Abstract
Objectives
The aim of this systematic literature review was to summarize the current knowledge regarding the prevalence of, time to recovery from, and influence of glucocorticoid dose and duration on glucocorticoid-induced adrenal insufficiency (AI).
Methods
Eligible studies were original research articles, which included adult patients with an indication for glucocorticoids and measured adrenal function following exposure to systemic glucocorticoids. Searches were performed in Web of Science and MEDLINE, with further articles identified from reference lists. Screening was performed in duplicate. Data were extracted for each group of glucocorticoid-exposed patients within eligible studies. The reported proportion of patients with AI was summarized as median and inter-quartile range. Results were then stratified by daily dose, cumulative dose, duration of exposure and time since last glucocorticoid use. The risk of bias within and across studies was considered: for randomised controlled trials risk of bias was assessed using the tool developed by the Cochrane Collaboration.
Results
Overall, 73 eligible studies were identified out of 673 screened. The percentage of patients with AI ranged from 0% to 100% with a median (IQR) = 37.4% (13–63%). Studies were small—median (IQR) group size 16 (9–38)—and heterogeneous in methodology. AI persisted in 15% of patients retested 3 years after glucocorticoid withdrawal. Results remained widely distributed following stratification. AI was demonstrated at <5 mg prednisolone equivalent dose/day, <4 weeks of exposure, cumulative dose <0.5 g, and following tapered withdrawal.
Conclusions
The heterogeneity of studies and variability in results make it difficult to answer the research questions with confidence based on the current literature. There is evidence of AI following low doses and short durations of glucocorticoids. Hence, clinicians should be vigilant for adrenal insufficiency at all degrees of glucocorticoid exposure.
Abbreviations: AI, adrenal insufficiency; IQR, inter-quartile range; GC, glucocorticoid; HPA-axis, hypothalamic–pituitary–adrenal axis; RCTs, randomised controlled trials; ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; IM, intra-muscular; IV, intravenous; HC, hydrocortisone
Keywords: Glucocorticoids, Adrenal insufficiency, Systematic literature review
Introduction
Adrenal insufficiency (AI) is failure of the adrenal cortex to produce sufficient levels of cortisol. Chronically low cortisol levels can cause non-specific symptoms such as fatigue and nausea whilst lack of the usual cortisol response to stress can lead to a potentially fatal adrenal crisis [1]. Taking glucocorticoids (GCs) can lead to suppression of the hypothalamic–pituitary–adrenal (HPA) axis. The HPA-axis may remain suppressed following cessation of GC therapy, leaving the patient with adrenal insufficiency [2].
GCs are widely used therefore many patients could potentially be at risk of AI. In 2008, almost 1% of the UK adult population were exposed to oral GCs [3], including 0.79% on long-term courses (longer than 3 months) [4], and over 8 million prescriptions for oral GCs were issued in England during April 2014–March 2015 [5]. While there is limited published data around the clinical impact, one recent study suggests 6% of patients presenting at hospital with AI may have glucocorticoid-induced AI [6]. Looking forward, novel treatments aimed at improving the benefit:risk ratio of glucocorticoids (selective glucocorticoid receptor agonists, SEGRAs), which are currently being trialed, are unlikely to alter the risk of adrenal insufficiency. This is because AI is believed to result from the same mechanism that mediates the beneficial anti-inflammatory effects of glucocorticoids [7], [8].
AI has been a recognized side-effect of GC therapy since the early 1950s [9], [10], [11]; despite this, the risk of developing GC-induced AI remains unclear. Prevalence estimates from some of the larger observational studies range from 14% to 63% [12], [13], [14], [15]. It is also unclear whether or not the dose and duration of GC use affects the risk of developing AI: Schlaghecke et al. [12] found no relationship between either the dose or duration of therapy and AI, while Jamilloux et al. [13] found both cumulative dose and treatment duration to be associated with an increased risk of AI.
The aim of this systematic review was to summarize the published literature investigating GC-induced AI in adults. Specific objectives were to investigate (1) the prevalence of GC-induced adrenal insufficiency, (2) the time course of adrenal recovery following cessation of GC therapy and (3) the association between the dose and duration of GC therapy and the prevalence of adrenal insufficiency.
Methods
Eligibility criteria
Original research articles that tested adrenal function following exposure to glucocorticoids were identified. The population of interest were adult patients treated for the most common glucocorticoid indications as reported by van Staa et al. [16] for UK primary care data. These indications were disorders of the respiratory system; skin or subcutaneous tissue; musculoskeletal system or connective tissue; nervous system; digestive system; circulatory system; or neoplasms. Glucocorticoid use was limited to systemic routes including oral, intravenous, intramuscular, and subcutaneous. Finally, eligible papers were required to report the numbers of patients with normal/sub-normal HPA-axis function test results rather than presenting the average cortisol levels for study groups.
During data extraction, the following additional eligibility criteria were applied: papers were excluded if (1) participants aged under 16 were included; (2) glucocorticoids were taken less than 12 h before the HPA-axis function test; (3) glucocorticoid indications were unspecified; (4) the daily dose, duration and cumulative dose of GCs were all unreported; (5) it was clear that patients were using local GCs (topical, inhaled or intra-articular) during the study window; and (6) patients were pregnant or critically ill, were being treated with megestrol acetate, had metastatic cancer or were being treated peri-operatively (all potential confounders). Where it was possible to remove individual patients who met these exclusion criteria from the results, the paper was retained.
Published or in-press articles were included. There was no time restriction and non-English language articles were translated using Google Translate [17].
Information sources
The search was performed in MEDLINE (1946-present) and Web of Science (1900-present). In addition, the reference lists of eligible papers were screened to identify further papers missed in the database search.
Search strategy
The search included terms for adult humans, the indications described in Section Information sources, systemic glucocorticoids/named drug substances, and adrenal insufficiency/HPA-axis tests. The full search strategy is available in Supplementary File 1. Papers with only local glucocorticoids, in children or adolescents, or where the glucocorticoid was used as steroid cover/replacement therapy (e.g., in patients with Addison′s disease) were excluded. In the Web of Science search, the explicit mention of the HPA-axis, adrenal gland, or cortisol in the title of the article was required due to a large number of results in initial searches. The SIGN strategy search filters [18] were used to limit the MEDLINE results to clinical trials or observational studies. The Web of Science search was limited to journal articles, abstracts and proceedings. The latest search was performed on November 25, 2014.
Study selection
The initial screening of search results by title and abstract was carried out in duplicate (R.J. and A.L.H.). Where there was disagreement, the article was discussed between the two reviewers and if there was still uncertainty the article was retained. Full texts were then assessed, in duplicate, for eligibility. Disagreements were resolved by discussion. The reference lists of eligible articles were then screened, and the eligibility of any articles identified was then checked in duplicate as before.
Data extraction
Data were extracted using pre-designed forms. If a paper included multiple groups of GC-exposed patients, data were extracted for each group rather than pooling the data. For example, Suzuki et al. [19] compared patients exposed to 9 mg budesonide to patients exposed to 15 mg budesonide: data were extracted for each of these groups separately. Information extracted included the study design, number of participants, the age/gender distributions and indication, about comorbidities or other medications used; the GC indication; drug substance, route, dose, and duration; the type of HPA-axis test performed, and the criteria used to define AI, and the numbers of patients tested and the number of patients with AI.
Summary measures
The data extracted are summarized in Table 1. The proportion of patients with AI in each group was extracted, and the data summarized using the median, inter-quartile range (IQR), and range. Summaries are presented for all groups and then stratified according to GC exposure, with dose converted to the prednisolone equivalent dose (Supplementary File 2). Groups were stratified by daily dose (<5 mg/day; 5–10 mg/day, 10–20 mg/day; 20+ mg/day), duration (<4weeks, 4–52weeks, 52+weeks) and cumulative dose (<0.5 g, 0.5–5 g, 5+ g). To investigate the time to adrenal recovery, groups were further stratified according to the timing of the test with respect to the last dose of GCs, limited to groups exposed to oral GCs only. These results were summarized as for the dose/duration; in addition, summaries of individual studies that tested patients multiple times after cessation of GCs are presented. Studies that used a tapered dose of GCs are also summarized individually.
Table 1.
Summary characteristics of the groups included in the review.
| Characteristics by group |
Overall (n = 100) |
Study design |
Observational (n = 76) |
|---|---|---|---|
| RCT (n = 24) | |||
| Total number of patients tested | 3166 | 795 | 2371 |
| Median (IQR) number of patients tested | 16 (9–38) | 25 (17–48) | 14.5 (8–36) |
| Range number of patients tested | 2–399 | 7–86 | 2–399 |
| Median (IQR) average age* | 45 (36–53) | 35.5 (33–37) | 48 (41–54) |
| Median (IQR) % female* | 51 (40–67) | 58.5 (32–67) | 50 (40–67) |
| Glucocorticoid indication | |||
| Musculoskeletal | 25 | 3 | 22 |
| Respiratory | 24 | 7 | 17 |
| Neoplasms | 4 | 0 | 4 |
| Digestive system | 19 | 14 | 5 |
| Nervous system | 3 | 0 | 3 |
| Transplant | 7 | 0 | 7 |
| Multiple | 18 | 0 | 18 |
| Drug substances | |||
| Prednisone | 17 | 4 | 13 |
| Prednisolone | 24 | 6 | 18 |
| Budesonide | 16 | 14 | 2 |
| Methylprednisolone | 8 | 0 | 8 |
| Triamcinolone | 5 | 0 | 5 |
| Dexamethasone | 4 | 0 | 4 |
| Other (hydrocortisone, paramethasone, fluocortolone) | 4 | 0 | 4 |
| Unclear | 5 | 0 | 5 |
| Multiple | 17 | 0 | 17 |
| Glucocorticoid Route | |||
| Oral | 87 | 24 | 63 |
| IM | 5 | 0 | 5 |
| IV | 3 | 0 | 3 |
| Multiple | 5 | 0 | 5 |
| Dose category** | |||
| <5 mg/day | 15 | 8 | 7 |
| 5–20 mg/day | 42 | 13 | 29 |
| 20+ mg/day | 23 | 3 | 20 |
| Unknown | 20 | 0 | 20 |
| Duration category | |||
| <4 weeks | 15 | 4 | 11 |
| 4–52 weeks | 36 | 20 | 16 |
| 52+ weeks | 37 | 0 | 37 |
| Unknown | 12 | 0 | 12 |
| Cumulative dose category** | |||
| <0.5 g | 27 | 19 | 8 |
| 0.5–5 g | 24 | 5 | 19 |
| 5+ g | 13 | 0 | 13 |
| Unknown | 36 | 0 | 36 |
| Adrenal function test | |||
| Short ACTH test | 57 | 17 | 40 |
| Insulin tolerance test | 14 | 0 | 14 |
| ACTH infusion | 8 | 5 | 3 |
| CRH test | 6 | 0 | 6 |
| Metyrapone test | 4 | 0 | 4 |
| Plasma cortisol | 8 | 2 | 6 |
| Urinary cortisol | 2 | 0 | 2 |
| Multiple | 1 | 0 | 1 |
RCT, randomised controlled trial; IQR, inter-quartile range; IM, intra-muscular; IV, intravenous; ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone.
Unless otherwise indicated, figures represent the number of studies with that characteristic.
Results may be for whole group not necessarily those tested. May be pooled for the whole study. 17/100 groups missing age; 15/100 groups missing gender.
Prednisolone equivalent dose.
Risk of bias/study quality
The Cochrane Collaboration′s tool for assessing the risk of bias [20] was applied to the randomised controlled trials (RCTs) included in the review, including an additional section to assess the risk of confounding in these studies. The quality of reporting and heterogeneity of the other included studies, and the potential for bias across studies, are commented on in the results and discussion.
Results
In total, 673 articles were screened and 73 studies were included in the final review (Fig. 1). Within the 73 studies there were 100 groups of GC-exposed patients and a total of 3166 patients. A summary of these groups is presented in Table 1, while a condensed version of the data extraction table can be seen in Supplementary File 3. There were thirteen RCTs [19], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33] with random allocation and blinding maintained throughout; the remaining 60 studies were classified as observational studies [12], [13], [14], [15], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84] with some open trials [85], [86], [87], [88] and pilot studies [89]. The majority of groups were small (median = 16 patients). The most frequent GC indications were musculoskeletal, respiratory, and digestive tract conditions and the most common GCs investigated were prednisolone, prednisone, and budesonide. The majority of studies investigated oral GCs.
Fig. 1.
Flow diagram showing the study selection process. Based on the PRISMA 2009 flow diagram [21]. Abbreviations: AI, adrenal insufficiency; GC, glucocorticoid.
Across all of the groups the median percentage of patients found to have adrenal insufficiency was 37.4% (IQR: 13–63%), and this was similar for RCTs (median = 37.4%, IQR: 11–60%) and observational studies (median = 37.2%, IQR: 13–63%). Results were widely distributed in each category, with a range of 0–92% for RCTs and 0–100% for observational studies.
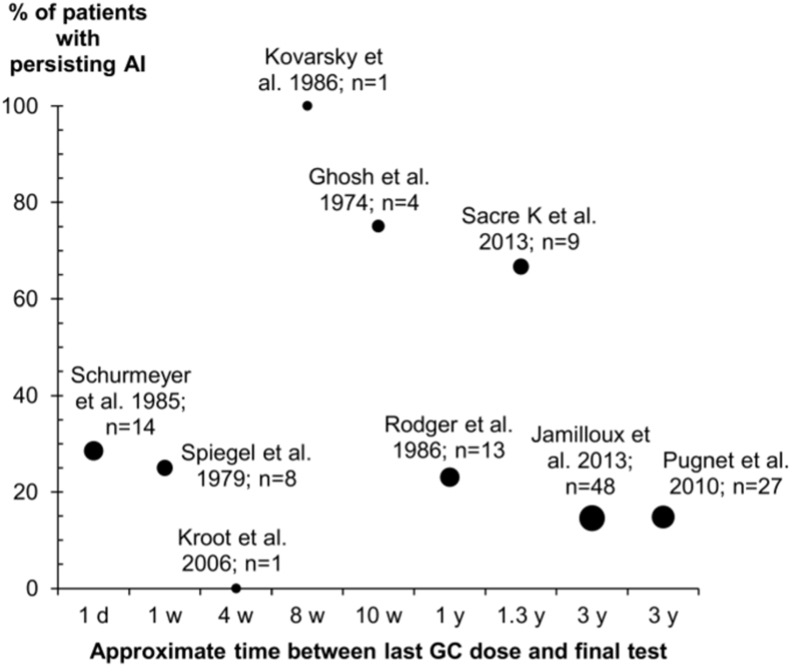
Table 2 shows the proportion of patients with AI for those taking oral GCs stratified by the timing of the test with respect to their most recent dose. Some patients were found to have adrenal insufficiency when tested more than 30 days after their last oral GC dose. In nine studies, patients were retested several times after GC withdrawal (Fig. 2). The number of patients retested ranged from 1 [61], [89] to 48 [13]. Excluding the studies retesting a single patient, the percentage of patients with AI persisting at the end of follow-up ranged from 75% (after 10 weeks of cessation) [48] to 15% (after 3 years of cessation) [13], [73]. Four studies followed patients for at least 1 year and in these studies the percentage of patients with persisting AI ranged from 15% to 67% [13], [14], [73], [75]. It was not always clear which patients had been selected for follow-up and two studies lost a large proportion of patients to follow-up (35–40%) [13], [73].
Table 2.
Percentage of patients per group with AI by time since last dose (oral glucocorticoids only).
| Time since last dose | Total number of patients | Number of groups | Median (range) group size | Median (IQR), % AI | Range, % AI |
|---|---|---|---|---|---|
| Up to 1 day | 2542 | 63 | 21 (3–399) | 40.9 (20–63) | 0–100 |
| 2–6 days | 323 | 20 | 10 (2–75) | 35.9 (0–71) | 0–90 |
| 7–29 days | 49 | 2 | 24.5 (4–45) | 16.7 (0–33) | 0–33 |
| 30+ days | 31 | 2 | 15.5 (15–16) | 47.9 (27–69) | 27–69 |
IQR, inter-quartile range; AI adrenal insufficiency.
Fig. 2.
Adrenal recovery over time. Abbreviations: AI adrenal insufficiency; GC, glucocorticoid; d, day; w, week; y, year. Each point represents a study in which HPA-axis tests were repeated at least once following withdrawal of GC therapy (in all patients, in patients with AI at the initial test, or in a subset of the latter). Horizontal axis: time between the final GC dose and the final test performed during the study. Vertical axis: percentage of patients found to have AI at the final test. Points are labeled with the total number of patients retested during the studies, including those who did and did not recover by the end of follow-up. The size of the point reflects the number of patients retested.
There was no obvious pattern when stratifying by the dose, duration or cumulative dose (Table 3). Across all strata, the median percentage of patients with adrenal insufficiency ranged from 14% (IQR: 0–40%) for a medium cumulative dose (0.5–5 g) to 50% (IQR: 35–66%) for a high cumulative dose (greater than 5 g).
Table 3.
Percentage of patients per group with AI by glucocorticoid dose, duration or cumulative dose.
| Total number of patients | Number of groups | Median (range) group size | Median (IQR), % AI | Range, % AI | |
|---|---|---|---|---|---|
| Average daily dose* | |||||
| <5 mg/day | 371 | 15 | 21 (6–63) | 22.7 (11–36) | 0–62 |
| 5–10 mg/day | 703 | 22 | 22 (7–86) | 43.7 (38–58) | 14–80 |
| 10–20 mg/day | 623 | 16 | 19 (3–279) | 33.3 (22–80) | 0–100 |
| 20+ mg/day | 527 | 26 | 8 (2–100) | 16.3 (0–71) | 0–100 |
| Duration | |||||
| <4weeks | 378 | 15 | 9 (4–86) | 36.4% (0–89%) | 0–100% |
| 4–52 weeks | 1533 | 36 | 20 (5–399) | 33.9% (12–55%) | 0–92% |
| 52+ weeks | 1093 | 37 | 19 (3–150) | 42% (26–65%) | 0–100% |
| Cumulative dose* | |||||
| <0.5g | 702 | 28 | 19 (2–86) | 35.4% (11–54%) | 0–100% |
| 0.5–5g | 804 | 23 | 10 (4–279) | 14% (0–40%) | 0–89% |
| 5+ g | 491 | 13 | 23 (3–150) | 50% (35–66%) | 0–100% |
IQR inter-quartile range; AI adrenal insufficiency
Prednisolone equivalent dose.
A total of 13 studies tested adrenal function after a GC taper and these are summarized in Table 4. The median percentage of patients with AI after a GC taper was 38% and ranged from 0% to 84%. The studies varied in the initial GC doses, duration of tapering and prior exposure pattern.
Table 4.
Summary of studies testing adrenal function after tapering GCs.
| References | Initial dose* (mg) | Final dose* (mg) | Duration of tapering | Rate of tapering | HC or ACTH cover? | Overall average daily dose* (mg) | Overall duration (weeks) | Overall total dose* (g) | N tested | % AI |
|---|---|---|---|---|---|---|---|---|---|---|
| Cydulka and Emerman [24] | 40 | 5 | 8 days | 5 mg/day | None | 22.5 | 1.1 | 0.18 | 8 | 0 |
| Miro et al. [70] | Mean = 62 | 0 | Mean = 30 days | 10 mg/3days then 2.5 mg/3days | None | NS | 5 | 3.9 | 8 | 12.5 |
| Miro et al. [70] | Mean = 61 | 0 | Mean = 30 days | 10 mg/3days then 2.5 mg/3days | None | NS | 4.3 | 0.78 | 14 | 14.3 |
| Barrier et al. [36] | 7 | 0 | NS | 1 mg/? | All | 7 | NS | NS | 22 | 31.8 |
| Boots et al. [39] | 20 | 10 | 4 weeks | 5 mg/2 weeks | None | 10 | 11 | 1.53 | 42 | 33.3 |
| Bacon et al. [35] | NS | 4–10 | NS | 1 mg/month | Some | NS | 393 | 24.8 | 23 | 34.8 |
| Havranek et al. [88] | 50 | 10 | 6 days | 8 mg/day | None | 7.5 | 17 | 0.87 | 8 | 38 |
| Pugnet et al. [73] | Mean = 65.5 | 5 | NS | NS | None | 65.5 | 142 | NS | 100 | 45 |
| Jamilloux et al. [13] | Mean = 51 | 5 | ~34 weeks | 10 mg/2 weeks, 5 mg/2 weeks, 2.5 mg/2 weeks, 1 mg/month | None | NS | 74.1 | 7.7 | 150 | 49.3 |
| Rutgeerts et al. [31] | 40 | 5 | 10 weeks | 3.5 mg/week | None | 24 | 10 | 1.68 | NS: <86 | 53 |
| Schlaghecke et al. [77] | 60 | 20 | 4 weeks | 10 mg/week | None | 45 | 4 | 12.6 | 9 | 55.6 |
| Desrame et al. [44] | NS | 4–7.5 | NS | 10 mg/10 days to 0.5 mg/7days | Some | 5.5 | 34 | 6.7 | 55 | 65.5 |
| Rodger et al. [75] | 7.5 | 2.5 | NS | 1.2–2.5 mg/month | Some | NS | 248 | 25.9 | 19 | 68.4 |
| Campieri et al. [23] | 40 | 5 | 9 weeks | 4 mg/week | None | 40 | 8.6 | 1.4 | 58 | 84 |
HC, hydrocortisone; ACTH, adrenocorticotropic hormone/corticotropin; N, number; AI, adrenal insufficiency.
Prednisolone equivalent dose.
The risk of bias assessment for RCTs is presented in Supplementary File 4. Where reported, studies scored a low risk in most domains. In many cases, however, insufficient information was provided on which to base a judgement. The risk of attrition bias scored poorly: three out of the 13 RCTs reported outcomes for all tested patients; in the remaining papers it was either impossible to calculate the number of patients tested or the outcome was missing for some patients with no explanations. The risk of confounding was high for many of the studies: in seven of the studies, some patients demonstrated abnormal HPA-axis function at baseline.
The risk of confounding is likely to be high in the observational studies: there was no information at all about concurrent medical conditions for 56 of the 76 groups, and no information about concurrent treatments in 46 groups. The exclusion of any local GC use was explicitly mentioned for 17 of the groups and therefore assumed in the remainder. The heterogeneity of these studies can be seen in Table 1, which shows the range of indications, drug substances, routes and adrenal function tests used. In general very few details were provided regarding patient selection, and loss to follow-up was difficult to ascertain.
Discussion
Across the 100 GC-exposed participant groups included in this review, the median prevalence of AI was 37%. Whilst this figure is in line with the estimates of the larger observational studies (14–63% [12], [13], [14], [15], it is unlikely to be a meaningful prevalence estimate given the wide distribution of our results and resultant imprecision. Most studies retesting patients after GC withdrawal found evidence of persisting AI, including 15% of patients retested after 3 years of cessation (Fig. 2), yet from the current literature it is not possible to describe a time course for adrenal recovery. There is also evidence that AI may persist for more than 3 years after GC withdrawal. When results were stratified according to the average dose, duration and cumulative dose of GCs used within groups, no clear trends were revealed. Within the stratifications, results were still widely distributed and AI was demonstrated even in the lowest exposure categories. AI was also found in studies which included a tapered reduction in dose.
It has not been possible to address any of our research questions adequately, based on over sixty years of published literature. There are several factors that would contribute to the wide distribution of the results. Firstly, the studies found were heterogeneous, including variation in indications and GC type, dose, and duration. There was also variability in methods of outcome assessment: while the short ACTH test (standard or low dose) was the most frequently used, a range of other tests including the insulin tolerance test (the gold-standard), ACTH infusion and CRH test were also used. Even within the same test, the cutoff values used to define AI varied from study to study. This heterogeneity in study methods is likely to increase the variability in the results and make it difficult to appropriately summarize the findings. Nonetheless, stratification led to no greater clarity in the results. Secondly, the group sizes included were small—50% had fewer than 16 patients—and so the individual prevalence estimates for each group are less precise. Another factor is having only group-level exposure data; for the majority of studies the dose and duration of GC use within the groups were variable and basing the stratification on average values may be one reason no obvious trends were apparent.
In addition to these, it is possible that bias was present within and across studies, although in most cases there was not enough evidence to be able to comment on the risk of bias. Only 6 of the 62 observational studies were published after the publication of the STROBE statement [90] in 2007 and few details were provided in any of the observational studies regarding the selection and recruitment of patients, how GC exposure was assessed, any loss to follow-up, or any comorbidities or therapies that may have confounded the results. While the majority of the RCTs were published after the publication of the CONSORT statement [91], many still lacked sufficient detail in some of the domains. This included incomplete outcome reporting making it difficult again to judge the risk of attrition bias for most studies. Importantly, many of the RCTs reported evidence of adrenal insufficiency prior to exposure to the study drug making it difficult to attribute AI to the effects of the study drug. There is also potential for bias across studies, although plotting the % AI against the group size did not reveal any obvious gaps (Supplementary File 5). It is possible that some patient groups are underrepresented in the literature: van Staa et al. [16] demonstrated that the most frequently recorded indication for oral GCs was respiratory disease (40% of GC users), yet 24% of the papers focused on respiratory disease, compared to 25% for musculoskeletal and 19% for gastro-intestinal conditions (Table 1).
While this manuscript was in preparation, Broersen et al. [92] published a systematic review on the same subject, with similar research questions and methods. The authors used a logistic regression model to generate pooled estimates of the percentage of patients with adrenal insufficiency; their overall result for patients using oral GCs was 48.7% (95% CI: 36.9–60.6). We decided at study inception, before our final search and data extraction, not to perform a meta-analysis, based on the heterogeneity shown in a few key references. Broersen et al. examined the association with GC dose and duration in asthma patients only, to create a more homogeneous population. Their results suggest an increased risk of AI associated with increased dose and duration of GCs, although confidence intervals overlapped. No such pattern was seen within our whole study population.
A strength of our study is the rigorous methodology followed, in duplicate, to identify suitable studies and extract data. It is reassuring that, despite slight differences in search strategy, including restricting our database searches to certain GC indications, the included studies overlap almost completely with those of another, independent, group [92]. This suggests that our search strategy was effective. We did not restrict results to English-language papers or a minimum study size, therefore we have included some additional papers compared to the study of Broersen et al. A possible weakness is the fact we tightened the eligibility criteria during data extraction, as described in the methods. The reason for this was the lack of detail and ambiguity in many of the articles, which became increasingly apparent when looking at papers in increasing detail and led us to write out a strict list of inclusion/exclusion criteria for this stage. We did not perform a risk of bias assessment for the observational studies because the quality of reporting was poor in the majority of studies, therefore, it would not have been possible to give a judgement of high/low risk in many cases.
From our results, it is clear that research is still needed into the risk of AI following GC exposure. Future studies should aim to have a sufficient sample size to estimate the prevalence of adrenal insufficiency with confidence. Studies could include patients who have been withdrawn from GCs as there remains a lack of published data regarding how long adrenal insufficiency persists. There is also a lack of published research regarding differing tapering schedules, a question potentially suited to a randomised controlled trial design. Finally, accurate patient-level data regarding GC exposure is important if the dose and duration is to be studied: ideally studies should include long-term prospectively collected data. Alongside these considerations, it is vital that quality of reporting is increased so that the generalizability, risk of bias and potential confounding can be assessed. Use of available guidelines, such as the STROBE [90] or CONSORT [91] statements, should ensure all important items are reported in future articles.
Our study raises questions for clinicians prescribing GC therapy. The major endocrinology societies (Endocrine Society [United States], Society for Endocrinology [United Kingdom], European Society of Endocrinology) do not currently issue clinical guidance on reducing GC-induced AI. UK clinicians can turn to the British National Formulary [93] and the National Institute for Health and Care Excellence Clinical Knowledge Summary [94], which both advise gradual GC withdrawal if patients have taken: more than 40 mg prednisolone (or equivalent) daily for more than one week; repeated GC doses in the evening; GCs for more than three weeks; a short course of GCs within one year of stopping long-term GC therapy; or have other risk factors for adrenal suppression. With regards to tapering, they advise rapid reduction to a physiological GC dose (7.5 mg prednisolone daily or equivalent), and slow reduction thereafter. However, our study suggests that the evidence base supporting these recommendations is not robust. Furthermore, we highlight that studies demonstrate AI at all levels of GC exposure, even low dose and after tapering. We therefore suggest that clinicians be vigilant for GC-induced AI with all degrees of GC exposure, and counsel patients accordingly. Anecdotally, testing for adrenal insufficiency in patients with chronic GC exposure is infrequent. Adrenal function can be tested using the short ACTH stimulation test (250 µg), as performed in 57% groups in this study; this produces equivalent results to the gold-standard insulin tolerance test (used in 14%), but is safer, and carries good long-term predictive value [95], [96]. Regarding who should be tested, and when, there is an imperative need for high quality, prospective studies to better guide clinicians, and reduce patient morbidity.
Contributorship statement
All authors participated in the design of the study. RJ and WD developed the search strategy. RJ and ALH performed screening, data extraction and analysis, and drafted the manuscript. All authors read and approved the final article.
Acknowledgments
This report includes independent research funded by the National Institute for Health Research Biomedical Research Unit Funding Scheme, UK. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Dr. Dixon was supported by an MRC Clinician Scientist Fellowship (G0902272). The work was further supported by the Arthritis Research UK Centre for Epidemiology (20380).
Footnotes
Supplementary data are available in the online version of this article at http://dx.doi.org/10.1016/j.semarthrit.2016.03.001
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Arlt W., Allolio B. Adrenal insufficiency. Lancet. 2003;361:1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 2.Dinsen S., Baslund B., Klose M., Rasmussen A.K., Friis-Hansen L., Hilsted L. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med. 2013;24:714–720. doi: 10.1016/j.ejim.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Fardet L., Petersen I., Nazareth I. Description of oral glucocorticoid prescriptions in general population. Rev Med Interne. 2011;32:594–599. doi: 10.1016/j.revmed.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Fardet L., Petersen I., Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford) 2011;50:1982–1990. doi: 10.1093/rheumatology/ker017. [DOI] [PubMed] [Google Scholar]
- 5.Health and Social Care Information Centre. Clinical Commissioning Group Prescribing Data. http://www.hscic.gov.uk/searchcatalogue [accessed 15.09.15].
- 6.Smans L.C., Van der Valk E.S., Hermus A.R., Zelissen P.M. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2016;84:17–22. doi: 10.1111/cen.12865. [DOI] [PubMed] [Google Scholar]
- 7.Buttgereit F., Burmester G.R., Lipworth B.J. Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet. 2005;365:801–803. doi: 10.1016/S0140-6736(05)17989-6. [DOI] [PubMed] [Google Scholar]
- 8.Schacke H., Schottelius A., Docke W.D., Strehlke P., Jaroch S., Schmees N. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc. Natl. Acad. Sci. U. S. A. 2004;101:227–232. doi: 10.1073/pnas.0300372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprague R.G., Power M.H., Mason H.L. Physiological effects of cortisone and pituitary adrenocorticotropic hormone (ACTH) in man. J Am Med Assoc. 1950;144:1341–1347. doi: 10.1001/jama.1950.02920160015003. [DOI] [PubMed] [Google Scholar]
- 10.Fraser C.G., Preuss F.S., Bigford W.D. Adrenal atrophy and irreversible shock associated with cortisone therapy. J Am Med Assoc. 1952;149:1542–1543. doi: 10.1001/jama.1952.72930340001009. [DOI] [PubMed] [Google Scholar]
- 11.Salassa R.M., Bennett W.A., Keating F.R., Jr., Sprague R.G. Postoperative adrenal cortical insufficiency: occurrence in patients previously treated with cortisone. J Am Med Assoc. 1953;152:1509–1515. doi: 10.1001/jama.1953.03690160009004. [DOI] [PubMed] [Google Scholar]
- 12.Schlaghecke R., Kornely E., Santen R.T., Ridderskamp P. The effect of long-term glucocorticoid therapy on pituitary–-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med. 1992;326:226–230. doi: 10.1056/NEJM199201233260403. [DOI] [PubMed] [Google Scholar]
- 13.Jamilloux Y., Liozon E., Pugnet G., Nadalon S., Heang Ly K., Dumonteil S. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS One. 2013;8:e68713. doi: 10.1371/journal.pone.0068713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacre K., Dehoux M., Chauveheid M.P., Chauchard M., Lidove O., Roussel R. Pituitary–adrenal function after prolonged glucocorticoid therapy for systemic inflammatory disorders: an observational study. J Clin Endocrinol Metab. 2013;98:3199–3205. doi: 10.1210/jc.2013-1394. [DOI] [PubMed] [Google Scholar]
- 15.Lubcke P., Poschke N. Plasma–cortisol levels during long-time treatment with steroids in patients with obstructive bronchopneumopathy. Med Welt. 1990;41:690–694. [Google Scholar]
- 16.van Staa T.P., Leufkens H.G., Abenhaim L., Begaud B., Zhang B., Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 17.Google. Google Translate. https://translate.google.co.uk/ [accessed 03.09.15].
- 18.Scottish Intercollegiate Guidelines Network. Search Filters. http://www.sign.ac.uk/methodology/filters.html [accessed 03.09.15].
- 19.Suzuki Y., Motoya S., Takazoe M., Kosaka T., Date M., Nii M. Efficacy and tolerability of oral budesonide in Japanese patients with active Crohn′s disease: a multicentre, double-blind, randomized, parallel-group Phase II study. J Crohns Colitis. 2013;7:239–247. doi: 10.1016/j.crohns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 20.The Cochrane Collaboration. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors) Cochrane handbook for systematic reviews of interventions; 2011.
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaronson D., Kaiser H., Dockhorn R., Findlay S., Korenblat P., Thorsson L. Effects of budesonide by means of the Turbuhaler on the hypothalmic–pituitary–adrenal axis in asthmatic subjects: a dose–response study. J Allergy Clin Immunol. 1998;101:312–319. doi: 10.1016/S0091-6749(98)70241-6. [DOI] [PubMed] [Google Scholar]
- 23.Campieri M., Ferguson A., Doe W., Persson T., Nilsson L.G. Oral budesonide is as effective as oral prednisolone in active Crohn′s disease. The Global Budesonide Study Group. Gut. 1997;41:209–214. doi: 10.1136/gut.41.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cydulka R.K., Emerman C.L. A pilot study of steroid therapy after emergency department treatment of acute asthma: is a taper needed? J Emerg Med. 1998;16:15–19. doi: 10.1016/s0736-4679(97)00227-8. [DOI] [PubMed] [Google Scholar]
- 25.Feiss G., Morris R., Rom D., Mansfield L., Dockhorn R., Ellis E. A comparative study of the effects of intranasal triamcinolone acetonide aerosol (ITAA) and prednisone on adrenocortical function. J Allergy Clin Immunol. 1992;89:1151–1156. doi: 10.1016/0091-6749(92)90299-h. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson A., Campieri M., Doe W., Persson T., Nygard G. Oral budesonide as maintenance therapy in Crohn′s disease—results of a 12-month study. Global Budesonide Study Group. Aliment Pharmacol Ther. 1998;12:175–183. doi: 10.1046/j.1365-2036.1998.00285.x. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg G.R., Feagan B.G., Martin F., Sutherland L.R., Thomson A.B., Williams C.N. Oral budesonide as maintenance treatment for Crohn′s disease: a placebo-controlled, dose-ranging study. Canadian Inflammatory Bowel Disease Study Group. Gastroenterology. 1996;110:45–51. doi: 10.1053/gast.1996.v110.pm8536887. [DOI] [PubMed] [Google Scholar]
- 28.Hellers G., Cortot A., Jewell D., Leijonmarck C.E., Lofberg R., Malchow H. Oral budesonide for prevention of postsurgical recurrence in Crohn′s disease. The IOIBD Budesonide Study Group. Gastroenterology. 1999;116:294–300. doi: 10.1016/s0016-5085(99)70125-3. [DOI] [PubMed] [Google Scholar]
- 29.Kirwan J.R., Hickey S.H., Hallgren R., Mielants H., Bjorck E., Persson T. The effect of therapeutic glucocorticoids on the adrenal response in a randomized controlled trial in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:1415–1421. doi: 10.1002/art.21747. [DOI] [PubMed] [Google Scholar]
- 30.Lofberg R., Rutgeerts P., Malchow H., Lamers C., Danielsson A., Olaison G. Budesonide prolongs time to relapse in ileal and ileocaecal Crohn′s disease. A placebo controlled one year study. Gut. 1996;39:82–86. doi: 10.1136/gut.39.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutgeerts P., Lofberg R., Malchow H., Lamers C., Olaison G., Jewell D. A comparison of budesonide with prednisolone for active Crohn′s disease. N Engl J Med. 1994;331:842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- 32.Sorkness C.A., LaForce C., Storms W., Lincourt W.R., Edwards L., Rogenes P.R. Effects of the inhaled corticosteroids fluticasone propionate, triamcinolone acetonide, and flunisolide and oral prednisone on the hypothalamic–pituitary–adrenal axis in adult patients with asthma. Clin Ther. 1999;21:353–367. doi: 10.1016/S0149-2918(00)88292-2. [DOI] [PubMed] [Google Scholar]
- 33.Vargas R., Dockhorn R.J., Findlay S.R., Korenblat P.E., Field E.A., Kral K.M. Effect of fluticasone propionate aqueous nasal spray versus oral prednisone on the hypothalamic–pituitary–adrenal axis. J Allergy Clin Immunol. 1998;102:191–197. doi: 10.1016/s0091-6749(98)70085-5. [DOI] [PubMed] [Google Scholar]
- 34.Ackerman G.L., Nolsn C.M. Adrenocortical responsiveness after alternate-day corticosteroid therapy. N Engl J Med. 1968;278:405–409. doi: 10.1056/NEJM196802222780801. [DOI] [PubMed] [Google Scholar]
- 35.Bacon P.A., Myles A.B., Beardwell C.G., Daly J.R. Corticosteroid withdrawal in rheumatoid arthritis. Lancet. 1966;2:935–937. doi: 10.1016/s0140-6736(66)90537-x. [DOI] [PubMed] [Google Scholar]
- 36.Barrier J.H., Larrose C., Magadur/Joly G., Hamidou M. Potential risks of the withdrawal of corticoids in Horton′s disease. Prospective study of 22 patients. Rev Med Interne. 1993;14:976. doi: 10.1016/s0248-8663(05)80094-3. [DOI] [PubMed] [Google Scholar]
- 37.Beris P., Burger A., Favre L., Riondel A., Miescher P.A. Adrenocortical responsiveness after discontinuous corticosteroid therapy. Klin Wochenschr. 1986;64:70–75. doi: 10.1007/BF01784132. [DOI] [PubMed] [Google Scholar]
- 38.Bloch-Michel H., Gorins A., Thevenet M. Hypothalamo-hypophyso-adrenal activity under corticotherapy. Comparative study of ACTH, synacthene, metopirone, lysine-vasopressin and pyrogen tests. Ann Endocrinol. 1969;30:470–479. [PubMed] [Google Scholar]
- 39.Boots J.M., van den Ham E.C., Christiaans M.H., van Hooff J.P. Risk of adrenal insufficiency with steroid maintenance therapy in renal transplantation. Transplant Proc. 2002;34:1696–1697. doi: 10.1016/s0041-1345(02)02987-1. [DOI] [PubMed] [Google Scholar]
- 40.Bromberg J.S., Alfrey E.J., Barker C.F., Chavin K.D., Dafoe D.C., Holland T. Adrenal suppression and steroid supplementation in renal transplant recipients. Transplantation. 1991;51:385–390. doi: 10.1097/00007890-199102000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Canafax D.M., Mann H.J., Sutherland D.E.R., Simmons R.L., Najarian J.S. The use of a cosyntropin stimulation test to predict adrenal suppression in renal-transplant patients being withdrawn from prednisone. Transplantation. 1983;36:143–146. doi: 10.1097/00007890-198308000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Cayton R.M., Howard P. Plasma cortisol and use of hydrocortisone in treatment of status asthmaticus. Thorax. 1973;28:567–573. doi: 10.1136/thx.28.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasgupta B., Gray J., Fernandes L., Olliff C. Treatment of polymyalgia rheumatica with intramuscular injections of depot methylprednisolone. Ann Rheum Dis. 1991;50:942–945. doi: 10.1136/ard.50.12.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desrame J., Sabate J.M., Agher R., Bremont C., Gaudric M., Couturier D. Assessment of hypothalamic–pituitary–adrenal axis function after corticosteroid therapy in inflammatory bowel disease. Am J Gastroenterol. 2002;97:1785–1791. doi: 10.1111/j.1572-0241.2002.05786.x. [DOI] [PubMed] [Google Scholar]
- 45.Droszcz W., Malunowicz E., Lech B., Krawczynska H., Madalinska M. Assessment of adrenocortical function in asthmatic patients on long-term triamcinolone acetonide treatment. Ann Allergy. 1979;42:41–43. [PubMed] [Google Scholar]
- 46.Flood L., Lofberg R., Stierna P., Wikstrom A.C. Glucocorticoid receptor mRNA in patients with ulcerative colitis: a study of responders and nonresponders to glucocorticosteroid therapy. Inflammatory Bowel Dis. 2001;7:202–209. doi: 10.1097/00054725-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Gellner R., Stange M., Schiemann U., Domschke W., Hengst K. CRH test prior to discontinuation of long-term low-dose glucocorticoid therapy. Exp Clin Endocrinol Diabetes. 1999;107:561–567. doi: 10.1055/s-0029-1232566. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh P., Taneja R.L., Malhotra S.C., Kumar V., Ahuja M.M. A study of diurnal variation of adrenocortical function and recovery of the functional integrity of pituitary adrenal axis after long term corticosteroid therapy. Indian J Med Res. 1974;62:246–253. [PubMed] [Google Scholar]
- 49.Goth E., Goergenyi G., Foevenyi J., Szanto E. Adrenocortical and pituitary function following protracted prednisolone treatment. Orv Hetil. 1964;105:2314–2317. [PubMed] [Google Scholar]
- 50.Han H.S., Shim Y.K., Kim J.E., Jeon H.J., Lim S.N., Oh T.K. A pilot study of adrenal suppression after dexamethasone therapy as an antiemetic in cancer patients. Support Care Cancer. 2012;20:1565–1572. doi: 10.1007/s00520-011-1248-z. [DOI] [PubMed] [Google Scholar]
- 51.Hedner P., Persson G. Suppression of the hypothalamic–pituitary–adrenal axis after a single intramuscular injection of methylprednisolone acetate. Ann Allergy. 1981;47:176–179. [PubMed] [Google Scholar]
- 52.Henzen C., Suter A., Lerch E., Urbinelli R., Schorno X.H., Briner V.A. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet. 2000;355:542–545. doi: 10.1016/S0140-6736(99)06290-X. [DOI] [PubMed] [Google Scholar]
- 53.Hicklin J.A., Wills M.R. Plasma “cortisol” response to synacthen in patients on long-term small-dose prednisone therapy. Ann Rheum Dis. 1968;27:33–37. doi: 10.1136/ard.27.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann H., Richter E.I., Seeligmuller K., Klingelhofer D., Mayer G. Plasma–cortisol levels in bronchial asthma, during attacks of asthma and during treatment with corticosteroids. Dtsch Med Wochenschr. 1972;97:1207–1210. doi: 10.1055/s-0028-1107530. [DOI] [PubMed] [Google Scholar]
- 55.Holub D.A., Jailer J.W., Kitay J.I., Frantz A.G. Direct and indirect estimation of pituitary adrenocorticotropin reserves in man following adrenal steroid therapy. J Clin Endocrinol Metab. 1959;19:1540–1552. doi: 10.1210/jcem-19-12-1540. [DOI] [PubMed] [Google Scholar]
- 56.Jasani M.K., Boyle J.A., Dick W.C., Williams J., Taylor A.K., Buchanan W.W. Corticosteroid-induced hypothalamo–pituitary–adrenal axis suppression—prospective study using 2 regimens of corticosteroid therapy. Ann Rheum Dis. 1968;27:352–359. doi: 10.1136/ard.27.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jasani M.K., Boyle J.A., Greig W.R., Dalakos T.G., Browning M.C., Thompson A. Corticosteroid-induced suppression of the hypothalamo–pituitary–adrenal axis: observations on patients given oral corticosteroids for rheumatoid arthritis. Q J Med. 1967;36:261–276. [PubMed] [Google Scholar]
- 58.Jasani M.K., Diver M.J., Bell A.M., Dalakos T.G., Buchanan W.W. Some clinical observations on the diurnal rhythm of plasma cortisol (11-OHCS) in patients with rheumatoid arthritis treated with oral corticosteroid drugs. Curr Med Res Opin. 1974;2:373–386. doi: 10.1185/03007997409112653. [DOI] [PubMed] [Google Scholar]
- 59.Kaden J., Egerer K., May G., Schreiner S., Wesslau C., Schonemann C. Postoperative endogenous serum cortisol level as indicator of the immunosuppressive efficacy of prednisolone in kidney allograft recipients. Transplant Proc. 1992;24:2622–2623. [PubMed] [Google Scholar]
- 60.Kane K.F., Emery P., Sheppard M.C., Stewart P.M. Assessing the hypothalamo–pituitary–adrenal axis in patients on long-term glucocorticoid therapy: the short synacthen versus the insulin tolerance test. QJM. 1995;88:263–267. [PubMed] [Google Scholar]
- 61.Kovarsky J., Pegram S.B. Bolus intramuscular methylprednisolone acetate as a therapeutic adjunct in rheumatoid-arthritis. South Med J. 1986;79:1521–1523. doi: 10.1097/00007611-198612000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Landon J., Wynn V., James V.H., Wood J.B. Adrenal response to infused corticotropin in subjects receiving glucocorticoids. J Clin Endocrinol Metab. 1965;25:602–611. doi: 10.1210/jcem-25-5-602. [DOI] [PubMed] [Google Scholar]
- 63.LaRochelle G.E., Jr., LaRochelle A.G., Ratner R.E., Borenstein D.G. Recovery of the hypothalamic–pituitary–adrenal (HPA) axis in patients with rheumatic diseases receiving low-dose prednisone. Am J Med. 1993;95:258–264. doi: 10.1016/0002-9343(93)90277-v. [DOI] [PubMed] [Google Scholar]
- 64.Levitt M., Sharma R.N., Faiman C. Normal metyrapone response after 1 month of high-dose methylprednisolone in cancer patients: a phase I study. Cancer Treat Rep. 1979;63:1327–1330. [PubMed] [Google Scholar]
- 65.Livanou T., Ferriman D., James V.H. Recovery of hypothalamo-pituitary-adrenal function after corticosteroid therapy. Lancet. 1967;2:856–859. doi: 10.1016/s0140-6736(67)92592-5. [DOI] [PubMed] [Google Scholar]
- 66.Maberly D.J., Gibson G.J., Butler A.G. Recovery of adrenal function after substitution of beclomethasone dipropionate for oral corticosteroids. Br Med J. 1973;1:778–779. doi: 10.1136/bmj.1.5856.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malone D.N., Grant I.W., Percy-Robb I.W. Hypothalamo–pituitary–adrenal function in asthmatic patients receiving long-term corticosteroid therapy. Lancet. 1970;2:733–735. doi: 10.1016/s0140-6736(70)90217-5. [DOI] [PubMed] [Google Scholar]
- 68.Meakin J.W., Tantongco M.S., Crabbe J., Bayles T.B., Nelson D.H. Pituitary–adrenal function following long-term steroid therapy. Am J Med. 1960;29:459–464. doi: 10.1111/j.1749-6632.1960.tb42866.x. [DOI] [PubMed] [Google Scholar]
- 69.Miozzari M., Ambuhl P.M. Steroid withdrawal after long-term medication for immunosuppressive therapy in renal transplant patients: adrenal response and clinical implications. Nephrol Dial Transplant. 2004;19:2615–2621. doi: 10.1093/ndt/gfh421. [DOI] [PubMed] [Google Scholar]
- 70.Miro J., Amado J.A., Pesquera C., Lopez-Cordovilla J.J., Berciano J. Assessment of the hypothalamic–pituitary–adrenal axis function after corticosteroid therapy for MS relapses. Acta Neurol Scand. 1990;81:524–528. doi: 10.1111/j.1600-0404.1990.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 71.Myles A.B., Bacon P.A., Daly J.R. Single daily dose corticosteroid treatment—effect on adrenal function and therapeutic efficacy in various diseases. Ann Rheum Dis. 1971;30:149–153. doi: 10.1136/ard.30.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pollok M., Worrlein B., Heppner C., MullerWieland D., Baldamus C.A., Kopp K.F. Long term steroid therapy in stable kidney transplant recipients does not imply secondary adrenal insufficiency. J Am Soc Nephrol. 1997;8:A3251. [Google Scholar]
- 73.Pugnet G., Sailler L., Astudillo L., Vernet J., Bennet A., Arlet P. Frequency and risk factors of biological adrenal insufficiency screened by the 250 microg Synacthene stimulation test after a prolonged course of systemic glucocorticoid therapy. A study of 100 patients. Rev Med Interne. 2010;31:332–336. doi: 10.1016/j.revmed.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Robinson B.H., Mattingly D., Cope C.L. Adrenal function after prolonged corticosteroid therapy. Br Med J. 1962;1:1579–1584. doi: 10.1136/bmj.1.5292.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodger R.S., Watson M.J., Sellars L., Wilkinson R., Ward M.K., Kerr D.N. Hypothalamic-pituitary-adrenocortical suppression and recovery in renal transplant patients returning to maintenance dialysis. Q J Med. 1986;61:1039–1046. [PubMed] [Google Scholar]
- 76.Santen R.T., Schlaghecke R., Schwalen A. Assessment of the hypothalamic–hypophyseal–adrenal axis in patients with chronic obstructive lung disease. Comparison of inhalant with systemic glucocorticoid therapy. Dtsch Med Wochenschr. 1997;122:497–503. doi: 10.1055/s-2008-1047643. [DOI] [PubMed] [Google Scholar]
- 77.Schlaghecke R., Ridderskamp P., Degner F.L., Juli E. Corticotropin-releasing hormone (CRH) test for monitoring glucocorticoid therapy. Dtsch Med Wochenschr. 1990;115:1136–1140. doi: 10.1055/s-2008-1065132. [DOI] [PubMed] [Google Scholar]
- 78.Schurmeyer T.H., Tsokos G.C., Avgerinos P.C., Balow J.E., D’Agata R., Loriaux D.L. Pituitary–adrenal responsiveness to corticotropin-releasing hormone in patients receiving chronic, alternate day glucocorticoid therapy. J Clin Endocrinol Metab. 1985;61:22–27. doi: 10.1210/jcem-61-1-22. [DOI] [PubMed] [Google Scholar]
- 79.Spiegel R.J., Vigersky R.A., Oliff A.I., Echelberger C.K., Bruton J., Poplack D.G. Adrenal suppression after short-term corticosteroid therapy. Lancet. 1979;1:630–633. doi: 10.1016/s0140-6736(79)91077-8. [DOI] [PubMed] [Google Scholar]
- 80.Then Bergh F., Kumpfel T., Schumann E., Held U., Schwan M., Blazevic M. Monthly intravenous methylprednisolone in relapsing-remitting multiple sclerosis—reduction of enhancing lesions, T2 lesion volume and plasma prolactin concentrations. BMC Neurol. 2006;6:19. doi: 10.1186/1471-2377-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Treadwell B.L., Salvage O., Sever E.D., Copeman W.S. Pituitary–adrenal function during corticosteroid therapy. Lancet. 1963;1:355–358. doi: 10.1016/s0140-6736(63)91383-7. [DOI] [PubMed] [Google Scholar]
- 82.Webb J., Clark T.J.H. Recovery of plasma corticotropin and cortisol-levels after a 3-week course of prednisolone. Thorax. 1981;36:22–24. doi: 10.1136/thx.36.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zamkoff K., Kirshner J., Cass D., Miller M. Adrenal response to serial cosyntropin stimulation after repeated high-dose prednisone administration in patients with lymphoma. Cancer Treat Rep. 1981;65:563–566. [PubMed] [Google Scholar]
- 84.Zuckner J., Uddin J., Ramsey R.H. Low dosage steroid therapy in rheumatoid arthritis-evaluation of effectiveness toxicity and adrenal–pituitary suppression. Arthritis Rheum. 1965;8:478. [Google Scholar]
- 85.Alten R., Doring G., Cutolo M., Gromnica-Ihle E., Witte S., Straub R. Hypothalamus–pituitary–adrenal axis function in patients with rheumatoid arthritis treated with nighttime-release prednisone. J Rheumatol. 2010;37:2025–2031. doi: 10.3899/jrheum.100051. [DOI] [PubMed] [Google Scholar]
- 86.Bakran I., Jr., Korsic M., Durakovic Z., Vrhovac B., Tajic M. The effect of alternate-day prednisone therapy on cortisol secretion rate in corticosteroid-dependent asthmatics. Int J Clin Pharmacol Biopharm. 1977;15:57–60. [PubMed] [Google Scholar]
- 87.D’Haens G.R., Kovacs A., Vergauwe P., Nagy F., Molnar T., Bouhnik Y. Clinical trial: preliminary efficacy and safety study of a new Budesonide-MMX(R) 9 mg extended-release tablets in patients with active left-sided ulcerative colitis. J Crohns Colitis. 2010;4:153–160. doi: 10.1016/j.crohns.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 88.Havranek V., Witek F., Haack D., Templ H., Dorda W., Kummer F. Lung-function and acth stimulation of adrenal-cortical function during oral and intramuscular therapy with triamcinolone. Atemwegs—Lungenkrankh. 1986;12:104–109. [Google Scholar]
- 89.Kroot E.J., Huisman A.M., Van Zeben J., Wouters J.M., Van Paassen H.C. Oral pulsed dexamethasone therapy in early rheumatoid arthritis: a pilot study. Ann N Y Acad Sci. 2006;1069:300–306. doi: 10.1196/annals.1351.028. [DOI] [PubMed] [Google Scholar]
- 90.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Begg C., Cho M., Eastwood S., Horton R., Moher D., Olkin I. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. J Am Med Assoc. 1996;276:637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 92.Broersen L.H., Pereira A.M., Jorgensen J.O., Dekkers O.M. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:2171–2180. doi: 10.1210/jc.2015-1218. [DOI] [PubMed] [Google Scholar]
- 93.Joint Formulary Committee, Glucocorticoid therapy . BMJ Group and Pharmaceutical Press; London: 2013. British National Formulary; p. 462. [Google Scholar]
- 94.National Institute for Health and Care Excellence. Corticosteroids—oral. http://cks.nice.org.uk/corticosteroids-oral#!topicsummary [accessed 15.09.20].
- 95.Stewart P.M., Corrie J., Seckl J.R., Edwards C.R., Padfield P.L. A rational approach for assessing the hypothalamo–pituitary–adrenal axis. Lancet. 1988;1:1208–1210. doi: 10.1016/s0140-6736(88)92020-x. [DOI] [PubMed] [Google Scholar]
- 96.Agha A., Tomlinson J.W., Clark P.M., Holder G., Stewart P.M. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic–pituitary–adrenal axis. J Clin Endocrinol Metab. 2006;91:43–47. doi: 10.1210/jc.2005-1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material