Abstract
Aims
Reflex bladder excitation has been demonstrated by stimulation of the pudendal nerve and several of its distal branches. However, excitation parameters have not been consistent and the relationship to anatomical locations within the urethra has not been fully investigated. An improved understanding of the lower urinary tract neurophysiology will improve human studies and neuroprosthetic device development.
Methods
Intraurethral stimulation was performed in nine cats at near isovolumetric bladder volumes before and/or after spinalization. Bladder excitability profiles were obtained for lower (2 Hz) and higher (33 Hz) frequency stimuli along the urethra between the bladder neck and external meatus.
Results
Higher frequency stimuli were excitable at all urethral locations prior to spinalization but only excitable in the middle and distal urethra after spinalization. Lower frequency stimuli were excitable at proximal and middle locations before spinalization but not excitable at any location after spinalization. In most evaluations, bursting pulse stimulation patterns evoked greater bladder pressures than the dominant continuous frequency (2 or 33 Hz).
Conclusions
These data indicate the potential presence of two distinct pathways for reflex bladder activation within the urethra: a supra-T10 circuit initiated in the proximal and mid urethra that responds to lower and higher frequency stimuli, and a sacral circuit initiated in the mid and distal urethra that responds to higher frequency stimuli. This work suggests potential anatomical targets and stimulus patterns for clinical evaluations of peripheral nerve-based neuroprostheses for bladder control.
Keywords: afferent pathways, bladder, electric stimulation, spinal cord injuries, urethra
INTRODUCTION
Pudendal nerve (PN) stimulation evokes reflex bladder contractions through activation of afferent fibers within it. These afferents are presumably the initial segment in a urethral fluid flow feedback loop,1,2 which evokes bladder contractions.1,3 The specific anatomical locations of afferents within the urethra to initiate this reflex circuit are not well defined. Although stretch receptors are prevalent in the external urethral sphincter (EUS)2 and pre-prostatic and EUS locations in the urethra may initiate the reflex,4 the relationship between stimulation patterns and the urethral end organs is unknown. For effective translation towards development of a neuroprosthesis for bladder control, an improved understanding of the lower urinary tract (LUT) neurophysiology and potential anatomical targets for stimulation is needed.
The ability to trigger reflex bladder contractions by stimulating PN afferent fibers has been reported.4–16 Most of these studies have focused on the PN trunk.7–11 Others evaluated PN branches: deep perineal nerve (dPN),12,13 dorsal nerve of the penis (DNP),13,14 urethral afferents,13,15 intra-urethral stimulation,4–6 and the perigenital region.16 Across these studies, different excitatory frequency bands have been observed: 20–40 Hz4–16 and 0.5–2 Hz4,5,11,12 or below 10 Hz,13 although stimuli below 10 Hz are generally thought to be inhibitory.8–10,16 While afferent firing rates of 20–40 Hz have been observed during urethral fluid flow,2 and different frequency preferences for PN branches have been observed,13 the basis for these two frequency ranges remains unanswered and the relationship to urethral end organs undefined. Additionally, many of these studies have focused on either spinal intact4,11,15 or spinal transected5,9,10,16 experiments while fewer have evaluated responses both before and after acute spinalization.7,8,12–14
Systematic analyses of the relationships between urethral location and stimulus parameters have not been performed. Stimulating within the urethra is an end-organ approach that serves as a segue towards clinical evaluations. In cats, 2 Hz prostatic urethra stimuli4 and 33 Hz distal urethra stimuli6 have evoked bladder contractions. Human trials indicated prostatic urethra excitability for 2 and 20 Hz.5 A more comprehensive evaluation of stimulus parameters at different urethral locations may provide a rationale for the observed PN stimulus frequency preferences.
Afferent evoked bladder recruitment and voiding efficiencies are currently less robust than for direct activation of motor fibers during sacral root stimulation.7 The use of variable patterned stimuli, mimicking action potential bursting, improved reflex bladder activation for PN stimulation.11 Application of this technique to intraurethral stimulation may similarly enhance bladder responses and therefore clinical potential.
This study evaluated intraurethral excitability for reflex bladder contractions before and after spinal transection using different stimulation patterns (frequency ranges and pulse bursting) and multiple urethral locations. The outcomes are expected to provide a better understanding of LUT neurophysiology, enhance clinical evaluations of urethral excitability, and advance clinical translation of PN-based neuroprostheses for bladder control.
MATERIALS AND METHODS
Animal Model
This study utilized nine mature, male cats. The Louis Stokes Cleveland Veterans Affairs Medical Center and the Case Western Reserve University Institutional Animal Care and Use Committees approved this study prior to initiation.
Experimental Preparation
A dual-lumen supra-pubic catheter was inserted into the bladder of alpha-chloralose anesthetized (65 mg/kg induction, 15 mg/kg maintenance) cats for fluid infusion and pressure monitoring. Buprenorphine (0.01 mg/kg) was administered every 12 hr to ensure analgesia. A 2–3.5 Fr (0.7–1.1 mm diameter) catheter mounted with one or two (1–3 mm) ring electrodes was used to excite the urethra. The use of three different catheters across experiments did not have a significant effect on the results (P = 0.97 by ANOVA). Stimulus trials were monopolar (monopolar catheter) referenced to a dorsal surface electrode or bipolar between electrodes on the catheter. Although the results did not suggest a difference based on stimulus polarity, an ANOVA indicated it was a significant factor (P < 0.01). The general setup, hardware and preparation have been described previously.11
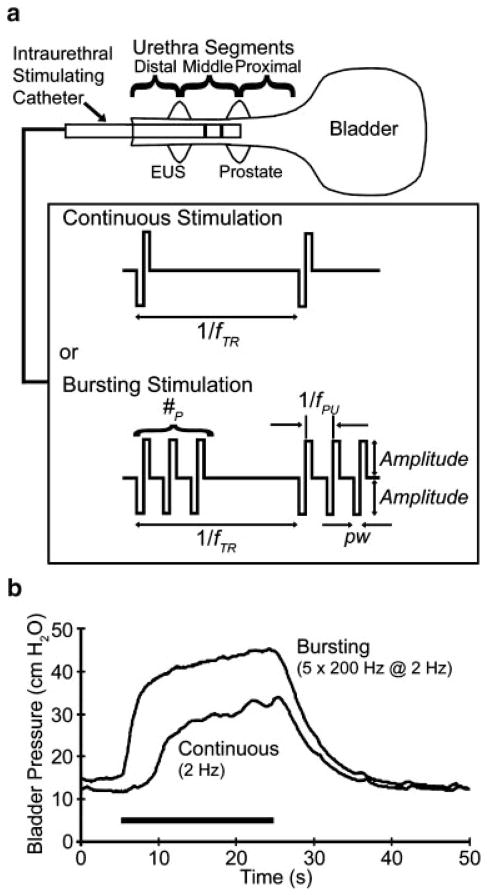
Stimulus patterns were arranged such that continuous single pulse stimulation occurred at a train rate frequency (fTR) while bursting stimulation added one or more pulses (#P) repeated at a pulse frequency (fPU) of 200 Hz at the fTR (Fig. 1). Stimulus nomenclature was #P by fPU at fTR (e.g., 5 × 200 Hz at 2 Hz). Stimuli were cathode-leading, current-controlled, balanced biphasic pulses, with pulse widths of 200 μsec.
Fig. 1.
Intraurethral stimulation for reflex bladder activation utilized either continuous or bursting stimulation patterns (a). Stimulus nomenclature: #P =number of pulses; fTR =train rate frequency; fPU =pulse frequency; pw =pulse width. EUS =external urethral sphincter. In most evaluations, the use of stimulus pulse bursting led to greater evoked bladder pressures than single-pulse continuous stimulation, as seen in (b) for two sequential stimulus trials at a proximal urethra location before spinalization. In these trials, 2 Hz stimulation led to an average evoked bladder pressure of 11.1 cm H2O (0.50 normalized to the maximum PAVE-EV within this experiment) while pulse bursting triggered an average evoked pressure of 20.6 cm H2O (0.92 normalized).
Experimental Protocol
The stimulating catheter was inserted into the distal urethra. A cystometrogram was performed; the bladder was filled at 0.5–2 ml/min while the urethra was stimulated with a 5–10 mA, 20 sec, 33 Hz continuous pattern until a reflex bladder contraction was evoked. At this point, fluid infusion was stopped although urine generation led to incremental volume changes. The excitatory bladder volume was defined as VSTIM. If urine generation led to distension evoked contractions, the bladder volume was returned to near VSTIM.
A urethral profile was performed to determine excitability. The stimulating catheter was inserted up to the bladder neck, and stimulus trials were performed for 2 and 33 Hz stimuli, with 45–75 sec between trials, consistent with other studies.14,16 Subsequently, the catheter was withdrawn by intervals of 1–2 cm, and 33 and 2 Hz stimulation trials were alternated at each location out to the external meatus.
At excitable locations additional continuous stimuli and pulse bursting were evaluated. Burst parameters were chosen based on effective PN stimulation results.11 For 2 Hz responding locations, 0.5–5 Hz stimuli and bursting of 2–30 #P for fTR of 1–5 Hz were tested. At 33 Hz responding locations, 10–50 Hz stimuli and bursting of 2–5 #P for fTR of 10–50 Hz were evaluated. In some cases, trials were repeated in a randomized order.
Marcaine was applied to the spinal cord surface before transection between T10–T12. Gelfoam was inserted between the cut ends and complete transection verified post-mortem. After spinalization, bladder responses were evaluated at regular intervals until they were observed to plateau (30–90 min; slightly lower than others12,13). Then, pre-spinalization steps were repeated to determine VSTIM, perform a urethral excitability profile and expand the parameter space at excitable locations.
The PN pathway was verified through nerve conduction block before spinalization in one cat and after spinalization in another cat. Stimulation trials were performed before and after bilateral application of Marcaine to the PNs, then both nerves were rinsed by saline irrigation (>50 ml) prior to response recovery trials. In one additional cat, before spinalization, the PNs were bilaterally transected.
In post-mortem dissections for all experiments, relative distances with respect to the external meatus were determined for the bladder neck, the center of the prostate, and the center of the EUS.
Data Analysis
The average evoked bladder pressure (PAVE-EV) was found for each trial by subtracting the 3-sec average pressure before stimulus initiation (PBASE) from the average while the pressure was greater than PBASE plus three times the PBASE standard deviation. Within each experimental spinalization section, PAVE-EV results were normalized to the maximum PAVE-EV. Urethral lengths were standardized to 10 cm, with the prostate at 6 cm, and experimental trial locations were standardized to their relative position within this range, similar to previous methodology.4 Individual trial results at 1 cm standardized intervals were pooled by stimulus parameters and spinalization status within experiments before averaging across experiments. Excel 2007 (Microsoft, Redmond, WA) and JMP 6 (SAS, Cary, NC) were used for data accumulation and analysis. An ANOVA was performed on the entire data set, using cat, fTR, #P, bladder volume, stimulus amplitude, catheter, standardized urethral location, stimulus duration, and PBASE as factors for PAVE-EV and normalized PAVE-EV as responses. Student’s t-test was used for comparison testing of pooled groups of trials, using two-tailed distributions and assuming equal variance. For evaluations of statistical significance, α was set to 0.05.
RESULTS
Intraurethral excitability was evaluated in nine cats (seven before and after spinalization; one only before; one only after). One cat, before and after spinalization, and another cat after spinalization did not exhibit reflex bladder excitation with urethral stimulation. This is consistent with other studies that reported several non-responders in their study groups.11,12,14 Results from the seven pre-spinalization and six post-spinalization excitable experiments were analyzed. The average VSTIM before spinalization (30.5 ± 18.3 ml) and after spinalization (35.8 ± 6.2 ml) were not significantly different (P = 0.52). The average stimulus levels necessary to elicit bladder responses before spinalization (5.4 ± 2.7 mA, 3–10 mA) were not significantly different than the average post-spinalization current levels (6.7 ± 1.3 mA, 5–8.5 mA, P > 0.31). Although neural signals were not recorded, these stimulus levels are consistent with activation of myelinated fibers. The EUS was 3.2 ± 0.5 cm from the external meatus, the prostate was 6.4 ± 0.9 cm, and the bladder neck was 10.2 ± 1.2 cm, consistent with measurements reported elsewhere.6,17 The standardized EUS location was 3.0 ± 0.5 cm.
Intraurethral Excitability
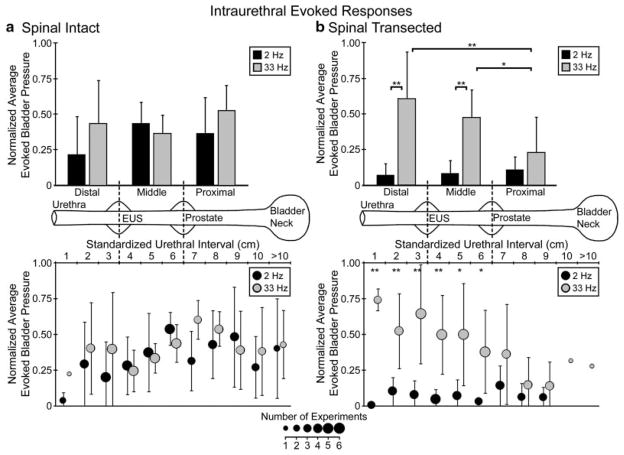
Before spinalization, bladder contractions were evoked with both lower and higher frequency intraurethral stimulation (Fig. 2a). Lower frequency stimuli excited the bladder at middle and proximal urethral locations, while higher frequency stimuli excited the bladder at proximal, middle, and distal locations. After spinalization, bladder contractions were only evoked with higher frequency intraurethral stimulation at middle and distal locations (Fig. 2b), while lower frequency stimuli were not excitable. Evoked responses across cats for each urethral section (Table I) mirrored the pooled evoked pressure results.
Fig. 2.
Across experiments, average evoked bladder responses normalized to maximum experimental values varied based on stimulus frequency, catheter position and spinal transection. Before spinalization (a), lower frequency (2 Hz) stimuli predominantly excited the bladder at proximal and middle urethral locations while higher frequency (33 Hz) stimuli excited the bladder at proximal, middle, and distal urethral locations. After spinalization (b), lower frequency stimuli did not excite the bladder at any location while higher frequency stimuli predominantly excited the bladder at middle and distal locations (*P < 0.10; **P < 0.05). In the upper panels, pooled trials for three urethral segments correspond to the anatomical landmarks indicated on the urethra diagram in the center of the figure. Two Hertz distal stimuli evoked the lowest pressures (P =0.26 vs. 33 Hz distal responses; not significantly different due to small sample size of experiments pooled together). In the lower panels, individual interval pooled results are given, with the symbol size corresponding to the number of experiments in which that specific interval was tested. Error bars in both panels indicate standard deviations. For pre-spinalization to post-spinalization same-frequency comparisons, 2 Hz stimuli in middle (P < 0.001) and proximal (P < 0.05) sections were significantly different while distal sections (P =0.26) were not different. Thirty-three Hertz results for proximal stimulation were significantly different (P < 0.05) while distal (P =0.42) and middle (P =0.35) sections were not different.
TABLE I.
Summary of Excitable Experiments per Location, Stimulus Frequency, and Spinalization
| Urethra segment
|
|||
|---|---|---|---|
| Distal | Middle | Proximal | |
| Spinal intact | |||
| 1–3 Hz | 2/6 | 7/7 | 2/5 |
| 20–50 Hz | 5/6 | 6/7 | 4/5 |
| Spinalized | |||
| 1–3 Hz | 0/6 | 0/6 | 0/5 |
| 20–50 Hz | 6/6 | 4/6 | 0/6 |
Pulse Bursting Effects
Stimulus pulse bursting increased the evoked bladder pressures in all lower frequency evaluations and most higher frequency evaluations. The burst stimulus parameter space was not evaluated in three cats before spinalization and three cats after spinalization due to small excitable bladder volume ranges, between VSTIM and the occurrence of distension evoked contractions, which limited the ability to perform trials beyond urethral excitability. Before spinalization, the best lower frequency bursting parameters evoked greater pressures than the best continuous stimulation (2 Hz) at all tested locations (four cats; four middle and two proximal locations). The average evoked pressure increase was 72 ± 27% (P < 0.002). Optimal lower frequency bursting parameters were 5–10 × 200 Hz at 2–3 Hz (Fig. 3). The best high frequency bursting parameters evoked greater pressures than the best continuous stimulation (20–40 Hz) in three of the four evaluations (two cats; two distal, one middle, and one proximal location). The average evoked pressure increase was 11 ± 20% (P =0.31). Optimal higher frequency bursting parameters before spinalization were 2 × 200 Hz at 20–25 Hz.
Fig. 3.
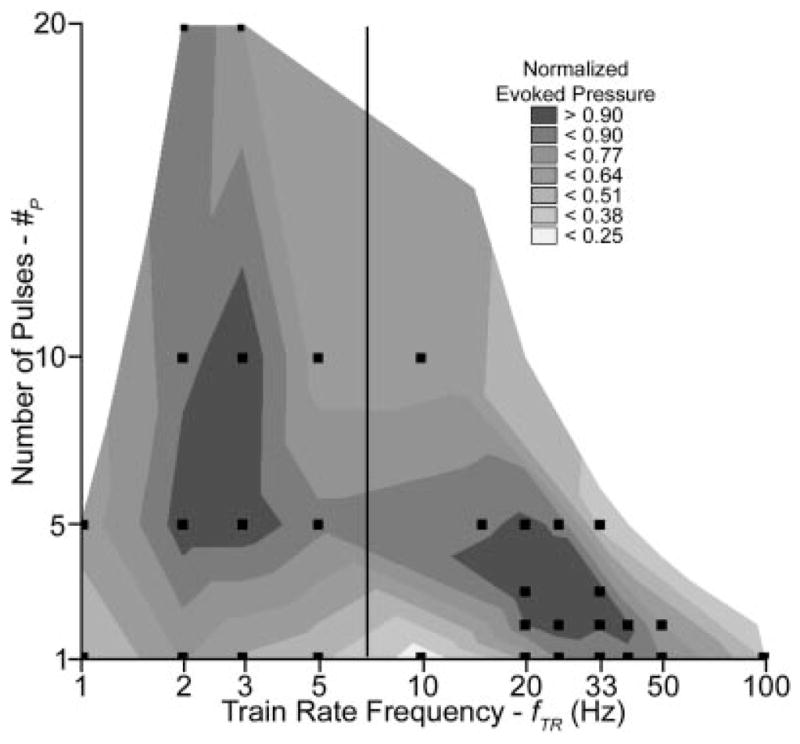
Pulse bursting evoked greater pressures for two stimulus parameter regions: 5–10 × 200 Hz at 2–3 Hz for proximal and middle urethra locations before spinalization and 2–3 × 200 Hz at 20–40 Hz for middle and distal urethra locations before and after spinalization. This figure is a side-by-side representation of lower frequency pre-spinalization proximal and middle urethra pooled bursting results (left of vertical bar) and higher frequency pre- and post-spinalization middle and distal urethra pooled bursting results (right of vertical bar). Normalized PAVE-EV values were pooled at each #P–fTR stimulus parameter combination within experiments prior to pooling across experiments for both lower frequency bursting evaluations (1–5 Hz fTR) and pre- and post-spinalization higher frequency evaluations (10–100 Hz fTR). Pooled values were then normalized to the maximum #P–fTR result with in the respective lower or higher frequency range. Parameter combinations in figure were evaluated in at least three experiments (indicated by squares).
After spinalization, high frequency bursting evoked greater bladder pressures than the best continuous stimulation (25–50 Hz) in six of the eight evaluations (three cats; five distal and three middle locations). The average evoked pressure increase was 34 ± 54% (P =0.09). Optimal bursting parameters were 2–4 × 200 Hz at 20–40 Hz. Lower frequency pulse bursting did not evoke contractions after spinalization.
PN Path Verification
In one spinal intact cat, incomplete Marcaine application reduced the evoked response for 2 Hz stimulation at a middle urethra location, while bilateral PN transection in another cat completely eliminated 2 Hz evoked bladder responses at a proximal urethra location. In a separate cat after spinalization, complete Marcaine application eliminated the evoked bladder response.
DISCUSSION
Two Reflex Micturition Pathways
Previous studies using direct PN stimulation demonstrated lower and higher frequency excitation before spinalization11 but only higher frequency excitation after spinalization,18 which suggested two reflex pathways are involved. The current study demonstrated an additional anatomical factor in the excitatory reflex circuits while supporting the frequency and spinalization effects previously noted.
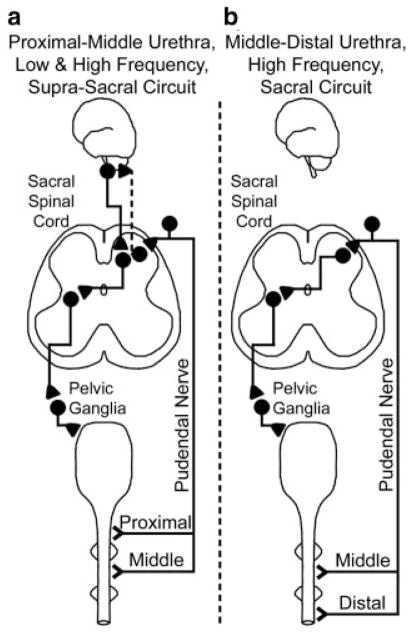
As lower frequency stimuli were primarily excitable in the proximal and middle urethra (Fig. 2a) and acute spinalization eliminated proximal higher frequency evoked responses and all lower frequency evoked responses (Fig. 2b), these results suggest two reflex micturition pathways: a middle and proximal urethra initiated pathway involving supra-T10 circuitry which responds to lower and higher frequency stimuli (Fig. 4a) and a higher frequency responding middle and distal urethra initiated pathway involving sub-T12 circuitry only (Fig. 4b). The middle and proximal urethra-initiated pathway may be triggered by A-fiber afferents19 and involve either descending projections from higher centers such as the pontine micturition center, or both ascending and descending projections. Clinicians evaluating subjects for reflex bladder excitability could potentially tailor the stimulation location and frequency based on subject pathology.
Fig. 4.
Reflex bladder activation occurs via two reflex pathways. The proximal and middle urethra responds to lower and higher frequency stimuli and evokes bladder contractions via supra-T10 circuitry (a). The supra-T10 circuitry in this path may involve either descending projections from higher levels such as the pontine micturition center or both ascending (dashed line) and descending projections. The middle and distal urethra responds to higher frequency stimuli and evokes bladder contractions via sub-T12 circuitry (b).
Lower frequency stimuli are effective at evoking bladder responses, in spinal intact preparations, for both direct PN11 and intraurethral stimulation. Studies that evaluated post-spinalization excitability and suggested that lower frequency stimuli are inhibitory9,10,16 could not excite the proximal and middle urethra-initiated pathway. Individuals with bladder dysfunction not related to complete spinal cord injuries are potential beneficiaries of this pathway. The use of lower frequency stimuli, in neuroprosthetic devices for these individuals, will have a reduced duty cycle and lower power requirements than for higher frequency stimuli. In spinal cord injured humans, 2 Hz has been shown to excite the bladder,5 which differs from the post-spinalization excitability seen here and suggests that thorough clinical evaluations are necessary.
The distal urethra-initiated pathway likely excites afferents in the dPN branch of the PN. Similar results before and after spinalization were obtained via higher frequency stimulation of the dPN12 (which contains afferents from the DNP20) and the DNP.13,14 Bilateral transection of the dPN has been shown to eliminate distal urethra higher frequency stimuli evoked contractions,6 further supporting this pathway.
Proximal urethra stimulation likely excites the urethral afferent or cranial sensory (CS) path, which has been shown to respond to lower frequency stimuli before spinalization.13 However, direct stimulation of the CS, suggested as the main innervation of the proximal urethra, with higher frequency stimuli failed to evoke bladder contractions.13 This conflicts with the observed proximal excitability for higher frequency stimuli. As bilateral pudendal transection eliminated this proximal pathway, it supports our hypothesis of a PN pathway.
Pulse Bursting Improved Responses
Pulse bursting evoked larger bladder contractions than continuous stimulation in most testing scenarios (Figs. 1 and 3). These results suggest that stimulus patterns similar to physiological burst timings are more successful for afferent-mediated bladder excitation. This result is consistent with direct PN stimulation results,11 and may improve clinical evaluations of reflex excitability.
The best bursting parameters for higher frequency stimulation were very similar before and after spinalization, although the post-spinalization fTR range increased. The inclusion of extra pulses for some excitable post-spinalization experiments suggests that increased post-synaptic recruitment or neurotransmitter release may be necessary after acute spinal transection. These bursting parameters are in agreement with the best bursting parameters in higher frequency responders for direct PN stimulation before11 and after spinalization.18
The best lower frequency intraurethral stimulation parameters were shifted 2–3 Hz higher than the best bursting parameters in lower frequency responders for direct PN stimulation.11 PN bursting stimulation was most effective at 1 Hz with little effect at 2–3 Hz,11 while intraurethral bursting had little effect at 1 Hz and was effective at 2–3 Hz (Fig. 3). This frequency shift may result from intraurethral stimulation selecting a subset of PN afferents.
In the three evaluations where pulse bursting did not evoke greater contractions than for continuous stimulation the parameter space was not thoroughly evaluated, thus an optimal bursting parameter may not have been tested.
CONCLUSIONS
Lower (proximal and middle urethra) and higher (proximal, middle and distal urethra) frequency stimuli evoked reflex bladder contractions with an intact spinal cord. Under acute spinalized conditions, only higher frequency stimuli at middle and distal locations evoked reflex bladder contractions. These results suggest the presence of two distinct reflex bladder excitation pathways in the cat. Pulse bursting led to greater bladder contractions than continuous stimulation. Sensory-based stimulation applications should utilize burst-patterned stimulation for evoking maximal responses. In clinical evaluations of bladder excitability using intraurethral stimulation, and the development of neuroprostheses for bladder control, the pathology and stimulus frequency should be considered.
Acknowledgments
Grant sponsor: Department of Veterans Affairs; Grant number: RR&D 3675R; Grant sponsor: NIH; Grant number: DK077089; Grant sponsor: Department of Education; Grant number: P200A040207.
The authors thank Tina Emancipator, Adam Boger, and Tim Mariano for assistance with experimental setup and preparation. This work was supported in part by the Department of Veterans Affairs RR&D 3675R, NIH DK077089, and by the Department of Education P200A040207.
Footnotes
Conflicts of interest: Dr. Gustafson is an inventor of 2 patents related to this work, owned by case Western Reserve University.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Barrington FJF. The component reflexes of micturition in the cat, Parts I and II. Brain. 1931;54:177–88. [Google Scholar]
- 2.Todd JK. Afferent impulses in the pudendal nerves of the cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258–67. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- 3.Robain G, Combrisson H, Mazieres L. Bladder response to urethral flow in the awake ewe. Neurourol Urodyn. 2001;20:641–9. doi: 10.1002/nau.1014. [DOI] [PubMed] [Google Scholar]
- 4.Gustafson KJ, Creasey GH, Grill WM. A catheter based method to activate urethral sensory nerve fibers. J Urol. 2003;170:126–9. doi: 10.1097/01.ju.0000070821.87785.14. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Boggs JW. PhD Diss. Cleveland, OH: Case Western Reserve University; 2005. Stimulation of the Pudendal Nerve for Restoration of Bladder Function. [Google Scholar]
- 7.Boggs JW, Wenzel BJ, Gustafson KJ, et al. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neural Eng. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- 8.Boggs JW, Wenzel BJ, Gustafson KJ, et al. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006;577:115–26. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai C, Smerin SE, de Groat WC, et al. Pudendal-to-bladder reflex in chronic spinalcord-injured cats. Exp Neurol. 2006;197:225–34. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Tai C, Wang J, Wang X, et al. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26:570–7. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 11.Bruns TM, Bhadra N, Gustafson KJ. Variable patterned pudendal nerve stimuli improves reflex bladder activation. IEEE Trans Neural Syst Rehabil Eng. 2008;16:140–8. doi: 10.1109/TNSRE.2007.914460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boggs JW, Wenzel BJ, Gustafson KJ, et al. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–97. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 13.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212:218–25. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1880–9. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol. 1999;517:599–605. doi: 10.1111/j.1469-7793.1999.0599t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai C, Shen B, Wang J, et al. Inhibitory and excitatory perigenital-to-bladder spinal reflexes in the cat. Am J Physiol Renal Physiol. 2008;294:F591–602. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Bhadra N, Grill WM. Functional anatomy of the male feline urethra: Morphological and physiological correlations. J Urol. 1999;161:654–9. [PubMed] [Google Scholar]
- 18.Bruns TM, Gustafson KJ, Bhadra N. Reflex bladder activation via pudendal nerve and intraurethral stimulation depends on stimulation pattern and location. Proc 30th Ann Intl IEEE EMBS Conf; Vancouver, BC, Canada. 2008. pp. 2760–3. [DOI] [PubMed] [Google Scholar]
- 19.de Groat WC. Integrative control of the lower urinary tract: Preclinical perspective. Br J Pharmacol. 2006;147:S25–40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariano TY, Boger AS, Gustafson KJ. The feline dorsal nerve of the penis arises from the deep perineal nerve and not the sensory afferent branch. Anat Histol Embryol. 2008;37:166–8. doi: 10.1111/j.1439-0264.2007.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]