Abstract
Purpose of review:
To review the literature and describe techniques to use ultrasound to guide performance of lumbar puncture (LP).
Recent findings:
Ultrasound evaluation of the lumbar spine has been shown in randomized trials to improve LP success rates while reducing the number of attempts and the number of traumatic taps.
Summary:
Ultrasound mapping of the lumbar spine reveals anatomical information that is not obtainable by physical examination, including depth of the ligamentum flavum, width of the interspinous spaces, and spinal bone abnormalities, including scoliosis. Using static ultrasound, the lumbar spine anatomy is visualized in transverse and longitudinal planes and the needle insertion site is marked. Using real-time ultrasound guidance, the needle tip is tracked in a paramedian plane as it traverses toward the ligamentum flavum. Future research should focus on efficient methods to train providers, cost-effectiveness of ultrasound-guided LP, and the role of new needle-tracking technologies to facilitate the procedure.
Point-of-care ultrasound is being increasingly used to guide bedside procedures. For certain bedside procedures, such as central venous catheterization, use of ultrasound guidance is now considered the standard of care. For other procedures, such as lumbar puncture (LP), ultrasound can facilitate performance of the procedure by revealing underlying anatomy. Although many clinicians can perform LP based on landmarks with high success rates, an increasing body of evidence supports the use of ultrasound guidance, particularly in obese patients with less palpable landmarks. A recent study demonstrated a 19% failure rate of LPs performed in a neurology clinic when landmarks alone were used. A strong correlation between body mass index (BMI) and procedure failure was shown with half of the failed LPs in patients with a BMI >35.1 Ultrasound can also reveal other anatomical information, such as depth of the ligamentum flavum and width of the interspinous spaces, which are useful to guide LP.
A training gap of health care providers in ultrasound techniques can partially account for the limited use of ultrasound to guide LPs in current practice. To help bridge this knowledge gap, we review the use of ultrasound guidance for LP, including a synopsis of the medical literature, procedural techniques, and research gaps.
Literature review
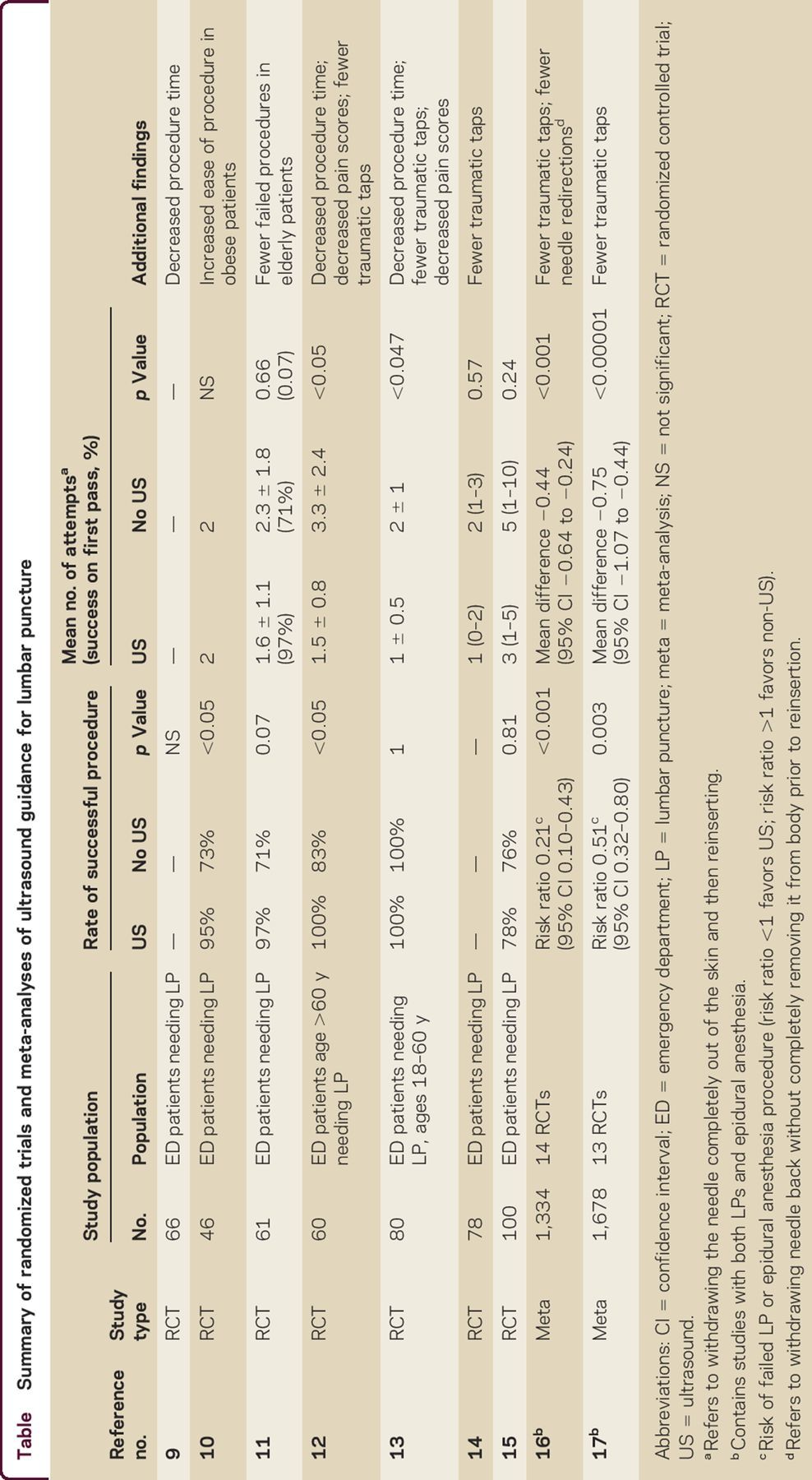
The value of using ultrasound to identify spinal anatomy prior to performance of neuraxial procedures has been recognized since its first description by Russian anesthesiologists in 1971.2 Nearly 30 years passed before Grau et al.3–8 published a series of articles describing the use of ultrasound to guide epidural anesthesia in the early 2000s. At least 20 randomized trials and 2 meta-analyses have since compared landmark-based vs ultrasound-guided techniques for lumbar puncture or epidural/spinal anesthesia. Key findings from these studies9–17 are summarized in the table. A systematic review and meta-analysis of 14 randomized controlled trials evaluated whether ultrasound decreased the risk of failed LPs or epidural catheterizations compared to standard palpation of landmarks. Additionally, the authors addressed whether ultrasound imaging decreased traumatic procedures, insertion attempts, and needle redirections. The 14 studies (5 on LPs and 9 on epidural catheterizations) included 1,334 patients (n = 674 in the ultrasound group and n = 660 in the control group). Ultrasound imaging decreased the risk of failed procedures, even when stratified into subgroups of LP or epidural catheterization. Ultrasound imaging also significantly reduced the risk of traumatic taps, number of insertion attempts, and number of needle redirections.16
Table.
Summary of randomized trials and meta-analyses of ultrasound guidance for lumbar puncture
Ultrasound provides additional clinical information for LP site selection that is not obtainable by physical examination. Ultrasound allows measurement of the distance from the skin to ligamentum flavum, allowing for selection of an appropriate length spinal needle and anticipation of the depth of needle insertion before obtaining CSF. The distance between the skin and ligamentum flavum in nonobese (BMI <25) vs morbidly obese patients (BMI >30) differs by an average of 2 cm (4.4 vs 6.4 cm) when measured using ultrasound.18 Depth of the ligamentum flavum can be accurately measured from either a longitudinal paramedian or transverse midline view, although identification and measurement of the ligamentum flavum may be easier from a longitudinal paramedian view.6,19,20 Most important, ultrasound allows the provider to select the widest interspinous space with the highest likelihood of a successful procedure.
Clinicians who are not radiologists can acquire high-quality images in <1 minute after brief, focused training.21 One training protocol to obtain consistently high-quality images required review of ultrasound anatomy images and 10 practice scans.21 Furthermore, use of ultrasound may improve the learning curve of trainees carrying out neuraxial procedures.22
LP spine mapping has generally shown the greatest benefit in patients with poorly palpable landmarks most commonly due to obesity.10,18,21 Other predictors of difficult LP include abnormal spinal anatomy, such as scoliosis, previous spinal surgery, or history of difficult LP. Ultrasound was superior to a landmark-guided approach in patients with one or more of these predictors undergoing spinal anesthesia.23 Palpation of lumbar spinous processes is inaccurate in both lean and obese patients, and inferior in prediction of lumbar level compared to ultrasound.24–28 It is not clear if routine use of ultrasound guidance in lean patients is superior to landmark-based LP.15,29
As a multidisciplinary panel of procedural experts from different institutions, we concur with a recent recommendation for routine use of ultrasound guidance for patients in whom risk of a failed procedure is high due to poorly palpable landmarks or atypical spinal anatomy.30 We would further advocate for the routine use of ultrasound in all patients because ultrasound reveals anatomical information, such as width of interspinous spaces and depth of ligamentum flavum, not elucidated by physical examination and can guide procedural decision-making.
Although ultrasound is most commonly used to mark the needle insertion site, use of real-time ultrasound guidance to track the needle tip has been described since 2001.31 Real-time ultrasound-guided LP is most often performed using a longitudinal (in-plane), paramedian approach.31–37 One prospective study using a conventional curvilinear transducer and a longitudinal paramedian approach demonstrated a 97% success rate with a median of 3 needle passes (interquartile range 1–6).34 Although small studies describing real-time ultrasound guidance appear promising, lumbar spine mapping to mark the needle insertion site is currently the most common technique with the largest body of supporting evidence.
Lumbar spine anatomy
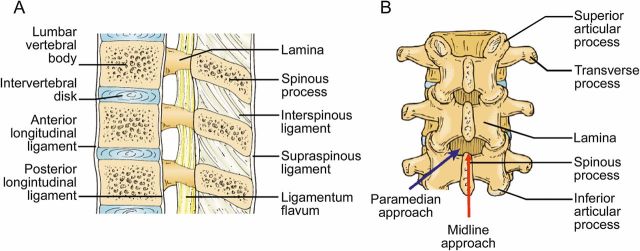
Using ultrasound, the 5 lumbar vertebrae can be sequentially identified and distinguished from the fused sacral bones. The spinal cord ends at L1–L2 in adult patients, and LP can be safely performed below the L2 vertebra. The L2, L3, and L4 spinous processes are the most superficial, and the L2–3 and L3–4 interspinous spaces are typically the widest. The L4–L5 interspinous space is generally narrower and deeper due to lordosis of the spine. The supraspinous ligament connects the tips of the spinous processes in the midline and the interspinous ligaments join the inferior and superior surfaces of adjacent spinous processes (figure 1A). The ligamentum flavum lies deep to the interspinous ligaments, connects the lamina, and lines the spinal canal in the interlaminar space. The posterior longitudinal ligament stretches as a continuous band along the posterior aspect of the vertebral bodies.38 LP is most commonly performed using a midline approach, although a paramedian approach may also be used (figure 1B).
Figure 1. Lumbar spine anatomy.
(A) Ligaments of the lumbar spine: The supraspinous ligament connects the tips of the spinous processes, the interspinous ligament connects the shafts of the spinous processes, and the ligamentum flavum connects the lamina. The posterior longitudinal ligament is a dense band that stretches along the posterior aspect of the vertebral bodies. (B) Midline vs paramedian approach to lumbar puncture: Using a traditional midline approach, the spinal needle is inserted in the narrow space in between spinous processes, while in a paramedian approach, the spinal needle is inserted lateral to the spinous processes and angled toward the center of the spinal canal. (Reprinted with permission from Point-of-Care Ultrasound by Nilam J. Soni, Robert Arntfield, and Pierre Kory, ch. 35, pp. 283–284, Elsevier Health Sciences, 2014).
Ultrasound-guided site marking
The patient can be positioned in an upright or lateral decubitus position to perform an ultrasound-guided LP. LP success rates tend to be higher in an upright position because the interspinous spaces are wider and the vertebral column does not twist or bow as may occur in a lateral decubitus position.39 If the patient is in a lateral decubitus position, ensure the shoulders and hips are perpendicular to the bed surface to avoid twisting of the spine.
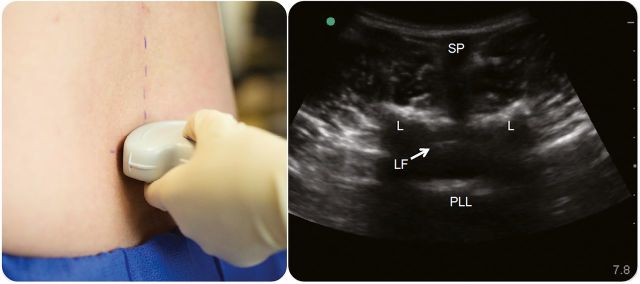
Ultrasound is used to assess the lumbar spine anatomy and identify the needle insertion site with the highest chance of a successful procedure. Techniques to map the lumbar spine have been described in published literature.10,13,38 A high-frequency, linear-array transducer generates high-resolution images, and is preferred in lean patients and for training novice providers. However, a low-frequency, curvilinear transducer is more often used because it provides deeper penetration to visualize the spinal structures in overweight and obese patients. In patients with poor landmarks, identify the sacrum first. Place the transducer in a transverse plane over the sacrum just above the intergluteal cleft. The sacrum has a distinct hyperechoic, serrated appearance due to its fused bones. Visualization of the sacrum orients the provider to then identify the lumbar spinous processes sequentially by sliding the transducer superiorly. Spinous processes appear as small, hyperechoic, superficial bone tips that cast a distinct vertical shadow that is readily recognized in the center of the screen (figure 2). The L5 spinous process is usually relatively deep with more overlying soft tissue compared to L4. Sliding the transducer superiorly allows sequential identification of L4, L3, and L2. Center the transducer exactly over each spinous process and make a mark perpendicular to the transducer denoting the midline of the spine.
Figure 2. Transverse midline view.
A lumbar spinous process is centered on the screen with the transducer in a transverse plane, and a mark is made perpendicular to the transducer. Sliding the transducer along the midline allows visualization of the spinous processes (SP), lamina (L), posterior longitudinal ligament (PLL), and ligamentum flavum (LF).
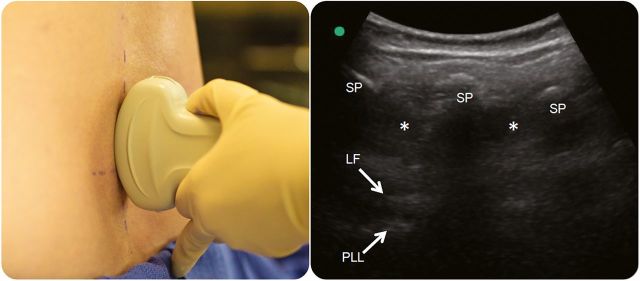
With the transducer over the L3 or L4 vertebra, rotate the transducer 90° clockwise to align the ultrasound beam longitudinally over the midline. The spinous processes in this orientation will appear rectangular, or as tombstones, with a shadow deep to the spinous processes (figure 3). Slide the transducer along the midline of the spine to identify the widest interspinous space. Center the transducer over the widest interspinous space in a longitudinal view and make a line perpendicular to the transducer. The intersection of the 2 markings of the spinal midline and interspinous space is the entry point for the spinal needle. If all the lumbar interspinous spaces are narrow, consideration should be given to using a paramedian approach or performing the procedure under fluoroscopic guidance.
Figure 3. Longitudinal midline view.
The transducer is centered over a lumbar interspinous space in a longitudinal plane, and a mark is made perpendicular to the center of the transducer. The spinous processes (SP) and interspinous spaces (*) are visualized in a longitudinal plane, and the ligamentum flavum (LF) and posterior longitudinal ligament (PLL) are visualized deep to the spinous processes.
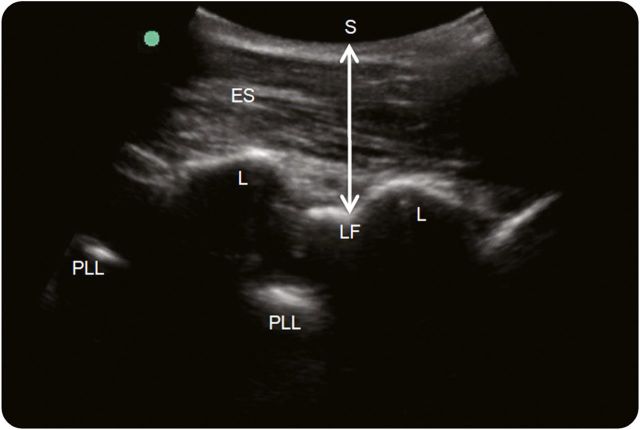
An alternative approach is to map the lumbar spine in a paramedian plane using the lamina to mark each lumbar vertebra (figure 4). With the transducer in a longitudinal plane over the sacrum, slide the transducer cephalad to identify the L5-sacrum interspinous space, which has a distinct appearance. The linear sacrum deepens as it approaches L5, and the L5 lamina appears as a sharp tooth or sail-like structure above the interlaminar space. After identifying the L5-S1 level, slide the transducer cephalad, and mark the lumbar level with the widest interlaminar space. Next, rotate the transducer 90° counterclockwise into a transverse plane, slide the transducer medially, center it over the spinous processes, and mark the midline of the spine. The intersection of the 2 lines denotes the needle insertion site.
Figure 4. Paramedian view.
With the transducer oriented longitudinally on one side of the midline, the erector spinae (ES) muscles are seen superficial to the lamina (L). From a paramedian view, the ligamentum flavum (LF) is easily visualized, and the skin (S)–LF distance (double-headed arrow) can be measured. The posterior longitudinal ligament (PLL) is seen deep to the ligamentum flavum.
Measuring the skin–ligamentum flavum distance guides selection of an appropriate length spinal needle, since the posterior dura is only a few millimeters deep to the ligamentum flavum. The skin–ligamentum flavum distance is best measured from a paramedian view. The ligamentum flavum appears as a hyperechoic horizontal line just deep to the lamina or articular processes (figure 4). The fluid-filled dural sac appears black (anechoic) in between the ligamentum flavum and posterior longitudinal ligament. The lamina can be mistaken for spinous processes in a paramedian view but there are 2 methods to avoid this pitfall. First, the transducer can be tilted in a longitudinal plane to differentiate the lamina from the spinous processes. Second, if muscle fibers of the erector spinae are seen superficial to the bony prominences, then the bony prominences are lamina because only skin and subcutaneous tissue are seen superficial to spinous processes. The patient should remain in the same position after marking the needle insertion site because changes in position may offset the lumbar spine markings. Selection of an appropriate length 20–22 gauge, atraumatic spinal needle can reduce the risk of a post–LP headache.40,41 As the spinal needle is inserted through the skin, resistance should be encountered as the needle traverses the dense supraspinous and interspinous ligaments. Despite careful site marking, minor needle redirections are often needed to advance the spinal needle into the spinal canal. Once the first pop or release of resistance is felt, the needle should be advanced 1–2 mm at a time until it enters the subarachnoid space and CSF is obtained.
Real-time ultrasound guidance
Real-time ultrasound guidance allows the provider to track the needle tip as it enters the ligamentum flavum. Both ultrasound-guided site marking (as described above) and real-time ultrasound guidance have been shown to have higher success rates than landmark-based techniques.
Real-time ultrasound guidance is performed using a paramedian approach.38 The ultrasound transducer must be placed in a sterile plastic sheath when using real-time guidance, and sterile gel is used. After centering the transducer over the widest lumbar interspinous space, rotate the transducer 45° toward the midline into an oblique paramedian view. The transducer is aligned in a plane from the spinous process of the superior vertebra to the lamina of the inferior vertebra. The lamina, ligamentum flavum, spinal canal, and posterior longitudinal ligament are visualized. Slide the transducer in the same plane 1–2 cm craniomedially to ease insertion of the needle underneath the transducer. The spinal needle is inserted in the plane of the ultrasound beam (figure 5). As the needle is advanced through the interlaminar space, resistance will increase, indicating the needle is traversing the ligamentum flavum. Once the ligamentum flavum has been penetrated, a loss of resistance is felt and the provider should begin to sequentially check for CSF while advancing the needle 1–2 mm at a time. The needle tip is usually not visualized as it penetrates the ligamentum flavum due to shadowing from the superior spinous process.
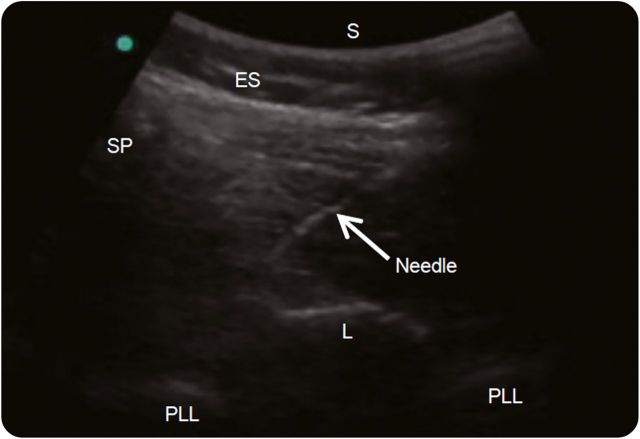
Figure 5. Real-time ultrasound guidance.
From an oblique paramedian view of lumbar spine, a spinal needle is inserted using an in-plane technique toward the lamina-ligamentum flavum junction. The transducer is oriented obliquely from the spinous process (SP) of the superior vertebra to the lamina (L) of the inferior vertebra. The needle is inserted underneath the transducer in a lateral to medial direction. ES = erector spinae muscles; PLL = posterior longitudinal ligament; S = skin.
Areas of uncertainty
Several randomized trials of unselected patients have demonstrated improved success rates of LP with ultrasound guidance, but it remains unclear whether routine use of ultrasound guidance for all patients is beneficial.15 Success of LP depends on patient factors (body habitus, spinal anatomy, previous spinal surgery, positioning, cooperation), operator factors (LP skills, ultrasound skills, experience), and system factors (ultrasound equipment, equipment availability, procedure supplies). Although randomized trials may control for most patient and system factors, the operator's ultrasound skill is an important and underappreciated potential source of bias in current studies. When comparing ultrasound vs landmark-based techniques, inexperienced operators may not use ultrasound accurately to map the lumbar spine, resulting in negative studies. A recent randomized trial comparing routine use of ultrasound guidance vs landmark-based techniques for LP did not demonstrate a benefit of using ultrasound; however, trainees were the primary operators and inexperience combined with convenience sampling may explain the negative results.15 On the contrary, studies with highly experienced operators may exaggerate benefits of ultrasound by demonstrating perfect success rates with only a small number of procedures.29 Therefore, a multicenter comparative trial using a standard protocol for routine use of ultrasound-guided vs landmark-based LP is needed.
Most published trials have compared ultrasound-guided site marking vs landmark-based site selection, and few studies have used real-time ultrasound guidance. No published trials have directly compared use of real-time ultrasound guidance vs ultrasound-guided site marking, and it is unknown whether real-time ultrasound guidance confers any additional benefit.
Training in ultrasound-guided LP, both site marking and real-time guidance, is another area of uncertainty. Simulation-based practice has been shown to improve acquisition of LP skills42 but how simulation-based practice affects performance of LP on actual patients in clinical settings is unknown. Further, no externally validated simulation-based training curriculum to teach ultrasound-guided LP skills has been published.
Future studies should address the effect of ultrasound on clinical decision-making and cost-effectiveness. Ultrasound can guide clinical decision-making by allowing the operator to select the interspinous space with the highest chance of a successful LP, or expedite referral for a fluoroscopically guided LP, if all interspinous spaces appear too narrow. By improving LP success rates, reducing needle redirections, and expediting care, ultrasound guidance may reduce health care costs and improve patient experience, although studies demonstrating this have yet to be conducted. Finally, the effect of ultrasound on common side effects, such as post-LP headache, and more serious adverse events, such as epidural bleeding, have yet to be investigated.
CONCLUSIONS
Ultrasound guidance for LP improves success rates, decreases needle redirections and traumatic taps, and can decrease total procedure time. When static ultrasound guidance is used, visualization of the spinous processes is the key element to mark the needle insertion site. When real-time ultrasound guidance is used, the needle is inserted under direct visualization using a paramedian approach. Future clinical studies should compare outcomes of site marking using static ultrasound vs real-time ultrasound guidance, as well as the effect of using ultrasound guidance on health services outcomes, including patient experience.
Take-home points
Ultrasound imaging of the lumbar spine improves success rates of LP, particularly in obese patients with less palpable landmarks.
Ultrasound reveals anatomic information that is not obtainable by physical examination, such as measurement of the depth of the ligamentum flavum, selection of the widest interspinous space, and detection of spinal abnormalities.
The most common technique of ultrasound guidance for LP is marking the needle insertion site using static ultrasound.
ACKNOWLEDGMENT
The authors thank Elizabeth Suelzer at the Medical College of Wisconsin libraries for assistance with the literature review.
AUTHOR CONTRIBUTIONS
N.J. Soni participated in the study conception and design, analysis and interpretation of the data, and drafting the manuscript for intellectual content. R. Franco-Sadud participated in the study conception and design, interpretation of data, and drafting the manuscript. D. Schnobrich participated in analysis and interpretation of the data and drafting the manuscript. R. Dancel participated in drafting the manuscript and figures. D.M. Tierney participated in analysis and interpretation of the data and drafting the manuscript. G. Salame participated in drafting the manuscript. M.I. Restrepo participated in analysis and interpretation of the data and drafting the manuscript. P. McHardy participated in drafting the manuscript and figures.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the NIH.
STUDY FUNDING
Partially supported by award K23HL096054 (MIR) from the National Heart, Lung, and Blood Institute.
DISCLOSURES
N.J. Soni receives publishing royalties for Point-of-Care Ultrasound, 1st ed. (Elsevier-Saunders, 2014). R. Franco-Sadud, D. Schnobrich, R. Dancel, D.M. Tierney, and G. Salame report no disclosures. M.I. Restrepo reports partial support of his time from award K23HL096054 from the National Heart, Lung, and Blood Institute. P. McHardy reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Edwards C, Leira EC, Gonzalez-Alegre P. Residency training: a failed lumbar puncture is more about obesity than lack of ability. Neurology 2015;84:e69–e72. [DOI] [PubMed] [Google Scholar]
- 2.Bogin IN, Stulin ID. Application of the method of 2-dimensional echospondylography for determining landmarks in lumbar punctures [in Russian]. Zh Nevropatol Psikhiatr Ime S S Korsakova 1971;71:1810–1811. [PubMed] [Google Scholar]
- 3.Grau T, Leipold R, Conradi R, Martin E, Motsch J. Ultrasonography and peridural anesthesia: technical possibilities and limitations of ultrasonic examination of the epidural space [in German]. Anaesthesist 2001;50:94–101. [DOI] [PubMed] [Google Scholar]
- 4.Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand 2001;45:766–771. [DOI] [PubMed] [Google Scholar]
- 5.Grau T, Leipold RW, Horter J, Conradi R, Martin E, Motsch J. The lumbar epidural space in pregnancy: visualization by ultrasonography. Br J Anaesth 2001;86:798–804. [DOI] [PubMed] [Google Scholar]
- 6.Grau T, Leipold RW, Horter J, Conradi R, Martin EO, Motsch J. Paramedian access to the epidural space: the optimum window for ultrasound imaging. J Clin Anesth 2001;13:213–217. [DOI] [PubMed] [Google Scholar]
- 7.Grau T, Leipold RW, Conradi R, Martin E, Motsch J. The visualisation of dura perforation and blood patches with ultrasound [in German]. Anasthesiol Intensivmed Notfallmed Schmerzther 2002;37:149–153. [DOI] [PubMed] [Google Scholar]
- 8.Grau T, Conradi R, Martin E, Motsch J. Ultrasound and local anaesthesia: part III: ultrasound and neuroaxial local anaesthesia [in German]. Anaesthesist 2003;52:68–73. [DOI] [PubMed] [Google Scholar]
- 9.Pisupati D, Heyming TW, Lewis RJ, Peterson MA. Effect of ultrasonography localization of spinal landmarks on lumbar puncture in the emergency department. Ann Emerg Med 2004;44:S83. [Google Scholar]
- 10.Nomura JT, Leech SJ, Shenbagamurthi S, et al. . A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med 2007;26:1341–1348. [DOI] [PubMed] [Google Scholar]
- 11.Lee WS, Jeong WJ, Yi HY, Ryu S, Lee JW, Kim SW. The usefulness of ultrasound-assisted lumbar puncture on adult patients in the emergency center: comparison with classic lumbar puncture. J Korean Soc Emerg Med 2008;19:562. [Google Scholar]
- 12.Cho YC, Koo DH, Oh SK, Jeong WJ, Lee WS, You YH. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med 2009;20:304. [Google Scholar]
- 13.Mofidi M, Mohammadi M, Saidi H, et al. . Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci 2013;18:303–307. [PMC free article] [PubMed] [Google Scholar]
- 14.Fox JC, Kinney A, Youssefian A, et al. . Success of lumbar puncture after using ultrasound to identify landmarks. Acad Emerg Med 2013;20:S11. [Google Scholar]
- 15.Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med 2014;21:130–136. [DOI] [PubMed] [Google Scholar]
- 16.Shaikh F, Brzezinski J, Alexander S, et al. . Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ 2013;346:f1720. [DOI] [PubMed] [Google Scholar]
- 17.Perlas A. Evidence for the use of ultrasound in neuraxial blocks. Reg Anesth Pain Med 2010;35:S43–S46. [DOI] [PubMed] [Google Scholar]
- 18.Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med 2007;25:331–334. [DOI] [PubMed] [Google Scholar]
- 19.Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg 2013;116:829–835. [DOI] [PubMed] [Google Scholar]
- 20.Gnaho A, Nguyen V, Villevielle T, Frota M, Marret E, Gentili ME. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol 2012;62:520–530. [DOI] [PubMed] [Google Scholar]
- 21.Ferre RM, Sweeney TW. Emergency physicians can easily obtain ultrasound images of anatomical landmarks relevant to lumbar puncture. Am J Emerg Med 2007;25:291–296. [DOI] [PubMed] [Google Scholar]
- 22.Grau T, Bartusseck E, Conradi R, Martin E, Motsch J. Ultrasound imaging improves learning curves in obstetric epidural anesthesia: a preliminary study. Can J Anaesth 2003;50:1047–1050. [DOI] [PubMed] [Google Scholar]
- 23.Chin KJ, Perlas A, Chan V, Brown-Shreves D, Koshkin A, Vaishnav V. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology 2011;115:94–101. [DOI] [PubMed] [Google Scholar]
- 24.Furness G, Reilly MP, Kuchi S. An evaluation of ultrasound imaging for identification of lumbar intervertebral level. Anaesthesia 2002;57:277–280. [DOI] [PubMed] [Google Scholar]
- 25.Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg 2008;106:538–540. [DOI] [PubMed] [Google Scholar]
- 26.Amin WA, Abou Seada MO, Bedair E, Elkersh MM, Karunakaran E. Comparative study between ultrasound determination and clinical assessment of the lumbar interspinous level for spinal anesthesia. Middle East J Anaesthesiol 2014;22:407–412. [PubMed] [Google Scholar]
- 27.Duniec L, Nowakowski P, Kosson D, Üazowski T. Use of ultrasound to determine the level of lumbar puncture in orthopaedic patients. Eur J Anaesthesiol 2012;29:119. [Google Scholar]
- 28.Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol 2010;60:13–19. [DOI] [PubMed] [Google Scholar]
- 29.Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth 2014;23:213–216. [DOI] [PubMed] [Google Scholar]
- 30.Rizzoli P. Taking the sting out of lumbar puncture. BMJ 2013;346:f1734. [DOI] [PubMed] [Google Scholar]
- 31.Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol 2004;21:25–31. [DOI] [PubMed] [Google Scholar]
- 32.Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS: a case series. Can J Anaesth 2013;60:896–901. [DOI] [PubMed] [Google Scholar]
- 33.Chin KJ, Chan VW, Ramlogan R, Perlas A. Real-time ultrasound-guided spinal anesthesia in patients with a challenging spinal anatomy: two case reports. Acta Anaesthesiol Scand 2010;54:252–255. [DOI] [PubMed] [Google Scholar]
- 34.Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract 2013;2013:525818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand 2014;58:875–881. [DOI] [PubMed] [Google Scholar]
- 36.Tran D, Kamani AA, Al-Attas E, Lessoway VA, Massey S, Rohling RN. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth 2010;57:313–321. [DOI] [PubMed] [Google Scholar]
- 37.Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS(R) needle tracking system: a case report. Can J Anaesth 2013;60:50–53. [DOI] [PubMed] [Google Scholar]
- 38.Soni N, Arntfield R, Kory P. Point-of-Care Ultrasound. 1st ed Philadelphia: Saunders; 2014. [Google Scholar]
- 39.Sandoval M, Shestak W, Sturmann K, Hsu C. Optimal patient position for lumbar puncture, measured by ultrasonography. Emerg Radiol 2004;10:179–181. [DOI] [PubMed] [Google Scholar]
- 40.Bradbury CL, Singh SI, Badder SR, Wakely LJ, Jones PM. Prevention of post-dural puncture headache in parturients: a systematic review and meta-analysis. Acta Anaesthesiol Scand 2013;57:417–430. [DOI] [PubMed] [Google Scholar]
- 41.Arendt K, Demaerschalk BM, Wingerchuk DM, Camann W. Atraumatic lumbar puncture needles: after all these years, are we still missing the point? Neurologist 2009;15:17–20. [DOI] [PubMed] [Google Scholar]
- 42.Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents' lumbar puncture skills. Neurology 2012;79:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]