Abstract
The prevalent human papillomaviruses (HPVs) infect human epithelial tissues. Infections by the mucosotropic HPV genotypes cause hyperproliferative ano-genital lesions. Persistent infections by high-risk (HR) HPVs such as HPV-16, HPV-18 and related types can progress to high grade intraepithelial neoplasias and cancers. Prophylactic HPV vaccines are based on DNA-free virus-like particles (VLPs) composed of the major capsid protein L1 of HPV-16, -18, -6 and -11 (Gardasil) or HPV-16 and -18 (Cervarix). Sera from vaccinated animals effectively prevent HPV pseudovirions to infect cell lines and mouse cervical epithelia. Both vaccines have proven to be highly protective in people. HPV pseudovirions are assembled in HEK293TT cells from matched L1 and L2 capsid proteins to encapsidate a reporter gene. Pseudovirions and genuine virions have structural differences and they infect cell lines or primary human keratinocytes (PHKs) with different efficiencies. In this study, we show that sera and isolated IgG from women immunized with Gardasil prevent authentic HPV-18 virions from infecting PHKs, whereas non-immune sera and purified IgG thereof are uniformly ineffective. Using early passage PHKs, neutralization is achieved only if immune sera are added within 2 to 4 h of infection. We attribute the timing effect to a conformational change in HPV virions, thought to occur upon initial binding to heparan sulfate proteoglycans (HSPG) on the cell surface. This interpretation is consistent with the inability of immune IgG bound to or taken up by PHKs to neutralize the virus. Interestingly, the window of neutralization increases to 12 to 16 h in slow growing, late passage PHKs, suggestive of altered cell surface molecules. In vivo, this window might be further lengthened by the time required to activate the normally quiescent basal cells to become susceptible to infection. Our observations help explain the high efficacy of HPV vaccines.
Keywords: HPV vaccine, HPV antibodies, HPV-18 neutralization, Primary human keratinocytes, FcRn
1. Introduction
The ubiquitous human papillomaviruses (HPVs) infect either mucosal or cutaneous epithelia [1, 2]. The mucosotropic HPVs are the most common sexually transmitted viral pathogens. The low-risk (LR) HPV types -6 and -11 cause 90% of ano-genital warts and all recurrent respiratory papillomatosis but only rarely initiate neoplastic changes. In contrast, the high-risk (HR) HPV types, notably HPV-16 and HPV-18 and closely related genotypes, are the causative agents of cervical cancers and most other HPV-associated ano-genital carcinomas as well as 20-25% of head and neck cancers [3]. Cervical carcinoma is the fourth leading cause of cancer death in women globally and accounts for 10-15% of cancer deaths [4, 5].
HPVs are non-enveloped small DNA viruses. The viral capsid is comprised of the major late protein L1 and the minor protein L2. HPVs establish infection in the basal stratum of squamous epithelia whereas viral DNA amplification, capsid protein expression and virion assembly take place sequentially in the differentiated keratinocytes of the mid and upper strata. Mature virions are shed as the cornified envelopes are sloughed off [6]. Organotypic raft cultures of primary human foreskin keratinocytes (PHKs) developed at the liquid medium/air interface generate differentiated squamous epithelia that closely resemble native tissues, and they support the productive viral program [7, 8]. The HPV particles generated can elicit a new round of productive infection in PHK raft cultures [9].
Two available HPV vaccines, Gardasil (Merck), directed against HPV-6, -11, -16, and -18, and Cervarix (GlaxoSmithKline), targeting HPV-16 and -18, provide highly efficacious protection of uninfected women and men for at least a decade [10, 11]. The vaccines are based on virus-like particles (VLPs) assembled exclusively from the major capsid protein L1. The FDA recently approved Gardasil-9, which protects against infections by 5 additional HR genotypes, HPV-31, -33, -45, -52 and -58 [12].
Pseudovirions harbor a reporter gene encapsidated in matched L1 and L2 proteins of various HPV genotypes [13]. The particles are assembled in HEK 293TT cells and have been used to study the HPV infection process and to characterize neutralizing antibodies elicited by the vaccines. However, pseudovirions have structural differences from authentic virions and require a maturation process in vitro to stabilize the particles [14]. Pseudovirions are infectious in cell lines but they have low infectivity in PHKs, the natural host for HPVs. The differential infectivity has been attributed to variations in the modifications of heparan sulfate proteoglycans (HSPG) on the cell surface [15]. In contrast, authentic HPV particles infect PHKs at a multiplicity of infection (MOI) as low as 2 and initiate early gene expression [9].
Plasma and external secretions contain virus-neutralizing antibodies as a consequence of infection or immunizations. Neutralizing antibodies in their free form bind to relevant epitopes and inhibit the attachment of viruses to cellular receptors. Several laboratories have reported that the ability of antibodies to neutralize certain viruses can be extended to intracellular interactions. Internalized antibodies of IgA or IgG isotypes effectively interfere with the replication of these viruses [16-19] due to the fact that mucosal epithelial cells express receptors specific for immunoglobulins (Ig), which mediate their internalization [20, 21]. Epithelial cells of the female genital tract express FcRn, which is responsible for the selective transport of IgG in mucosal secretions [16, 22, 23]. The FcRn-mediated transcellular transport of IgG effectively inhibits genital tract infection by the herpesvirus in a murine model [16].
The availability of genuine HPV-18 virions produced in organotypic epithelial raft cultures allowed us to re-examine the infection process in PHKs. Importantly, it has been of great interest to estimate the “window of neutralization”, which could help us understand the high efficacy of HPV vaccines. Accordingly, we examined human sera collected from women immunized with Gardasil for their ability to neutralize infection of PHKs by genuine HPV-18 virions generated in organotypic epithelial raft cultures. We also explored the possibility that HPV-specific antibodies of the IgG isotype might exert their protective effect through FcRn-mediated internalization of IgG by PHKs.
2. Methods
2.1. Cells and culture conditions
PHKs were isolated from neonatal foreskins following elective circumcision according to an IRB-approved protocol at the University of Alabama at Birmingham (UAB). They were grown in keratinocyte serum-free medium (K-SFM) (Life Technologies, Grand Island, NY) in the presence of mitomycin C-treated J2 feeder cells (Swiss 3T3 J2 fibroblasts, a gift of Dr. Elaine Fuchs, Rockefeller University) [9, 24]. PHKs were split 1:3 at 90% confluence. Unless otherwise specified, all assays used PHKs at passage 2. The human colon carcinoma epithelial cell line HT-29 (ATCC Cat# HTB-38) and endometrial carcinoma cell line HEC-1-A (ATCC Cat# HTB-112) were grown in complete RPMI 1640 medium. The TZM-bl cell line (NIH AIDS Reagent Program Cat# 8129) was maintained in complete Dulbecco’s modified Eagle’s medium.
2.2. Isolation of RNA and RT-PCR analysis for FcRn
Total RNA was isolated from PHKs, HT-29, HEC-1-A, and TZM-bl cells using standard phenol-chloroform extraction with RNA-STAT60 (Tel-Test Inc., Friendwoods, TX), followed by treatment with Turbo DNase I (Life Technologies) and reverse transcription with SuperScript III (Life Technologies). Primers for FcRn cDNA to amplify a 326 bp product were: Forward U190 5’-TGGGCGCAGAAAGCCACCTCTC-3’, Reverse L494 5’-GGTGGGCACCGAGGTGTTGTCA-3’.
2.3. Serum samples and determination of total and HPV-specific IgG and IgA antibodies
Four matched pre-immune and post-immune (7 months after the first of three vaccinations according to protocol) sera of Gardasil-vaccinated women were provided by Merck & Co., Inc., Kenilworth, NJ. Sixteen additional sera collected from Gardasil-vaccinated women (1 to 3 years post-immunization) were obtained from the Department of Obstetrics and Gynecology, UAB. In lieu of matched pre-immune sera, serum from a healthy, unimmunized individual was used as a negative control.
ELISA for total IgG and IgA was performed as previously described [25-27]. Titers of HPV-specific IgG and IgA antibodies were measured in all sera by an ELISA similar to that performed for total Ig, with the exception that Gardasil vaccine at a dilution 1:80 was used to coat the ELISA plates. Two-fold serial dilutions of sera starting from 1/100 for IgA and 1/500 for IgG were used. The endpoint titer was defined as the dilution of serum which gave an optical density above the cut-off value (mean value of background +/− 3 × standard deviation).
2.4. IgG isolation and fluorescein labeling of IgG
IgG was purified from sera collected before and after Gardasil immunization using Protein G Sepharose according to the manufacturer’s instructions (GE Healthcare Bio-sciences Corp., Piscataway, NJ) [27]. The concentration of isolated IgG was adjusted to match the levels present in the corresponding serum sample. Aliquots of IgG were labeled with fluorescein (Fl) using a labeling kit (Solulink, San Diego, CA). The Fl to IgG molar ratio ranged between 3.0 and 4.6.
2.5. Binding and uptake of fluorescein-labeled IgG by PHKs
PHKs (6 × 104) were cultured overnight at 37°C. Fl-IgG in amounts of 75, 27 or 18 μg was added and incubated for up to 18 h. PHKs were then trypsinized and fixed in 2% paraformaldehyde for flow cytometry. Data acquisitions were performed on a BD-LSRII flow cytometer (BD Biosciences, San Jose, CA). To ascertain that the IgG internalization was Fc-mediated, PHKs were incubated with 25 μg of FITC-labeled, intact rabbit IgG or the F(ab’)2 fragment of IgG (SouthernBiotech, Birmingham, AL) for 3 h at 37°C and analyzed by flow cytometry.
2.6. HPV-18 virion production and neutralization assays
HPV-18 virions were produced, partially purified and titered as described [9, 24]. For neutralization assays, PHKs (6 × 104) were seeded in each well of 12-well plates in K-SFM and grown overnight. Cell number was determined before wells were infected with HPV-18 at the specified MOI. Dilutions of sera or purified IgG were added at the same time in a total incubation volume of 500 μl. The cells were then cultured for 24 h or 6 h, washed twice, and refreshed with K-SFM. At confluence, total RNA was harvested and reverse transcribed. Nested PCR or PCR targeted, respectively, a portion of the cDNA spanning the splice sites of the HPV-18 E1^E4 mRNA (521 bps) or the control β-actin cDNA (642 bps) [28, 29]. The cDNA products were electrophoresed in a 2% agarose gel and imaged after staining with ethidium bromide. To reveal the window of virus neutralization by immune sera, PHKs were infected with HPV-18 (MOI of 20) at 0 h and challenged with a 1:250 dilution of sera at 0, 2, 4, 6, 8, 10, 12, 16, 20 and 24 h post-infection. Detection of E1^E4 cDNA was conducted as described above. To examine the possibility of intracellular neutralization, PHKs were grown for 18 h in the presence of 150 μg of purified IgG prior to HPV-18 infection.
3. Results
3.1. Titers of HPV-specific IgG and IgA in sera of Gardasil-immunized women
The titers of HPV-specific IgG were higher than the IgA isotype in all sera collected after immunization (Table 1 and Table S1). Four paired pre- and post-immune sera provided by Merck along with two post-immune sera from UAB with the highest antibody titers were selected for evaluating their HPV-neutralizing activity. The UAB sera were collected after 1 to 3 years post-vaccination, and the IgG titers varied among immunized individuals. In a previous report, the HPV-specific titer reached a peak after 7 months and then declined after 12 months [30]. However it is not possible to compare IgG ”titers” among different studies, because the methods of titering are completely different among laboratories, as were the times of sera collection.
Table 1.
HPV specific IgG and IgA antibody titers in sera of women immunized with Gardasil.
| Name | Source | Immunization Status |
End-Point Titer by ELISA |
|
|---|---|---|---|---|
| IgG | IgA | |||
| WH 102 | UAB | Post | 64,000 | 8,000 |
|
|
|
|||
| WH 113 | Post | 32,000 | 600 | |
|
| ||||
| Control | Volunteer | None | — | — |
|
| ||||
| 4851 | Merck & Co., Inc. |
Pre, Day 1 | 4,000 | 1,900 |
|
|
|
|||
| 4861 | Post, Month 7 | 64,000 | 2,400 | |
|
|
|
|||
| 4871 | Pre, Day 1 | 4,000 | 1,600 | |
|
|
|
|||
| 4881 | Post, Month 7 | 80,000 | 3,200 | |
|
|
|
|||
| 4891 | Pre, Day 1 | 2,000 | 2,200 | |
|
|
|
|||
| 4901 | Post, Month 7 | 60,000 | 4,800 | |
|
|
|
|||
| 4911 | Pre, Day 1 | 1,000 | 800 | |
|
|
|
|||
| 4921 | Post, Month 7 | 64,000 | 12,800 | |
End-point titers of Gardasil-specific IgG and IgA were determined by ELISA in 16 immune sera received from UAB (see also Table S1) and 4 pairs of pre- and post- immune sera provided by Merck & Co., Inc. Only two sera (WH102, WH113) from UAB with the highest antibody titers were used in this study.
3.2. Neutralization of HPV-18 by immune human sera
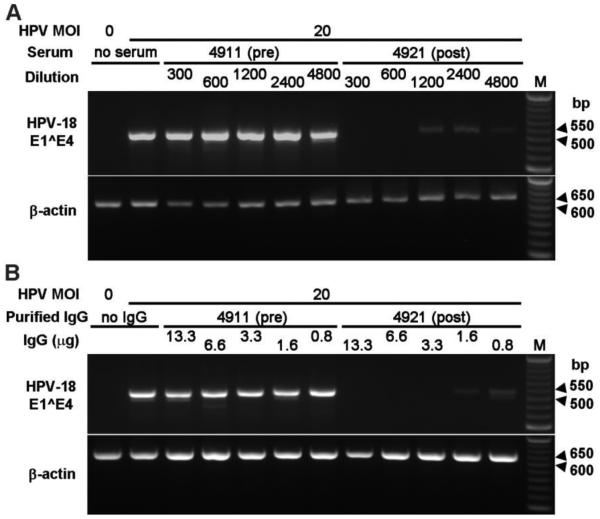
Unless otherwise specified, all neutralization experiments were conducted with passage 2 PHKs. To determine the efficacy of the Gardasil-immune sera in preventing HPV-18 infection of PHKs, we added HPV-18 virions along with different dilutions of immune, pre-immune or control sera to submerged PHKs. All four immune sera from Merck abrogated HPV-18 infection at an MOI 20 at a dilution of more than 1:600 (Fig. 1A and Table 2). None of the matched pre-immune sera were able to neutralize the infection at a dilution of 1:300 (Fig. 1A). Similarly, HPV-18 infection was successfully prevented by a dilution between 1:1000 and 1:5000 of immune sera WH102 and WH113 at MOI 50 (Fig. S1 and Table 2). At MOI 10, neutralization was evident down to a dilution of 1:5000 (Table 2). The negative control serum had no activity even at a 1:40 dilution (Fig. S1). Although we selected only two UAB immune sera with the highest IgG titers (64,000 and 32,000) for testing, we expect that sera with lowest IgG titer of 2,000 would still be able to neutralize the infection at MOI of 50 at a dilution of 30- 50 or higher. Thus, we conclude that Gardasil-immune sera neutralize HPV-18 infection of PHKs with high efficacy.
Fig. 1.
Immune human sera and IgG neutralize HPV-18 and show high efficacy in preventing infection of PHKs. (A) HPV-18 neutralization by four matched pairs of sera collected pre- and post-immunization with Gardasil. One example of pre-immune (4911) and immune (4921) sera is shown here. Additional data are presented in Table 2. PHKs at passage two were infected with HPV-18 at MOI 20 in the presence of diluted sera (1:300, 1:600, 1:1200, 1:2400 and 1:4800). Infection was detected by the presence of a cDNA fragment of a spliced E1^E4 mRNA via RT-nested PCR. β-actin cDNA served as a control. (B) IgG was responsible for the HPV-neutralizing activity in the serum. IgG was isolated from pre-immune (4911) and immune (4921) sera. PHKs were infected with HPV-18 at MOI 20 in the presence of purified IgG. IgG of 13.3, 6.6, 3.3, 1.6 and 0.8 μg corresponded to the serum serial dilutions in Fig. 1A. Uninfected PHKs and PHKs infected in the absence of serum were negative and positive infection controls, respectively (A & B, left two lanes). M, 50 bp DNA ladder.
Table 2.
End-point dilution of immune sera capable of neutralizing HPV18 at the specified MOI.
| Name | HPV MOI | Serum Dilution for HPV Neutralization (fold) |
|---|---|---|
| WH 102 | 10 | >5000 |
|
| ||
| 50 | 1000 to 5000 | |
|
| ||
| WH 113 | 10 | >5000 |
|
| ||
| 50 | 1000 to 5000 | |
|
| ||
| 4861 | 20 | 600 to 1200 |
|
| ||
| 4881 | 20 | 1200 to 2400 |
|
| ||
| 4901 | 20 | 600 to 1200 |
|
| ||
| 4921 | 20 | 600 to 1200 |
3.3. HPV-specific IgG is responsible for virus neutralization
To confirm that HPV-neutralizing activity was mainly attributable to IgG, PHKs were infected with HPV-18 at MOI 20 in the presence of different amounts of purified IgG from matched pre- and post-immune sera (4911 and 4921). The amounts of IgG added ranged from 0.8 μg to 13.3 μg in 2-fold increments, equivalent to 1:4800 to 1:300 dilution of sera in experiments shown in Fig. 1A. The results indicate that the immune IgG had a comparable neutralizing efficacy as the original immune serum (compare 3.3 μg in Fig. 1B to 1:1200 serum dilution in Fig. 1A). As expected, the pre-immune IgG did not prevent infection (Fig. 1B).
3.4. FcRn expressed on PHKs mediates the uptake of IgG
To examine whether PHKs express FcRn, RT-PCR analyses were performed on total RNA isolated from PHKs and human cell lines positive (HT-29 and HEC-1-A) or negative (TZM-bl) for this receptor [16]. The results demonstrated the presence of FcRn mRNA in PHKs (Fig. 2A). To determine whether FcRn is able to bind and internalize IgG, PHKs were incubated with Fl-IgG in K-SFM at 37°C and the percentage of Fl-IgG-positive cells was determined by flow cytometry. The percentage of Fl-IgG-positive PHKs was dependent on the incubation period and amount of Fl-IgG. When the incubation time with 27 μg of Fl-IgG was increased from 1 to 2 h, the percentage of Fl-IgG-positive PHKs increased 2.5 fold (Fig. 2B left and middle columns). When Fl-IgG was increased from 18 μg to 27 μg, the percentage of Fl-IgG-positive PHK increased from 16.1% to 30.8% after a 2 h incubation (Fig. 2B middle and right columns). Since FcRn binds IgG in a pH-dependent manner [16, 20], we also assessed the IgG binding at pH 6.0, 6.5, and 7.0. However, PHKs did not survive the somewhat acidic K-SFM for more than 1-2 h. Finally, we cultured PHKs (6 × 104) in 250 μl of K-SFM in the presence of 75 μg of Fl-IgG for 18 h. Under this condition, cells maintained normal viability and over 99% of the PHKs became positive for Fl-IgG (Fig. 2C).
Fig. 2.
Binding and internalization of IgG by PHKs are mediated by FcRn. The pre-bound or internalized IgG did not neutralize HPV. (A) PHKs express FcRn RNA transcripts, as determined by RT-PCR. (B) Percentage of Fl-IgG+ PHKs following 1 and 2 h incubation with 18 or 27 μg Fl-IgG. (C) FACS profile of 6×104 PHKs cultured for 18 h in 250 μl K-SFM in the presence of 75 μg of immune Fl-IgG (4921; blue) vs. PHKs cultured in K-SFM only (pink). Greater than 99% of cells were positive for Fl-IgG. Pre-immune Fl-IgG (4911) also showed the same profiles (data not shown). (D) Binding and internalization of rabbit IgG by PHKs is mediated by the Fc domain of IgG through FcRn. PHKs were incubated with 25 μg of FITC-labelled rabbit IgG or F(ab’)2 of rabbit IgG. About 60% of PHKs were IgG positive whereas only 2.4% of PHKs were positive for IgG F(ab’)2. (E) Pre-incubation with immune IgG did not prevent infection. PHKs (6×104 cells/well) were cultured in K-SFM for 18 h in the presence of 150 μg of IgG isolated from pre-immune serum 4911 or immune serum 4921. Cells were washed and then infected with HPV-18 at MOI 20. After 6 or 24 h, the infected cells were washed, cultured and then processed for viral cDNA detection (lanes, 4, 5, 7, 8). For comparison, PHKs not previously exposed to IgG were infected for 24 h with HPV in the presence of 150 μg of pre-immune IgG (lane 3) or 6.6 μg of immune IgG (lane 6). As a positive control, PHKs not previously exposed to IgG were infected with HPV for 6 h in the absence of IgG (lane 2). Uninfected PHKs served as a negative control (lane 1). M, 50 bp DNA ladder.
Human FcRn binds not only human IgG, but also IgG derived from different species, such as rabbit and guinea pig [31]. To ascertain that the binding of IgG to FnRn was mediated by the Fc region of IgG, we used FlTC-labeled rabbit IgG or F(ab’)2 preparations in uptake and internalization studies. About 30% of PHKs internalized human IgG when incubated with ~25 μg of Fl-IgG (Fig. 2B). For comparison, when 25 μg of rabbit FlTC-IgG was used, about 60% of the PHKs were positive. In contrast, fewer than 3% of the cells were positive after incubation of PHKs with FlTC-labeled F(ab’)2 (Fig. 2D). These data indicate that IgG uptaken by PHKs is Fc-dependent, most likely mediated by FcRn.
3.5. Bound or internalized immune IgG did not neutralize HPV
Next we asked whether the IgG antibodies can neutralize HPV intracellularly. After an 18 h preincubation of 6 × 104 PHKs with 150 μg purified, unlabeled pre-immume or immune IgG, the cells were washed to remove any unbound IgG. HPV virions were added at MOI 20 for 6 h or 24 h prior to removal of unbound virus particles. Cells were cultured and total RNA was isolated for analyses. No HPV neutralization was detected (Fig. 2E, lanes 4, 5, 7, 8). In the control experiment, 6.6 μg of immune IgG added at the time of infection neutralized HPV infection effectively (lane 6), whereas 150 μg of pre-immune IgG added at the time of infection had no neutralizing effect (lane 3). As expected, uninfected PHKs did not have any signal (compare lanes 1, 2). These results demonstrate that immune IgG was not able to neutralize HPV after it was bound to or gained entry into PHKs.
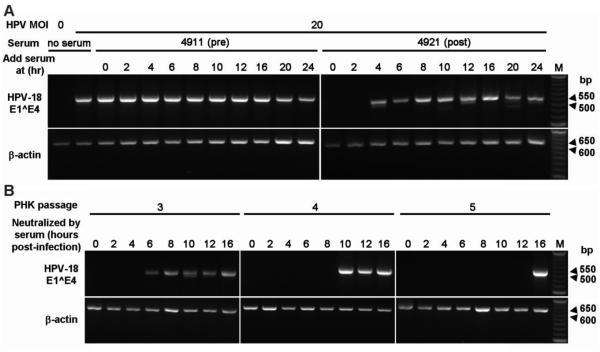
3.6. Window of virus neutralization by immune sera increases with PHKs passages
We next determined the window during which the immune sera were able to neutralize the virus. PHKs were infected with HPV-18 at MOI 20 and challenged with 1:250 dilutions of immune sera at 0, 2, 4, 6, 8, 10, 12, 16, 20 or 24 h post-infection. Using 5 immune sera, we consistently observed that neutralization was achieved only when the serum was added within 2 to 4 h of infection (Table 3). One such experiment is shown in Fig. 3A. As before, the pre-immune serum was ineffective. To investigate whether PHK growth characteristics might affect the window of neutralization, we repeated the experiments in PHKs grown to passages 3, 4 or 5 (P3, P4 and P5). The P4 and P5 PHKs grew more slowly than P3 PHKs, and the P5 PHKs were slightly enlarged in morphology, suggestive of moving toward senescence, as expected. The results show that the window for the immune sera to neutralize the virus increases significantly in slow growing, later passage PHKs. In P3 PHKs, the window increased to 4 to 6 h (Fig. 3B, left panel). It extended further to 8 - 10 h and 12 - 16 h in P4 and P5 PHKs, respectively (middle and right panels). As before, the pre-immune sera were not able to neutralize the virus.
Table 3.
Window of HPV18 neutralization by immune sera.
| Name | Serum Dilution (fold) |
Window of Neutralization (hour) |
|---|---|---|
| WH 102 | 1000 | <2 |
| 4861 | 250 | <2 |
| 4881 | 250 | <4 |
| 4901 | 250 | <2 |
| 4921 | 250 | <4 |
The experiments were all conducted with passage 2 PHKs at MOI 20. Immune sera added after 2 or 4 h post-infection did not neutralize the virus. An example can be seen in Fig. 3A.
Fig. 3.
The window of HPV neutralization increases with PHK passage number. (A) PHKs at passage 2 were infected (0 h) with HPV-18 MOI 20. 1:250 dilutions of pre-immune serum 4911 or immune serum 4921 were added at 0, 2, 4, 6, 8, 10, 12, 16, 20 or 24 h post-infection. Negative and positive infection controls are presented in the left two lanes of the left panel. M, 50 bp DNA ladder is shown only for the right panel. Data from additional sera are summarized in Table 3. (B) PHKs at passage 3, 4, and 5 were infected with HPV-18 (MOI 20) at 0 h followed by the addition of 1:250 diluted immune human serum 4881 at 0, 2, 4, 6, 8, 10, 12, or 16 h post-infection. M, 50 bp DNA ladder is shown only for the right panel.
4. Discussion
In vitro studies of HPV pseudovirions have suggested a 2-stage HPV infection process [32-35]. The virus particles initially bind to the HSPG on the cell surface. The virion undergoes a conformational change, exposing the amino-terminus of the L2 protein. Furin cleavage of the L2 amino-terminus exposes a conserved L2 epitope, the subject of cross-reactive HPV vaccine development [36]. The “processed” virus particle then binds to an unknown non-HSPG receptor for internalization. It takes from 24 h to 48 h for the majority of the virions [34] or pseudovirions to enter immortalized HaCaT cells [37]. This 2-stage infection process has been confirmed in pseudovirion infection of the mouse cervical epithelium [38].
In this study, we used genuine, mature HPV-18 virus particles harvested from organotypic epithelial raft cultures of PHKs, the native host for HPVs, to obtain information concerning the infection and virus neutralization processes. Unlike pseudovirions, these HPV-18 virus particles infect PHKs effectively at an MOI as low as 2 [9]. Furthermore, our data suggest that most of the virions have gained entry into early passage PHKs within 6 h of infection, because prolonged infection (24 h) did not produce higher viral cDNA signals (Fig. 2E, compare lane 2, 4, 7 to lanes 3, 5, 8).
Using this system, we examined sera and isolated IgG from Gardasil-immunized women for their ability to neutralize HPV-18 extracellularly or intracellularly and to define the temporal window of neutralization. Expression of receptors for Ig on the surface of epithelial cells is essential for selective internalization, transcellular Ig transport, and intracellular neutralization of several viruses [19-21]. Epithelial Ig receptors can also internalize and transport Igs complexed with viruses or other antigens [23]. In contrast to the protective role of internalized Ig amply demonstrated in vitro [39-41], recent studies indicate that Ig-receptor-dependent internalization of antibody-coated viruses could result in enhanced infectivity [23]. The difference in the outcome thus depends on the virus species, phenotypes of epithelial target cells and whether the antibody is neutralizing or non-neutralizing.
HPVs infect human mucosal epithelial cells that also express Ig receptors. This system represents a suitable experimental model to explore the potential protective effect of internalized antibodies. Our results show that epidermal PHKs from foreskins express FcRn and can take up IgG (Fig. 2A to D). However, isolated IgG taken up and internalized by PHKs was not able to neutralize HPV (Fig. 2E). Our observation is consistent with the proposed conformational changes occurring during infections with HPV pseudovirions. After a conformation change induced by binding to HSPG on the cell surface, virions that gain entry into cells are no longer recognized by intracellular IgG antibodies. Thus, the window of neutralization represents the time line of this conformation change.
The time required to induce the conformational change experienced by the virions upon binding to HSPG may depend on the cell types (immortalized or transformed cells vs. PHKs) or the virus particles used (pseudovirions vs. genuine HPV). These factors could contribute to the slow infection process in previous reports [42]. In contrast, we show that, in early passage fast growing PHKs, the window of neutralization is as short as 2 to 4 h after virions are added to the cells (Fig. 3A, Table 3). The possibility that the viruses have already entered into the cells within 2 to 4 h can be ruled out, as the window of neutralization by the L2-specific antibody was longer (H-K. Wang, R.B. Roden, T.R. Broker and L.T. Chow, unpublished observations). Our data further show that cell growth properties are a major determinant, as the window of neutralization greatly lengthened in slow growing late passage cells (Fig. 3B), possible attributed to changes in HSPG. Alternatively, the amount of furin or the numbers of HPV-specific receptors may have decreased in these cells.
It is generally accepted that wounding is necessary to permit HPV to gain entry into the basal cells, as also observed with pseudovirions infecting the mouse cervix [35]. In vivo, the basal cells are usually quiescent and, upon wounding, these cells at the wound edge are reactivated into the cell cycle necessary for virus particles to establish infection [43]. The length of time required for these processes might vary with the wound site and size, as well as the age and the hormonal status of the individual. We suggest that this activation process also involves changes on the cell surface to make them susceptible to HPV infection, consistent with the expanded window of neutralization observed with slow growing cells (Fig. 3). These considerations could significantly increase the window for the immune sera to reach the wound site and to neutralize the HPV before it establishes infection.
5. Conclusions
By using genuine HPV-18 virions and PHKs, we have demonstrated that sera of Gardasil-immunized individuals possess strong extracellular neutralization activity. Our results support the previously proposed 2-stage infection process. We observed that the window of neutralization by the HPV-specific antibodies is limited to 2 to 4 h in early passage PHKs, likely corresponding to period prior to the conformation change induced in the virion upon initial binding to the HSPG on the cell surface. Once the conformational change has taken place, the virus is no longer susceptible to neutralization by extracellular or intracellular antibodies. Our data further demonstrate that the window of neutralization varies with the growth properties of the cells and is extended to 12 - 16 h in late passage, slow growing PHKs. Thus, characteristics of PHKs influence the infection process and are important factors in the highly efficacious protection of individuals immunized with Gardasil.
Supplementary Material
Acknowledgments
The project was supported by Merck IISP 36110 and 36111 to LTC and JM and by NIH grant CA83679 to LTC. The collection of foreskins and the use of sera were performed with IRB approval. We are indebted to the nurses in the UAB Well-Baby Nursery for collection of neonatal foreskins from elective circumcisions.
Footnotes
Conflict of interest statement
The authors have no financial conflicts of interest.
References
- [1].Bravo IG, Felez-Sanchez M. Papillomaviruses: Viral evolution, cancer and evolutionary medicine. Evol Med Public Health. 2015;2015:32–51. doi: 10.1093/emph/eov003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bzhalava D, Eklund C, Dillner J. International standardization and classification of human papillomavirus types. Virology. 2015;476:341–4. doi: 10.1016/j.virol.2014.12.028. [DOI] [PubMed] [Google Scholar]
- [3].zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- [4].World Health Organization/International Agency for Research on Cancer GLOBOCAN 2012: Estimated cancer incidence, moratlity and prevalence worldwide in 2012. 2012 Available at http://globocan.iarc.fr/Default.aspx.
- [5].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [6].Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010;118:422–49. doi: 10.1111/j.1600-0463.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- [7].Dollard SC, Wilson JL, Demeter LM, Bonnez W, Reichman RC, Broker TR, et al. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. Genes Dev. 1992;6:1131–42. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- [8].Wilson JL, Dollard SC, Chow LT, Broker TR. Epithelial-specific gene expression during differentiation of stratified primary human keratinocyte cultures. Cell Growth Differ. 1992;3:471–83. [PubMed] [Google Scholar]
- [9].Wang HK, Duffy AA, Broker TR, Chow LT. Robust production and passaging of infectious HPV in squamous epithelium of primary human keratinocytes. Genes Dev. 2009;23:181–94. doi: 10.1101/gad.1735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10:681–92. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bosch FX, Broker TR, Forman D, Moscicki AB, Gillison ML, Doorbar J, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against Infection and Intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- [13].Buck CB, Thompson CD. Production of papillomavirus-based gene transfer vectors. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb2601s37. Chapter 26:Unit 26.1. [DOI] [PubMed] [Google Scholar]
- [14].Buck CB, Thompson CD, Pang Y-YS, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005;79:2839–46. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, et al. The L1 major capsid protein of human papillomavirus type L1 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–22. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- [16].Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci USA. 2011;108:4388–93. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci USA. 2010;107:19985–90. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bidgood SR, Tam JC, McEwan WA, Mallery DL, James LC. Translocalized IgA mediates neutralization and stimulates innate immunity inside infected cells. Proc Natl Acad Sci USA. 2014;111:13463–8. doi: 10.1073/pnas.1410980111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].van Egmond M, Bakema JE, Woof JM. Fc receptors in mucosal immunology. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroute H, Lambrecht BN, editors. Mucosal immunology. 4th Elsevier/Academic Press; Waltham, MA: 2015. pp. 409–28. [Google Scholar]
- [20].Baker K, Blumberg RS, Kaetzel CS. Immunoglobulin transport and immunoglobulin receptors. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroute H, Lambrecht BN, editors. Mucosal immunology. 4th Elsevier/Academic Press; Waltham, MA: 2015. pp. 349–408. [Google Scholar]
- [21].Russell MW, Kilian M, Mantis NJ, Corthésy B. Biological activities of IgA. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroute H, Lambrecht BN, editors. Mucosal immunology. 4th Elsevier/Academic Press; Waltham, MA: 2015. pp. 429–54. [Google Scholar]
- [22].World Health Organization Human papillomavirus vaccines WHO position paper. 2009;84:117–32. [Google Scholar]
- [23].Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, et al. The neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang HK, Broker TR, Chow LT. Robust HPV-18 production in organotypic cultures of primary human keratinocytes. Methods Mol Biol. 2015;1249:93–109. doi: 10.1007/978-1-4939-2013-6_7. [DOI] [PubMed] [Google Scholar]
- [25].Mestecky J, Wright PF, Lopalco L, Staats HF, Kozlowski PA, Moldoveanu Z, et al. Scarcity or absence of humoral immune responses in the plasma and cervicovaginal lavage fluids of heavily HIV-1-exposed but persistently seronegative women. AIDS Res Hum Retrov. 2011;27:469–86. doi: 10.1089/aid.2010.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moldoveanu Z, Huang WQ, Kulhavy R, Pate MS, Mestecky J. Human male genital tract secretions: both mucosal and systemic immune compartments contribute to the humoral immunity. J Immunol. 2005;175:4127–36. doi: 10.4049/jimmunol.175.6.4127. [DOI] [PubMed] [Google Scholar]
- [27].Wei Q, Moldoveanu Z, Huang WQ, Alexander RC, Goepfert PA, Mestecky J. Comparative evaluation of HIV-1 neutralization in external secretions and sera of HIV-1-Infected women. Open AIDS J. 2012;6:293–302. doi: 10.2174/1874613601206010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nasseri M, Hirochika R, Broker TR, Chow LT. A human papilloma virus type 11 transcript encoding an E1--E4 protein. Virology. 1987;159:433–9. doi: 10.1016/0042-6822(87)90482-x. [DOI] [PubMed] [Google Scholar]
- [29].Meyers C, Bromberg-White JL, Zhang J, Kaupas ME, Bryan JT, Lowe RS, et al. Infectious virions produced from a human papillomavirus type 18/16 genomic DNA chimera. J Virol. 2002;76:4723–33. doi: 10.1128/JVI.76.10.4723-4733.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009;8:1663–79. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- [31].Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–9. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- [32].Day PM, Schiller JT. The role of furin in papillomavirus infection. Future Microbiol. 2009;4:1255–62. doi: 10.2217/fmb.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118:S12–7. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Culp TD, Christensen ND. Kinetics of in vitro adsorption and entry of papillomavirus virions. Virology. 2004;319:152–61. doi: 10.1016/j.virol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [35].Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- [36].Jagu S, Kwak K, Schiller JT, Lowy DR, Kleanthous H, Kalnin K, et al. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J Virol. 2013;87:6127–36. doi: 10.1128/JVI.03218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by L2 cross-neutralizing and L1 type-specific antibodies. J Virol. 2008;82:4638–46. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA. 2009;106:20458–63. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alfsen A, Iniguez P, Bouguyon E, Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J Immunol. 2001;166:6257–65. doi: 10.4049/jimmunol.166.10.6257. [DOI] [PubMed] [Google Scholar]
- [40].Mestecky J. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J Reprod Immunol. 2007;73:86–97. doi: 10.1016/j.jri.2007.01.006. [DOI] [PubMed] [Google Scholar]
- [41].Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, et al. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–26. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- [42].Day PM, Lowy DR, Schiller JT. Heparan sulfate-independent cell binding and infection with furin-precleaved papillomavirus capsids. J Virol. 2008;82:12565–8. doi: 10.1128/JVI.01631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009;5:e1000318. doi: 10.1371/journal.ppat.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.