Letter to the Editor
Ibrutinib (PCI-32765) is an irreversible BTK (Bruton’s tyrosine kinase) kinase inhibitor that has been extensively used as a tool compound to validate the role of BTK kinase in B-Cell related malignances.1, 2 Ibrutinib has been shown in preclinical studies to inhibit the proliferation of Diffuse Large B-Cell Lymphoma (DLBCL) cells, Mantle Cell Lymphoma (MCL) cells, Chronic Lymphocytic Leukemia (CLL) cells and Multiple Myeloma (MM) cells by blocking BTK kinase activity; ibrutinib was recently approved for the clinical application on MCL and CLL.3, 4, 5, 6, 7 Ibrutinib has also exhibited anti-inflammatory effects in preclinical models.8, 9 Recently, it has been reported that ibrutinib is also effective against EGFR mutant-positive non-small cell lung cancers through inhibition of EGFR kinase activities.10 In addition, there is evidence showing that BTK is also an important target for Acute Myeloid Leukemia (AML).11
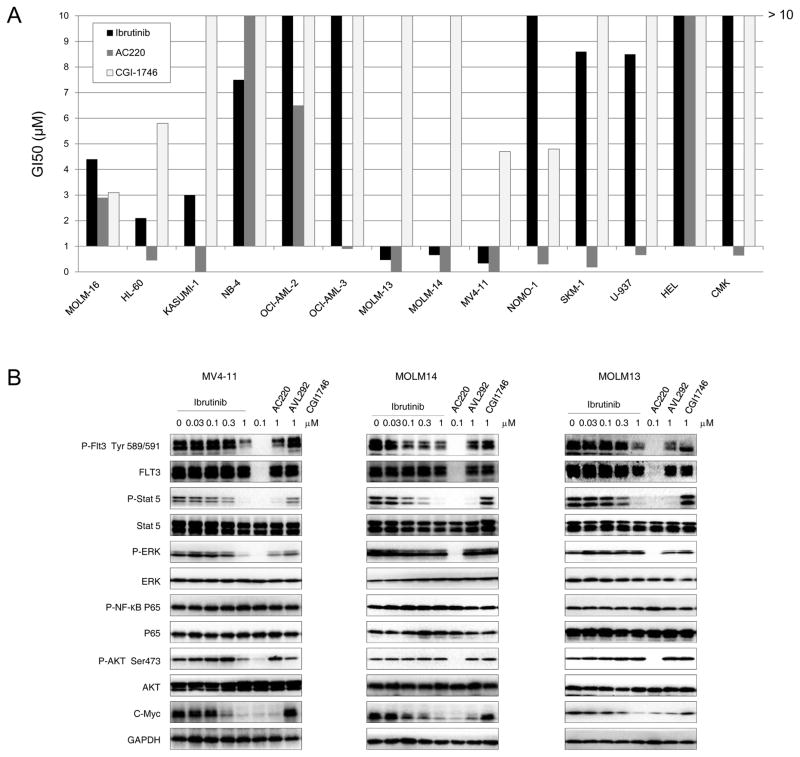
Despite the evidence that BTK knock down impaired AML cancer cell growth, which suggested that BTK was important for AML cell proliferation, BTK kinase inhibition through use of a small molecule inhibitor like ibrutinib led only to moderate inhibition of proliferation of U937 cells with no apparent activity against other AML cell lines such as HL60, TF-1 and THP-1.11 To further investigate the potency and activity of ibrutinib against AML, we screened a panel of AML cell lines spanning M0–M7 disease stages. Interestingly, we found that only FLT3-ITD mutant AML cell lines (MOLM13, MOLM14 and MV4-11) were sensitive to ibrutinib. (Fig. 1A and Supplemental Table 1) This is similar to what has been observed with the highly potent and selective FLT3 inhibitor, AC220, but differs from that observed for the targeted BTK kinase inhibitor, CGI-1746.12, 13 The selective anti-proliferative activity was further confirmed in the clonogenic assay (GI50: 170 nM – 478 nM). (Supplemental Fig. 1) This suggests that ibrutinib might target FLT3 kinase in addition to BTK kinase.
Fig. 1.
Ibrutinib effect on AML cell proliferation and FLT3-mediated signaling.
(A) Ibrutinib inhibits proliferation of AML cell lines. (B) Ibrutinib inhibits FLT3-ITD mediated signaling in MV4-11, MOLM14 and MOLM13 cell lines.
We then investigated the effect of ibrutinib on FLT3-ITD mediated signaling in drug-sensitive cell lines. (Fig. 1B) Ibrutinib potently inhibited FLT3-ITD auto-phosphorylation (EC50: 318 nM, 39 nM and 356 nM respectively for MOLM13, MOLM14 and MV4-11). (Supplemental Fig. 2A) Phosphorylation of STAT5, a well-established downstream target, also was significantly inhibited with an EC50 between 100 nM and 200 nM. (Supplemental Fig. 2B) In addition, c-Myc expression was inhibited with an EC50 between 185 nM and 315 nM. (Supplemental Fig. 2C) However, in FLT3 low expression cell lines such as OCI-AML3 and NOMO-1 cells, the related signaling was not affected. (Supplemental Fig. 2D, E). The FLT3 inhibitor AC220 demonstrated similar inhibitory activity to ibrutinib in FLT3-mediated signaling pathways in the FLT3-ITD positive cell lines. However, the BTK inhibitors, CGI-1746 and AVL-292, did not exhibit any effect on the signaling.14 (Fig. 2B and supplemental Fig. 2D, E)
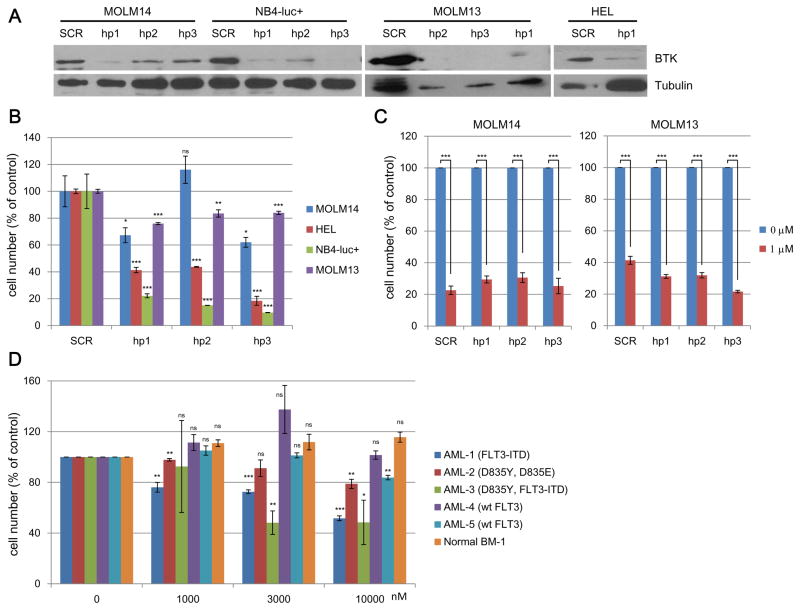
Figure 2. BTK-independent inhibition of proliferation of mutant FLT3-positive AML cells.
(A) BTK knockdown in MOLM14 (FLT3-ITD-dependent), MOLM13 (FLT3-ITD-dependent), NB4-luc+ (BTK-dependent, FLT3 wt) and HEL (mutant Jak2-dependent, FLT3 wt): validation of BTK knockdown efficiency. (B) Effect of BTK knockdown on growth of wt FLT3-expressing HEL and NB4-luc+ cells versus FLT3-ITD-expressing MOLM13-luc+ and MOLM14. (C) Effects of ibrutinib on treatment of MOLM13-luc+ and MOLM14 cells with normal BTK expression (scrambled controls) and reduced BTK expression (due to BTK knockdown). (D) Ibrutinib anti-proliferation effect on FLT3 wt and FLT3-mtuant patient primary cells (P values were calculated using GraphPad software with normalized raw data from CellTiter-Glo experiments done in qudruplicates.(*, P value ≤ 0.05; **, P value ≤ 0.01; ***, P value ≤ 0.001)
As all of these cell lines express BTK kinase, we then studied the effect of ibrutinib on BTK signaling. We found that only in the FLT3 wild-type (wt)-expressing cell lines (NOMO-1 and OCI-AML3), downstream mediators of BTK kinase were affected despite BTK phosphorylation having been inhibited in both FLT3 wt and FLT3-ITD cell lines. This suggests that FLT3-ITD mutant cell lines might not rely on BTK signaling for growth. (Supplemental Fig. 3) Again, AC220 did not affect BTK kinase activity and its direct downstream target PLCγ but instead inhibited phosphorylation of AKT and ERK. The BTK inhibitors, CGI-1746 and AVL292, were effective against BTK kinase and PLCγ kinase phosphorylation, but had no effect on the phosphorylation of AKT and ERK. These results suggest that ibrutinib may exert its inhibitory activity through FLT3 kinase but not BTK kinase in the FLT3-ITD positive cells. To further confirm this, we knocked down BTK kinase in MOLM14 (FLT3-ITD), MOLM13 (FLT3-ITD), HEL (FLT3 wt) and NB4 (FLT3 wt) cells and found that the growth of MOLM14 and MOLM13 was only minimally affected; however NB4 cells were significantly inhibited and HEL cells were moderately inhibited. (Fig. 2 A, B) The fact that BTK knock down in HEL cells did not lead to suppressed growth may be due to the fact that mutant JAK2 is the oncogenic driver in this cell line.15 In addition, the finding that ibrutinib still significantly blocked the growth of BTK-KD MOLM14 and MOLM13 cells further validated that the growth inhibitory effect of ibrutinib was likely independent of BTK kinase. (Fig. 2 C)
Previous kinase selectivity profiling revealed that FLT3 wt might be an additional target of ibrutinib.9 The biochemical ADP-Glo assay confirmed that ibrutinib has an IC50 of 205.8 nM/65.65 nM against FLT3 wt and FLT3-ITD, respectively. (Supplemental Fig. 4A) In the FLT3-ITD-dependent isogenic BaF3 cell line, ibrutinib demonstrated a GI50 of 120 nM; this inhibitory effect could be completely rescued by IL-3 treatment. (Supplemental Fig. 4B) Interestingly, it also potently inhibited the FLT3-D835H-BaF3 isogenic cell (GI50: 0.063 μM) and moderately inhibited FLT3-D835Y-BaF3 as well as TEL-FLT3-wt-BaF3 isogenic cells (GI50: 0.5 μM/0.46 μM), however was less active against ITD-D835Y, ITD-F691L and FLT3-K663Q mutations. (Supplemental Fig. 5A) In addition, ibrutinib did not affect the growth of other oncogene-expressing Ba/F3 cells, such as c-Kit, PDGFRα, BCR-ABL and NRAS. (Supplemental Fig. 5B) Although there are cysteine residues in the ATP binding pocket, binding mode examination with molecular modeling demonstrates that unlike the mode of inhibition against BTK kinase, ibrutinib inhibits FLT3 kinase through reversible binding and this is further confirmed by the washing-out experiment. (Supplemental Fig. 4 C, D)
Ibrutinib induced apoptotic cell death in a concentration- and time-dependent manner in FLT3-ITD cell lines but not FLT3 wt-expressing cell lines. (Supplemental Fig. 6) Cell cycle analysis revealed that ibrutinib, but not the highly selective BTK kinase inhibitor, CGI-1746, could- induce a concentration-dependent manner- arrest cells in G0/G1 phase. (Supplemental Fig. 7) In addition, ibrutinib exhibited dose-dependent anti-proliferation activity against FLT3-ITD mutant-expressing primary patient cells but not wt-expressing samples. (Fig. 2D)
AML still remains a serious unmet medical need and the FLT3-ITD mutant has been found in approximately 30% of AML patients. Besides standard chemotherapy, there is no targeted therapy approved in the clinic to date despite several inhibitors that are presently under clinical investigation.16 Currently, ibrutinib is under extensive clinical investigation against a variety of different B-Cell malignancies. Given the fact that the safety profile of ibrutinib is tolerated in the patients, our results might help to expand the application of this drug to AML patients harboring the FLT3-ITD mutant.
Interestingly, despite the fact that the effective concentration of ibrutinib in the in vitro studies in the FLT3-ITD positive cell lines falls within the range of the peak plasma concentration used in human clinical trials (around 150 ng/mL at 560mg/day dosage), the concentration required for the induction of apoptosis and cell cycle arrest for ibrutinib is higher, which suggests that an alternative formulation might be needed for the potential clinical application. 17
Supplementary Material
Acknowledgments
J. Liu, Q. Liu and W. Wang are supported by the grant of “Cross-disciplinary Collaborative Teams Program for Science, Technology and Innovation (2014–2016)” from Chinese Academy of Sciences. Z. Zhao is supported by Anhui Province Natural Science Foundation Annual Key Program (grant number: 1301023011). We want to thank China “Thousand Talents Program” support for Prof. Q. Liu and “Hundred Talents Program” of The Chinese Academy of Sciences support for Prof. J. Liu, and W. Wang.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at Leukemia’s website
References
- 1.Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. Chem Med Chem. 2007 Jan;2(1):58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 2.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014 Apr;14(4):219–232. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 3.Woyach JA, Bojnik E, Ruppert AS, Stefanovski MR, Goettl VM, Smucker KA, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL) Blood. 2014 Feb 20;123(8):1207–1213. doi: 10.1182/blood-2013-07-515361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012 Mar 15;119(11):2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 5.Tai YT, Chang BY, Kong SY, Fulciniti M, Yang G, Calle Y, et al. Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma. Blood. 2012 Aug 30;120(9):1877–1964. doi: 10.1182/blood-2011-12-396853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013 Aug 8;369(6):507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013 Jul 4;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, et al. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther. 2011;13(4):R115. doi: 10.1186/ar3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao W, Wang M, Wang L, Lu H, Wu S, Dai B, et al. Selective antitumor activity of ibrutinib in EGFR-mutant non-small cell lung cancer cells. J Natl Cancer Inst. 2014 Sep;106(9) doi: 10.1093/jnci/dju204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rushworth SA, Murray MY, Zaitseva L, Bowles KM, MacEwan DJ. Identification of Bruton’s tyrosine kinase as a therapeutic target in acute myeloid leukemia. Blood. 2014 Feb 20;123(8):1229–1238. doi: 10.1182/blood-2013-06-511154. [DOI] [PubMed] [Google Scholar]
- 12.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009 Oct 1;114(14):2984–3076. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Paolo JA, Huang T, Balazs M, Barbosa J, Barck KH, Bravo BJ, et al. Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat Chem Biol. 2011 Jan;7(1):41–50. doi: 10.1038/nchembio.481. [DOI] [PubMed] [Google Scholar]
- 14.Evans EK, Tester R, Aslanian S, Karp R, Sheets M, Labenski MT, et al. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. J Pharmacol Exp Ther. 2013 Aug;346(2):219–228. doi: 10.1124/jpet.113.203489. [DOI] [PubMed] [Google Scholar]
- 15.Quentmeier H, MacLeod RA, Zaborski M, Drexler HG. JAK2 V617F tyrosine kinase mutation in cell lines derived from myeloproliferative disorders. Leukemia. 2006 Mar;20(3):471–477. doi: 10.1038/sj.leu.2404081. [DOI] [PubMed] [Google Scholar]
- 16.Kayser S, Levis MJ. FLT3 tyrosine kinase inhibitors in acute myeloid leukemia: clinical implications and limitations. Leuk Lymphoma. 2014 Feb;55(2):243–298. doi: 10.3109/10428194.2013.800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Has Significant Activity in Patients With Relapsed/Refractory B-Cell Malignancies. J Clin Oncol. 2013 Jan 1;31(1):88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.