Abstract
The plastid genome (plastome) of heterotrophic plants like mycoheterotrophs and parasites shows massive gene losses in consequence to the relaxation of functional constraints on photosynthesis. To understand the patterns of this convergent plastome reduction syndrome in heterotrophic plants, we studied 12 closely related orchids of three different lifeforms from the tribe Neottieae (Orchidaceae). We employ a comparative genomics approach to examine structural and selectional changes in plastomes within Neottieae. Both leafy and leafless heterotrophic species have functionally reduced plastid genome. Our analyses show that genes for the NAD(P)H dehydrogenase complex, the photosystems, and the RNA polymerase have been lost functionally multiple times independently. The physical reduction proceeds in a highly lineage-specific manner, accompanied by structural reconfigurations such as inversions or modifications of the large inverted repeats. Despite significant but minor selectional changes, all retained genes continue to evolve under purifying selection. All leafless Neottia species, including both visibly green and nongreen members, are fully mycoheterotrophic, likely evolved from leafy and partially mycoheterotrophic species. The plastomes of Neottieae span many stages of plastome degradation, including the longest plastome of a mycoheterotroph, providing invaluable insights into the mechanisms of plastome evolution along the transition from autotrophy to full mycoheterotrophy.
Keywords: heterotrophy, plastid genome reduction, Neottieae, Orchidaceae, relaxed selection
Introduction
Plastids are essential cell organelles and the site of many metabolic processes, including photosynthesis and carbon fixation, the biosynthesis of starch, lipids, pigments, and amino acids, among others (Neuhaus and Emes 2000; McNeal et al. 2007; Green 2011; Rousseau-Gueutin et al. 2011; Wicke et al. 2011). The importance of these functions, especially of photosynthesis, is also reflected in the plastid genome (plastome). Because of the constantly high selection pressure to suppress of eliminate potentially disadvantageous mutations, most land plant plastomes are highly conserved concerning structure and gene contents. The two single copy regions (large and small single-copy region, i.e. LSC and SSC) and the two identical large inverted repeats (IRs) separating LSC and SSC normally encode 30 unique transfer RNAs, two sets of four ribosomal RNAs, and less than 100 protein-coding genes (Douglas 1998; Green 2011). And several hypotheses have been proposed to understand why chloroplasts retain their own genomes and genetic systems (Race et al. 1999; Barbrook et al. 2006; Allen 2015).
In heterotrophic land plants (including parasitic and mycoheterotrophic [MH] plants), photosynthesis-associated genes are no longer required, resulting in an extensive and rapid functional and physical reduction of their plastomes (Wolfe et al. 1992; Delannoy et al. 2011; Logacheva et al. 2011; Braukmann et al. 2013; Wicke et al. 2013). There appears to be a clear trend that genes for the thylakoid NAD(P)H dehydrogenase (ndh genes), the plastid-encoded polymerase (PEP; rpo genes), RuBisCO (rbcL locus), photosystems I and II (psa and psb genes, respectively), and the cytochrome b6f complex (pet genes) are lost or pseudogenized soon after the transition to a heterotrophic and nonphotosynthetic lifeform, whereas the thylakoid ATP synthase complex (atp genes) and some ribosomal protein genes (rpl and rps genes) as well as tRNAs are lost later (Barrett and Davis 2012; Barrett et al. 2014; Cusimano and Wicke 2016). A case in point, the rbcL locus is a critical photosynthesis gene and therefore highly conserved in autotrophic plants (Kellogg and Juliano 1997). However, it displays a rather unexpected evolutionary pattern in MH plants (Barrett and Freudenstein 2008; Cafasso and Chinali 2012). In leafless Corallorhiza, rbcL is putatively functional in some visible green species and pseudogenized in other species (Barrett and Freudenstein 2008; Barrett et al. 2014). In Neottia nidus-avis, genomic rearrangements in the rbcL region and three distinct rbcL sequences had been detected (Cafasso and Chinali 2012). In Epipogium and Rhizanthella, rbcL has been completely lost in plastomes (Delannoy et al. 2011; Schelkunov et al. 2015).
Although mycoheterotrophs are very rare in nature, they are relatively common in Orchidaceae (Merckx et al. 2013a,b). About 235 leafless species in 43 genera across Orchidaceae are putatively full or nearly mycoheterotrophs (Merckx et al. 2013a,b). The tribe Neottieae (Epidendroideae, Orchidaceae) comprises about 100 species in six recognized genera, that is Aphyllorchis, Cephalanthera, Epipactis, Limodorum, Neottia, Palmorchis, out of which 50 species in four genera are putatively full or nearly mycoheterotrophs (Pridgeon et al. 2005; Xiang et al. 2012; Merckx et al. 2013a,b; Chase et al. 2015). There are three growth forms and trophic specializations in Neottieae: (1) autotrophic or partially mycoheterotrophic species with green leaves (Abadie et al. 2006; Liebel et al. 2010; Merckx et al. 2013a,b; Bellino et al. 2014; Gonneau et al. 2014) carrying out photosynthesis (e.g. Neottia pinetorum), (2) partially or entirely mycoheterotrophic leafless (the definition of leaf, see Beentje 2012) species with photosynthetic pigmentation (e.g. Limodorum abortivum (Girlanda et al. 2006; Merckx et al. 2013a,b; Bellino et al. 2014)), and (3) fully mycoheterotrophic and visibly nongreen taxa that have lost the ability to photosynthesize (e.g. N. nidus-avis (Zimmer et al. 2008)). It remains to be tested if all leafless species in Aphyllorchis, Cephalanthera, and Neottia truly are full mycoheterotrophs (Merckx et al. 2013a,b). While putatively fully mycoheterotrophic species of Cephalanthera, such as Cephalanthera exigua, are mostly visibly nongreen, containing only trace photosynthetic pigments (Cummingsand Welschmeyer 1998), spontaneous visibly nongreen variants (albinos) occur time and again in otherwise photosynthetic species such as Cephalanthera damasonium (Preiss et al. 2010; Stoeckel et al. 2011; Roy et al. 2013). These observations in Neottieae strongly suggest that plastomes of these partial mycoheterotrophs may be at the very beginning of degradation.
The tribe Neottieae is an excellent model system to understand the mechanisms underlying plastome degradation in heterotrophic plants and the series of functional losses, as this requires comparative analyses of closely related autotrophic and heterotrophic species, which, ideally, differ in their trophic specialization within a narrow phylogenetic lineage. Here, we compare the complete plastome sequences of 12 species from four genera of the Neottieae. Our sampling covers all three growth forms and trophic specializations within this orchid tribe (fig. 1) and allows us to infer the general modes and mechanisms of early-stage plastome reduction. Using comparative plastid genomics, we aim to (1) investigate the extent, progression, and tempo of reductive plastome evolution in Neottieae, and especially the molecular evolution of rbcL; (2) test the hypothesis that the leafless species in Aphyllorchis, Cephalanthera, and Neottia are fully mycoheterotrophic, (3) to know whether plastomes of leafless Cephalanthera, such as Cephalanthera humilis, are at the very beginning of degradation.
Fig. 1.—
Growth forms in Neottieae. (A) Leafy and photosynthetic E. veratrifolia, and (B) N. pinetorum. (C) Leafless and visibly green N. camtschatea. (D) Leafless and visible nongreen (nonphotosynthetic) N. acuminata, and (E) Ce. humilis.
Materials and Methods
Taxon Sampling, DNA Isolation, Library Preparation, and Sequencing
We sampled 11 species from four Neottieae genera, including the leafy Cephalanthera longifolia, Epipactis veratrifolia, Epipactis mairei, Neottia ovata, Neottia fugongensis, N. pinetorum, the leafless but green Neottia camtschatea and Neottia listeroides, and the leafless visible nongreen Aphyllorchis montana, Ce. humilis, and Neottia acuminata (table 1). The previously published plastomes of leafless, visibly nongreen, and holo-mycoheterotrophic N. nidus-avis (Logacheva et al. 2011) and leafy, green, and autotrophic Calanthe triplicata (with 3–4 plicate leaves) (GenBank ID: NC_024544) complemented our analyses.
Table 1.
Length and GC Content of Plastid Genomes in Neottieae
| Length (in bp) |
GC Content (in %) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Length | LSC | SSC | IR | Total | LSC | SSC | IR | One IR | cds | rrn | trn | Noncoding |
| A. montana | 94,559 | 37.1 | 37.1 | 36.8 | 55.5 | 52.6 | 35.3 | ||||||
| Ce. humilis | 157,011 | 86,908 | 15,133 | 27,485 | 37.3 | 34.9 | 30.8 | 42.9 | 36.1 | 37.9 | 55.2 | 52.7 | 32.9 |
| Ce. longifolia | 161,877 | 88,806 | 19,187 | 26,942 | 37.2 | 35.0 | 30.6 | 43.2 | 36.0 | 37.9 | 55.2 | 52.7 | 31.7 |
| E. mairei | 159,019 | 86,377 | 18,816 | 26,913 | 37.3 | 35.1 | 30.5 | 43.2 | 36.1 | 38.0 | 55.2 | 52.8 | 31.6 |
| E. veratrifolia | 159,719 | 87,043 | 18,854 | 26,911 | 37.3 | 35.2 | 30.7 | 43.2 | 36.2 | 38.0 | 55.3 | 52.8 | 31.8 |
| N. ovata | 156,978 | 85,433 | 18,071 | 26,737 | 37.6 | 35.4 | 31.3 | 43.2 | 36.4 | 37.9 | 55.3 | 52.9 | 32.3 |
| N. fugongensis | 156,536 | 85,357 | 18,311 | 26,434 | 37.4 | 35.3 | 30.6 | 43.3 | 36.3 | 37.9 | 55.3 | 52.9 | 32.0 |
| N. pinetorum | 155,959 | 84,449 | 18,104 | 26,703 | 37.5 | 35.4 | 30.8 | 43.1 | 36.4 | 37.9 | 55.2 | 52.9 | 32.2 |
| N. acuminata | 83,190 | 51,145 | 5,371 | 13,337 | 36.6 | 33.1 | 26.7 | 45.2 | 34.9 | 34.3 | 54.9 | 52.1 | 32.0 |
| N. camtschatea | 106,385 | 52,960 | 9,273 | 22,076 | 37.2 | 33.5 | 28.8 | 43.3 | 35.6 | 35.3 | 54.9 | 52.5 | 33.3 |
| N. listeroides | 110,246 | 45,021 | 95,97 | 27,814 | 37.2 | 33.6 | 28.9 | 41.6 | 35.8 | 35.4 | 55.1 | 52.3 | 33.5 |
| N. nidus-avis | 92,060 | 36,423 | 78,21 | 23,908 | 34.4 | 29.2 | 25.3 | 39.9 | 32.5 | 33.2 | 54.4 | 51.0 | 28.4 |
total—total GC content, one IR—plastome excluding one IR, cds—protein-coding regions, rrn—ribosomal RNA genes, trn—tRNA genes; gray shading highlights leafless taxa.
Total genomic DNA was extracted from silica-dried materials using a modified CTAB-method (Li et al. 2013). DNA with a concentration higher than 100 ng/µl were sheared to 500 bp, and sequencing libraries were prepared using the NEBNext Ultra DNA Library Prep Kit (according to the manufacturer's protocol) for sequencing on an Illumina Hiseq 2500.
Read Mapping, Sequence Assembly, and Plastome Finishing
The raw reads (100 bp paired-end sequences) were filtered using NGSQCTOOLKIT v 2.3.3 (Patel and Jain 2012). We trimmed all bases with a quality lower than PHRED 20 and discarded all reads shorter than 70 bp after the trimming. The cleaned reads were mapped to Ca. triplicata in GENEIOUS 8.0 (Biomatters, Inc., Auckland, New Zealand; http://www.geneious.com, last accessed May 8, 2016) with medium-low sensitivity in five iterations. Used reads were exported and assembled using SOAPDENOVO2 (Luo et al. 2012), for which we chose a k-mer size from 37 to 67. Scaffolding was carried out in SSPACE (Boetzer et al. 2011). We additionally assembled all fully mycoheterotrophs de novo using VELVET (Zerbino and Birney 2008) with a k-mer size ranging from 37 to 45 and an auto-adjustment of the coverage cut-offs. The plastid sequences were extracted from the contigs pool using BLASTN 2.2.29+ (Camacho et al. 2009) and the Ca. triplicata plastome (Genbank ID: NC_024544) as the subject (reference) sequence. The extracted contigs were re-assembled and scaffolded using SSPACE, finished as described above, and reoriented to match the Ca. triplicata plastome reference; IR boundaries were determined by BLAST. All reads were mapped to the plastome scaffold to correct for assembly errors. Remaining gaps and stretches of undetermined bases were closed by PCR and Sanger sequencing. We also corrected a one bp-deletion in the matK gene of the N. nidus-avis plastome by Sanger resequencing (voucher: Jin X.H. 14261, PE; primers and PCR conditions: Raskoti et al. 2016).
Annotation
We annotated the reconstructed plastomes of Neottieae using DOGMA with an e value of 5, a percent identity cutoff for protein coding genes and tRNAs of 60 or 80, respectively (Wyman et al. 2004), as well as the GENEIOUS annotation tool with the plastomes of Ca. triplicata and Oncidium Gower Ramsey (Genbank ID: NC_014056.1) as references. We annotated genes as pseudogenes if they showed disruptions of the reading frame, premature stop codons or frameshifts caused by nontriplet insertions and deletions; their assemblies were inspected by eye to confirm that the pseudogenes were not due to assembly errors. We used OGDRAW (Lohse et al. 2013) to draw linear plastome maps for all species (Lohse et al. 2013). All fully annotated plastome sequences were deposited in NCBI Genbank under accession numbers KU551262–KU551272.
Repeat Analyses
We removed one IR region for all repeat analyses, for which we employed REPUTER to quantify forward and palindromic repeats longer than 25 nt, setting the Hamming distance to 3; all duplicate hits were removed. We quantified tandem repeats with MREPS (Kolpakov et al. 2003), which was run with a minimal and maximal period of 5 and 30, respectively. Statistical analyses and plots were performed using the R statistical software (http://www.r-project.org, last accessed May 8, 2016).
GC Content and Codon Usage
We analyzed the GC content using GENEIOUS and/or a custom Perl script. Codon usage, base composition, and the effective number of codons (ENC) in protein-coding genes were computed using CODONW (http://codonw.sourceforge.net/, last accessed May 8, 2016). The subsequent statistical analyses and hypothesis testing were performed in the R statistical framework. Pairwise Wilcoxon tests were employed to evaluate differences in GC content at different codon positions (GCn) between pairs of species for 27 genes commonly retained in the investigated species. We specifically tested if the change of GC content influences the codon usage in the retained genes of Neottieae. Published plastomes of Epidendroideae (Orchidaceae) were analyzed and the well-annotated Ca. triplicata (Genbank ID: NC_024544) was selected as a reference to build a relative distance of GC content at the different codon positions. The results were illustrated using R in combination with the ggplot2 package (http://ggplot2.org/, last accessed May 8, 2016).
Phylogenetic Analyses and Molecular Dating
For our phylogenetic analyses using complete plastid genome sequences, we used Ca. triplicata (Genbank ID: NC_024544), Dendrobium officinale (Genbank ID: KJ862886), and Erycina pusilla (Genbank ID: NC_018114) as outgroups. We subdivided the plastomes into 26 conserved and unique segments based on the position of housekeeping genes and the deletion of photosynthetic genes of holo-mycoheterotrophic species; the orientation of these segments were adjusted accurately. All segments were aligned using MAFFT under the automatic model selection option (Katoh et al. 2002) with some manual adjustments, and subsequently combined into a single plastome matrix in SEQUENCEMATRIX (Vaidya et al. 2011). We analyzed the aligned data with maximum likelihood (ML) using RAXML (Stamatakis 2014) under the GTR + Γ model on the CIPRES SCIENCE GATEWAY webserver (www.phylo.org, last accessed May 8, 2016), and with Bayesian inference (BI) using MrBayes 3.2.3 (Ronquist et al. 2012). The best model (Lset nst = 6 rates = invgamma; Prset statefreqpr = dirichlet(1,1,1,1)) for BI analyses was selected using MrModeltest v2.3 (Nylander et al. 2004). We used two separate MrBayes runs with four parallel Monte Carlo Markov chains (MCMC), sampling every 1000th generation over ten million replicates, and computed a majority rule (>50%) consensus tree after removing 25% of the samples as ‘burn-in’.
Molecular dating among Neottieae lineages was assessed using r8s 1.7 under a penalized likelihood (PL) method (Sanderson 2003) and the truncated Newton algorithm on the ML tree. A previous molecular phylogenetic dating of the Orchidaceae estimated the split between Palmorchis and remaining Neottieae at 30.9 Ma (Xiang et al. 2016).
Analyses of Selective Regimes
The synonymous and nonsynonymous substitution rates (dS, dN) of the concatenated 27 protein-coding housekeeping genes present in the plastome of each species were analyzed in PAML v.4.8 using the CODEML module (Yang et al. 2007). Selective regimes among branches were analyzed in PAML v.4.8, using the “branch” models in the CODEML module to test if the ratio of nonsynonymous to synonymous changes (dN/dS = ω) differs between branches in the 27 commonly retained genes (Yang 1997, 2007). We specifically tested for differences in ω between (1) Neottieae and autotrophic outgroups, (2) leafless Neottieae and leafy Neottieae plus outgroups, (3) visibly nongreen Neottieae and visibly green Neottieae plus leafy outgroups, and (4) Neottia s.l. and autotrophic outgroup. We performed a likelihood ratio tests (LRTs) against a null model that assumes a global ω as described earlier (Barrett et al. 2014); LRT P values were Bonferroni-corrected to account for multiple comparisons (Yang 2007). To assess the extent of relaxed selection, that is either relaxed purifying or positive selection, in retained plastid genes during and after the transition to mycoheterotrophy in orchids in general, we used the RELAX branch-site random effects likelihood method (Wertheim et al. 2015), implemented in the Datamonkey web server (Delport et al. 2010). To this end, plastid gene data of all orchid plastomes published in Genbank as of December 05, 2015 were downloaded (47 species) and complemented with our Neottieae data, and a dataset comprising all housekeeping genes was prepared as described earlier (Cusimano and Wicke 2016). We ran two tests with different branch-partitioning patterns to evaluate, firstly, if the relaxation of selection occurs only in nonphotosynthetic species compared to photosynthetic ones, and, secondly, if the relaxation of selection already occurs with the transition to a mycoheterotrophic lifestyle and irrespective of the photosynthetic capability.
Results
Size, Gene Content, and Structure of Plastomes of Neottieae
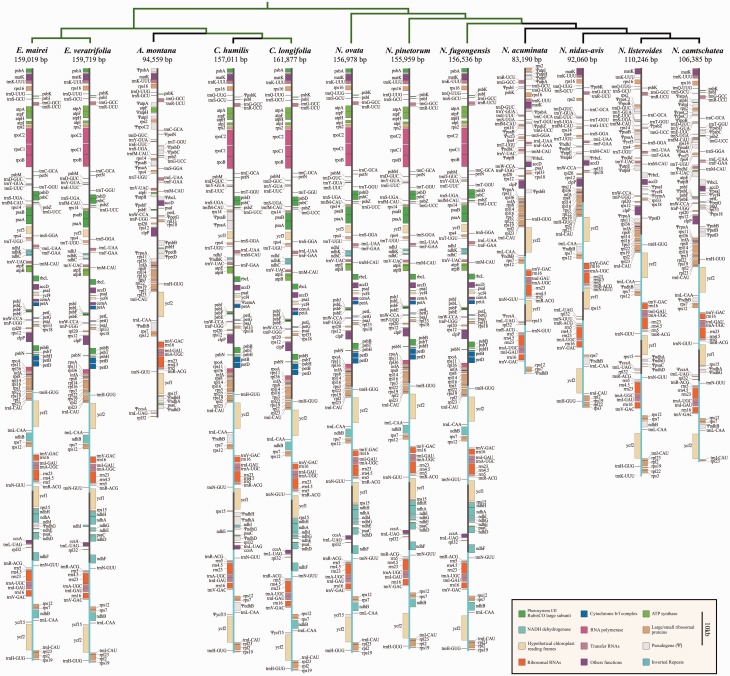
Plastid genomes of Neottieae range between 83,190 bp in N. acuminata and 161,877 bp in C. longifolia (fig. 2, table 1). The fully mycoheterotrophic Ce. humilis retains a plastome of 157,011 bp in length, and, hence, the longest plastome of any fully mycoheterotrophic species sequenced so far. The proportion of plastid reads in whole-genome shotgun data amounted to usually >1% in leafy species (except for N. ovata, 0.43%), whereas reads of plastid origin were less than 0.4% in the leafless taxa (supplementary table S1, Supplementary Material online).
Fig. 2.—
Plastid genome structures of 12 Neottieae species. Genes are colored by function. Pseudogenes are shown in gray and marked with a ‘Ψ’.
Plastomes of Neottieae are mostly collinear with those of autotrophic orchids, with only a few exceptions. For example, the plastome of N. acuminata has an 11 kb inversion in the LSC between its psbA pseudogene (ΨpsbA) and rps2. Considerably shorter inversions occur in the SSC of C. humilis between rpl32 and ΨccsA, and in the intergenic spacer between atpA and atpF of A. montana. Variations of the IRs is especially common in leafless Neottieae (supplementary fig. S1, Supplementary Material online). Whereas A. montana has lost one copy of the IR (fig. 2), N. listeroides has a slightly expanded IR that extends to the rpl16 region. In N. acuminata, IRs range beyond the trnL-CAA gene but no longer enclose the ycf2 gene. Similar to other plants, plastomes of Neottieae are rare in dispersed repeats in general, with only a handful of repeats longer than 60 bp (supplementary fig. S2, Supplementary Material online). Only the plastome of N. listeroides harbors a considerably higher number of tandem repeats.
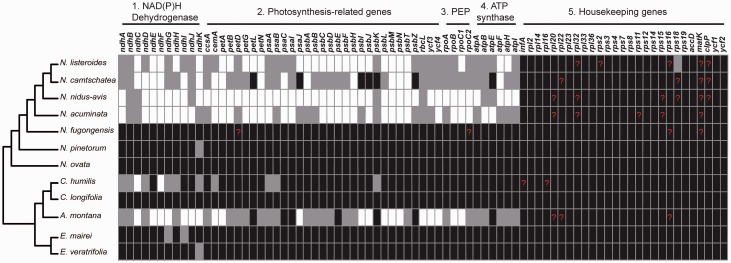
Pseudogene and Gene Loss
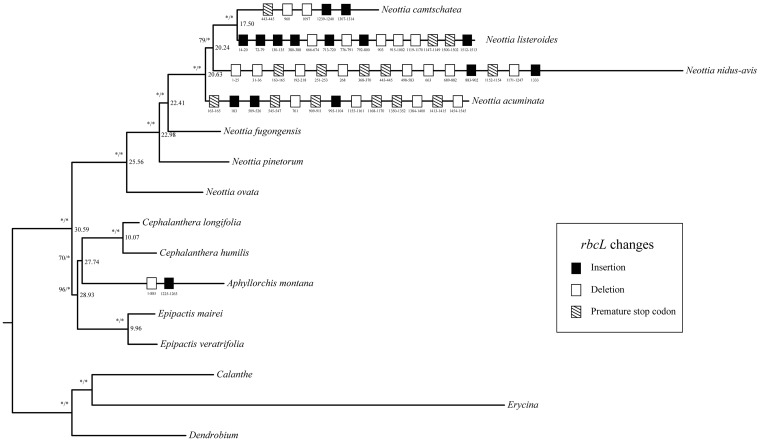
Similar to the structural changes to the plastome, physical deletions of genes are lineage-specific despite a common pattern in the series of losses (fig. 3 and supplementary fig. S3, Supplementary Material online). Except Ce. humilis (fig. 4 and supplementary table S2, Supplementary Material online), leafless Neottieae have functionally lost genes related to photosynthesis (i.e. ndh, pet, psa, psb, rpo, atp genes, plus ccsA, cemA, rbcL, ycf3, and ycf4) and PEP (rpo genes) (fig. 4 and supplementary table S2, Supplementary Material online). In Ce. humilis, most psa and psb genes have intact open reading frames (ORFs), except for psaA, psaB, psbK, whereas A. montana and N. camtschatea both retain only five intact psa and psb genes, respectively (fig. 4 and supplementary table S2, Supplementary Material online). The rbcL locus is intact in Ce. humilis but exhibits many indels, frameshift mutations or premature stop codons in the plastomes of the other five leafless Neottieae species. There are six premature stop codons in the rbcL region of N. acuminata and 12 indels in N. listeroides. Aphyllorchis montana retains only a truncated copy of rbcL (1–885 bp) (fig. 5).
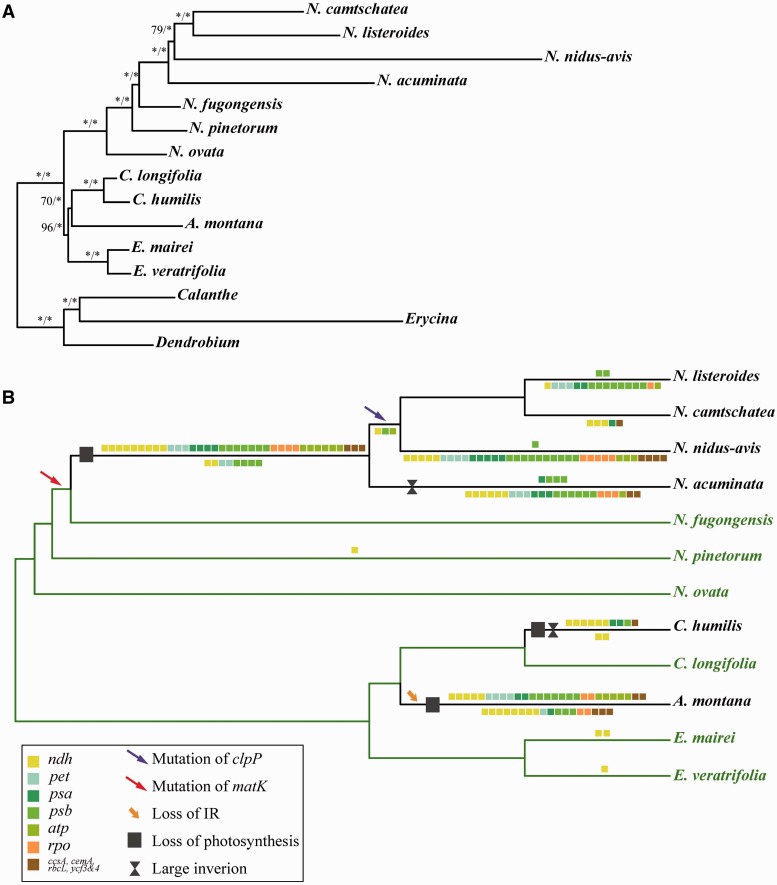
Fig. 3.—
Phylogenetic results and the evolution of plastid genomes in Neottieae. (A) The maximum likelihood (ML, BI) trees inferred from whole plastomes. Number above branches are the bootstrap support (ML) and posterior probabilities (BI), respectively. (B) Functionally and physically lost genes are mapped on the plastome tree, showing pseudogenes above branches and genes that were deleted from the plastome below.
Fig. 4.—
Protein-coding genes in Neottieae. Intact genes per species are indicated by black boxes, whereas gray and white boxes mark functional and or physical losses, respectively. Red question marks highlight uncertain calls. PEP—plastid-encoded RNA polymerase.
Fig. 5.—
Changes in the rbcL locus over time in leafless Neottieae. Changes such as insertions (black boxes), deletions (white), and premature stop codons (striped) in rbcL are mapped on the branches of all leafless taxa. Divergence ages (in million years) inferred from a PL analysis are given in bold on the right-hand side of all Neottieae internodes.
Housekeeping genes such as accD, matK, clpP, and ycf1 have intact ORFs in most Neottieae, except for N. nidus-avis in which also rps16 and rps18 both have premature stop codons. Some housekeeping genes such as rps4, rps18, and rpl16 have accumulated unique indels or frameshift mutations towards their 3' ends in various leafless or nonphotosynthetic species (supplementary table S2, Supplementary Material online). The clpP genes of N. camtschatea, N. listeroids, and N. nidus-avis has a premature stop codon 30 bp upstream of its known gene stop, thus possibly producing a 10 amino acid shorter protein in these species. Although all seven group-IIA introns that require matK for splicing are present in Neottieae, this plastid-encoded maturase gene shows a premature stop codon 33 bp downstream of the initiator in some Neottia species (supplementary fig. S3, Supplementary Material online). This premature stop is, however, followed immediately by another potential start codon (supplementary fig. S3, Supplementary Material online).
Leafy and green Neottieae (E. mairei, N. pinetorum, and E. veratrifolia) have functionally lost one or two ndh genes. Some of these species (e.g. N. fugongensis) also show evidence for the nonfunctionalization of PEP, although no genes have been physically deleted from their plastomes.
GC Content and Codon Usage
The GC content differs notably in Neottieae, ranging from 34.4% in N. nidus-avis up to 37.6% in N. ovata (table 1). In protein-coding regions, GC reduction amounts to between 0.06% and 4.3% in the leafless species, while in structural RNAs it remains nearly unaltered (table 1). The IR-lacking fully mycoheterotrophic A. montana possesses the highest GC content in its noncoding regions (35.3%), compared to 32.5% in the leafy species and <34% in other leafless species (table 1). The three leafless species N. acuminata, N. camtschatea, and N. nidus-avis show a marked AT richness in both the first and second position, whereas the GC content in A. montana is significantly elevated only in the second and third position (supplementary fig. S4, Supplementary Material online).
Most leafless Neottieae utilize codons just like the leafy species, although we observe some minor under- or overuses (e.g. ValGUC, ThrACG in A. montana; ArgAGA, AsnAAU, GlyGGA, ProCCG in N. acuminata; CysUGU in N. camtschatea; AlaGCU, AspGAU, IleAUA, SerUCA, TerUAA in N. nidus-avis; TerUAG in N. listeroides). The most pronounced differences are found for the IleAUC, whose usage rises from <14% to >21% in A. montana, N. nidus-avis, and N. listeroides, as well as for the terminator tRNA TerUAA, which is used <42% in A. montana compared to >59% in N. nidus-avis (supplementary table S3, Supplementary Material online).
Phylogenetic Analyses and Molecular Dating
The topology of the trees from our ML inference and BI results are mostly congruent with the MP results, with only one exception in the Neottia crown group. The relationships among Neottieae are mostly well resolved with high supported, except for the Cephalanthera–Aphyllorchis clade (ML = 70) (fig. 3A). Neottia species are sister to a clade formed by Cephalanthera and Aphyllorchis, which themselves are sister to Epipactis. Within Neottia, both ML and BI analyses show that the three leafy species N. ovata, N. pinetorum, and N. fugongensis are successive sisters to a grade of the four leafless Neottia (pp = 1, ML = 100). N. acuminata and N. nidus-avis are successive sisters to the two visibly green N. listeroides and N. camtschatea in ML and BI results (fig. 3A). In our MP analysis, N. acuminata and N. nidus-avis together form a clade that is sister to N. listeroides and N. camtschatea (supplementary fig. S5, Supplementary Material online).
The split between Neottia and the remaining three genera was immediately followed by the divergence of Epipactis and the Aphyllorchis–Cephalanthera clade at 28.98 and 27.74 million years ago (Ma). We inferred the age of the four leafless species of Neottia at 20.63 Ma, A. montana at 27.74 Ma, and Ce. humilis at 10.07 Ma (fig. 5).
Analyses of Selective Regimes
Species of Neottia, including both leafy and leafless ones, have a much higher synonymous and nonsynonymous substitution rates (dS, dN) in the concatenated housekeeping genes than the remaining Neottieae (supplementary table S4, Supplementary Material online). Leafless and visibly nongreen Neottia have the highest dS and dN (supplementary table S4 and fig. S6, Supplementary Material online), and leafless Ce. humilis exhibits similar dS and dN as leafy Ce. longifolia (supplementary table S4 and fig. S7, Supplementary Material online). The ratio of dN/dS (ω) of Neottieae is <1, A. montana has the highest ω (supplementary table S4 and fig. S6, Supplementary Material online).
A “two-ratio” likelihood model (M1), which allows all Neottieae to adopt a different ω than the outgroups, has a significantly better fit for rpl16, rpl33, rps18, and rps2 than the basic “one-ratio” model, M0 (Bonferroni-corrected LRTs between M0 and M1, P values all <0.05; supplementary table S4 Supplementary Material online). The “three-ratio” model, M2, separating the leafless Neottieae from other Neottieae and from the outgroup applies only for ycf1 (LRT M2 vs. M1: P < 0.01), but is outperformed by both a three- and a six-ratio model (M3 and M4, respectively) allowing for different ω between Ce. humilis, A. montana, and the leafless Neottia, or between Ce. humilis, A. montana, green and leafless Neottia, nongreen Neottia, and leafless Neottia (M3 vs. M2 < 0.001; M2 vs. M4: P < 0.001) (supplementary table S5, Supplementary Material online). When all housekeeping genes are concatenated for analyses, no model is significantly better than one-ratio model (supplementary table S5, Supplementary Material online).
The multiple transitions to a mycoheterotrophic lifestyle within orchids (supplementary fig. S7, Supplementary Material online) coincide with significant changes in the selective pressures of the retained plastid genes. Housekeeping genes in the ten visibly nongreen lineages show a slightly relaxed purifying selection (selection intensity parameter k = 0.75, likelihood ratio (LR) = 9.06, P = 0.003). However, mean ω is only slightly different between visibly green (ω = 0.417) and visibly nongreen orchids (ω = 0.553), suggesting that housekeeping genes continue to evolve under purifying selection in the latter. A comparison of both visibly green and visibly nongreen mycoheterotrophs versus autotrophic orchids also indicates a weak trend for relaxed purifying selection (k = 0.99), which is, however, not significant (P = 0.118).
Discussion
Phylogenetic Relationships in Neottieae
The phylogeny of Neottieae remained unresolved in previous molecular systematics based on several loci (Bateman et al. 2004; Roy et al. 2009; Selosse and Roy 2009; Xiang et al. 2012). Cephalanthera sometimes was placed as sister to the clade formed by Neottia + Aphyllorchis + Epipactis, albeit with no statistic support (Bateman et al. 2004; Xiang et al. 2012). Our data resolves the phylogeny of Neottieae well, showing that Neottia is sister to a clade of Epipactis, Aphyllorchis, and Cephalanthera. These results are corroborated by several vegetative and floral traits (Burnsbalogh et al. 1987; Pridgeon et al. 2005; Chen et al. 2009). A substitution in the plastid clpP gene shared by three of the investigated Neottia species further supports our inferred close relationships of N. camtschatea, N. listeroides, and N. nidus-avis. The minor incongruency of the four mycoheterotrophic Neottia species seen in the ML and BI trees compared to the MP analyses may be the result of a long-branch attraction phenomenon in MP.
Plastome Reduction in Neottieae and Orchidaceae
Leafless members of Neottieae, including the two leafless green species, all show evidence of the degradation of genes that are directly involved in photosynthetic processes (fig. 3 and supplementary fig. S3, Supplementary Material online), albeit with an extraordinary variation in the number and nature of the genes lost or pseudogenized. Despite several intact photosynthesis genes (e.g. petL, psaI, psbI, psbJ, psbK, and psbZ), most species have lost subunits required for light harvesting and CO2 fixation. Unlike in Corallorhiza striata var. vreelandii (Barrett et al. 2014) and N. nidus-avis (Logacheva et al. 2011), ccsA, cemA, psaA, and psaB are among the first to be lost in mycoheterotrophic Neottieae. These findings suggest that, despite the presence of chlorophyll, these plants likely are unable to carry out photosynthesis anymore (Girlanda et al. 2006; Cameron et al. 2009). Moreover, recent results indicate that the degradation of plastid genomes is independent of chlorophyll synthesis in some nonphotosynthetic parasites (Cameron et al. 2009; Wickett et al. 2011). The photosynthesis genes that have escaped deleterious mutations normally are short in length or benefit from some protection by nearby essential genes (Wicke et al. 2013). Genes for the thylakoid ATP synthase, which appears to play a significant role in some nonphotosynthetic plants (Kamikawa et al. 2015), are either pseudogenes or deleted from the plastomes of leafless Neottieae, except in Ce. humilis.
Although required under stress conditions in many plants (Peltier and Cournac 2002), ndh genes are often functionally or even physically lost in Orchidaceae (Wu et al. 2010; Lin et al. 2015), including in all mycoheterotrophic orchids studied so far. Although leafless Neottieae retain only a few ndh genes, the sets of retained ndh genes genes imply that the loss of the NAD(P)H dehydrogenase complex seems to have occurred at least four times independently within the tribe (fig. 4 and supplementary table S2, Supplementary Material online). For example, in E. mairei the genes ndhG and ndhI are pseudogenes, whereas intact ORFs of these exist in E. veratrifolia. In this species, we find a pseudogene of ndhK, which is, however, intact in E. mairei.
The transition to mycoheterotrophy, the progressive trophic specialization and the eventual loss of photosynthesis also alters the selective regimes in several housekeeping genes of Neottieae (supplementary tables S4, S5, and fig. S6, Supplementary Material online), although the pattern of selectional shifts itself is rather erratic. It seems that purifying selection has been relaxed in leafless non-Neottieae species than in leafless Neottieae. The loss of housekeeping genes often occurs rather late during the reductive plastome evolution in nonphotosynthetic plants. However, some Neottieae show evidence for the pseudogenization of genes for transcription (e.g. rpoC2), translation (e.g. rps18), and RNA maturation (e.g. matK). The latter has been shown recently to be expressed in Neottieae (Barthet et al. 2015), but our data indicate that some of the mycoheterotrophs may utilize an alternative start codon. The gene clpP encoding a subunit of the Clp protease), which is retained also in most other nonphotosynthetic lineages except for Apodanthaceae (Bellot and Renner 2015), may produce a protein that is ten amino acids shorter in the three leafless Neottia species than in other Neottieae (fig. 3A), but otherwise apparently intact. Most of the remaining housekeeping genes, such as rpl, rps, trn, rrn, rpo, rpl, ycf1, and ycf2, are intact and putatively functional in most Neottieae (but see Logacheva et al. 2011).
Our results of the molecular dating and the analysis of plastid genome structures indicate that C. humilis might be one of the most recently evolved completely mycoheterotrophic species in Neottieae. C. humilis clearly is at the earliest stage of plastome reduction with only a few functional losses in the plastid photosystem genes. Its GC content is still very similar to that of close photosynthetic relatives (table 1). Although Aphyllorchis and Cephalanthera split ca. 27.74 Ma ago (fig. 5), A. montana may also be a recently evolved species. It, too, shows plastome reduction at an early stage, which we characterize as having a rather high GC content and a large number of putatively functional photosynthesis genes. It needs more work to elucidate if the loss of one copy of the IR in A. montana has occurred already in the common ancestor of Aphyllorchis. In contrast to Cephalanthera and Aphyllorchis, N. acuminata, N. camtschatea, N. listeroides, and N. nidus-avis are already at rather late stages. Neottia species already have begun to reduce the proportion of nonessential DNA after the massive pseudogenization of most photosynthesis-related genes. Their plastomes' GC contents decrease gradually alongside the physical reduction. Plastomes size varies considerably among different species, with reductions of up to 47% compared to the leafy congenerics.
Nuclear copies may have replaced a few plastid housekeeping genes (rps16 and rps18), which seem to have been lost functionally from the plastome. The molecular evolution of the rbcL locus is highly lineage-specific in Neottieae. While rbcL is putatively functional in C. humilis, it is a pseudogene in the four leafless Neottia species and A. montana. There are about 44 indels in the rbcL locus of the four leafless Neottia, however, none of them is shared (fig. 5).
The Evolution of Mycoheterotrophy in Neottieae and Orchidaceae
Our findings indicate that the transition to a fully mycoheterotrophic lifestyle has evolved at least three times independently during the evolution of Neottieae (fig. 2A), that is, once in Aphyllorchis, in Cephalanthera, and in Neottia. In Neottieae, most investigated leafy species are partial mycoheterotrophic species (Bidartondo et al. 2004; Julou et al. 2005; Abadie et al. 2006; Girlanda et al. 2006; Selosse and Roy 2009; Liebel et al. 2010), and leafless species are full mycoheterotrophs (Cameron et al. 2009; Logacheva et al. 2011). In Neottia, the leafy partially mycoheterotrophic N. ovata is sister to the four fully mycoheterotrophs, which together nest deeply in the leafy clade (fig. 2A). It, thus, seems that the ancestor of Neottia was a leafy and partial mycoheterotroph from which the nonphotosynthetic mycoheterotrophic lifestyle evolved only once. In Cephalanthera, the visibly nongreen C. austinae also nests within leafy and partially mycoheterotrophic species (Abadie et al. 2006). In support of an earlier hypothesis (Abadie et al. 2006), we may thus assume that the last common ancestor of Aphyllorchis and Cephalanthera was partially mycoheterotrophic, too (Merkx et al. 2013).
All investigated plastomes of leafless partially or fully mycoheterotrophic orchids such as N. nidus-avis. (Logacheva et al. 2011), Epipogium roseum (Schelkunov et al. 2015), and Epipogium aphyllum [19] have lost genes for the photosynthesis machinery, with the notable exception of Corallorhiza trifida (Zimmer et al. 2008). The plastome of C trifida retains many intact photosynthesis-related genes (e.g. psa, psb, pet genes, and rbcL) (Barrett et al. 2014), although it physiologically resembles the fully mycoheterotrophic condition (Cameron et al. 2009). This observation tempts us to speculate that the loss of leaves precedes the reduction of plastomes in some partially mycoheterotrophic orchids.
In sum, our study demonstrates that the degradation of plastomes in Neottieae not only agrees with the previously proposed general trends (Barrett and Davis 2012; Wicke et al. 2013; Barrett et al. 2014), but also underlies highly lineage-specific patterns of physical and functional reductions with many potentially independent gene losses. The shift to a leafless growth form in Neottieae per se appears to be closely associated with a significant decrease in plastome size and changes of the selectional regimes in retained housekeeping genes. The loss of leaves in combination with the onset of plastome degeneration including several functional gene losses and relaxed purifying selection in some genes imply that the transition to an obligate mycoheterotrophic lifestyle relaxes selection on plastid gene function rather than only the eventual loss of photosynthesis. By shedding new light on the earliest functional changes during the transition from partial to fully mycoheterotrophy, the complete plastid genome sequences of Neottieae contribute significantly to our understanding of the various routes of plastome degeneration in heterotrophic plants in general.
Supplementary Material
Supplementary figures S1–S7 and tables S1–S5 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 31470299), and the Chinese Special Fund for Medicine Research in the Public Interest (201407003).
Literature Cited
- Abadie J-C, et al. 2006. Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Can J Bot.-Revue Canadienne De Botanique 84:1462–1477. doi: 10.1139/b06-101 [Google Scholar]
- Allen JF. 2015. Why chloroplasts and mitochondria retain their own genomes and genetic systems: Colocation for redox regulation of gene expression. Proc Natl Acad Sci U S A. 112:10231–10238. doi: 10.1073/pnas.1500012112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbrook AC, Howe CJ, Purton S. 2006. Why are plastid genomes retained in non-photosynthetic organisms? Trends Plant Sci. 11:101–108. doi: 10.1016/j.tplants.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Barrett CF, Davis JI. 2012. The plastid genome of the mycoheterotrophic Corallorhiza striata (Orchidaceae) is in the relatively early stages of degradation. Am J Bot. 99:1513–1523. doi: 10.3732/ajb.1200256 [DOI] [PubMed] [Google Scholar]
- Barrett CF, Freudenstein JV. 2008. Molecular evolution of rbcL in the mycoheterotrophic coralroot orchids (Corallorhiza Gagnebin, Orchidaceae). Mol Phylogenet Evol. 47:665–679. doi: 10.1016/j.ympev.2008.02.014 [DOI] [PubMed] [Google Scholar]
- Barrett CF, et al. 2014. Investigating the path of plastid plastome degradation in an early-transitional clade of heterotrophic orchids, and implications for heterotrophic angiosperms. Mol Biol Evol. 31:3095–3112. doi: 10.1093/molbev/msu252 [DOI] [PubMed] [Google Scholar]
- Barthet MM, Moukarzel K, Smith KN, Patel J, Hilu KW. 2015. Alternative translation initiation codons for the plastid maturase MatK: unraveling the pseudogene misconception in the Orchidaceae. BMC Evol Biol. 15:210. doi: 10.1186/s12862-015-0491-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RM, Hollingsworth PM, Squirrell J, Hollingsworth ML. 2004. Tribe Neottieae: phylogenetics In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera Orchidacearum (4): Epidendroideae (Part 1). Oxford, UK: Oxford University Press; p. 487–515. [Google Scholar]
- Beentje H. 2012. The Kew Plant Glossary: an illustrated dictionary of plant terms. London: Kew Publishing. [Google Scholar]
- Bellino A, et al. 2014. Nutritional regulation in mixotrophic plants: new insights from Limodorum abortivum. Oecologia 175:875–885. doi: 10.1007/s00442-014-2940-8 [DOI] [PubMed] [Google Scholar]
- Bellot S, Renner SS. 2015. The plastomes of two species in the endoparasite genus Pilostyles (Apodanthaceae) each retain just five or six possibly functional genes. Genome Biol Evol. 8:189–201. doi:10.1093/gbe/evv1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidartondo MI, et al. 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc R Soc B-Biol Sci. 271:1799–1806. doi: 10.1098/rspb.2004.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. 2011. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27:578–579. doi: 10.1093/bioinformatics/btq683 [DOI] [PubMed] [Google Scholar]
- Braukmann T, Kuzmina M, Stefanovic S. 2013. Plastid genome evolution across the genus Cuscuta (Convolvulaceae): two clades within subgenus Grammica exhibit extensive gene loss. J Exp Bot. 64:977–989. doi: 10.1093/jxb/ers391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnsbalogh P, Szlachetko DL, Dafni A. 1987. Evolution, pollination, and systematics of the tribe Neottieae (Orchidaceae). Plant Syst Evol. 156:91–115. doi: 10.1007/bf00937204 [Google Scholar]
- Cafasso D, Chinali G. 2012. Multiple and different genomic rearrangements of the rbcL gene are present in the parasitic orchid Neottia nidus-avis. Genome 55:629–637. doi: 10.1139/g2012-057 [DOI] [PubMed] [Google Scholar]
- Camacho C, et al. 2009. BLAST plus: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DD, Preiss K, Gebauer G, Read DJ. 2009. The chlorophyll-containing orchid Corallorhiza trifida derives little carbon through photosynthesis. New Phytol. 183:358–364. doi: 10.1111/j.1469-8137.2009.02853.x [DOI] [PubMed] [Google Scholar]
- Chase MW, et al. 2015. An updated classification of Orchidaceae. Bot J Linn Soc. 177:151–174. doi: 10.1111/boj.12234 [Google Scholar]
- Chen SC, et al. 2009. Flora of China. Beijing: Science Press. [Google Scholar]
- Cummings MP, Welschmeyer NA. 1998. Pigment composition of putatively achlorophyllous angiosperms. Plant Syst Evol. 210:105–111. doi: 10.1007/bf00984730 [Google Scholar]
- Cusimano N, Wicke S. 2016. Massive intracellular gene transfer during plastid genome reduction in nongreen Orobanchaceae. New Phytol. 210:680–693. doi: 10.1111/nph.13784 [DOI] [PubMed] [Google Scholar]
- Delannoy E, Fujii S, des Francs-Small CC, Brundrett M, Small I. 2011. Rampant gene loss in the underground orchid Rhizanthella gardneri highlights evolutionary constraints on plastid genomes. Molecular Biology and Evolution 28:2077–2086. doi: 10.1093/molbev/msr028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delport W, Poon AFY, Frost SDW, Pond SLK. 2010. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26:2455–2457. doi: 10.1093/bioinformatics/btq429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SE. 1998. Plastid evolution: origins, diversity, trends. Curr Opin Genet Dev. 8:655–661. doi: 10.1016/s0959-437x(98)80033-6 [DOI] [PubMed] [Google Scholar]
- Girlanda M, et al. 2006. Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol Ecol. 15:491–504. doi: 10.1111/j.1365-294X.2005.02770.x [DOI] [PubMed] [Google Scholar]
- Gonneau C, et al. 2014. Photosynthesis in perennial mixotrophic Epipactis spp. (Orchidaceae) contributes more to shoot and fruit biomass than to hypogeous survival. J Ecol. 102:1183–1194. doi: 10.1111/1365-2745.12274 [Google Scholar]
- Green BR. 2011. Chloroplast genomes of photosynthetic eukaryotes. Plant J. 66:34–44. doi: 10.1111/j.1365-313X.2011.04541.x [DOI] [PubMed] [Google Scholar]
- Julou T, et al. 2005. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol. 166:639–653. doi: 10.1111/j.1469-8137.2005.01364.x [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30:3059–3066. doi: 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA, Juliano ND. 1997. The structure and function of RuBisCO and their implications for systematic studies. Am J Bot. 84:413–428. doi: 10.2307/2446015 [PubMed] [Google Scholar]
- Kolpakov R, Bana G, Kucherov G. 2003. mreps: efficient and flexible detection of tandem repeats in DNA. Nucleic Acids Res. 31:3672–3678. doi: 10.1093/nar/gkg617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang S, Yu J, Wang L, Zhou S. 2013. A modified CTAB protocol for plant DNA extraction. Chin Bull Bot. 48:72–78. [Google Scholar]
- Liebel HT, et al. 2010. C and N stable isotope signatures reveal constraints to nutritional modes in orchids from the Mediterranean and Macaronesia. Am J Bot. 97:903–912. doi: 10.3732/ajb.0900354. [DOI] [PubMed] [Google Scholar]
- Lin C-S, et al. 2015. The location and translocation of ndh genes of chloroplast origin in the Orchidaceae family. Sci Rep. 5:9040. doi: 10.1038/srep0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logacheva MD, Schelkunov MI, Penin AA. 2011. Sequencing and analysis of plastid genome in mycoheterotrophic orchid Neottia nidus-avis. Genome Biol Evol. 3:1296–1303. doi: 10.1093/gbe/evr102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41:W575–W581. doi: 10.1093/nar/gkt289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, et al. 2012. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1. doi: 10.1186/2047-217x-1-18. p.1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal JR, Kuehl JV, Boore JL, de Pamphilis CW. 2007. Complete plastid genome sequences suggest strong selection for retention of photosynthetic genes in the parasitic plant genus Cuscuta. BMC Plant Biol. 7:57. doi: 10.1186/1471-2229-7-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx VSFT, et al. 2013a. Taxonomy and classification In: Merckx VSFT, editor. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer; p. 19–101. [Google Scholar]
- Merkx VSFT, Mennes CB, Peay KG, Geml J. 2013b. Taxonomy and diversification In: Merckx VSFT, editor. Mycoheterotrophy: the biology of plants living on fungi. New York: Springer; p. 215–244. [Google Scholar]
- Neuhaus HE, Emes MJ. 2000. Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol. 51:111–140. doi: 10.1146/annurev.arplant.51.1.111 [DOI] [PubMed] [Google Scholar]
- Nylander JAA, Ronquist F, Huelsenbeck JP, Nieves-Aldrey JL. 2004. Bayesian phylogenetic analysis of combined data. Syst Biol. 53:47–67. doi: 10.1080/10635150490264699 [DOI] [PubMed] [Google Scholar]
- Patel RK, Jain M. 2012. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS One 7: doi: 10.1371/journal.pone.0030619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G, Cournac L. 2002. Chlororespiration. Annu Rev Plant Biol. 53:523–550. doi: 10.1146/annurev.arplant.53.100301.135242 [DOI] [PubMed] [Google Scholar]
- Preiss K, Adam IKU, Gebauer G. 2010. Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proc R Soc B-Biol Sci. 277:1333–1336. doi: 10.1098/rspb.2009.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN. 2005. Genera Orchidacearum. Vol. 4. Epidendroideae (Part 1). Oxford, UK: Oxford University Press. [Google Scholar]
- Race HL, Herrmann RG, Martin W. 1999. Why have organelles retained genomes? Trends Genet. 15:364–370. doi: 10.1016/s0168-9525(99)01766-7 [DOI] [PubMed] [Google Scholar]
- Raskoti BB, et al. 2016. A phylogenetic analysis of molecular and morphological characters of Herminium (Orchidaceae, Orchideae): evolutionary relationships, taxonomy, and patterns of character evolution. Cladistics 32:198–210. doi: 10.1111/cla.12125 [DOI] [PubMed] [Google Scholar]
- Ronquist F, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61:539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau-Gueutin M, Ayliffe MA, Timmis JN. 2011. Conservation of plastid sequences in the plant nuclear genome for millions of years facilitates endosymbiotic evolution. Plant Physiol. 157:2181–2193. doi: 10.1104/pp.111.185074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, et al. 2013. Why do mixotrophic plants stay green? A comparison between green and achlorophyllous orchid individuals in situ. Ecol Monogr. 83:95–117. doi: 10.1890/11-2120.1 [Google Scholar]
- Roy M, et al. 2009. Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol. 7:51. doi: 10.1186/1741-7007-7-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikawa R, et al. 2015. Proposal of a twin arginine translocator system-mediated constraint against loss of ATP synthase genes from nonphotosynthetic plastid genomes. Mol Biol Evol. 32:2598–2604. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ. 2003. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19:301–302. doi: 10.1093/bioinformatics/19.2.301 [DOI] [PubMed] [Google Scholar]
- Schelkunov MI, et al. 2015. Exploring the limits for reduction of plastid genomes: a case study of the mycoheterotrophic orchids Epipogium aphyllum and Epipogium roseum. Genome Biol Evol. 7:1179–1191. doi: 10.1093/gbe/evv019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse M-A, Roy M. 2009. Green plants that feed on fungi: facts and questions about mixotrophy. Trends Plant Sci. 14:64–70. doi: 10.1016/j.tplants.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel M, Meyer C, Gebauer G. 2011. The degree of mycoheterotrophic carbon gain in green, variegated and vegetative albino individuals of Cephalanthera damasonium is related to leaf chlorophyll concentrations. New Phytol. 189:790–796. doi: 10.1111/j.1469-8137.2010.03510.x [DOI] [PubMed] [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–180. doi: 10.1111/j.1096-0031.2010.00329.x [DOI] [PubMed] [Google Scholar]
- Wertheim JO, Murrell B, Smith MD, Pond SLK, Scheffler K. 2015. RELAX: Detecting relaxed selection in a phylogenetic framework. Mol Biol Evol. 32:820–832. doi: 10.1093/molbev/msu400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicke S, et al. 2013. Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the Broomrape family. Plant Cell 25:3711–3725. doi: 10.1105/tpc.113.113373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. 2011. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 76:273–297. doi: 10.1007/s11103-011-9762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickett NJ, et al. 2011. Transcriptomes of the parasitic plant family Orobanchaceae reveal surprising conservation of chlorophyll synthesis. Curr Biol. 21:2098–2104. doi: 10.1016/j.cub.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Wolfe KH, Morden CW, Palmer JD. 1992. Function and evolution of a minimal plastid genome from a nonphotosynthetic parasitic plant. Proc Natl Acad Sci U S A. 89:10648–10652. doi: 10.1073/pnas.89.22.10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F-H, et al. 2010. Complete chloroplast genome of Oncidium Gower Ramsey and evaluation of molecular markers for identification and breeding in Oncidiinae. BMC Plant Biol. 10:68. doi: 10.1186/1471-2229-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 20:3252–3255. doi: 10.1093/bioinformatics/bth352 [DOI] [PubMed] [Google Scholar]
- Xiang X-G, et al. 2012. Phylogenetic placement of the enigmatic orchid genera Thaia and Tangtsinia: evidence from molecular and morphological characters. Taxon 61:45–54. [Google Scholar]
- Xiang XG, et al. 2016. Biogeographical diversification of mainland Asian Dendrobium (Orchidaceae) and its implications for the historical dynamics of evergreen broad-leaved forests. J Biogeogr. doi:10.1111/jbi.12726. [Google Scholar]
- Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 24:1586–1591. doi: 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- Yang ZH. 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 13:555–556. [DOI] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. doi: 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer K, Meyer C, Gebauer G. 2008. The ectomycorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytol. 178:395–400. doi: 10.1111/j.1469-8137.2007.02362.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.