Abstract
Purpose
Chemokines are implicated in T cell trafficking. We mapped the chemokine landscape in advanced stage ovarian cancer and characterized the expression of cognate receptors in autologous DC-vaccine primed T cells in the context of cell-based immunotherapy.
Experimental design
The expression of all known human chemokines in patients with primary ovarian cancer was analyzed on two independent microarray datasets and validated on tissue microarray. Peripheral blood T cells from five HLA-A2 patients with recurrent ovarian cancer, who previously received autologous tumor DC vaccine, underwent CD3/CD28 costimulation and expansion ex vivo. Tumor-specific T cells were identified by HER2/neu pentamer staining and were evaluated for the expression and functionality of chemokine receptors important for homing to ovarian cancer.
Results
The chemokine landscape of ovarian cancer is heterogeneous with high expression of known lymphocyte-recruiting chemokines (CCL2, CCL4 and CCL5) in tumors with intraepithelial T cells, whereas CXCL10, CXCL12 and CXCL16 are expressed quasi-universally, including in tumors lacking tumor infiltrating T cells. DC-vaccine primed T cells were found to express the cognate receptors for the above chemokines. Ex vivo CD3/CD28 costimulation and expansion of vaccine-primed T cells upregulated CXCR3 and CXCR4, and enhanced their migration toward universally expressed chemokines in ovarian cancer.
Conclusions
DC-primed tumor specific T cells are armed with the appropriate receptors to migrate towards universal ovarian cancer chemokines, and these receptors are further upregulated by ex vivo CD3/CD28 costimulation, which render T cells more fit for migrating towards these chemokines.
Introduction
Despite therapeutic advances in the treatment of ovarian cancer, survival of patients with late stage disease remains low. Increased infiltration of cytotoxic T cells in tumor islets correlates with significantly longer survival (1), while increased numbers of immunosuppressive cells such as CD4+CD25+FoxP3+ regulatory T cells (Treg) or B7-H4 expressing tumor macrophages predict poor survival (2, 3). Our group has focused on autologous whole tumor lysate dendritic cell (DC)-based immune therapy strategies for patients with recurrent ovarian cancer. In a recent pilot clinical trial (UPCC-11807), patients showed clinical benefit from a personalized vaccine manufactured with freeze-thawed lysate of autologous tumor cells pulsed on autologous DCs after they were pretreated with systemic anti-VEGF antibody bevacizumab and oral metronomic cyclophosphamide (4). In addition to clinical benefit, in four out of six patients, a significant increase in circulating tumor-reactive T-cells was detected after vaccination. Furthermore, vaccine-primed T cells expanded efficiently in response to CD3/CD28 bead stimulation ex vivo while retaining tumor-reactive specificities. Following completion of this clinical trial, patients who had not progressed but had residual measurable disease, received an infusion of 5x109 of autologous vaccine-primed, ex vivo CD3/CD28 costimulated peripheral blood T-cells. Importantly, tumor-reactive T-cells reconstituted effectively in vivo after adoptive transfer and resulted in complete response in one patient and stable disease in another (4).
Following further optimization of the DC vaccine (5), we next opened a clinical trial for recurrent stage III/IV ovarian cancer (UPCC19809, NCT01132014) (6). In this trial, subjects are vaccinated five times intranodally with autologous DCs loaded with HOCl-oxidized autologous tumor lysate in combination with bevacizumab, low-dose cyclophosphamide and therapeutic dose acetyl salicylic acid (ASA) to inhibit tumor VEGF, attenuate Treg cells, and suppress tumor prostaglandin production, respectively. Preliminary results show that vaccination produces clinical benefit, which correlates with the induction of anti-tumor immune response (7). Following DC vaccination, patients undergo apheresis to collect vaccine primed Tcells, retaining the option to enrol in a subsequent adoptive T cell therapy study (UPCC-26810, NCT01312376) using ex vivo CD3/CD28 costimulated autologous vaccine-primed T-cells, in an attempt to boost the efficacy of the autologous cancer vaccine.
Successful immunotherapy depends on the ability of T cells to home into tumors. Infiltration of tumors by T cells is a complex multistep process involving adhesive interactions with vascular cells and migration within the stroma, much of which is regulated by chemotactic gradients (8, 9). Chemokines are structurally similar chemotactic cytokines, with overlapping receptor specificity and functions (10–12) and have a multifaceted role in tumor biology (13–16). The chemokine landscape of the tumor microenvironment may differ significantly among tumors, and can affect immune cell composition, tumor growth and metastasis (17). Since leukocyte infiltration into tumors is controlled by chemokine gradients in the tumor microenvironment and cognate chemokine receptors expressed on immune cells (13, 18–20), decreased expression of appropriate chemokines can contribute to a lack of effector T cell infiltration and resistance to immunotherapy (21). Thus, successful immunotherapy should achieve an optimal match between the chemokine landscape of targeted tumors and the chemokine receptor repertoire expressed by the elicited effector T cells.
The heterogeneity of tumors with respect to their chemokine expression represents a major challenge to overcome. For example, although tumors with pre-existing intraepithelial T cell infiltrate exhibit a microenvironment that is conducive to T cell accumulation, tumors lacking T cells at the steady state could be resistant to immunotherapy. Considering that vascular normalization and reduction of Tregs would be two important maneuvers to enhance T cell homing in tumors and the impact of immunotherapy, we have designed a clinical trial that combines the optimized DC vaccine with low-dose cyclophosphamide and bevacizumab (4, 6). However, it remains uncertain whether the chemokines expressed by these tumors can pair with the chemokine receptors expressed by tumor-reactive T cells generated by immunotherapy and thus remains a potentially important issue in the design of effective immunotherapeutic strategies.
This study aimed to map the chemokine microenvironment in advanced stage papillary serous ovarian cancer in order to understand the requirements for chemokine receptor expression by tumor-reactive T cells. We also sought to characterize the chemokine receptors expressed by peripheral blood T lymphocytes after DC vaccination and after ex vivo CD3/CD28 costimulation in preparation for adoptive transfer. We found that ovarian cancers, including tumors lacking intraepithelial T cells, express specific lymphocyte attracting chemokines, such as CXCL10 and CXCL12, which are quasi-universally prevalent across patients and across tumor sites within patients. Importantly, vaccine-primed tumor-specific peripheral blood T cells expressed functional cognate receptors for these chemokines, which enabled them to migrate towards these common ovarian cancer chemokines. Furthermore, CD3/CD28 costimulation of vaccine-primed T cells significantly enhanced the expression of the relevant chemokine receptors and augmented the migration of vaccine-induced tumor-specific T cells to the common ovarian cancer chemokines.
Materials and methods
Gene expression analysis
To analyze chemokine expression patterns in ovarian cancer, we performed gene expression profiling on 63 stage III–IV papillary serous primary (but not metastatic) ovarian cancer samples resected during debulking at the University of Turin, Italy. The clinicopathological characteristics of patients in this cohort are presented in Table 1. All patients received standard platinum-taxane based chemotherapy. Gene-expression profiling was performed using Affymetrix GeneChip® Human Gene ST 1.0 Arrays (GSE62873) as previously described (22). Publicly available Affymetrix array expression data set (GSE9891) covering 222 matching patients with papillary serous ovarian cancer (with similar clinicopathological characteristics – Table 1) from the Australian Ovarian Cancer Study was used as a validation cohort (23). Following normalization, technical outlier samples were identified and excluded.
Table 1.
| Clinicopathologic characteristics | Gene array training cohort n=63 (%) | Gene array validation cohort n=222 (%) | Penn TMA cohort n=50 (%) |
|---|---|---|---|
| Mean age at diagnosis (years) | 57.2 | 60.44 | 59.58 |
| Age SD | 10.41 | 10.05 | 11.7 |
| Stage III | 57 (90.5) | 201 (90.5) | 44 (88) |
| Stage IV | 6 (9.5) | 21 (9.5) | 6 (12) |
| Grade 1 | 6 (9.5) | 4 (1.8) | 1 (2) |
| Grade 2 | 11 (17.5) | 80 (36) | 7 (14) |
| Grade 3 | 42 (66.7) | 136 (61.2) | 31 (62) |
| Unknown grade | 4 (6.3) | 2 (1) | 11 (22) |
| Optimally debulked | 31 (49) | 112 (50.5) | 29 (58) |
| Suboptimally debulked | 32 (51) | 110 (49.5) | 21 (42) |
Gene expression analysis was also performed on 13 primary and established human ovarian cancer cell lines using Affymetrix GeneChip® Human Gene ST 1.0 Arrays (GSE63553). OV7M and OV95 primary ovarian cancer cell lines were provided by Dr. Richard Carroll at the University of Pennsylvania (UPENN) and were derived from ovarian cancer patients as described (24). All other cell lines (A2008, OAW42, OVCAR2, OVCAR3, OVCAR4, OVCAR5, OVCAR8, OVCAR10, PE01, PE04, SKOV3) were obtained from the Ovarian Cancer Research Center cell bank at UPENN with documented and cited origin, thus were not re-authenticated at the time of the study (25). The detailed origin and characteristics of the cell lines are listed in Supplementary table I.
Tissue microarrays construction
A tissue microarray (TMA) was constructed at the Department of Pathology at UPENN from 50 treatment-naive patients with stage IIIC or IV papillary serous ovarian cancer who underwent primary resection at UPENN between 2005 and 2008, and whose clinical characteristics were similar to the two cohorts of patients used in the microarray data analysis (Table 1). Both primary tumor samples and matched metastatic deposits from the same patients were included in the TMA, as previously reported (26). Briefly, a total of 207 tumor sites were represented on the array with a mean of 3.8 sites per patient, including one or two ovarian sites and one to seven metastatic sites. TMA blocks were selected by a trained pathologist. For each block, triplicate 0.6-mm cores of tumor were placed on a TMA slide using a manual arrayer.
Immunohistochemistry and TMA Scoring
Commercially available chemokine antibodies were validated and titrated on positive (i.e lymph node, prostate, lung or colon cancer) and negative control tissues (i.e cerebellum, stomach or testis) - as recommended by the manufacturers and described in the literature - prior to immunohistochemical analysis of the TMA samples. TMA immunostaining was performed with the following antibody clones: CCL2 (HPA019163, Sigma-Aldrich), CCL4 (1738-1, Epitomics), CCL5 (AF-278-NA, R&D Systems), CCL28 (MAB7171, R&D Systems), CXCL10 (ab9807, Abcam), CXCL12 (SC-28876, Santa Cruz), CXCL16 (ab101404, Abcam), CX3CL1 (HPA040361, Sigma-Aldrich). Lymphocytes were stained for CD3 (0452, Dakocytomation), CD8 (C8/144B, M7103, Dakocytomation) and FoxP3 (206D, 320102, Biolegend).
Tumor cores were imaged using Aperio ImageScope and were independently scored at 20X magnification by two experienced pathology observers (RF and MDF) who were blinded to clinical and pathological parameters. An H-score was calculated by using intensity (score of 3: strongly staining, score of 2: moderately staining, score of 1: weakly staining, score of 0: no staining) x percentage of tumor tissue stained (score of 1: 0–25%, score of 2: 26–50%, score of 3: 51–75%, score of 4: 76–100%) for each core (maximum score was 12). When multiple fields were available for review, the median of their scores was recorded for that core. In the few cases where there was a discrepancy, a review was performed and a consensus reached. The immunostained microarrays were also scored for tumor-infiltrating lymphocytes (TIL) by an experienced pathologist (ISH). Lymphocytes infiltrating the tumor islets (i.e. the malignant epithelial compartment) and stromal lymphocytes (all other lymphocytes) were graded according to the following quantitative criteria: 0 - absent; 1 - rare [1–10/400x high-power field (hpf)]; 2 - moderate (11–20/hpf); 3 - numerous (>20/hpf). The lymphocyte density in partial/incomplete fields was normalized based on a visual assessment of the percentage that consisted of tumor epithelium or stroma.
Procurement of T cells from ovarian cancer subjects undergoing vaccination (UPCC-19809) followed by adoptive T cell transfer in (UPCC-26810)
We analyzed T cells from five HLA-A*0201 subjects with recurrent ovarian cancer, who were enrolled in a phase-I clinical protocol at UPENN UPCC-19809 (NCT01132014) and administered therapeutic vaccination using autologous DCs loaded in vitro with oxidized autologous tumor lysate. Vaccine-primed peripheral blood T cells were obtained in all subjects as part of study procedures through apheresis 10–15 days after the 4th or 5th vaccination. A ~15L apheresis was performed at the Apheresis Unit of the Hospital of the University of Pennsylvania (HUP). Cells were transferred fresh to the Clinical Cell and Vaccine Production Facility (CVPF) at HUP where they were washed to remove plasma, platelets and red blood cell contamination using the Baxter CytoMate or Haemonetics CellSaver5 with X-VIVO™ 15 based media (Lonza, Walkersville, MD). T cells were isolated by counterflow centrifugal elutriation (Terumo ElutraTM Cell Separation System) to eliminate monocyte contamination. Cells were then counted and cryopreserved in liquid nitrogen vapor phase in cryopreservation media containing 10% DMSO.
Following completion of vaccination, the same subjects as above were enrolled in a follow-on phase I clinical protocol at UPENN (UPCC-26810, NCT01312376) administering adoptive transfer of ex vivo CD3/CD28 costimulated vaccine-primed autologous peripheral blood T cells. Elutriated vaccine-primed T cells were thawed and seeded into gas permeable flasks in X-VIVO™ 15 media supplemented with 5% pooled human AB serum. Anti-CD3/anti-CD28 antibody-coated Dynal microbeads were added at a ratio of 3:1 beads to cell. To maintain appropriate T cell density, fresh media with low level of IL-2 (100 IU/ml) was added throughout the 11 day expansion. Cells were then harvested for adoptive transfer, while aliquots were cryopreserved after microbead removal. For this study, we analyzed freshly thawed elutriated T cells from the apheresis product (vaccine-primed T cells) as well as freshly thawed CD3/CD28 costimulated T cells.
Chemotaxis assays
T cells number and viability was assessed using Guava Viacount reagent followed by quantitative capillary flow cytometry (Millipore Guava). Cells were rested in fresh media (RPMI 1640, Mediatech) supplemented with 10% FBS, 100 IU/ml penicillin, 100 mg/ml streptomycin and 20 IU/ml IL-2 for 24 hours prior to the chemotaxis assay. Following assay optimization and manufacturers’ guidelines, the following chemokines were plated either alone or in combinations on the bottom of Corning plate: 10 ng/ml CCL28 (SRP3112, Sigma-Aldrich), 50 ng/ml CXCL10 (300-12, Peprotech) and 100 ng/ml CXCL12 (300-28A, Peprotech). Vaccine primed and costimulated T cells were seeded at a concentration of 3-5x105 cells at the top transwell migration chamber (Corning HTS Transwell®-96, 5 μm pore polycarbonate membrane). The average number of live loaded CD3+ cells was determined by cell counter for each patient. After 3 hours of migration the total number of migrated CD3+ was counted and these cells were further analyzed for CD4, CD8 or HER2/neu expression by flow cytometry. All experiments were performed in duplicates and repeated in three independent experiments. Results of migration were reported as the average number of migrated CD4+, CD8+ or HER2/neu specific T cells divided by the average loaded live CD3+ cells for that patient and then multiplied by 100.
To block CXCL10 and CXCL12-mediated migration, T cells were pre-stained with anti-CXCR3 (MAB160, R&D Systems) and anti-CXCR4 antibodies (MAB170, R&D Systems) respectively. To block CCL28-mediated migration, anti-CCL28 antibody (MAB717, R&D Systems) was directly added to the chemokine containing chamber and incubated at 37° C for an hour.
Statistical analysis
Statistical analysis of gene expression and the significance of associations between categorical variables were performed using R. Pearson’s χ2-tests. Heat maps and cluster analysis of the 8 most highly expressed chemokines in ovarian cancer TMAs were done using R. Paired t-test was used to compare chemokine receptor expression and migration data. P-values <0.05 were considered statistically significant.
Results
Specific chemokine genes are expressed by advanced ovarian cancer
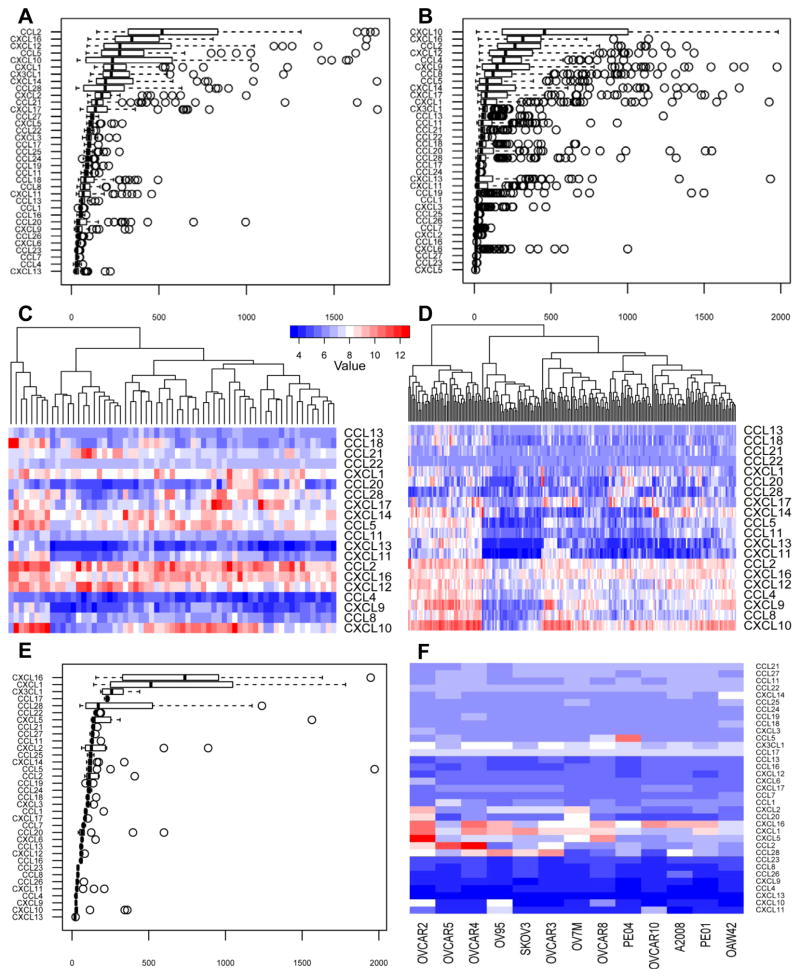
In order to determine the chemokines that are important in ovarian cancer for T cell homing, the expression of all known human chemokines was analyzed on a gene expression array dataset from 63 patients with primary ovarian cancer from the University of Turin, and validated on a publicly available microarray dataset from 222 ovarian cancer samples from the Australian Ovarian Cancer study. Most chemokines were expressed at low levels on average in ovarian cancer, although there was significant heterogeneity in the expression levels among patients for approximately half of the chemokine genes (Figure 1A–D). The five most highly expressed chemokines in both the training and validation datasets were CCL2, CCL5, CXCL10, CXCL12 and CXCL16.
Figure 1. Chemokine expression in ovarian cancer.
A and B: Gene expression analysis of chemokines in the training cohort (A) and in the validation cohort (B), showing the median expression and the four quartiles with the outliers presented as individual circles. C and D: Heat map generated from the training cohort (C: n=63) and the validation cohort (D: n=222) showing the individual patients’ chemokine expression level for the top 20 most highly expressed chemokines from both data sets. Color code shows the expression level compared to the mean gene expression, where the white color shows the mean expression level for all genes. E: Gene expression analysis of all known chemokines on 13 papillary serous ovarian cancer cell lines, showing the median expression and the four quartiles with the outliers presented as individual circles. F: Heat map generated from the 13 ovarian cancer cell lines showing each cell line separately with the expression level for all known chemokines.
We further validated the chemokines expressed by ovarian cancer cells by examining the gene expression data of 13 established ovarian cancer cell lines with papillary serous origin. We found that few were constitutively expressed in vitro, including CXCL16, CXCL1, CX3CL1, CCL17 and CCL28.
Among the chemokines most highly expressed in ovarian cancer in vivo, we found high constitutive expression of only CXCL16 in cancer cell lines in vitro, while CXCL10, CXCL12, CCL2 and CCL5 were not expressed or were expressed at low levels (Figure 1E, F). Thus, ovarian tumors express specific chemokines, and their expression seems to be associated with in vivo tumor conditions rather than being a constitutive feature of ovarian cancer cells.
Ovarian cancers express quasi-universal chemokines
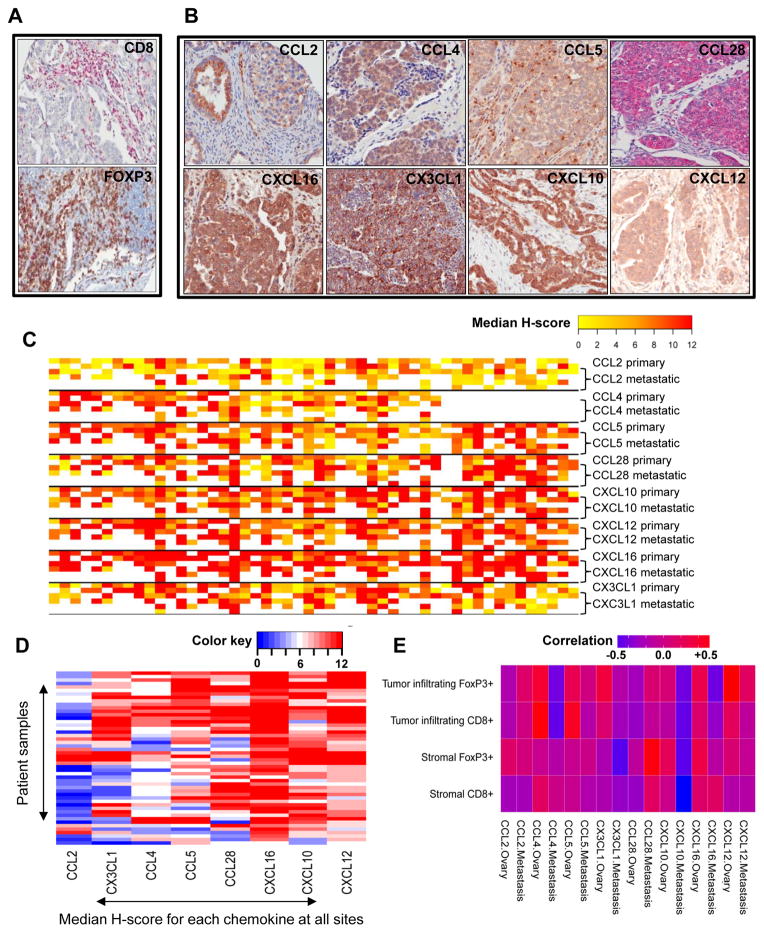
Next, we validated the chemokine expression by immunohistochemistry using commercially available antibodies on an ovarian serous carcinoma tissue microarray from an independent cohort of 50 patients from UPENN (Table 1). This patient cohort was similar to the two previous cohorts of patients analyzed by Affymetrix arrays: the most important clinical factors associated with survival (age, stage of disease, tumor grade, and ratio of optimally/suboptimally debulked patients) were not statistically different among the three cohorts, and all cohorts received standard adjuvant chemotherapy that reflected the best current practice. All samples were collected during primary surgery from chemotherapy-naïve patients. We selected for validation the chemokine genes with the most high and/or prevalent expression in any of the three above microarray datasets, as well as chemokines with expression level showing large variation across patients. This final list included 9 chemokines: CCL2, CCL4, CCL5, CCL28, CXCL1, CXCL10, CXCL12, CXCL16 and CX3CL1. With the exception of CXCL1 (where staining with all the commercially available antibodies appeared to be nonspecific), staining of the selected chemokines was successfully titrated and validated on positive and negative control tissues prior to immunohistochemical analysis of the TMA samples. To associate the expression of the above chemokines with the presence of effector cytotoxic lymphocytes (CTLs) and Treg cells at the time of diagnosis, TMA samples were also stained for CD3, CD8 and FoxP3 (Figure 2A). Median expression levels of chemokines and T cells were correlated at primary and metastatic sites (Figure 2E).
Figure 2. Correlation of chemokine expression and infiltration of CD8+ and FoxP3+ T cells in ovarian cancer.
A: Representative images of papillary serous ovarian cancer specimens that stained strongly positive for chemokine expression (20X). B: Representative images of staining for CD8 and FoxP3 (20X). C: Heterogeneity heat map demonstrating the median H-score calculated for every single evaluable tumor core. The first two rows represent tumor samples from the primary disease site (ovaries), while the last 4 rows represent tumor samples from available metastatic sites. Missing values are coded with white. D: Heat map demonstrating the average median H-scores from all sites (ovaries and metastasis combined) calculated for every patient sample on TMA. E: Heat map showing the correlation between chemokine expression and CD8+ and FoxP3+ cells at the primary and metastatic site.
Immunohistochemistry confirmed the expression of all above chemokines at the protein level in ovarian cancer (Figure 2B). Importantly, expression of all chemokines was confirmed within tumor islets in association with the tumor cells. In addition, expression of the same chemokines was found at both primary and metastatic sites, indicating high degree of similarities among sites with no statistical staining differences for any of these chemokines (Figure 2C).
However, there was heterogeneity of chemokine expression among samples. This heterogeneity was mostly restricted to CCL2, CCL4, CCL5, CCL28 and CX3CL1, which showed no expression in a proportion of patients (Figure 2C). On the other hand, we could identify a group of chemokines that appeared to be quasi-universally expressed in ovarian cancer (CXCL10, CXCL12, CXCL16) (Figure 2C). Based on the median expression, the calculated mean H-score for chemokine staining was the highest for CXCL10, CXCL12 and CXCL16, demonstrating that these chemokines showed the strongest staining and stained the highest percentage of ovarian cancer tissue (Figure 2D).
Tumors with intraepithelial T cells expressed many of the prior mentioned variable chemokines: CCL2, CCL4, CCL5, CCL28 and CX3CL1, and in addition, they expressed most of the quasi-universal chemokines CXCL10, CXCL12 and CXCL16. The expression of CCL4 and CCL5 showed the strongest correlation with the presence of tumor-infiltrating CD8+ cells, whereas CCL4, CX3CL1 and CXCL12 expression showed correlation with increased numbers of FoxP3+ cells in tumor islets (correlation 0.4–0.5) in the primary tumor tissues (Figure 2E). In addition, CCL28 and CXCL16 were correlated with FoxP3+ cells present in the stromal tumor tissue. These data are in agreement with previous observations (2, 27, 28). Importantly, the above correlations were substantially reduced or lost in the metastatic tumor sites (Figure 2E).
Vaccine-primed and costimulated T cells used in adoptive therapy express appropriate receptors for ovarian cancer chemokines
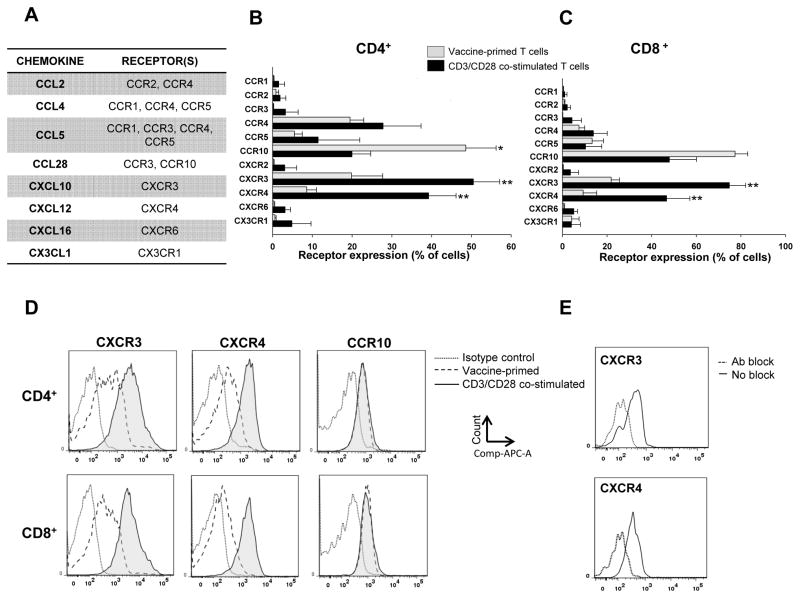
Based on the above data, we hypothesized that ovarian cancers express ligands for the following chemokine receptors: CCR1, CCR2, CCR3, CCR4, CCR5, CCR10, CXCR3, CXCR4, CXCR6, CX3CR1 (Figure 3A). We thus asked whether our two immunotherapy protocols, i.e. the whole tumor lysate-pulsed DC vaccine approach (UPCC-19809 protocol) and the following adoptive transfer of ex vivo CD3/CD28 costimulated vaccine-primed peripheral blood T cells (UPCC-26810 protocol), generate T cells expressing the appropriate chemokine receptors for being attracted by the ovarian cancer chemokine microenvironment. In protocol UPCC-19809, vaccine-primed peripheral blood T cells were collected through apheresis and elutriation following 4–5 vaccinations. A portion of these vaccine-primed cells was then subjected to ex vivo costimulation and expansion using anti-CD3/anti-CD28 Ab coated beads, in preparation for adoptive transfer to the same patient. As expected, CD3/CD28 costimulation resulted in preferential expansion of CD4+ cells compared to the apheresis samples (79% SD=13.7 vs. 59% SD=21.4 of total CD3+ cells, respectively, not shown). We analyzed the expression of the above chemokine receptors by flow cytometry in matched samples of vaccine-primed peripheral blood CD4+ and CD8+ cells T cells collected at the completion of vaccination and following ex vivo expansion with CD3/CD28-coated beads from five HLA A*0201+ subjects (Figure 3B and C). Figures 3D and E show representative examples of FACS analysis and staining specificity.
Figure 3. Chemokine receptor expression on vaccine-primed and CD3/CD28 costimulated CD4+ and CD8+ T cells.
A: Table showing the most highly expressed chemokines from the 3 microarray data set selected for staining with their matching chemokine receptors. B and C: Chemokine receptor expression on peripheral blood vaccine-primed peripheral blood CD4+ (B) and CD8+ T cells (C) at the steady state and after ex vivo CD3/CD28 costimulation. CD4+ cells mainly express CCR4, CCR10, CXCR3 and CXCR4 receptors. The number of CD4+ T cells expressing CCR10 was significantly decreased, while the number of cells expressing CXCR3 and CXCR4 was significantly increased by CD3/CD28 costimulation. CD8+ cells mainly express CCR10, CXCR3 and CXCR4 receptors. The number of CD8+ T cells expressing CXCR3 and CXCR4 was significantly increased by CD3/CD28 costimulation. (*p<0.05, **p<0.01). D: Representative FACS figures of CCR10, CXCR3 and CXCR4 receptor density expression on the different cell types. E: Representative FACS figures showing CXCR3 and CXCR4 before and after the blockade with specific antibodies.
In peripheral blood CD4+ T cells collected following vaccination the most commonly expressed chemokine receptors were CCR10 (CCL27 and CCL28 receptor), expressed on 48.5% (SD=17.1) of cells; CXCR3 (CXCL9-11 receptor), expressed on 19.8% (SD=17.5) of cells; CXCR4 (CXCL12 receptor), expressed on 8.5% (SD=5.4) of cells; and CCR4 (CCL2, CCL4, CCL5, CCL17 and CCL22 receptor), expressed on 19.4% (SD=7.7) of cells (Figure 3C). A low proportion (5.5% (SD=4.4)) of vaccine-primed CD4+ cells also expressed CCR5 (CCL3, CCL4 and CCL5 receptor). Vaccine-primed CD8+ cells similarly expressed CCR10 (77.3% SD=12.7), CXCR3 (21.8% SD=8.2), CXCR4 (9.3% SD=13.7) and CCR5 (13.3% SD=11.4) (Figure 3D). CCR1, CCR2, CCR3, CXCR2, CXCR6, CX3CXR1 receptors were all expressed at very low levels in vaccine-primed elutriated peripheral CD4+ and CD8+ T cells. In addition, CCR4 expression in the CD8+ cell population was low (7.4%, SD=5.7).
Interestingly, CD3/CD28 costimulation induced a significant upregulation in CXCR3 and CXCR4 expression as well as the number of CD4+ and CD8+ cells expressing these receptors.. Specifically, 40–75% of CD4+ and CD8+ cells expressed CXCR3 and CXCR4, compared to 8–21% of the peripheral vaccine primed T cells. The receptor density for CXCR3 and CXCR4 also increased on the cells expressing these receptors, while the receptor density remained stable for CCR10 (Figure 3C, D). Thus, vaccine-primed peripheral blood T cells express appropriate chemokines for homing to ovarian cancer, and costimulation increases expression of relevant receptors (both receptor density and number of cells) to quasi-universal ovarian cancer chemokines.
CD3/CD28 costimulation enhances migration of vaccine-primed peripheral blood T cells to ovarian cancer chemokines
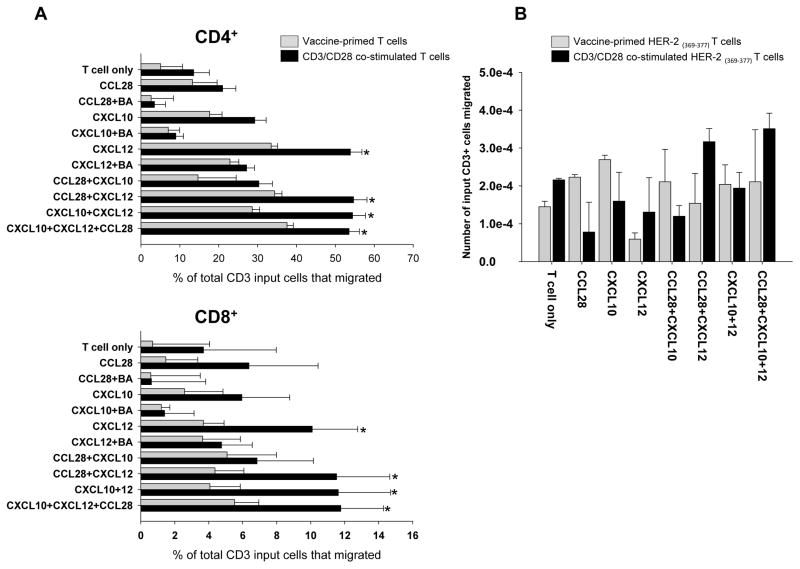
Next, we tested whether peripheral blood T cells collected directly from vaccinated subjects or following ex vivo costimulation can migrate towards chemokine ligands cognate to the most highly expressed chemokine receptors, CCR10, CXCR3 and CXCR4. We chose CCL28, CXCL10 and CXCL12 as the cognate chemokines.
Considering vaccine-primed peripheral blood T cells, CCL28 (at 10 ng/ml) exerted a modest chemoattractant effect on bulk CD4+ and minimal effect on CD8+ cells, while CXCL10 (at 50 ng/ml) recruited both CD4+ and CD8+ cells (Figure 4A). Migration of bulk CD4+ as well as CD8+ cells was highest in the presence of CXCL12 (100 ng/ml). Blocking antibodies reversed the pro-migratory effect of all 3 chemokines. The combination of chemokines did not enhance significantly the recruitment of T cells relative to individual chemokines, and most of the migration was attributed to the presence of CXCL12, which recruited the largest population of T cells.
Figure 4. Chemokine-induced migration of vaccine-primed and CD3/CD28 costimulated HER2/neu369–377 specific T cells.
A: Chemotaxis of CD4+ and CD8+ T cells T cells in the presence of tested chemokines. CCD3/CD28-costimulated T cells show increased migration even at baseline compared to vaccine primed T cells. In both CD4+ and CD8+ cells CCL28 induced very little migration compared to baseline chemotaxis measured in the T cell only group. CXCL10 induced slightly increased migration in the CD4+ cell population; however it was not statistically significant. CXCL12 induced significantly increased migration compared to baseline. Also the CD3/CD28 costimulated T cells migrated significantly more in the presence of CXCL12 compared to elutriated T cells. The various combination of chemokines only induced strong migration if CXCL12 was present, and the addition of CCL28 and CXCL10 did not further enhance chemotaxis. * p<0.05 B: Migration of HER2/neu369–377 positive cells in the presence of various chemokines. Her2/ neu369–377 positive cells were detected in both the elutriated and the adoptive T cell population and also showed enhanced migration in the presence of CXCL12.
CD3/CD28 costimulation enhanced the baseline motility of T cells and, importantly, induced a 2-fold increase in chemotaxis to CXCL10 and a 4-fold increase in chemotaxis to CXCL12 compared to non-costimulated elutriated T cells, both in CD4+ and CD8+ cells (Figure 4A). This increase was consistent with CXCR3 and CXR4 upregulation in these cells. Thus, costimulation appears to enhance the homing ability of vaccine-primed T cells towards the chemokines expressed in the ovarian tumor microenvironment. Supplementary Figure 1 shows an example of FACS analysis from one patient’s sample.
Tumor-specific T cells respond to chemokines expressed in ovarian cancer
Finally, we analyzed whether tumor-specific T cells elicited by the DC vaccine in protocol UPCC-19809 or the same T cells costimulated and expanded with CD3/CD28 in protocol UPCC-26810 migrated towards the cognate ovarian cancer chemokines. Costimulation of vaccine-primed T cells increased their migration toward the quasi-universal ovarian cancer chemokines (CXCL10, CXCL12, CCL28) compared to non-costimulated vaccine-primed T cells. We previously reported that advanced ovarian cancers ubiquitously express the Her2/neu protein (25) and that the DC based whole tumor lysate vaccine elicits T cells directed against known HLA-A*0201 restricted epitopes (7). We thus tested whether among T cells attracted by the quasi-universal ovarian cancer chemokines we could identify pentamer-positive T cells specific to A*0201-restricted Her2/neu peptide 369–377. HER2/neu-specific T cells were detected both in the vaccine primed and expanded T cell population. Expanded HER2/neu-specific T cells migrated the most in the presence of all three chemokines (CCL28+CXCL10+CXCL12) compare to baseline (T cell only). Due to the very low frequency of these cells we are only able to show the absolute number of migrated tumor specific cells among the loaded live CD3+ cells (Figure 4B). Additional studies with more patient samples are needed to further validate these findings.
Discussion
Ovarian cancer patients with pre-existing CD3+ or CD8+ intraepithelial T cells (TILs) at the time of diagnosis live significantly longer (1), suggesting that activation of antitumor immunity can enhance their survival. To test this hypothesis, we are conducting two related clinical studies, in which patients with recurrent ovarian cancer undergo vaccination with DC in combination with bevacizumab and low dose cyclophosphamide, followed by adoptive transfer of CD3/CD28 costimulated vaccine-primed T cells. Although in patients with pre-existing TILs it is intuitive that lymphocytes elicited by the vaccine or transferred adoptively should be able to home to tumors, such assumption may not be justified in patients lacking pre-existing T cells, since their tumor at the steady state may not have the necessary conditions for lymphocyte homing. As chemokines can regulate immune cell trafficking, we sought to understand the chemokine microenvironment of ovarian cancer and test whether this environment can support the homing of effector lymphocytes generated with cell-based immunotherapy.
Although the overall ovarian cancer chemokine landscape was found to be quite heterogeneous with high expression of known lymphocyte-recruiting chemokines in tumors with TILs, few chemokines such as CXCL10, CXCL12 and CXCL16, were expressed quasi-universally, including in tumors with T cells in the stroma but lacking TILs. We also observed high expression of CXCL1 mRNA in all three gene expression data sets, however could not validate its presence at the protein level due to the lack of reliable commercial antibodies. CXCL1 is a highly pro-angiogenic ELR+ chemokine (29), which mainly recruits neutrophils and thus could be less relevant with respect to T cell homing. Importantly, and with relevance to immune therapy, CXCL10, CXCL12 and CXCL16, are important lymphocyte chemoattractants. Their expression was localized to the epithelial component of the tumor, i.e. in association with tumor cells, and it was preserved across all metastatic sites, suggesting that T cells elicited by immunotherapy can home to the tumor cell compartment and potentially to all metastatic deposits.
The expression of T cell-recruiting chemokines by cancer cells could appear paradoxical. For example, although tumor derived CXCL12 has been reported to attract T cells (30), (31) it also exerts important tumor-promoting functions. In fact, CXCL12 and CXCL16 are overexpressed in epithelial ovarian carcinomas (32, 33), and other solid tumors (34, 35). CXCL12 is a powerful activator of the MAP kinase cascade in ovarian cancer cells. Cross-talk between the CXCL12/CXCR4 and the EGFR pathways promotes ovarian cell proliferation (36) and CXCL12/CXCR4 expression is associated with peritoneal metastasis and ascites formation (36, 37). High CXCL16 expression has also been correlated with poor prognosis in colorectal and prostate cancer (38, 39). Unlike CXCL10 and CXCL12, which were expressed only in vivo, CXCL16 seems constitutively activated in ovarian cancer cells, as it was also detected in primary and established cell lines in vitro. This could be driven in part by the commonly activated Akt/mTOR pathway (40, 41).
Although CXCL10, CXCL12 and CXCL16 are known to recruit T cells, their expression did not correlate with T cell infiltration in ovarian tumor islets at the time of diagnosis. In fact, approximately half of the tumors did not exhibit intraepithelial T cells, in agreement with our previous observations (1), despite expressing one or more of these chemokines. This could raise the possibility that such chemokines in fact do not contribute to recruiting T cells. For example, tumors can secrete an antagonistic N-terminally cleaved CXCL10 variant, which leads to early impairment of the immune response (42). CXCL12 expression by tumor fibroblasts was recently associated with lack of T cells in a pancreatic adenocarcinoma model, while the use of a CXCR4 small molecule inhibitor ameliorated T cell homing to tumors and tumor immune attack (43). It was hypothesized that CXCL12 bound on tumor cells through CXCR4 could directly eliminate tumor-reactive T cells. CXCL12 can also recruit suppressive plasmacytoid DCs to ovarian cancer (44) and we found a correlation of CXCL12 expression with the presence of Treg cells. These are indirect CXCL12-dependent mechanisms, which could drive peripheral tolerance through deregulation of tumor antigen presentation or attenuation of tumor-reactive T cells. Furthermore, CXCR4 ligation may downregulate tumor cell MHC-I expression (45) leading to the inability of T cells to recognize the tumor and engraft in tumor islets (46).
Additional mechanisms to prevent T cell homing and engraftment are likely to operate in these tumors. For example, we have described the tumor endothelial barrier, which prevents T cell extravasation in tumors in part due to endothelin B receptor-mediated downregulation of endothelial ICAM-1 expression (47), and through the upregulation of death-inducing molecules on surface endothelium such as Fas ligand (48). In addition, tumor derived soluble factors and surface inhibitory ligands can attenuate T cell function, further preventing their engraftment (49). Successful immunotherapy must address these multiple mechanisms to maximize clinical efficacy.
A small fraction of peripheral blood CD4+ and CD8+ T cells from ovarian cancer patients vaccinated with DC vaccine expressed chemokine receptors to the quasi-universal chemokines CXCL10 (CXCR3) and CXCL12 (CXCR4) and a higher fraction of peripheral blood CD4+ and CD8+ T cells expressed CCR10, the receptor for CCL28. In addition, a small fraction expressed CCR4 and CCR5, the receptors for heterogeneous ovarian cancer chemokines such as CCL2, CCL4 and CCL5. Thus, peripheral blood CD4+ and CD8+ T cells have the potential ability to traffic to ovarian cancer sites, which was corroborated by chemotaxis experiments, demonstrating migration towards recombinant CCL28, CXCL10, or CXCL12 alone or combined with the above. Among vaccine-primed T cells migrating towards the above cytokines, we found CD8+ T cells specific to the Her2/neu peptide 369–377, which as we demonstrated previously, are undetectable at base line and are specifically induced by DC-lysate vaccine (4, 7). Importantly, CD3/CD28 costimulation induced a significant upregulation of CXCR3 and CXCR4 in both CD4+ and CD8+ T cells from vaccine-primed peripheral blood T cells. We believe this enhanced migration in the CD3/CD28 costimulated cells was mainly attributed to upregulated CXCR3 and CXCR4 receptors however, it is known that activated T lymphocytes undergo a metabolic reprogramming with increased aerobic glycolysis and comparatively low rates of oxidative phosphorylation, factors that could also contribute to migration (50)
In summary, we demonstrate that ovarian cancers express a variety of lymphocyte recruiting chemokines. Tumors with pre-existing intraepithelial T cells express high levels of CCL4, CCL5 or CCL8, which can recruit vaccine-primed T cells expressing CCR5 or CCR10. The expression of CCL4 and CCL5, which bind to CCR1, CCR3, CCR5 and CCR4, showed the strongest correlation with the presence of tumor-infiltrating CD8+ T cells. One could hypothesize that upregulation of CCR5, CCR4 and CCR3 in ex vivo CD3/CD28 activated vaccine primed T cells could improve tumor homing in cancers expressing CCL4 and CCL5. In addition, these tumors express also one or more of the quasi-universal chemokines CXCL10 and CXCL12, which could recruit relevant vaccine-primed tumor-reactive T cells expressing CXCR3 or CXCR4. Given the ability of these tumors to recruit T cells at baseline, and based on their chemokine repertoire, these tumors are predictably readily infiltrated by vaccine-primed or ex vivo costimulated T cells. On the other hand, tumors lacking pre-existing T cells were found to express one or both of the quasi-universal lymphocyte recruiting chemokines CXCL10 and CXCL12. T cells elicited by DC vaccine expressed the appropriate receptors to home to these chemokines, and ex vivo CD3/CD28 costimulation further enhanced expression of these chemokine receptors and migration towards these chemokines. Further work is required to assess whether CXCL12 or CXCL10 can promote or rather suppress recruitment of T cells in tumors lacking intraepithelial T cells, but additional factors could certainly prevent T cell homing and function in these tumors. In our clinical trial design the addition of vascular normalization with bevacizumab and the attenuation of Treg with low dose cyclophosphamide are addressing two such important factors. Immunotherapy approaches that can expand the available pool of tumor-reactive T cells such as vaccines and adoptive transfer of vaccine-primed T cells could obviate the lack of intraepithelial T cells in many ovarian cancers. The ongoing studies with the tumor lysate-pulsed DC vaccine and adoptive transfer of vaccine-primed costimulated T cells will provide us an opportunity to test this hypothesis.
Supplementary Material
Translational Relevance.
Chemokines are key regulators of circulation, homing and retention of T cells, thus the characterization of the tumor microenvironment chemokine milieu is key to developing effective immunotherapy against solid tumors. Our data show that DC-primed and ex vivo CD3/CD28 costimulated peripheral blood T cells are endowed with appropriate chemokine receptors and show enhanced migration towards commonly expressed chemokines in ovarian cancer.
Acknowledgments
We thank Dr. Kathleen Montone, Li-Ping Wang and Amy Ziober for their assistance with validating and performing the chemokine staining on tissue microarray.
Grant Support
This study was supported by NCI P01-CA83638 SPORE in Ovarian Cancer, R01 FD003520, and the Ovarian Cancer Research Fund (GC) and the CA127334 (HL). The study was also supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology (Young Investigator Award granted to Davide Bedognetti).
Footnotes
Conflicts of interest:
The authors declare no conflict of interest to disclose.
References
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 2.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 3.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer research. 2007;67:8900–5. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 4.Kandalaft LE, Powell DJ, Jr, Chiang CL, Tanyi J, Kim S, Bosch M, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang CL, Hagemann AR, Leskowitz R, Mick R, Garrabrant T, Czerniecki BJ, et al. Day-4 myeloid dendritic cells pulsed with whole tumor lysate are highly immunogenic and elicit potent anti-tumor responses. PloS one. 2011;6:e28732. doi: 10.1371/journal.pone.0028732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandalaft LE, Chiang CL, Tanyi J, Motz G, Balint K, Mick R, et al. A Phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer. Journal of translational medicine. 2013;11:149. doi: 10.1186/1479-5876-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4801–15. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Mukai S, Kjaergaard J, Shu S, Plautz GE. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer research. 1999;59:5245–9. [PubMed] [Google Scholar]
- 10.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 11.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annual review of immunology. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 12.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. The New England journal of medicine. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 13.Kulbe H, Chakravarty P, Leinster DA, Charles KA, Kwong J, Thompson RG, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer research. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer letters. 2008;267:226–44. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 15.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. Journal of immunological methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 16.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–16. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balkwill FR. The chemokine system and cancer. The Journal of pathology. 2012;226:148–57. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 18.Owen JL, Criscitiello MF, Libreros S, Garcia-Areas R, Guthrie K, Torroella-Kouri M, et al. Expression of the inflammatory chemokines CCL2, CCL5 and CXCL2 and the receptors CCR1–3 and CXCR2 in T lymphocytes from mammary tumor-bearing mice. Cellular immunology. 2011;270:172–82. doi: 10.1016/j.cellimm.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang TL, Lee LY, Wang CC, Liang Y, Huang SF, Wu CM. CCL7 and CCL21 overexpression in gastric cancer is associated with lymph node metastasis and poor prognosis. World journal of gastroenterology : WJG. 2012;18:1249–56. doi: 10.3748/wjg.v18.i11.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinader V, Afarinkia K. The emerging role of CXC chemokines and their receptors in cancer. Future medicinal chemistry. 2012;4:853–67. doi: 10.4155/fmc.12.48. [DOI] [PubMed] [Google Scholar]
- 21.Oh SM, Oh K, Lee DS. Intratumoral administration of secondary lymphoid chemokine and unmethylated cytosine-phosphorothioate-guanine oligodeoxynucleotide synergistically inhibits tumor growth in vivo. Journal of Korean medical science. 2011;26:1270–6. doi: 10.3346/jkms.2011.26.10.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Tomei S, Ascierto ML, De Giorgi V, Bedognetti D, Dai C, et al. Melanoma NOS1 expression promotes dysfunctional IFN signaling. The Journal of clinical investigation. 2014;124:2147–59. doi: 10.1172/JCI69611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 24.Bertozzi CC, Chang CY, Jairaj S, Shan X, Huang J, Weber BL, et al. Multiple initial culture conditions enhance the establishment of cell lines from primary ovarian cancer specimens. In vitro cellular & developmental biology Animal. 2006;42:58–62. doi: 10.1290/0512084.1. [DOI] [PubMed] [Google Scholar]
- 25.Lanitis E, Dangaj D, Hagemann IS, Song DG, Best A, Sandaltzopoulos R, et al. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PloS one. 2012;7:e49829. doi: 10.1371/journal.pone.0049829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagemann AR, Hagemann IS, Cadungog M, Hwang WT, Patel P, Lal P, et al. Tissue-based immune monitoring II: multiple tumor sites reveal immunologic homogeneity in serous ovarian carcinoma. Cancer biology & therapy. 2011;12:367–77. doi: 10.4161/cbt.12.4.16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 28.Facciabene A, Santoro S, Coukos G. Know thy enemy: Why are tumor-infiltrating regulatory T cells so deleterious? Oncoimmunology. 2012;1:575–7. doi: 10.4161/onci.19401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiefer F, Siekmann AF. The role of chemokines and their receptors in angiogenesis. Cellular and molecular life sciences : CMLS. 2011;68:2811–30. doi: 10.1007/s00018-011-0677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunussi-Joannopoulos K, Zuberek K, Runyon K, Hawley RG, Wong A, Erickson J, et al. Efficacious immunomodulatory activity of the chemokine stromal cell-derived factor 1 (SDF-1): local secretion of SDF-1 at the tumor site serves as T-cell chemoattractant and mediates T-cell-dependent antitumor responses. Blood. 2002;100:1551–8. [PubMed] [Google Scholar]
- 31.Franciszkiewicz K, Boutet M, Gauthier L, Vergnon I, Peeters K, Duc O, et al. Synaptic release of CCL5 storage vesicles triggers CXCR4 surface expression promoting CTL migration in response to CXCL12. J Immunol. 2014;193:4952–61. doi: 10.4049/jimmunol.1401184. [DOI] [PubMed] [Google Scholar]
- 32.Guo L, Cui ZM, Zhang J, Huang Y. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chinese journal of cancer. 2011;30:336–43. doi: 10.5732/cjc.010.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer biology & therapy. 2007;6:1302–12. doi: 10.4161/cbt.6.8.4506. [DOI] [PubMed] [Google Scholar]
- 34.Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. American journal of respiratory and critical care medicine. 2003;167:1676–86. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- 35.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer research. 2003;63:3833–9. [PubMed] [Google Scholar]
- 36.Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecologic oncology. 2006;103:226–33. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 37.Machelon V, Gaudin F, Camilleri-Broet S, Nasreddine S, Bouchet-Delbos L, Pujade-Lauraine E, et al. CXCL12 expression by healthy and malignant ovarian epithelial cells. BMC cancer. 2011;11:97. doi: 10.1186/1471-2407-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hershberger PM, Peddibhotla S, Sugarman E, Maloney P, Key D, Suyama E, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Probing the CXCR6/CXCL16 Axis: Targeting Prevention of Prostate Cancer Metastasis. [Google Scholar]
- 39.Matsushita K, Toiyama Y, Tanaka K, Saigusa S, Hiro J, Uchida K, et al. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Annals of surgical oncology. 2012;19(Suppl 3):S518–27. doi: 10.1245/s10434-011-1993-8. [DOI] [PubMed] [Google Scholar]
- 40.Deng L, Chen N, Li Y, Zheng H, Lei Q. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochimica et biophysica acta. 2010;1806:42–9. doi: 10.1016/j.bbcan.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Zhang L, Greshock J, Colligon TA, Wang Y, Ward R, et al. Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes, chromosomes & cancer. 2011;50:606–18. doi: 10.1002/gcc.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rainczuk A, Rao JR, Gathercole JL, Fairweather NJ, Chu S, Masadah R, et al. Evidence for the antagonistic form of CXC-motif chemokine CXCL10 in serous epithelial ovarian tumours. International journal of cancer Journal international du cancer. 2013;134:530–41. doi: 10.1002/ijc.28393. [DOI] [PubMed] [Google Scholar]
- 43.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nature medicine. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Zhang L, Qiao A, Watson K, Zhang J, Fan GH. Activation of CXCR4 triggers ubiquitination and down-regulation of major histocompatibility complex class I (MHC-I) on epithelioid carcinoma HeLa cells. The Journal of biological chemistry. 2008;283:3951–9. doi: 10.1074/jbc.M706848200. [DOI] [PubMed] [Google Scholar]
- 46.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, et al. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:3372–9. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nature medicine. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 48.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nature medicine. 2014 doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaber T, Strehl C, Sawitzki B, Hoff P, Buttgereit F. Cellular Energy Metabolism in T-Lymphocytes. International reviews of immunology. 2014 doi: 10.3109/08830185.2014.956358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.