Abstract
Aims
Uncoordinated reflex contractions of the external urethral sphincter (EUS) are a major component of voiding dysfunction after neurologic injury. Patterned stimulation of sacral afferent pathways can reduce abnormal EUS reflexes after acute SCI; however, effectiveness following chronic SCI is unknown.
Methods
Four adult male cats were implanted with bilateral extradural sacral root electrodes to allow bladder activation and underwent subsequent spinal transection (T10–12). Nine weeks after SCI urethral and bladder pressures were recorded with and without sacral afferent stimulation. Surface electrodes were applied to sacral and lumbar dermatomes and stimulus amplitude set below the muscle fasciculation threshold. The stimulation pattern was varied by on/off times of fixed frequency at each location.
Results
Reflexive EUS contractions were observed in all animals after chronic SCI. Patterned sacral dermatome stimulation reduced EUS reflex rate and amplitude in 2 of 4 cats. Suppression was dependent on both the stimulus location and pattern. Sacral locations and a stimulation pattern of (0.75 seconds on, 0.25 seconds off, 20 Hz) were effective in both responder animals.
Conclusions
Patterned sacral dermatome stimulation can reduce urethral abnormal reflexes following chronic SCI. Reflex suppression is dependent on both the stimulation location and stimulus pattern. Reduction of reflexive EUS activity after chronic SCI with this non-destructive and non-invasive approach may provide an advance for the treatment of detrusor-sphincter-dyssynergia.
INTRODUCTION
Clinical Impact of SCI on Micturition
Bladder hyperreflexia and detrusor-sphincter-dyssynergia (DSD) affect over 90% of individuals with spinal cord injuries (1). In the urinary tract, fluid flow in the urethra incites a “guarding reflex” that increases urethral sphincter tone to prevent leakage. This reflex is normally suppressed during voluntary micturition, however is disorganized after SCI (2). Abnormal external urethral sphincter (EUS) reflexes can obstruct voiding. The resulting high-pressure or high-residual voiding reduces patient quality of life, incurs high medical costs, and can produce long-term damage to the bladder and kidneys if not managed effectively (1). Present clinical standards of bladder maintenance include clean intermittent catheterization, anticholinergic pharmacotheraputics, sphincterotomy, urethral stents, or pudendal nerve block (3,4). Such management methods are limited by factors such as severe spasticity, poor upper limb function, intolerable systemic effects or urinary incontinence (4).
Electrical Stimulation for Control of LUT Function
The advent of sacral anterior root stimulation combined with dorsal rhizotomy in the 1970s provided a means of abolishing reflexes and producing bladder evacuation (5,6). It has resulted in an improved quality of life for many; however, rhizotomy that eliminates residual bowel and sexual functions is undesirable and limits wider patient acceptance (6,7). A means of obtaining effective bladder evacuation without interfering with any existing neural capacities would improve Lower Urinary Tract (LUT) management after neurological injury.
Electrically stimulating afferent pathways can interrupt or excite LUT reflexes, such as inhibition of bladder hyperreflexia. Sensory stimulation of the dorsal genital nerve (8,9), posterior tibial nerve (10), and sacral spinal nerves (11,12) all provide inputs to the lumbosacral spinal cord and produce functional improvements in bladder capacity and low pressure urine storage. Reflex bladder contractions can be activated using stimulation of pudendal afferent fibers (13,14). The amplitude of the evoked bladder contractions is dependent on the stimulus frequency (15,16). In addition, different stimulus patterns are more effective at different locations within the urethra (17,18).
It is unknown whether afferent stimulation can specifically inhibit urethral sphincter reflexes. Our group previously demonstrated that patterned dermatome stimulation reduces spastic urethral spike-activity in cats after acute spinal transection (19). Surface stimulation of sacral dermatomes provides a reversible, non-invasive technique that may be able to interrupt the aberrant urethral reflex pathway while retaining desirable bowel and sexual reflexes that are lost with rhizotomy. To be of clinical relevance, sacral surface stimulation must be able to suppress urethral sphincter spasms after chronic SCI, with the resultant changes in the spinal circuits (20). This study sought to determine if afferent reflex suppression can be achieved following neural reorganization in cats after chronic SCI.
MATERIALS & METHODS
Chronic SCI animal model
Four sexually intact adult male cats had extradural sacral electrodes implanted for on-demand bladder drive; a second surgery to transect the spinal cord was carried out after the electrodes had stabilized and animals were deemed behaviorally suitable for chronic SCI maintenance. All procedures were carried out under general anesthesia (ketamine induction, isoflurane maintenance), with prior approval from the Case Western Reserve University IACUC. Animals first underwent a laminectomy at the L7–S2 level. Tripolar spiral cuff electrodes (Ardiem Medical, Indiana, PA) of 1.25 mm diameter were implanted on the extradural sacral roots eliciting the greatest bladder pressures intraoperatively (verified S2 roots post mortem). Electrode leads were tunneled subcutaneously to exit in the inter-scapular region. Animals were fitted with jackets (Lomir Biomedical Inc., Malone, NY) to protect the lead exit sites.
Animals underwent surgical spinal transection at the T10–T12 vertebral level (after 17 weeks in animals 1–2, 6 weeks in animals 3–4). The dura was exposed through a laminectomy, and the cord cut through a small incision made in the dura with local application of intradural Marcaine .25% (Hospira Inc., Lake Forest, IL). Completeness of transection was visually confirmed prior to closure. Following recovery, animals received manual bladder expression 2–3 times daily; reflex defecation circumvented the need for assistive bowel care. A 9 week survival period established a chronic model of abnormal LUT reflexes.
Post mortem dissection was carried out to confirm the spinal root levels, identify any damage to the sacral root or electrode implants, and verify completeness of spinal transection.
Terminal Procedure
Nine weeks post-SCI animals were anesthetized with an IV infusion of alpha-chloralose (75 mg/kg induction, 19 mg/kg supplemental maintenance as needed) (Sigma Alderitch, St. Louis, MO). Sub-cutaneous buprenorphine (.01 mg/kg) was given every 12 hours. Each animal was instrumented with a suprapubic bladder catheter for bladder filling and draining, and measuring bladder pressure. External sphincter and proximal urethral pressures were measured using a 3.5 French catheter (Gaeltec, Isle of Skye, Scotland) mounted with two microtransducer pressure sensors placed into the urethra. The transducer was zeroed to atmospheric pressure prior to use. The gain of the transducer was calculated using a mercury manometer and two-point linear slope. Active urethral pressure profiles (UPP) were conducted to determine the position of the EUS from the external meatus.
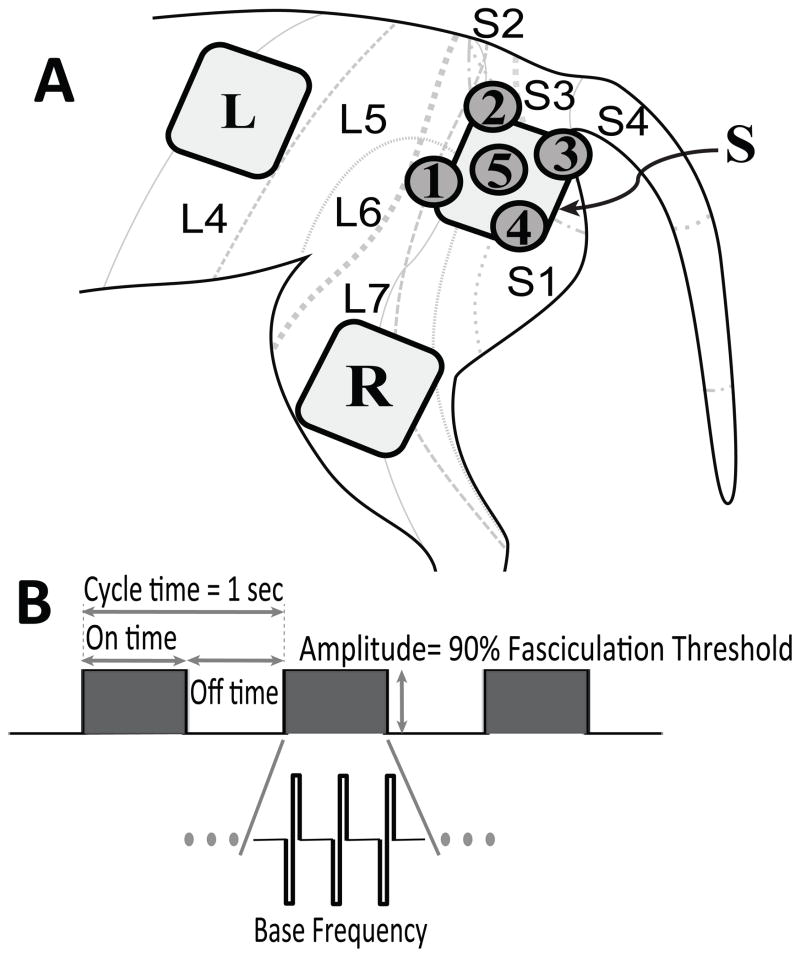
Sacral dermatome levels more effectively deliver sensory stimulation to the spinal circuits generating unwanted urethral reflexes (19,21). To investigate surface localization effects, the L4 through S4 dermatomes were shaved and prepped with a medical depilatory prior to application of surface electrode patches. Dermatome locations were estimated from published dermatome maps (22,23). “Large” surface electrodes (4cmx4cm square, Re-Ply Unipatch; Covidien, San Francisco CA) were used for gross dermatome localization effects (lumbar vs. sacral dermatomes). “Small” electrodes (2 cm diameter round, Cardinal Health) were used for finer resolution within lumbar and sacral dermatomes. Figure 1 shows placement of large and small electrodes for spatial differentiation and the general pattern of electrical stimulation.
Figure 1. Surface electrode placement and patterned stimulus waveform.
A: Large 4 cm square electrode patches were used to stimulate sacral dermatomes [S] (L7–S3) and lumbar dermatomes [L] (L4–L5). Smaller 2 cm round patches were used to improve spatial resolution within the sacral dermatomes [1–5]. Return patch [R] for anodal stim was used for all electrodes. Feline dermatome map adapted from Kuhn (22), showing the approximate boundaries and overlap of L4–S4 dermatomes. Electrode [S] overlaps the L7–S3 dermatomes; [1]: L5–L7; [2]: L7/S2; [3]: L7–S1; [4]: S3; [5]: L7–S2 dermatomes.
B: The stimulus waveform was defined in terms of ON/OFF time, base frequency, and amplitude. On/ off times always summed to a total cycle time of 1 sec. Base frequency was fixed at 20 Hz for duty cycles less than 100%; other base frequencies were tested for continuous stimulation.
Data Collection: Stimulation Protocol
Bladder contractions were evoked using 5–10 seconds of 20 Hz stimulation on sacral root electrodes. Control trials consisting of bladder drive without any dermatome stimulation were used to consistently evoke EUS reflex activity. Baseline EUS spasticity was defined as the pressure spikes following root stimulation.
Dermatome stimulation consisted of monophasic constant-current pulses of 100 μs (DS7A, Digitimer, Hertfordshire, England). Afferent neurons typically utilize bursts of action potentials with varying burst durations, frequency and inter-burst intervals suggesting that particular stimulation patterns will provide more effective reflex modulation than others. We used cycle time (s), duty cycle (%), and base frequency (Hz) to generate patterned stimuli. Cycle time was fixed at 1 s for all patterns. Duty cycle was varied (25%, 50%, 75%, 100%) for a fixed base frequency (20 Hz). Cycle time and duty cycle are simultaneously described though stimulation ON time and OFF time (Figure 1). At 100% duty cycle, representing continuous stimulation, base frequency was varied between 10, 20 and 40 Hz for all animals (except animal 2, which did not respond to 20 Hz). Stimulation amplitude was determined by visible muscle fasciculation; all trials reported were conducted at 90% of fasciculation threshold.
Voiding improvement was investigated by adding (.75 ON .25 OFF, 20 Hz) dermatome stimulation to intermittent sacral root stimulation (2.0 ON 4.0 OFF, 20 Hz) in one animal (#2). Surface stimulation was limited to the “middle” 2cm round surface electrode (electrode [5], Figure 1). Voiding was limited to 4 paired, bladder volume matched control/ dermatome runs. Voiding percentages were calculated for each run; voiding to completion was not attempted.
In the same animal a 22 gauge stainless steel needle was inserted through the same location and stimulated with (.75 ON .25 OFF, 20 Hz) to test the effect of on urethral reflexes. All data was recorded in a custom designed data-acquisition program (Labview, National Instruments, Austin, TX).
Data Analysis
Urethral sphincter spasms were observed as EUS pressure “spikes” in all animals, due to high-fidelity microtransducer recording (Figure 2). Spike rate and spike amplitude were used as the primary measures of reflex EUS activity (19). Spikes were defined as pressure increases 1 standard deviation above a 0.5 second moving average, quantified in Matlab (Mathworks, Natick, MA). Absolute pressure was used rather than pressure evoked above baseline. Baseline urethral pressure was consistent at ~15 cmH2O. Variables reduced from identified spikes include: Pur= Average urethral spike pressure amplitude (cmH2O) and Rur= Average urethral spike rate (number/sec) calculated over the period of dermatome stimulation (typically 60 seconds), with a corresponding length chosen for comparative control trials.
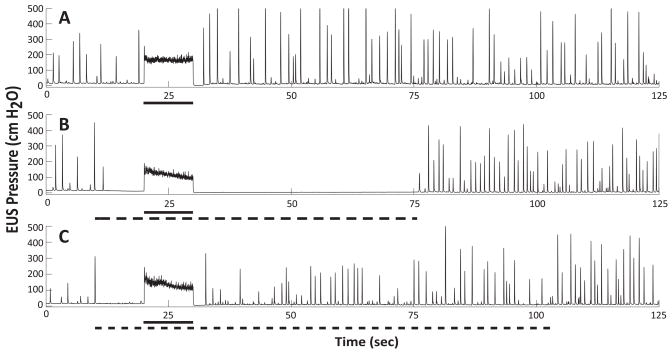
Figure 2. Suppression of aberrant urethral reflexes after chronic SCI.
Representative examples of aberrant urethral reflex activity with and without dermatome stimulation. All panels: Sacral root stimulation (20 Hz, solid bar) was applied to directly evoke bladder pressure and provoke DSD in a consistent, repeatable manner. Panel A: Control trial showing baseline reflex activity. Panel B: The addition of patterned electrical stimulation to the sacral dermatomes (.75 ON .25 OFF, 20 Hz, dashed bar) suppressed the aberrant EUS reflexes. Panel C: Applying dermatome stimulation to the same electrode with different stimulus pattern (.5 ON .5 OFF, 20 Hz, dashed bar) did not significantly reduce urethral activity. This parameter space specificity is representative across both responder animals.
For each animal one-way ANOVAs were used to analyze the effect of dermatome stimulation on reflex activity when compared to control trials. Additionally ANOVAs were calculated for stimulus patterns at each electrode location, and across locations for each pattern. The Tukey-Kramer method was used to determine which parameter combinations differed significantly; only reductions from baseline which reached (P<.05) significance were labeled “suppression”.
RESULTS
Reflex Activity Following Chronic SCI
Prior to SCI, no animals displayed urethral reflex activity under light isoflurane anesthesia. Neither EUS nor bladder activity was evoked when applying dermatome stimulation at sub-fasciculation (4.5–12.5 mA) threshold levels, and current amplitudes high enough to produce urethral pressure changes were inseparable from motion artifact due to hind limb muscle contractions.
The bulbocavernosus reflex reappeared in all animals within 12 hours after SCI and LUT reflexes appeared within 1 week. All animals displayed distention evoked bladder reflexes and the progressive development of spastic urethral sphincter reflexes that were observed under light isoflurane or alpha-chloralose. These EUS reflexes were consistently evoked following sacral root stimulation, however they also occurred spontaneously or with urethral catheter movement. Baseline reflex activity with effective and ineffective patterned surface stimulation are shown in Figure 2.
Three animals had confirmed complete transection of the spinal cord. Animal 1 had partial voluntary stepping and visceral sensation caudal to transection, suggesting incomplete injury. All animals had at least one working sacral root electrode capable of producing greater than 50 cm H2O intra-vesicular pressure and greater than 70 cm H2O urethral sphincter pressure. The right S2 root was damaged in animal 2 during the week after spinal transection.
Effect of Dermatome Stimulation on Urethral Reflex Suppression
Significant, reproducible suppression of urethral reflexes was achieved in 2 of 4 animals when effective stimulus parameters were used (Figure 2). Reflex reduction was observed within 2 seconds of the start of dermatome stimulation, and was able to be maintained for over 60 seconds. Statistically significant suppression was only achieved for a limited combination of electrode locations and stimulus patterns. Urethral reflexes were reduced by dermatome stimulation at all locations tested in responder animals (1 and 2), but only sacral dermatome locations significantly reduced urethral activity compared to control and were classified as suppression (Figure 3).
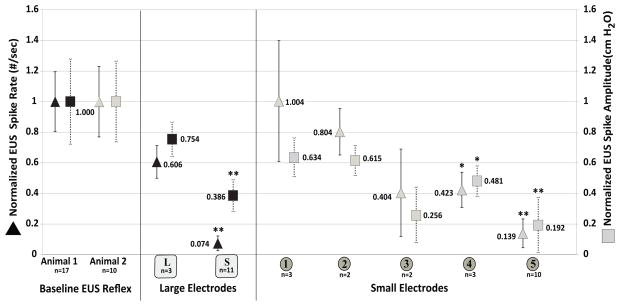
Figure 3. Spatial selectivity of stimulus location.
Sacral dermatome stimulation reduced urethral reflexes. Data from large lumbar [L] and sacral [S] electrode locations (Black symbols, animal 1) show sacral locations [S] reduce urethral reflexes. Finer resolution at location [S] using smaller electrodes [1–5] (gray symbols, animal 2) demonstrates further location specificity within the sacral dermatomes. Data are normalized to the baseline control evoked urethral activity Pur and Rur. EUS reflex spike rate (Rur) is shown on the left axis (■). EUS reflex spike amplitude is shown on the right axis (▲). Stimulus pattern shown for [L] and [S] is 20 Hz continuous; for [1–5] is [.75 ON .25 OFF, 20 Hz]. The number of trials (n) is shown for each location. *P<.05; ** P<.01.
Rur was reduced 92.6% and Pur reduced 61.4% (P<.001) using the large electrode location [S] (animal 1, 20 Hz continuous). Sacral [S] stimulation with (.75 ON .25 OFF, 20 Hz) produced comparable suppression to 20 Hz continuous stimulation (see Figure 4); 20 Hz continuous data are presented as lumbar stimulation [L] was not attempted using (.75 ON .25 OFF, 20 Hz). Finer resolution of the sacral skin beneath [S] using smaller electrodes [1–5] (animal 2) demonstrated finer spatial resolution within the sacral dermatomes. Locations 4 and 5 both significantly suppressed urethral reflexes. Location [5] reduced Pur 80.8% and Rur 86.1% (P <.001) using (.75 ON .25 OFF, 20 Hz). Suppression was not achieved at electrodes [1–4] for any other patterns tested (data not shown).
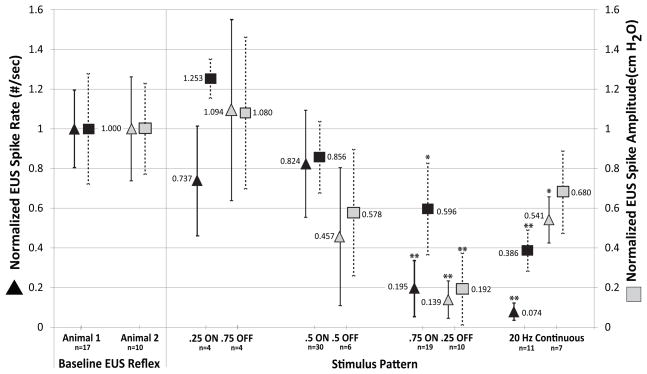
Figure 4. Stimulus pattern affects reflex suppression.
Urethral reflex suppression was dependent on stimulus pattern. Only (.75 ON .25 OFF, 20 Hz) patterned stimulation was effective in both responder animals. Increasing duty cycle led to progressive reductions in spike rate Rur and amplitude Pur. 20 Hz continuous stimulation was effective only in animal 1. Data are normalized to the baseline control evoked urethral activity Pur and Rur. EUS reflex spike rate (Rur) is shown on the left axis (■). EUS reflex spike amplitude is shown on the right axis (▲). Data from animal 1 (black symbols, location [S]) and animal 2 (gray symbols, location [5]) are shown separately for each stimulation pattern. The number of trials (n) is shown for each location. *P<.05; ** P<.01.
Data shown in Figures 3 and 4 are normalized to the control for each animal. The Pur and Rur, control values for all animals are shown in Table I. The pooled values for all dermatome stimulation trials are also shown for animals 3 and 4, where no differences were observed between surface stimulation and control for any stimulus locations or patterns tested. Only (.75 ON .25 OFF, 20 Hz) patterned stimulation produced suppression in both responder animals, when applied at effective locations. Increasing duty cycle from 25% to 75% led to progressive reductions in spike rate Rur and amplitude Pur, however only 75% produced suppression compared to control and were classified as suppression. Figure 4 shows patterned stimuli tested at electrode locations [S] (animal 1) and [5] (animal 2). Data are normalized to the control for each animal.
Table I.
Table I lists the individual and pooled baseline urethral reflex measures for all four animals. Urethral spike rate (Rur) was significantly different for all four animals (P<.05). Urethral reflex amplitude (Pur) was significantly different only for animal 2 (P<.05). Pooled data are shown for all dermatome trials for non-responder animals 3 and 4. No difference was seen between baseline activity and any subset of dermatome stimulation.
| ANIMAL | CONTROL | DERMATOME | ||||
|---|---|---|---|---|---|---|
| Trials (n) | Rur (spikes/ sec) | Pur (cm H2O) | Trials (n) | Rur (spikes/ sec) | Pur (cm H2O) | |
| 1 | 20 | 0.438 ± 0.171 | 167.8 ± 93.4 | ** | ||
| 2 | 24 | 0.088 ± 0.041 | 355.5 ± 186.4 | |||
| 3 | 21 | 0.188 ± 0.012 | 167.2 ± 100.8 | 83 | 0.199 ± 0.099 | 157.3 ± 121.1 |
| 4 | 24 | 0.054 ± 0.031 | 140.3 ± 59.0 | 49 | 0.078 ± 0.032 | 205.2 ± 79.3 |
| Pooled | 89 | 0.181 ± 0.061 | 210.8 ± 110.9 | 132 | 0.154 ± 0.100 | 175.1 ± 109.7 |
Continuous (20 Hz) stimulation produced suppression in animal 1, but not animal 2. In animal 1, 10 Hz continuous reduced Rur by 82.4% (P<.001) and Pur by 28.2%, (P= .053) and 40 Hz continuous reduced Pur 38.1% (P=.289) and Rur 60.1% (P= .084).
Bladder emptying was improved from 30.3%±3.8% (8.95± 2.2mL) without dermatome stimulation to 41.93% ±6.6% (12.43 ± 2.9 mL) with stimulation (P<.05) in single bladder drive-limited stimulus runs. When normalized to control voided volumes, this represents a 38.3% ±21.7% increase with the addition of effective surface stimulation. Bladder volume for all trials was 29.3 mL ±3.5 mL.
Subcutaneous stimulation reduced Rur (P<.01) and Pur (P<.001) when compared with control trials. Subcutaneous stimulation was not significantly different from surface stimulation using the same (.75 ON .25 OFF, 20 Hz) pattern (Rur (P= .362), Pur (P= .771)). In 7 subcutaneous trials, (Rur= .0208 ±.019 /sec; Pur= 83.95 ± 88.21 cm H2O).
DISCUSSION
Reduction of Urethral Spasm after Chronic SCI
This study provides the first evidence that patterned sensory stimulation of sacral dermatomes can reduce spastic urethral activity after chronic spinal cord injury. These data provide a key step in validating previous results after acute SCI (19) and justify work to evaluate the voiding effectiveness of this novel approach to treat urethral sphincter spasticity.
Location of the stimulation electrode significantly impacts the extent of reduction of EUS spasticity, both at a large-scale level (lumbar vs. sacral dermatomes) and within the sacral dermatomes (see Figures 1 and 3). Effective skin locations corresponded to the expected overlap of the L7 through S2 dermatomes, while higher lumbar levels or levels below S2 were ineffective. Location specificity is consistent with our expectation that sensory stimulation must be directed to specific spinal interneurons in the sacral spinal cord. The skin locations are consistent with neurophysiology studies placing spinal circuits controlling the external sphincter in cats at the S1–S2 level (21). Pharmacological work in spinal transected rats (24) demonstrating that local delivery of GABA agonists to the lumbosacral spinal cord could eliminate dyssynergic EUS contractions suggests that afferent stimulation could similarly act through a GABAergic inhibitory response.
EUS reflex reduction is dependent on the electrical stimulus pattern. Specific patterns reduced aberrant sphincter reflexes while others were ineffective. (.75 ON .25 OFF, 20 Hz) was effective in both responder animals, while 20 Hz continuous was only effective in one animal. This consistency suggests that pattern is an important factor. Other stimulus features such as pulse width or wave shape may provide more optimal sensory input. While stimulation location and pattern significantly affected the ability to reduce urethral sphincter activity, these specific locations and patterns may not generalize to all animals.
Stimulus parameters found effective after chronic SCI were not tested in acute SCI, but the (.5 ON .5 OFF, 20 Hz) that was successful in acute animals (19) did not have a significant effect after chronic SCI. This suggests that the changes in the spinal reflex pathways from after chronic SCI could impact the afferent input required for reflex suppression. Demonstration that patterned afferent neuromodulation reduces urethral spasms and improves functional outlet resistance after SCI is the first step towards realizing a functional neuroprosthesis. Although complete bladder emptying was not attempted, improvement in the first run (of a potential series) demonstrates that a reduction in EUS spike pressure and frequency is functionally relevant to reducing the outlet pressures in animals with DSD. Evidence that a wire in the subcutaneous space can reduce sphincter pressures equivalent to surface stimulation, combined with existing afferent neuromodulation technology for bladder inhibition, suggests a fully implantable system is feasible.
Non-Responder Animals
Reflex suppression was only obtained in 2 of the 4 animals, which is consistent the 50% (3 of 6) success rate previously demonstrated in acute SCI animals (19). There is no clear pattern in the baseline activity of the four animals that would suggest why suppression was achieved in some and not others. Other applications of afferent stimulation have shown similar variability in individual responses (14,15,17). It is currently unknown if we have not appropriately mapped the parameter space and missed the correct input, if dermatome overlap results in too diffuse spinal input, or that the neurophysiologic pathways were different in the non-responder animals after SCI (25).
CONCLUSIONS
This study demonstrates proof-of-concept that afferent stimulation of the sacral dermatomes can significantly reduce urethral reflexes after chronic spinal cord injury. Suppression is dependent on the location and pattern of the sensory input. Stimulation of sacral dermatomes provides a minimally-invasive method for reducing urethral sphincter spasms after chronic spinal cord injury.
Acknowledgments
This work was supported by NIH DK077089, EB004314, Department of Veterans Affairs RR&D668 and the Cleveland FES Center. The authors thank Manfred Franke, Tina Emancipator, Jennifer Mikulan, and Julie Murphy for technical assistance, and Drs. Steven Brose and Dennis Bourbeau for manuscript review.
References
- 1.Weld KJ, Graney MJ, Dmochowski RR. Clinical significance of detrusor sphincter dyssynergia type in patients with post-traumatic spinal cord injury. Urology. 2000;56(4):565–8. doi: 10.1016/s0090-4295(00)00761-5. [DOI] [PubMed] [Google Scholar]
- 2.Park JM, Bloom DA, McGuire EJ. The guarding reflex revisited. Br J Urol. 1997;80(6):940–5. doi: 10.1046/j.1464-410x.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 3.Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31(1):12–28. 48. quiz 29. [PubMed] [Google Scholar]
- 4.Ahmed HU, Shergill IS, Arya M, Shah PJ. Management of detrusor-external sphincter dyssynergia. Nat Clin Pract Urol. 2006;3(7):368–80. doi: 10.1038/ncpuro0521. [DOI] [PubMed] [Google Scholar]
- 5.Brindley GS. An implant to empty the bladder or close the urethra. J Neurol Neurosurg Psychiatry. 1977;40(4):358–69. doi: 10.1136/jnnp.40.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martens FMJ, Heesakkers JP. Clinical results of a brindley procedure: sacral anterior root stimulation in combination with a rhizotomy of the dorsal roots. Adv Urol. 2011;2011:709708. doi: 10.1155/2011/709708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders PMH, Ijzerman MJ, Roach MJ, Gustafson KJ. Patient preferences for next generation neural prostheses to restore bladder function. Spinal Cord. 2011;49(1):113–9. doi: 10.1038/sc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman HB, Amundsen CL, Mangel J, Grill J, Bennett M, Gustafson KJ, et al. Dorsal Genital Nerve Stimulation for the Treatment of Overactive Bladder Symptoms. Neurourol Urodyn. 2008;27(6):499–503. doi: 10.1002/nau.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y-hee, Creasey GH, Lim H, Song J. Detrusor and Blood Pressure Responses to Dorsal Penile Nerve Stimulation During Hyperreflexic Contraction of the Bladder in Patients With Cervical Cord Injury. Arch Phys Med Rehabil. 2003;84(1):136–40. doi: 10.1053/apmr.2003.50075. [DOI] [PubMed] [Google Scholar]
- 10.MacDiarmid SA, Peters KM, Shobeiri SA, Wooldridge LS, Rovner ES, Leong FC, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol. 2010;183(1):234–40. doi: 10.1016/j.juro.2009.08.160. [DOI] [PubMed] [Google Scholar]
- 11.Bosch JL, Groen J. Neuromodulation: urodynamic effects of sacral (S3) spinal nerve stimulation in patients with detrusor instability or detrusor hyperflexia. Behav Brain Res. 1998;92(2):141–50. doi: 10.1016/s0166-4328(97)00186-1. [DOI] [PubMed] [Google Scholar]
- 12.Kirkham PS, Knight SL, Craggs MD, Casey AT, Shah PJ. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002;40(6):272–81. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- 13.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiology. 2006;577(Pt 1):115–26. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woock JP, Yoo PB, Grill WM. Intraurethral stimulation evokes bladder responses via 2 distinct reflex pathways. J Urol. 2009;182(1):366–73. doi: 10.1016/j.juro.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruns TM, Bhadra N, Gustafson KJ. Variable patterned pudendal nerve stimuli improves reflex bladder activation. IEEE Trans Neural Syst Rehabil Eng. 2008;16(2):140–8. doi: 10.1109/TNSRE.2007.914460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Liu H, Shen B, Roppolo JR, Groat WCD. Bladder Inhibition or Excitation by Electrical Perianal Stimulation in the Chronic SCI Cat. BJU Int. 2009;103(4):530. doi: 10.1111/j.1464-410X.2008.08029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruns TM, Bhadra N, Gustafson KJ. Intraurethral Stimulation for Reflex Bladder Activation Depends on Stimulation Pattern and Location. Neurourol Urodyn. 2009;28(6):561–6. doi: 10.1002/nau.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury. J Urol. 2011;185(2):737–43. doi: 10.1016/j.juro.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariano TY, Bhadra N, Gustafson KJ. Suppression of Reflex Urethral Responses by Sacral Dermatome Stimulation in an Acute Spinalized Feline Model. Neurourol Urodyn. 2010;29(3):494–500. doi: 10.1002/nau.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2011 May 9; doi: 10.1016/j.expneurol.2011.05.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blok BF. Central pathways controlling micturition and urinary continence. Urology. 2002;59(5 Suppl 1):13–7. doi: 10.1016/s0090-4295(01)01633-8. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn RA. Organization of tactile dermatomes in cat and monkey. J Neurophysiol. 1953;16(2):169–82. doi: 10.1152/jn.1953.16.2.169. [DOI] [PubMed] [Google Scholar]
- 23.Brown PB, Koerber HR. Cat hindlimb tactile dermatomes determined with single-unit recordings. J Neurophysiol. 1978;41(2):260–7. doi: 10.1152/jn.1978.41.2.260. [DOI] [PubMed] [Google Scholar]
- 24.Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, et al. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R336–42. doi: 10.1152/ajpregu.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Groat WC, Yoshimura N. Changes in Afferent Activity After Spinal Cord Injury. Neurourol Urodyn. 2010;29(1):63–76. doi: 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]