Summary
The cat has been used extensively as an animal model for urogenital studies involving the pudendal nerve. However, discrepancies persist in the literature regarding the origin of the dorsal nerve of the penis (DNP). This study used gross dissections and serial histological cross sections to demonstrate that the DNP arises from the deep perineal nerve and not the sensory afferent branch as previously reported. This finding indicates a better than previously appreciated neuroanatomical homology between the cat and human.
Introduction
The pudendal nerve (PN) and its branches are critical to proper urogenital function and a primary research targets for understanding and treating bladder sphincter dyssynergia resulting from lower spinal cord injury (Boggs et al., 2005; Bhadra et al., 2006). The cat has been a widely used model for these systems level neural studies because of similarities between the feline and human nervous systems. Previous experience in our laboratory has shown the deep perineal nerve (DPN) giving rise to the dorsal nerve of the penis (DNP), an observation that differs from extant literature such as Martin et al.’s ‘typical’ feline sacral plexus, which showed the dorsal genital nerves arising from a dedicated sensory afferent (AFF) branch of the PN (Martin et al., 1974).
The purpose of this study was to clarify the course of the DPN and DNP as they arose from the PN in the male cat and compare this pattern to known human anatomy. Closer neuroanatomical homology between cat and human has potential clinical repercussions as it would extend the suitability of the feline as an electrophysiological model for human neurourological studies.
Materials and Methods
Dissections were performed on cadavers of sexually intact adult male cats (n = 8) with body weight 3.3–5.5 kg (Harlan–Sprague–Dawley, Madison, WI, USA; Liberty Research, Waverly, NY, USA). The PNs (left: n = 8; right: n = 1) had been exposed pre-mortem for an electrophysiological study by surgical section and lateral reflection of the gluteus superficialis and gluteofemoralis muscles using methods previously described (Boggs et al., 2005). Cadavers were then refrigerated or frozen for between three and 32 days until dissection. Blunt dissection was used to trace the PN and its branches as distally as possible, usually including much of the penile shaft. The PN was traced rostrally in all cadavers – and in one to the sacral roots. In an additional cadaver, the pubic symphysis was removed to reveal ventral pelvic structures. Dissection was aided by use of a surgical microscope (Storz Urban US-1; Urban Engineering Inc., Burbank, CA, USA) and head-mounted magnifier (OptiVISOR DA-3; Donegan Optical Company, Inc., Lenexa, KS, USA). Digital photographs were taken using a 3.3 megapixel camera (Canon PowerShot G1; Canon, Inc., Tokyo, Japan).
Six PNs (left: n = 5; right: n = 1) and their branches were then excised from five cadavers with the branches transected as far distally as possible and the main trunk as far rostrally as possible. The harvested segments were photographed, placed in 10% buffered formalin, and refrigerated at approximately 7°C.
Histological processing for confirmation of gross dissection findings was performed by the Histology Core Facility, University Hospitals, Cleveland, OH, USA. Three complete specimens were labelled with histopathology tissue marking dyes and cut at 5- to 10-mm intervals with a razor blade. The segments were enclosed in Histo-Wrap© (Obex Industries, Buffalo, NY, USA), placed in tissue cassettes, again stored in 10% formalin, and refrigerated. Samples were embedded in paraffin, cut in 5-μm transverse sections, stained with haematoxylin and eosin, and mounted on glass slides. Digital photomicrographs were taken with a binocular microscope (Axioskop D-7082; Carl Zeiss, Jena GmbH, Jena, Germany) and a camera (spot 1.1.0) connected to a PC running commercial image acquisition software (spot 4.0.9). The resulting images were rotated or flipped as necessary with software (gimp 2.2.6) to establish consistent image orientation using careful examination of fascicular structure and arrangement.
Results and Discussion
The PN was found to arise primarily from the S1 and S2 sacral roots. In all cases, the origin of the DPN was identified visually by noting the bifurcation of the PN into a branch supplying the external anal sphincter (EAS) and another giving rise to the DPN. The AFF branch was most clearly observed in three cats – later processed histologically – arising 10–15-mm proximal to the PN bifurcation. The EAS branch (or inferior rectal nerve) was easily identified by noting ramifications disappearing into the EAS muscle. In all cats, blunt dissection showed the DPN running inferior to the ipsilateral inferior pubic ramus; in five cats, the dissection was continued distally to show the DPN giving rise to the DNP as it coursed along the dorsal surface of the penis beneath a thick fascia layer analogous to Buck’s fascia in the human (Yang and Bradley, 1998c). For the cadaver in which the pubic symphysis was removed, the DPN was also observed sending a branch that innervated the external urethral sphincter (EUS) muscle (Fig. 1). A representative specimen appears in Figs 2 and 3.
Fig. 1.
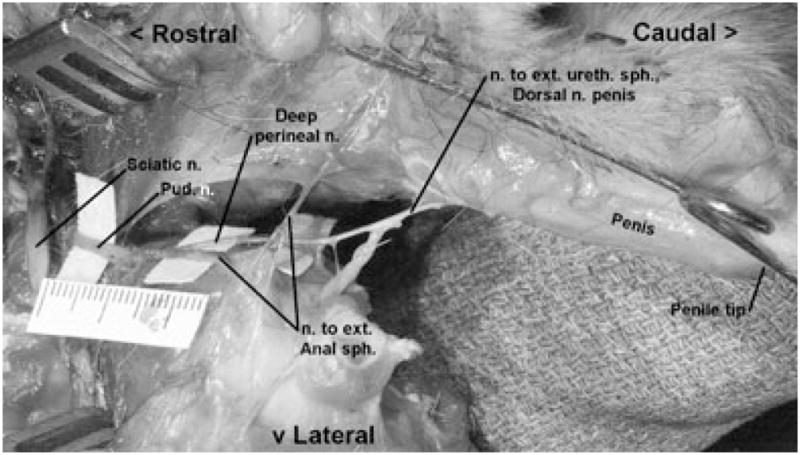
Dorsolateral view of the left pudendal nerve (PN) and major branches (scale in cm). The dissection involved a ventral approach to remove the pubic symphysis, exposing branches of the left deep perineal nerve (DPN) that supplied the external urethral sphincter muscle and the dorsal nerve of the penis, and was continued proximally. The left testicle was removed and the perineum divided to demonstrate the left PN giving rise to the left DPN.
Fig. 2.
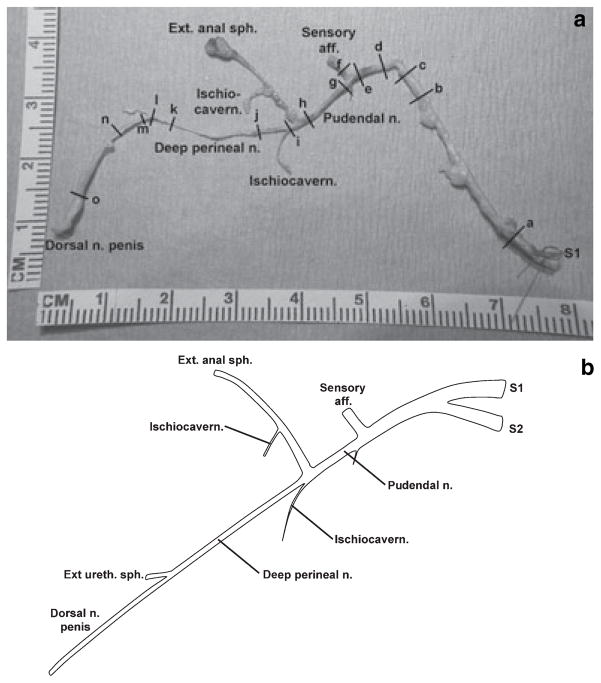
(a) Gross image of a nerve sample from a different cat than that of Fig. 1 (scale in cm). The external urethral sphincter was not identified. All other branches have been labelled. Transverse sections taken at marks (a)–(o) correspond to the panels of Fig. 3. (b) Revised pudendal nerve branching diagram showing the deep perineal nerve giving rise to the dorsal nerve of the penis. Figure adapted from Martin et al. (1974).
Fig. 3.
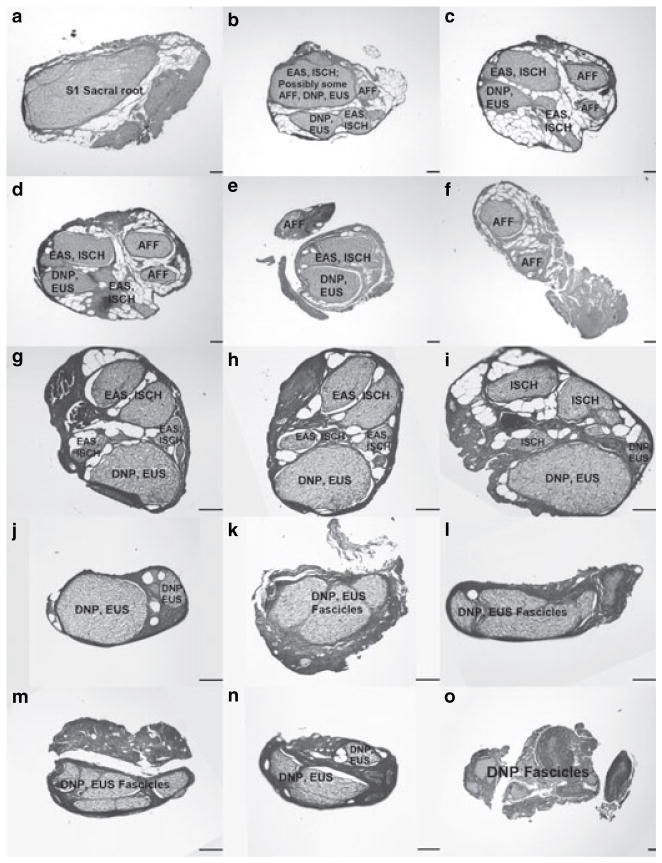
Serial histological cross sections taken as indicated in Fig. 2a. Black vertical bars in each panel indicate 100 μm. Note that three distinct scales are used to best show structural detail: panels (a)–(f) are 1700-μm wide in total, panels (g)–(n) are 900-μm wide in total, and panel (o) is 2550-μm wide in total. In slices (k)–(n), the precise division between dorsal nerve of the penis (DNP) and external urethral sphincter fascicles is unclear. The small projection seen between (l) and (m) in Fig. 2a was found not to contain nerve tissue and is not represented here. Slice (o) shows only DNP fascicles because of its distal location.
In Fig. 2a, the DPN was clearly identified as arising distal to the AFF branch at a bifurcation of the PN with the other fork supplying the EAS branch. Two candidates for the ischiocavernosus (ISCH) muscle’s innervation are also shown. The EUS was not identified in this cadaver; the small projection arising between cuts (l) and (m) of Fig. 2 was found not to contain nerve tissue upon histological examination. Figure 2b shows the typical PN branching pattern based on all dissections in this study, with special attention paid to the origins of the DNP.
Figure 3 shows serial cross sections corresponding to the gross image in Fig. 2a and was representative of the other two histologically processed specimens. In the proximal cross sections, fascicles were found to have a mixed role as axons supplying multiple distal structures were enclosed within one perineurial sheath. The AFF branch arose from dedicated fascicles in two specimens (including that depicted in Fig. 3). In each specimen, fascicles giving rise to the DPN, in turn, divided into smaller fascicles supplying the DNP and EUS branches, mirroring the observed gross anatomy of Fig. 1. Moving distally along the penis, the DNP divided into many small fascicles as seen in (o) of Fig. 3. The precise origin of the EUS branch could not be identified in any of the histologically processed samples, but is expected near (n) as this was the approximate location of the EUS muscle during gross dissection. In multiple slides, large voids were observed between the perineurial sheathes and neuron cell bodies, suggesting shrinkage or mechanical damage during preparation. As a result of these artefacts, cross sectional areas of individual fascicles could not be reliably obtained.
The human DNP arises from the PN usually distal to the EAS branch but separately from the perineal nerve (Juenemann et al., 1988; Shafik et al., 1995; Schraffordt et al., 2004; Gustafson et al., 2005). This is largely analogous to the described feline neuroanatomy, in which the DNP arises distal to the EAS and AFF branches but is itself a distal continuation of the DPN as opposed to a separate terminal branch of the PN. The distal human urethra is innervated by the DNP (Yang and Bradley, 1998a,c), a finding that can be extended to the feline based on urethral stimulation results (Bradley et al., 1973) when taken in light of the present study.
The serial histological cross sections also confirm the results of the gross dissections, demonstrating that the DPN supplies both the DNP and EUS, thus suggesting a mixed afferent and efferent function of the feline DPN. This finding is inconsistent with the Martin et al. branching pattern, as it showed a dedicated sensory PN branch giving rise to the DNP and a dedicated motor branch supplying the DPN (Martin et al., 1974). However, Martin et al. examined the distribution of myelinated fibres in the DPN and DNP and found the former to be 46% afferents, a result consistent with it giving rise to a large afferent nerve such as the DNP – itself found to be 96% afferents. Therefore, segregating the male feline PN into anatomically separate sensory and motor branches is an oversimplification.
The findings of this study extend the validity of the feline as an appropriate model for human neurourological studies. Furthering our knowledge of sensory innervation of the penis could aid in restoration of micturition and sexual function especially given the proposed role of penile afferents in bladder excitation and ejaculation (Yang and Bradley, 1998b; Gustafson et al., 2004).
Acknowledgments
The authors thank Narendra Bhadra, M.D., Ph.D., Yanina Grinberg, and Jake Parks for their assistance. This work was supported by the NIH DK077089, HD40298, EB004314, the VA RR&D B3675R, and The State of Ohio BRTT03-10.
References
- Bhadra N, Bhadra N, Kilgore K, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. J Neural Eng. 2006;3:180–187. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- Bradley W, Griffin D, Teague C, Timm G. Sensory innervation of the mammalian urethra. Invest Urol. 1973;10:287–289. [PubMed] [Google Scholar]
- Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gustafson KJ, Zelkovic PF, Feng AH, Draper CE, Bodner DR, Grill WM. Fascicular anatomy and surgical access of the human pudendal nerve. World J Urol. 2005;23:411–418. doi: 10.1007/s00345-005-0032-4. [DOI] [PubMed] [Google Scholar]
- Juenemann KP, Lue TF, Schmidt RA, Tanagho EA. Clinical significance of sacral and pudendal nerve anatomy. J Urol. 1988;139:74–80. doi: 10.1016/s0022-5347(17)42297-x. [DOI] [PubMed] [Google Scholar]
- Martin WD, Fletcher TF, Bradley WE. Innervation of feline perineal musculature. Anat Rec. 1974;180:15–29. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- Schraffordt SE, Tjandra JJ, Eizenberg N, Dwyer PL. Anatomy of the pudendal nerve and its terminal branches: a cadaver study. ANZ J Surg. 2004;74:23–26. doi: 10.1046/j.1445-1433.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- Shafik A, el-Sherif M, Youssef A, Olfat ES. Surgical anatomy of the pudendal nerve and its clinical implications. Clin Anat. 1995;8:110–115. doi: 10.1002/ca.980080205. [DOI] [PubMed] [Google Scholar]
- Yang CC, Bradley WE. Innervation of the human anterior urethra by the dorsal nerve of the penis. Muscle Nerve. 1998a;21:514–518. doi: 10.1002/(sici)1097-4598(199804)21:4<514::aid-mus10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Yang CC, Bradley WE. Peripheral distribution of the human dorsal nerve of the penis. J Urol. 1998b;159:1912–1916. doi: 10.1016/S0022-5347(01)63194-X. [DOI] [PubMed] [Google Scholar]
- Yang CC, Bradley WE. Neuroanatomy of the penile portion of the human dorsal nerve of the penis. Br J Urol. 1998c;82:109–113. doi: 10.1046/j.1464-410x.1998.00669.x. [DOI] [PubMed] [Google Scholar]