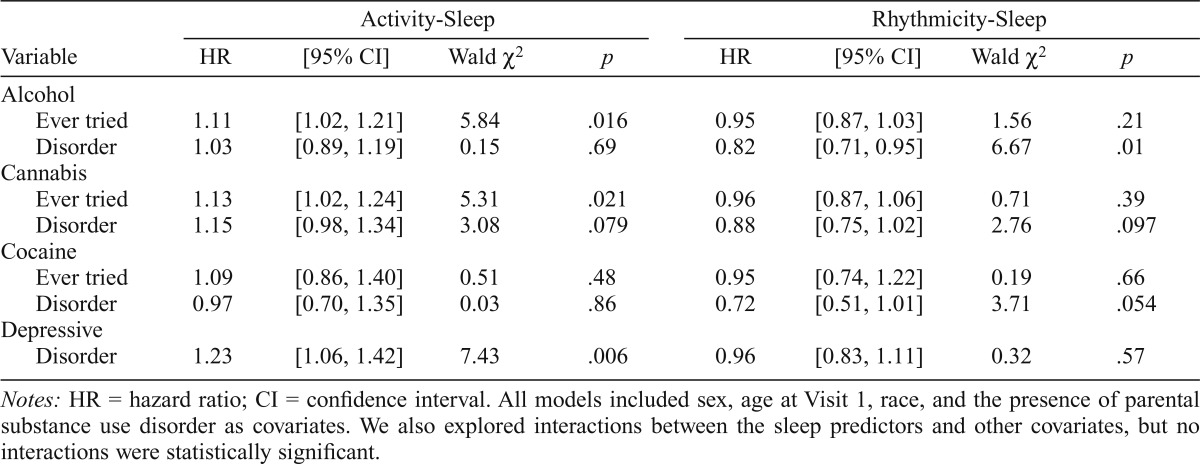
Based on the Cox proportional models with variance accounted for by covariates, more restless sleep predicted an earlier onset of alcohol use and cannabis use (Figures 1A and 1B) but not cocaine use. A 1-SD increase in the Activity- Sleep scale (3.45 points) was associated with an 11% faster onset to alcohol use and a 13% faster onset to cannabis use (Table 1). More restless sleep also showed a trend toward predicting an earlier onset of cannabis use disorder (15% faster rate per SD) but did not predict AUD or cocaine use disorder. More restless sleep also predicted an earlier depressive disorder onset; specifically, a 1-SD increase in the Activity-Sleep scale was associated with a 23% faster onset to depressive disorder (Table 1).
Figure 1.
The cumulative frequency of substance outcomes over time as predicted by sleep during late childhood. Specifically, more restless sleep predicted an earlier onset of alcohol use (A) and cannabis use (B), while more variable sleep timing predicted an earlier onset of alcohol use disorder (C). For the sake of illustration, participants were classified into four groups for each sleep measure: participants with scores less than -1 SD from the mean, participants with scores in between -1 SD and the mean, participants with scores in between the mean and +1 SD, and participants with scores greater than +1 SD from the mean. DOTS-R = Dimensions of Temperament Survey-Revised.
Table 1.
Results from Cox proportional hazards models
| Activity-Sleep |
Rhythmicity-Sleep |
|||||||
| Variable | HR | [95% CI] | Wald χ2 | P | HR | [95% CI] | Wald χ2 | P |
| Alcohol | ||||||||
| Ever tried | 1.11 | [1.02, 1.21] | 5.84 | .016 | 0.95 | [0.87, 1.03] | 1.56 | .21 |
| Disorder | 1.03 | [0.89, 1.19] | 0.15 | .69 | 0.82 | [0.71, 0.95] | 6.67 | .01 |
| Cannabis | ||||||||
| Ever tried | 1.13 | [1.02, 1.24] | 5.31 | .021 | 0.96 | [0.87, 1.06] | 0.71 | .39 |
| Disorder | 1.15 | [0.98, 1.34] | 3.08 | .079 | 0.88 | [0.75, 1.02] | 2.76 | .097 |
| Cocaine | ||||||||
| Ever tried | 1.09 | [0.86, 1.40] | 0.51 | .48 | 0.95 | [0.74, 1.22] | 0.19 | .66 |
| Disorder | 0.97 | [0.70, 1.35] | 0.03 | .86 | 0.72 | [0.51, 1.01] | 3.71 | .054 |
| Depressive | ||||||||
| Disorder | 1 .23 | [1.06, 1.42] | 7.43 | .006 | 0.96 | [0.83, 1.11] | 0.32 | .57 |
Notes: HR = hazard ratio; CI = confidence interval. All models included sex, age at Visit 1, race, and the presence of parental substance use disorder as covariates. We also explored interactions between the sleep predictors and other covariates, but no interactions were statistically significant.
Variability of sleep timing did not predict the onset of alcohol, cannabis, or cocaine use. More variable sleep timing predicted an earlier onset of AUD (Figure 1C; also see Table 1) and showed trends toward predicting earlier onset of cannabis use disorder and cocaine use disorder. Specifically, a 1-SD increase in the Rhythmicity-Sleep scale (3.65 points) was associated with an 18% faster onset to AUD, a 12% faster onset to cannabis use disorder, and a 28% faster onset to cocaine use disorder (Table 1). Variability of sleep timing did not predict onset of depressive disorder.
Full models with statistics for all variables are shown in Supplemental Tables S1 and S2.
Discussion
Our findings indicate that sleep characteristics during late childhood are predictive of subsequent alcohol and drug involvement during adolescence and young adulthood; that is, more disturbed sleep appears to accelerate the onset of not only initial substance use, but also the development of clinically defined SUDs. Complementing prior studies in other age groups (Breslau et al., 1996; Wong et al., 2004, 2010), restless sleep in late childhood predicted an accelerated alcohol and cannabis use onset that was not seen for cocaine use. We also report a more novel finding: more variable sleep timing predicts an accelerated AUD onset. Together, these findings add to the growing literature suggesting that childhood sleep disturbance is a risk factor for later substance involvement during adolescence.
Self-reported restless sleep predicted an earlier onset of alcohol and cannabis use but not cocaine use. Interpreting restless sleep as an indicator of insomnia or difficulty sleeping, this finding is consistent with other research showing that sleep difficulty predicts the earlier onset of alcohol and other drug use (Breslau et al., 1996; Wong et al., 2004, 2010). This may be explained in part by individuals with difficulty sleeping turning to alcohol and cannabis for their sedating properties. Based on this explanation, the lack of predictive association with the onset of cocaine use may be attributable to cocaine’s stimulating effects, although said effects could potentially be used to counteract the daytime fatigue typical of insomnia (Riedel & Lichstein, 2000). Our findings are similarly in line with a small study conducted with actigraphy in children of alcoholics (COAs) that found that the COAs had higher average nocturnal activity as compared with non-COAs (Conroy et al., 2015). Restless sleep also predicted an earlier onset of major depression in the current sample, consistent with a large body of research (Baglioni et al., 2011). Sleep disturbance may be dysregulating positive and/or negative affectivity (Buysse et al., 2007), which could provide an alternative, not mutually exclusive, mechanistic explanation for accelerated onset to substance use.
We also found that more variable sleep timing predicted an earlier AUD diagnosis. This complements our previous finding that larger weekday-weekend differences in sleep duration during adolescence predicted increases in AUD symptoms at 3- and 5-year follow-ups (Hasler et al., 2014). Together, these findings are coherent with a larger crosssectional literature indicating that variability in sleep timing during adolescence is associated with a host of problems, including substance involvement, mood disorders, and academic problems (Fuligni & Hardway, 2006; Haraszti et al., 2014; O’Brien & Mindell, 2005; Pasch et al., 2010). Variable sleep timing, particularly the marked differences in weekday and weekend sleep, is presumed to result in part from a mismatch between teens’ predisposition toward later sleep timing and the earlier schedules imposed by secondary education (Hasler & Clark, 2013; Wittmann et al., 2006). This predisposition is not driven purely by social concerns but has a biological basis in later timing of circadian rhythms and apparent developmental differences in the homeostatic regulation of sleep (Hagenauer et al., 2009). This explanation remains speculative, as the current study did not include measures of circadian rhythms (e.g., melatonin) or homeostatic pressure (e.g., electroencephalography) that would allow us to test this. Last, the variability in sleep timing could also reflect a lack of parental monitoring and control over sleep schedules, which would still be developmentally appropriate during early adolescence.
Some limitations of the present study are important to consider. First, the sleep variables were based on self-report items that have not been validated against more widely used subjective (e.g., sleep diaries) and objective (e.g., actigraphy) sleep measures. In addition, the Rhythmicity-Sleep scale had relatively low internal consistency. That said, these are limitations of the child and adolescent sleep literature more broadly, given the lack of validated sleep measures for these age groups, and questionable psychometrics among some existing measures (reviewed in Lewandowski et al., 2011; Spruyt & Gozal, 2011). A logical follow-up study would include actigraphic sleep measures, particularly given the conceptual overlap between the restless sleep measure in this study and the fragmentation index in actigraphy. Of note, the significant risk association between the Activity-Sleep scale and onset of depressive disorders provides predictive validity for this measure, given overwhelming evidence that insomnia predicts the onset of first depressive episodes (Baglioni et al., 2011).
Next, although our high-risk sample provides a more efficient route to examining associations with significant substance involvement in a smaller sample than those used in population-based samples, it is necessarily limited in terms of population-wide generalizability. Last, the present study focused on a limited range of risk factors, precluding examination of how sleep problems might associate and/or interact with other demonstrated risk factors. For example, sleep problems show heritability during childhood and adolescence (Gehrman et al., 2011; Moore et al., 2011), and it remains unknown how various genetic risk factors for substance abuse (Vanyukov et al., 2003) may overlap with those for sleep problems and/or confer additive vulnerability. Furthermore, as noted, lack of parental monitoring may contribute to sleep problems and is also a well-established risk factor for initiation of substance use (e.g., Chilcoat & Anthony, 1996). Follow-up studies should investigate whether parental monitoring serves as a common risk factor for both sleep and substance use problems or whether a mediation pathway through sleep problems might exist.
In conclusion, restless sleep and variable sleep timing during late childhood was associated with an accelerated onset of substance use and clinically defined SUD during adolescence and early adulthood. These findings buttress a growing literature indicating that childhood and adolescent sleep disturbance is a vulnerability factor for substance involvement, possibly operating through mood disorders or altered reward mechanisms (Hasler & Clark, 2013; Hasler et al., 2015). Fortunately, sleep problems are a modifiable risk factor; the means to address this vulnerability are available at both the individual and systemic levels. Effective approaches to managing adolescent sleep at the individual level include cognitive-behavioral therapies for insomnia, melatonin, and bright light (Gradisar et al., 2011; van Geijlswijk et al., 2010). Furthermore, districts that have delayed school start times have demonstrated corresponding benefits such as improved grades, reduced depression, and decreased automobile accidents (Danner & Phillips, 2008; Owens et al., 2010). Sleep-focused preventative efforts during late childhood and early adolescence may be an important and effective means to reduce the incidence of mood disorders and SUDs that can persist well into adulthood.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Washington, DC: Author; 1987. [Google Scholar]
- Baglioni C., Battagliese G., Feige B., Spiegelhalder K., Nissen C., Voderholzer U., Riemann D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. doi:10.1016/j. jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Blank M., Zhang J., Lamers F., Taylor A., Hickie I., Merikangas K. Health correlates of insomnia symptoms and comorbid mental disorders in a nationally representative sample of US adolescents. Sleep. 2015;38:197–204. doi: 10.5665/sleep.4396. doi:10.5665/sleep.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Roth T., Rosenthal L., Andreski P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biological Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. doi:10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Brower K. J., Aldrich M. S., Robinson E. A., Zucker R. A., Greden J. F. Insomnia, self-medication, and relapse to alcoholism. American Journal of Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. doi:10.1176/appi. ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D. J., Thompson W., Scott J., Franzen P. L., Germain A., Hall M., Kupfer D. J. Daytime symptoms in primary insomnia: A prospective analysis using ecological momentary assessment. Sleep Medicine. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. doi:10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat H. D., Anthony J. C. Impact of parent monitoring on initiation of drug use through late childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:91–100. doi: 10.1097/00004583-199601000-00017. doi:10.1097/00004583-199601000-00017. [DOI] [PubMed] [Google Scholar]
- Clark D. B., Moss H. B., Kirisci L., Mezzich A. C., Miles R., Ott P. Psychopathology in preadolescent sons of fathers with substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:495–502. doi: 10.1097/00004583-199704000-00012. doi:10.1097/00004583-199704000-00012. [DOI] [PubMed] [Google Scholar]
- Clark D. B., Parker A. M., Lynch K. G. Psychopathology and substance-related problems during early adolescence: A survival analysis. Journal of Clinical Child Psychology. 1999;28:333–341. doi: 10.1207/S15374424jccp280305. doi:10.1207/ S15374424jccp280305. [DOI] [PubMed] [Google Scholar]
- Clark D. B., Pollock N. K., Mezzich A., Cornelius J., Martin C. Diachronic substance use assessment and the emergence of substance use disorders. Journal of Child & Adolescent Substance Abuse. 2001;10:13–22. doi:10.1300/J029v10n04_02. [Google Scholar]
- Conroy D. A., Arnedt J. T. Sleep and substance use disorders: An update. Current Psychiatry Reports. 2014;16:487. doi: 10.1007/s11920-014-0487-3. doi:10.1007/s11920-014-0487-3. [DOI] [PubMed] [Google Scholar]
- Conroy D. A., Hairston I. S., Zucker R. A., Heitzeg M. M. Sleep patterns in children of alcoholics and the relationship with parental reports. Austin Journal of Sleep Disorders. 2015;2:1009. Retrieved from http://austinpublishinggroup.com/sleep-disorders/fulltext/ajsdv2-id1009.php. [Google Scholar]
- Danner F., Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. Journal of Clinical Sleep Medicine. 2008;4:533–535. [PMC free article] [PubMed] [Google Scholar]
- Drummond S. P., Gillin J. C., Smith T. L., DeModena A. The sleep of abstinent pure primary alcoholic patients: Natural course and relationship to relapse. Alcoholism: Clinical and Experimental Research. 1998;22:1796–1802. doi:10.1111/j.1530-0277.1998.tb03983.x. [PubMed] [Google Scholar]
- First M. B., Spitzer R. C., Gibbon M., Williams J. B. W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fuligni A. J., Hardway C. Daily variation in adolescents’ sleep, activities, and psychological well-being. Journal of Research on Adolescence. 2006;16:353–378. doi:10.1111/j.1532-7795.2006.00498.x. [Google Scholar]
- Gehrman P. R., Meltzer L. J., Moore M., Pack A. I., Perlis M. L., Eaves L. J., Silberg J. L. Heritability of insomnia symptoms in youth and their relationship to depression and anxiety. Sleep. 2011;34:1641–1646. doi: 10.5665/sleep.1424. doi:10.5665/sleep.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M., Dohnt H., Gardner G., Paine S., Starkey K., Menne A., Trenowden S. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep. 2011;34:1671–1680. doi: 10.5665/sleep.1432. doi:10.5665/sleep.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer M. H., Perryman J. I., Lee T. M., Carskadon M. A. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental Neuroscience. 2009;31:276–284. doi: 10.1159/000216538. doi:10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszti R. Á., Ella K., Gyöngyösi N., Roenneberg T., Káldi K. Social jetlag negatively correlates with academic performance in undergraduates. Chronobiology International: The Journal of Biological and Medical Rhythm Research. 2014;31:603–612. doi: 10.3109/07420528.2013.879164. doi:10.3109/074205 28.2013.879164. [DOI] [PubMed] [Google Scholar]
- Hasler B. P., Clark D. B. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism: Clinical and Experimental Research. 2013;37:558–565. doi: 10.1111/acer.12003. doi:10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler B. P., Martin C. S., Wood D. S., Rosario B., Clark D. B. A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2014;38:2225–2233. doi: 10.1111/acer.12474. doi:10.1111/acer.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler B. P., Soehner A. M., Clark D. B. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2015;49:377–387. doi: 10.1016/j.alcohol.2014.06.010. doi:10.1016/j.alcohol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. O., Breslau N. Sleep problems and substance use in adolescence. Drug and Alcohol Dependence. 2001;64:1–7. doi: 10.1016/s0376-8716(00)00222-2. doi:10.1016/S0376-8716(00)00222-2. [DOI] [PubMed] [Google Scholar]
- Johnson E. O., Roth T., Schultz L., Breslau N. Epidemiology of DSM-IV insomnia in adolescence: Lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117:e247–e256. doi: 10.1542/peds.2004-2629. doi:10.1542/peds.2004–2629. [DOI] [PubMed] [Google Scholar]
- Kessler R. C., Berglund P., Demler O., Jin R., Merikangas K. R., Walters E. E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. doi:10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lewandowski A. S., Toliver-Sokol M., Palermo T. M. Evidence- based review of subjective pediatric sleep measures. Journal of Pediatric Psychology. 2011;36:780–793. doi: 10.1093/jpepsy/jsq119. doi:10.1093/jpepsy/jsq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. S., Kaczynski N. A., Maisto S. A., Bukstein O. M., Moss H. B. Patterns of DSM-IV alcohol abuse and dependence symptoms in adolescent drinkers. Journal of Studies on Alcohol. 1995;56:672–680. doi: 10.15288/jsa.1995.56.672. doi:10.15288/jsa.1995.56.672. [DOI] [PubMed] [Google Scholar]
- Martin C. S., Pollock N. K., Bukstein O. G., Lynch K. G. Interrater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug and Alcohol Dependence. 2000;59:173–176. doi: 10.1016/s0376-8716(99)00119-2. doi:10.1016/S0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Moore M., Slane J., Mindell J. A., Burt S. A., Klump K. L. Genetic and environmental influences on sleep problems: A study of preadolescent and adolescent twins. Child: Care, Health and Development. 2011;37:638–641. doi: 10.1111/j.1365-2214.2011.01230.x. doi:10.1111/j.1365-2214.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H. B., Vanyukov M., Yao J. K., Kirillova G. P. Salivary cortisol responses in prepubertal boys: The effects of parental substance abuse and association with drug use behavior during adolescence. Biological Psychiatry. 1999;45:1293–1299. doi: 10.1016/s0006-3223(98)00216-9. doi:10.1016/S0006-3223(98)00216-9. [DOI] [PubMed] [Google Scholar]
- O’Brien E. M., Mindell J. A. Sleep and risk-taking behavior in adolescents. Behavioral Sleep Medicine. 2005;3:113–133. doi: 10.1207/s15402010bsm0303_1. doi:10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Owens J. A., Belon K., Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Archives of Pediatrics & Adolescent Medicine. 2010;164:608–614. doi: 10.1001/archpediatrics.2010.96. doi:10.1001/archpediatrics.2010.96. [DOI] [PubMed] [Google Scholar]
- Pasch K. E., Laska M. N., Lytle L. A., Moe S. G. Adolescent sleep, risk behaviors, and depressive symptoms: Are they linked? American Journal of Health Behavior. 2010;34:237–248. doi: 10.5993/ajhb.34.2.11. doi: 10.5993/ AJHB.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B. W., Lichstein K. L. Insomnia and daytime functioning. Sleep Medicine Reviews. 2000;4:277–298. doi: 10.1053/smrv.1999.0074. doi:10.1053/smrv.1999.0074. [DOI] [PubMed] [Google Scholar]
- Roane B. M., Taylor D. J. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31:1351–1356. [PMC free article] [PubMed] [Google Scholar]
- Skinner H. A., Allen B. A. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. doi:10.1037/0021-843X.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner H. A., Horn J. L. Alcohol Dependence Scale (ADS) user’s guide. Toronto, Ontario: Addiction Research Foundation; 1984. [Google Scholar]
- Spruyt K., Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: A review of currently available instruments. Sleep Medicine Reviews. 2011;15:19–32. doi: 10.1016/j.smrv.2010.07.005. doi:10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geijlswijk I. M., Korzilius H. P., Smits M. G. The use of exogenous melatonin in delayed sleep phase disorder: A meta-analysis. Sleep. 2010;33:1605–1614. doi: 10.1093/sleep/33.12.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov M. M., Tarter R. E., Kirisci L., Kirillova G. P., Maher B. S., Clark D. B. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neuroscience and Biobehavioral Reviews. 2003;27:507–515. doi: 10.1016/j.neubiorev.2003.08.002. doi:10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Windle M., Lerner R. M. Reassessing the dimensions of temperamental individuality across the life span: The Revised Dimensions of Temperament Survey (DOTS-R) Journal of Adolescent Research. 1986;1:213–229. doi:10.1177/074355488612007. [Google Scholar]
- Wittmann M., Dinich J., Merrow M., Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. doi:10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wong M. M., Brower K. J., Fitzgerald H. E., Zucker R. A. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism: Clinical and Experimental Research. 2004;28:578–587. doi: 10.1097/01.alc.0000121651.75952.39. doi:10.1097/01.ALC.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- Wong M. M., Brower K. J., Nigg J. T., Zucker R. A. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcoholism: Clinical and Experimental Research. 2010;34:1033–1044. doi: 10.1111/j.1530-0277.2010.01178.x. doi:10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. M., Robertson G. C., Dyson R. B. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcoholism: Clinical and Experimental Research. 2015;39:355–362. doi: 10.1111/acer.12618. doi:10.1111/acer.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]