Abstract
Background
Neck pain is a frequent complaint in office workers. This pain can be caused by myofascial trigger points (MTrPs) in the trapezius muscle. This study aimed to determine the effectiveness of deep dry needling (DDN) of active MTrPs in the trapezius muscle.
Methods
A randomized, single blinded clinical trial was carried out at the Physical Therapy Department at Physiotherapy in Women's Health Research Group at Physical Therapy Department of University of Alcalá, in Alcalá de Henares, Madrid, Spain. Forty-four office workers with neck pain and active MTrPs in the trapezius muscle were randomly allocated to either the DDN or the control group (CG). The participants in the DDN group were treated with DDN of all MTrPs found in the trapezius muscle. They also received passive stretch of the trapezius muscle. The CG received the same passive stretch of the trapezius muscle only. The primary outcome measure was subjective pain intensity, measured using a visual analogue scale (VAS). Secondary outcomes were pressure pain threshold (PPT), cervical range of motion (CROM) and muscle strength. Data were collected at baseline, after interventions and 15 days after the last treatment.
Results
Differences were found between the DDN group and the CG for the VAS (P < 0.001), PPT (P < 0.001), range of motion (AROM) (P < 0.05) and strength (P < 0.05) after intervention and at the 15-day follow-up.
Discussion
Deep dry needling and passive stretch seems to be more effective than passive stretch only. The effects are maintained in the short term. The results support the use of DDN in the management of trapezius muscle myofascial pain syndrome in neck pain.
Keywords: Myofascial pain syndromes, Physical therapy, Muscle stretching exercises, Neck pain, Dry needling, Myofascial trigger point
Introduction
Neck pain is often found in physical therapy practice and it is a frequent cause of absenteeism in office workers.1 Thirty per cent of office workers report back pain.2 The most commonly affected area is the neck/shoulder producing 50% of work absenteeism.3 Moreover, continuous computer work, jobs with static neck/shoulder positions and high visual stressors may be related to the occurrence of myofascial trigger points (MTrPs),4 or associated with radiculopathy5 as a phenomenon of referred pain and risk factors for the incidence of MTrPs in the trapezius muscle.4,6–8
An MTrP is a hyperirritable nodule within a taut band of a muscle that is thought to be caused by a dysfunction of the motor endplates in that muscle. The area is painful on compression and can present a characteristic referred pain pattern, tenderness on pressure, motor dysfunction and autonomic phenomena.9 Myofascial trigger points were categorized by Simons et al.9 as either active or latent. While active MTrPs produce a spontaneous clinical complaint of pain, latent MTrPs are clinically silent and are only painful when properly stimulated. Both types of MTrPs may cause restricted range of motion (ROM) and weakness of the muscles harbouring them.9 Myofascial pain syndrome (MPS) is the set of sensory, motor and autonomic symptoms caused by MTrPs.9
According to the most accepted hypothesis, the aetiology of MTrP is a dysfunction of motor endplates characterized by excessive acetylcholine activity.10 New research suggests an abnormal depolarization of motor endplates as a source of provocation.11 The motor endplate dysfunction can be triggered by different mechanisms, such as direct trauma to the muscle,9 acute or chronic overload,12 postural deficiencies, visceral diseases, nerve entrapments, etc. Chronic overload of muscles, such as sustained contraction, as in computer work, or inadequate rest, has been widely reported by different authors as a cause of myalgia.6–8,13 Some authors report the existence of a continuous activity of specific motor units in the trapezius during low-level muscle contraction, such as in computer use.6,7,13 Kostopoulos and Rizopoulos4 state that active MTrPs in the trapezius muscle are frequently found in patients with mechanical neck pain. In addition, the most frequently active MTrPs found in patients with episodic tension-type headache were in the upper trapezius, temporalis and sternocleidomastoid muscles, associated with static head and neck positions.14 Palmer et al.3 concluded that the risk of suffering from neck pain in office workers who use a computer for >4 hours per day is 23.2% for women and 16% for men.
Deep dry needling (DDN) is a physical therapy technique indicated for the treatment of MTrPs.15–18 Several systematic reviews have determined that, although DDN seems to be useful for MTrP pain management, more high quality research is needed to support the recommendation for its use.19–22 Two clinical trials have reported that DDN of MTrP improves joint ROM and pressure pain threshold (PPT) of MTrP in treated muscles and attains the same effects in MTrPs located in the referred pain area.23,24 Furthermore, DDN of MTrP improves PPT, ROM and reduces the intensity of pain from MTrP in proximal muscles.24 Although both studies show positive results, the lack of proper controls, the small sample size and the lack of follow-up in the short and medium term and methodological issues regarding blinding mean that it is not possible unequivocally to establish the effectiveness of DDN in the treatment of MTrPs.23,24 A recent study with an interesting placebo methodology, proper blinding but a small sample size shows promising results for DDN in MTrP pain after total knee replacement surgery and warrants the need for more high quality research in MPS.25
The purpose of this randomized clinical trial (RCT) was to determine the effectiveness of the DDN of active MTrPs in the trapezius muscle in office workers with neck pain.
Methods
The authors carried out a randomized, single-blinded, clinical trial of office workers aged over 18 years, who presented neck pain and used the computer mouse for >4 hours/day.26 The study was undertaken at the Physiotherapy in Women's Health Research Group at Physical Therapy Department of University of Alcalá, in Alcalá de Henares, Madrid, Spain, between January 2011 and September 2013. The ‘Principe de Asturias’ Hospital Human Research Ethics Committee in Alcalá de Henares approved the study. The study was registered at the ClinicalTrials.gov register (Trial Registration: NCT02219386 https://register.clinicaltrials.gov/).
Participants were included if they had one or more active MTrPs in the trapezius muscle according to the diagnostic criteria established by Simons et al.9 Participants were required to sign an informed consent form prior to participating in this study. In line with Simons et al.,9 participants were excluded if they were under anti-inflammatory, analgesic, anticoagulant, muscle relaxant or antidepressant medication at the start of the study or 1 week before it,10 had fibromyalgia syndrome or had any contraindication to conservative or invasive physical therapy (infection, fever, hypothyroidism, fear of needles, wounds in the area of the puncture, metal allergy, cancer or systemic disease).27
Participants were enrolled by free-to-read print advertisements and emails. The free-to-read print advertisements were posted in the Physical Therapy Research Unit at University of Alcalá de Henares, Madrid, Spain, and the emails were sent to administrative personnel at the University of Alcalá by the researchers.
Participants willing to take part in the study were screened by the blinded assessor for the presence of active MTrPs in the trapezius muscle using the MTrP standardized protocol of assessment described by Simons et al.9
All participants were randomized to either the treatment group: DDN+passive stretch (n = 22), or to the control group (CG): passive stretch only (n = 22). The 44 participants completed all assessments.
All physical therapists taking part in the study had more than 9 years of experience in the diagnosis and treatment of MPS. Eligible participants were equally randomized into two groups between January 2011 and September 2013 using the computer programme, EPIDAT version 3.1 (Xunta de Galicia, 15703, Santiago de Compostela. A Coruña, Spain, 2008) to one of two groups: DDN of active MTrPs found in the trapezius muscle together with passive stretch of the muscle (DDN group, n = 22), or only passive trapezius muscle stretch (CG, n = 22). The final number of eligible participants was 44.
Both interventions lasted 3 weeks and were undertaken twice a week during 2 weeks and once a week the third week (with 3 days between adjacent sessions), comprising five sessions in all.
Assessments
The main outcome was pain intensity measured by the visual analogue scale (VAS). Secondary outcomes were PPT, active range of motion (AROM) and muscle strength. Every outcome was recorded at baseline (A0), after interventions (4 days after the fifth treatment) (A1), and at 2 weeks follow-up after A1 (A2).
The blinded assessor (ECT) screened the participants for the presence of active MTrPs in the trapezius muscle and did the baseline assessment (A0) and the two follow-up assessments (A1 and A2) of all participants. ECT remained blinded to group allocation. Participants were instructed not to reveal their allocation.
Trigger point assessment
Each active MTrP was diagnosed according to the diagnostic criteria provided by Simons et al.9 and marked with a permanent dermographic pencil (Richard-Allan Marking Surgical Pen, Aspen Surgical, 6945 Southbelt Dr. SE, Caledonia, MI 49316, United Kingdom), then covered with tape (Leukotape sport – BSM Medical GmbH, Quickbornstraße 24 . 20253 Hamburg, Germany) so that both DDN and PPT measurements were performed on the same point throughout the study.
Subjective pain intensity
Participant current neck pain intensity was scored using a VAS.28
Pressure pain threshold
The MTrP PPT was recorded in kg/cm2 using an analogue algometer (Wagner Instruments, Post Office Box 1217, Greenwich, CT 06836-1217, USA).29,30 The pressure of compression was increased gradually at a speed of ∼1 kg/s. The participant was asked to say ‘yes’ as soon as pain or discomfort occurred and at this moment the compression was stopped. Three repetitive measurements were recorded at every MTrP. The highest reading was discarded and the mean of the two remaining readings was used for analysis.9,31
Neck AROM
Active range of flexion and extension, rotation and side bending of the cervical spine were also recorded by means of a cervical range of motion (CROM) goniometer (Performance Attainment Associates, Roseville, Netherlands).32 The participant was sitting down, wearing the CROM goniometer and was asked to perform active cervical flexion, extension, rotation (left and right) and side bending (left and right); the degrees were recorded three times and the mean was calculated after discarding the lowest readings.
Muscle strength
Muscle strength was measured using a digital dynamometer (Hoggan Health Industries microFET 2 MT Digital Handheld Dynamometer, West Draper, UT, USA). These devices are more sensitive to small differences in muscle strength than manual muscle testing.33 The participant was seated and asked to perform maximal cervical isometric contractions in order in every measured direction (flexion, extension, rotation and side bending). Three separate measurements were recorded for each test consisting of a maximal isometric contraction during 3 seconds, with a rest period of 10 seconds between contractions to minimize variability due to fatigue.33
The VAS,28 algometry29,30 and goniometry31 have good validity and reproducibility.
Other variables
In the baseline assessment (A0), demographic and identification data were compiled: age, sex, occupation, family status, body mass index, sport practice frequency and antecedents related to the problem, such as the duration of symptoms and previous trauma.
Power calculations and sample size
Power analysis, using statistical programme GRANMO version 7.11 (Barcelona, Spain, 2011), showed that 22 participants per group would give a power of 80% to detect a difference in pain intensity between groups of 20 mm on the VAS, assuming a standard deviation of 25 mm on the VAS (according to findings in Ga et al.34 study), an alpha level of 0.05 and 8% dropout rate.
Interventions
Intervention in each group was applied by a different physical therapist. They were the only study members aware of group allocation but did not participate in the data analysis. Before the study, both physical therapists received special training in how to carry out the passive stretch of the trapezius muscle, so that both groups received the same type of passive stretch.
If participants no longer reported pain (VAS value = 0) before the end of the scheduled physical therapy sessions, treatment was finished, but follow-up examinations were conducted as planned.
Deep dry needling (DDN) group
The intervention included DDN of every active MTrP found in the trapezius muscle using a 4 cm × 0.32 mm acupuncture needle with guided tube (Ener-Qi Suzhou Huanqiu Acupuncture Medical Appliance Co., Suzhou City, China). In the case of the upper trapezius active MTrPs, DDN was performed in the prone position. For middle and lower trapezius muscle MTrPs, DDN was performed in a side-lying position as described by Simons et al.9 Once the needle was inserted into the MTrP previously marked by the blinded assessor, local twitch responses (LTRs) were obtained by using Hong's35 pistoning technique, which involves rapid movements of the needle in and out of the MTrP. After four LTRs, the needle was withdrawn and the area was disinfected with alcohol again.9 Then, passive stretch was performed on the trapezius muscle.
Passive stretch of the trapezius muscle (both groups)
The stretch applied was as described by Simons et al.9 The participant was in a supine position for the upper trapezius muscle stretch and in a side-lying position for the middle and lower trapezius muscle stretch. During the stretch, the physical therapist took up the slack, avoiding pain elicitation, maintaining the tension for 4 seconds and releasing the tension for 8 seconds; this cycle was repeated three times.
Data Analysis
The Statistical Package for the Social Sciences software (15.0 version) (SPSS Inc, Chicago, IL, USA, 2008) was used for the statistical analysis. Descriptive statistics were calculated to describe baseline data. The Shapiro–Wilk test was used to assess normal distribution and the chi-squared test and Mann–Whitney U-test or parametric t-test were conducted to determine whether the two groups differed on the demographic variables (age, sex) and day 0 (pre-intervention) characteristics: body mass index, hours of physical activity per week, work hours per day and assessment 0 pain, neck AROM, PPT and muscle strength (outcomes, dependent variables).
Either the ANOVA or the Friedman test was conducted to analyse dependent variables over time. Bonferroni and Dunn tests were used for multiple comparisons. Differences between the groups were analysed using the Mann–Whitney U-test (for VAS scores) and Student's t-test (for PPT, AROM and muscle strength scores) to assess the relationship between group status (DDN/CG). The association between dichotomous variables was examined using Fisher's exact test. Differences were considered statistically significant when P < 0.05.
Results
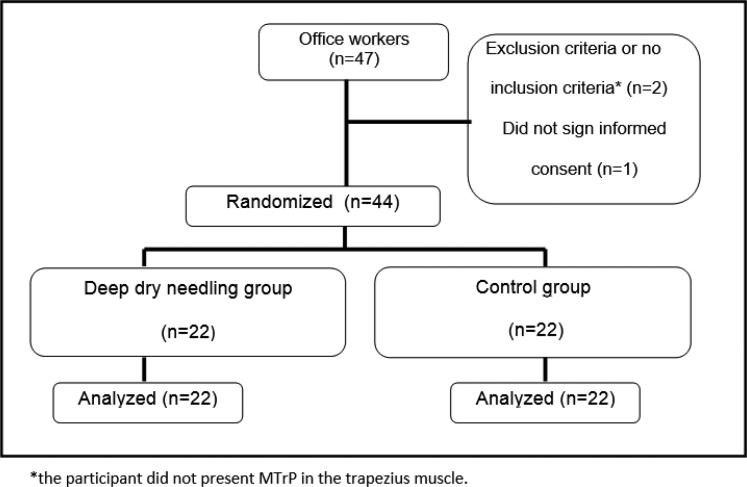
Figure 1 shows the flow of the participants throughout the study. The mean number of treatments received was three in the DDN group and five in the CG. Once the participant presented a VAS value = 0, no more treatment sessions were performed. There were no dropouts.
Figure 1.
Progress of participants throughout the study.
Table 1 shows the baseline demographics and descriptive statistics (pre-intervention frequencies and pre-intervention mean values [SD] and median values [IQR]) for the outcome variables of each group. All variables were similarly distributed between the groups at randomization. No significant differences between groups were found for any of the variables analysed regarding age, sex, body mass index, hours of work per day, sport practice frequency, pain, AROM, PPT, or muscle strength at baseline (Table 1).
Table 1.
Comparison between randomized groups at baseline. Values are numbers (percentages) unless stated otherwise
| Variables | Deep dry needling group n = 22 | Control group (CG) n = 22 | Total sample values n = 44 | P values |
|---|---|---|---|---|
| Demographic data | ||||
| Women | 77.3 | 86.4 | 81.8 | 0.698 |
| Men | 22.7 | 13.6 | 18.2 | 0.698 |
| Mean (SD) age (years) | 40.1 (13.1) | 47 (16.2) | 43.6 (15) | 0.152 |
| Mean (SD) body mass index | 24 (4.6) | 24.4 (3) | 23.97 (3.9) | 0.119 |
| Physical activity | 90.1 | 84.1 | 84.1 | 0.412 |
| Mean hours/week of physical activity (SD) | 7.5 (1.4) | 7 (1.1) | 4.16 (3.2) | 0.323 |
| Mean hours/day work (SD) | 7.18 (1.3) | 6.68 (1.6) | 6.93 (1.5) | 0.387 |
| Symptoms | ||||
| Median (IQR) VAS scores | 5.8 (1) | 5 (1.7) | 5.6 (1.5) | 0.784* |
| Mean (SD) PPT (kg/m2) | 1.9 (0.7) | 1.9 (0.7) | 1.9 (0.6) | 0.889 |
| Mean (SD) ROM flexion/extension (deg) | 104.4 (15) | 95.1 (24.4) | 99.8 (20.5) | 0.134 |
| Mean (SD) ROM rotation (deg) | 110.8 (23.8) | 113.2 (26) | 112 (24.7) | 0.751 |
| Mean (SD) ROM inclination (deg) | 73.6 (21) | 65.9 (21.3) | 70 (21.3) | 0.233 |
| Mean (SD) strength right rotation (N) | 67.1 (20.1) | 55.7 (13.6) | 61.4 (17.9) | 0.032 |
| Mean (SD) Strength Left Rotation (N) | 61.7 (20.1) | 57.7(12.9) | 59.7 (16.8) | 0.439 |
| Mean (SD) strength right inclination (N) | 75.5 (25) | 63.4 (15.9) | 69.5 (21.6) | 0.060 |
| Mean (SD) strength left inclination (N) | 72 (28.3) | 65.1 (17) | 68.5 (23.3) | 0.038 |
| Mean (SD) strength flexion (N) | 61.7 (22.9) | 62.3 (20.6) | 62 (21.5) | 0.923 |
| Mean (SD) strength extension (N) | 80.2 (25.8) | 77.4 (28.4) | 78.8 (26.8) | 0.735 |
deg: degrees in range of motion; ROM: range of motion; N: strength expressed in newtons; SD: standard deviation; VAS: visual analogue scale.
Effects of interventions
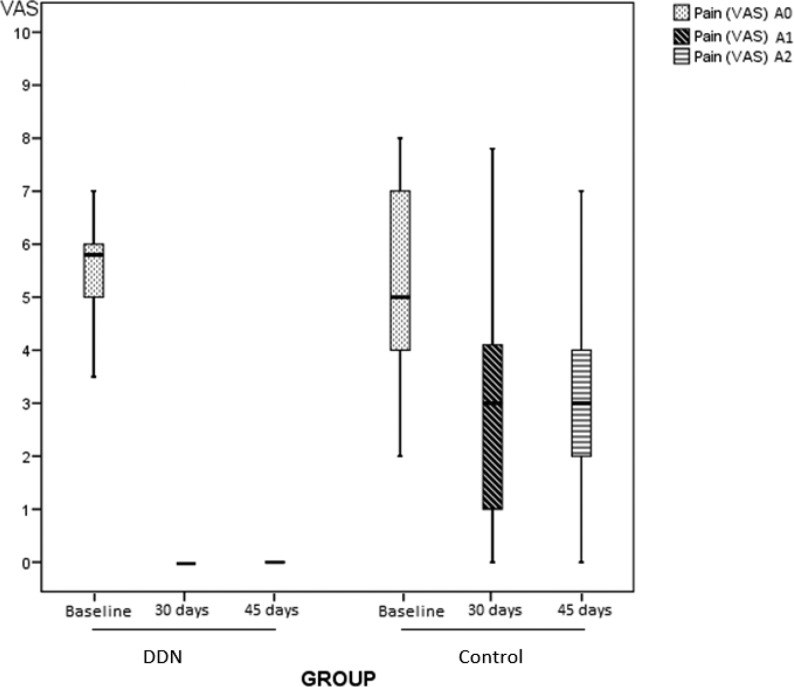
Regarding subjective pain (Fig. 2), the DDN group VAS median decreased from 5.8 to 0 after treatment (P < 0.001) and follow-up (P < 0.001). In the CG, VAS changed from 5 to 3 after treatment (P < 0.001). This value was maintained throughout the follow-up assessments (A0 vs A1/A0 vs A2; P < 0.001). In the DDN group, most participants reported not having neck pain at the end of the study. After the five sessions scheduled, only 9.1% of participants reached a VAS equal to 0 in the CG, obtaining a median VAS value of 3.5 in 50% of participants in both final and follow-up assessments (A1 & A2). DDN was significantly superior to passive stretch (P < 0.001) in decreasing pain. The median and interquartile ranges of subjective pain in each group are shown in Table 2.
Figure 2.
Evolution of pain throughout the study in both groups. Comparison of pain scores (VAS) at baseline (A0), at 30 days (A1) and at 45 days follow-up (A2).
Table 2.
Comparison intra- and inter-group [results expressed as mean (SD) except VAS expressed as median (IQR)] at baseline (A0), at 30 days (A1) and at 45 days (A2)
| Deep dry needling (DDN) | Control group (CG) | DDN vs CG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessments | Baseline (A0) | Post-intervention (A1) | Follow-up (A2) | P value (A0–A1) | P value (A0–A2) | Basal (A0) | Post-intervention (A1) | Follow-up (A2) | P value (A0–A1) | P value (A0–A2) | P value (A0–A0) | P value (A1–A1) | P value (A2–A2) |
| Pain | |||||||||||||
| Median (IQR) | 5.8 (1) | 0 (0.3) | 0 (0.3) | < 0.001** | < 0.001** | 5 (3) | 3 (3.2) | 3 (2.2) | < 0.001** | < 0.001** | 0.784* | < 0.001* | < 0.001* |
| PPT (Kg/cm2) | 1.9 (0.7) | 4.4 (1.3) | 4.3 (1.5) | < 0.001 | < 0.001 | 1.9 (0.7) | 2.9 (1.3) | 2.8 (1.4) | < 0.001 | 0.001 | 0.889 | < 0.001 | < 0.001 |
| AROM (deg) | |||||||||||||
| Flexion–extension | 104.4 (15) | 116.9 (17.4) | 119.6 (13.9) | 0.003 | 0.001 | 95.9 (24) | 102.7 (24.2) | 100.7 (22.7) | 0.120 | 0.261 | 0.134 | 0.031 | 0.002 |
| Inclination | 73.6 (21.1) | 88.1 (15) | 86.9 (15.6) | 0.006 | 0.006 | 65.9 (21.3) | 67.9 (17.6) | 67.1 (18.4) | 0.507 | 0.733 | 0.233 | < 0.001 | < 0.001 |
| Rotation | 110.8 (23.8) | 134.5 (5.7) | 133.6 (5.6) | < 0.001 | < 0.001 | 113.2 (26) | 121.4 (5.7) | 121.6 (5.3) | 0.034 | 0.057 | 0.751 | 0.029 | 0.037 |
| Strength (N) | |||||||||||||
| Flexion | 61.7 (22.9) | 86.3 (28.4) | 87.6 (29.8) | < 0.001 | < 0.001 | 62.3 (20.6) | 69.8 (21.3) | 65.8 (24.6) | 0.048 | 0.339 | 0.923 | 0.036 | 0.011 |
| Extension | 80.2 (25.8) | 103.1 (33.9) | 106.3 (31.1) | 0.001 | < 0.001 | 77.4 (28.4) | 80.3 (26.4) | 80.6 (22) | 0.356 | 0.441 | 0.735 | 0.017 | 0.003 |
| Right inclination | 75.5 (25) | 90.5 (26.7) | 93 (26.8) | 0.004 | 0.005 | 63.4 (15.8) | 72.5 (26.7) | 67.6 (24.6) | 0.114 | 0.368 | 0.060 | 0.031 | 0.003 |
| Left inclination | 72 (28) | 88.5 (26.8) | 89.1 (28.6) | < 0.001 | 0.002 | 65.1 (17) | 70.5 (24.2) | 71.3 (21.3) | 0.253 | 0.112 | 0.038 | 0.024 | 0.024 |
| Right rotation | 67.1 (20.1) | 85.2 (24.4) | 86.5 (26.1) | < 0.001 | < 0.001 | 55.7 (13.6) | 63.9 (18.3) | 61.7 (17.8) | 0.030 | 0.093 | 0.032 | 0.002 | 0.001 |
| Left rotation | 61.7 (20.1) | 83.8 (25.3) | 84.5 (25.3) | < 0.001 | < 0.001 | 57.7 (12.8) | 64.5 (18.4) | 63.6 (18.7) | 0.003 | 0.057 | 0.439 | 0.006 | 0.003 |
All outcomes used Student's t-test, except pain outcome. *Mann–Whitney U-test or **Wilcoxon test.
AROM: active range of motion; PPT: pressure pain threshold; VAS: visual analogue scale. Significant values bolded.
In the PPT comparison, both groups presented statistically significant differences after the interventions (A1 vs A1; P < .001): a PPT increase of 2.5 kg/cm2 in the DDN group (A0 vs A1; P < .001) and 1 kg/cm2 in the CG (A0 vs A1; P = .069). In follow-up assessment (A2 vs A2; P < .001), the DDN group was superior to the CG regarding the increase in PPT, with statistically significant differences (P < .001).
A statistically significant improvement in AROM in rotation, inclination and flexoextension flexion/extension was found in the DDN group (P < 0.001; P < 0.05; P < 0.05, respectively) in the A1 and A2 assessments and in rotation in CG (P < 0.05 in A1). No statistically significant differences were found either in flexion/extension or in side bending in the CG. The DDN group showed a higher improvement than the CG in AROM after interventions (A1 vs A1; P < 0.05) and at follow-up (A2 vs A2; P < 0.05).
Muscle strength in flexion, extension, right and left rotations increased significantly in the DDN group (P < .001) and in side bending (P < .05) but not in the CG (P >.05). No statistically significant differences were found in the comparisons A0 vs A1 or A0 vs A2 as paired databases in the CG, except flexion and left rotation (P < .05) in A1. The results showed statistically significant differences between both groups in post-intervention (A1) and follow-up (A2) assessments in all the values of muscle strength (P < .05) in favor to DDN group.
Mean and standard deviations of neck AROM, PPT and muscle strength in each group are shown in Table 2.
No side effects following MTrP needling were reported by participants or observed by the physical therapist.36
Discussion
Deep dry needling is a technique widely used by health professionals because of its effects in the restoration of the mechanical dynamic balance,37 improvement of local microcirculation10 and its clinical success in MTrP treatment. In this study, the authors aimed to determine whether DDN of trapezius muscle MTrPs in office workers is effective in reducing subjective neck pain, both in the short term and at two-week follow-up. Other studies have previously failed to show any improvement of pain with dry needling when the results were measured immediately after the intervention.38
Several authors discuss the relationship between the presence of MTrPs in the upper trapezius muscle and computer work.4,8,13,39 Our study seems to support this relationship as 96% of our participants presented active MTrPs in the trapezius muscle, which could indicate that maintained static positions of the neck and shoulder during computer work are precipitating and perpetuating factors of trapezius muscle MTrPs.8,14,39,40
Two systematic reviews on the needling management of MTrPs concluded that, although dry needling seemed to have a treatment effect on MTrP pain, the limited sample size and poor quality of most studies suggested the need for better research in this area.19,20 However, our results seem to give confirmatory evidence in agreement with the idea that DDN is effective in diminishing pain, at least in short term. The use of a proper blinding, control and randomization may account for the results obtained.
A recent systematic review on the use of dry needling in cervicogenic or tension-type headache argues the likelihood that dry needling is effective.22 Furthermore, another systematic review on the effectiveness of dry needling for upper-quarter myofascial pain reports better results and recommends dry needling compared to sham or placebo treatment in this region.21 However, both state the need for more well-designed studies to support those results.
Our findings add, to these promising results, dry needling better results in pain relief. Our results are in line with those of Edwards and Knowles31 regarding the convenience of deactivating active MTrPs with dry needling prior to stretching the muscle in order to reduce MTrP sensitivity or to relieve the condition. This could explain the better results obtained by DDN plus stretch when compared with stretch alone. In accordance with our results, they also found that patients in the dry needling group required a lower mean number of treatment sessions than patients in the stretching group.7,10,13,14
Ma et al.41 also found that DDN together with stretching was more effective than self-stretching alone in MPS treatment. Nevertheless, they gave no indication of the number of treatments applied to each patient, or about what they did when the participant scored 0 in the VAS scale. The fact that they used a self-stretching programme in the CG could question the differences observed between intervention groups and CG, since it is difficult to be sure that the stretching was being properly self-applied by the patient. In addition, they did not specify the adherence to the self-stretching protocol in the groups and, consequently, the authors do not know if all patients did the self-stretching protocol as recommended. Our study avoids this bias by making expert physical therapists apply the stretching which, in our opinion, could make differences among groups more reliable.
Simons et al.9 stated that the critical therapeutic factor for the effective treatment of MTrPs using dry needling is the mechanical disruption of the MTrP by the needle. According to these authors, the contraction areas that form an MTrP are located in dysfunctional motor endplates, in the endplate zone of the muscle.9 It has been proven that multiple insertions of dry needling in the endplate zone of mice muscles cause a neuromuscular injury that mechanically damages muscle fibres and motor endplates.42 There was complete regeneration in between three (neuromuscular injury) and seven (muscular injury) days.42 It is conceivable that the precise location of the MTrP with the needle, confirmed by the LTR elicitation, could contribute to the destruction of the dysfunctional muscle fibres and motor endplates that cause the MTrP, accounting for the good results observed in the DDN group.
The importance of LTRs has previously been stated and could account for the better results observed in the DDN group.43,44 Local twitch responses have been reported to decrease the concentration of sensitizing substances in the MTrP and are considered very important in breaking the centrally mediated vicious cycle of the MTrP phenomena.9,35 Thus, according to Hong,35 an LTR elicited during needling is the most definitive objective indication that the needle has been inserted precisely in the MTrP. Local twitch responses were elicited in 100% of participants in the DDN group through the entire course of treatment.
On the other hand, the therapeutic goals of the stretch technique are to reduce pain, restore the muscle to normal length and improve the range of both active and passive motion45; the increased ROM in neck movements is also consistent with Hsieh et al.'s23 findings. The fact that AROM was increased in the stretch group agrees with two other studies, and the same mechanism described may be applied to the DDN group.31,41 As pain decreases, muscle fibres recover their original length, and hence AROM improves. The fact that the changes found in rotation were higher than in the other components of AROM could be explained by the additional involvement of other muscles that were not included in the study and by the functional contribution of the trapezius muscle to the extreme rotation of the head towards the opposite side.9
Our results show that strength significantly improved in the DDN group, but not in the CG. Muscle strength is seldom assessed in MTrP studies. The mechanical factor may diminish neck strength, which agrees with our study.46–48 On the basis of the currently available evidence, it could be stated that neck strength is important to understand the potential relationship between muscle function and pathology, and that in the assessment of neck–pain patients, neck strength is often decreased in all the directions tested.48,49 In addition, strength training is associated with pain reduction.50,51 Furthermore, cervical flexor muscles present a greater fatigability in patients with neck pain.52
However, the available evidence shows that neck strength is limited to measurement of strength moment53,54 depending on the anatomic axes,54 dysfunction of a particular muscle may cause differences in the relative strengths in the three dimensions. Future studies on the treatment of MTrPs should include this variable to check if our results are confirmed.
Regarding the difficulty of controlling external interventions, such as self-administration of analgesics, anti-inflammatories, muscle relaxants or other drugs, it was solved by reminding and instructing all participants about this fact at each assessment visit. In addition, on every treatment session, participants were asked to fill out a form explaining if they had taken any drug or received any physical therapy treatment since the last visit. No external interventions were registered in our study.
The possible loss of MTrP locator marks was avoided by re-marking the location with a permanent felt-tip marker and immediately covering the mark with adhesive tape at every visit to prevent its erasure.
The fact that the passive stretch of the trapezius muscle was administered by different physical therapists may have influenced the outcomes, although consensus meetings were carried out before starting the study to ensure that both physical therapists applied the passive stretch of the trapezius muscle in the same way.
To our knowledge, the present study is the first one which considered the presence of other MTrPs perpetuating office–worker–participant's neck pain. Our study provides confirmatory evidence that an important number of our patients presented MTrPs not only in the trapezius muscle, but also in other cervical muscles (splenius, multifidus, levator scapulae), which were not treated. These muscles are considered to refer pain to the head and neck and could act as perpetuating factors of pain in our participants.9 Furthermore, since all of these muscles are related to the static position and stabilization of the head, neck and shoulder girdle, the assessment and treatment of their MTrPs should also be taken into account in future RCTs in patients with pain in these regions.
Huang et al.55 stated that long pain duration, high pain intensity, poor quality of sleep and repetitive stress are associated with poor outcomes. Some of these variables (e.g. quality of sleep, quality of life) were not registered in this RCT and should be included in future studies. In addition, taking into account the possibility that excessive computer work and static positions of the neck and head can contribute to the start and perpetuation of neck pain, the development of prevention strategies should be tested and implemented.
Most studies on DDN focus on its immediate effects.23,24,38,43,56 Although the authors did a 2-week follow-up, future studies should include longer term follow-up.
Finally, the sample was composed of participants from just one centre, and the recruitment was done by two recruitment methods, which could result in bias for this study's external validity.57
Conclusions
Deep dry needling plus passive stretch seems to be more effective in decreasing pain than passive stretch alone, increasing the PPT and cervical AROM and muscle strength in the trapezius muscle in office workers with neck pain. This would support the use of DDN in the management of MPS of the trapezius muscle. Further clinical trials with larger sample sizes, including other cervical muscles and a longer follow-up, are needed to uphold the results obtained in this clinical trial.
Disclaimer Statements
Contributors Conception and design: ECT, MTL and OMM. Provision of participants: MTL. Provision of interventions: ECT (blind assessor), BRM (passive stretch of the trapezius muscle in the CG), IFG (deep dry needling and passive stretch of the trapezius muscle in the DDN group). Data analysis and interpretation: CGO (blind analyst), ECT and MTL. Collection and assembly of data: OMM. Manuscript writing: ECT, MTL and OMM.
Funding
Conflicts of interest The authors declare no conflict of interest.
Ethics approval This clinical trial is approved by our local Research Ethics Committee. Code PI 44/11.
Acknowledgements
The authors thank the Physical Therapy in Women's Health Research Group of the Physical Therapy Department at University of Alcalá which provided the facilities for the study.
References
- 1.Côte P, Kristman V, Vidmar M, Van Eerd D, Hogg-Johnson S, Beaton D, et al. The prevalence and incidence of work absenteeism involving neck pain: a cohort of Ontario lost-time claimants. Spine (Phila Pa 1976). 2008;33:S192–8. [DOI] [PubMed] [Google Scholar]
- 2.Merllié D, Paoli P. Diez Años de condiciones de trabajo en la Unión Europea. Available from: http://www.eurofound.europa.eu/pubdocs/2000/128/es/1/ef00128es.pdf 2009.
- 3.Palmer KT, Walker-Bone K, Griffin MJ, Syddall H, Pannett B, Coggon D, et al. Prevalence and occupational associations of neck pain in the British population. Scand J Work Environ Health. 2001;27:49–56. [DOI] [PubMed] [Google Scholar]
- 4.Kostopoulos D, Rizopoulos K.. The manual of trigger point and myofascial therapy. Thorofare, NJ: Slack Inc. 2001. [Google Scholar]
- 5.Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989;151:157–60. [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahara T, Schnoz M, Laubli T, Wellig P, Krueger H. Motor-unit activity in the trapezius muscle during rest, while inputting data, and during fast finger tapping. Eur J Appl Physiol. 2000;83:181–9. [DOI] [PubMed] [Google Scholar]
- 7.Strom V, Knardahl S, Stanghelle JK, Roe C. Pain induced by a single simulated office-work session: time course and association with muscle blood flux and muscle activity. Eur J Pain. 2009;13:843–52. [DOI] [PubMed] [Google Scholar]
- 8.Zennaro D, Laubli T, Krebs D, Klipstein A, Krueger H. Continuous, intermitted and sporadic motor unit activity in the trapezius muscle during prolonged computer work. J Electromyogr Kinesiol. 2003;13:113–24. [DOI] [PubMed] [Google Scholar]
- 9.Simons DG, Travell JG, Simons LS. Myofascial pain and dysfunction: the trigger point manual: upper half of body. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 10.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14:95–107. [DOI] [PubMed] [Google Scholar]
- 11.McPartland JM, Simons DG. Myofascial trigger points: translating molecular theory into manual therapy. J Man Manip Ther. 2006;14:232–9. [Google Scholar]
- 12.Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons' integrated hypothesis of trigger point formation. Curr Pain Headache Rep. 2004;8:468–75. [DOI] [PubMed] [Google Scholar]
- 13.Kadefors R, Forsman M, Zoega B, Herberts P. Recruitment of low threshold motor-units in the trapezius muscle in different static arm positions. Ergonomics. 1999;42:359–75. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-de-Las-Penas C, Cuadrado ML, Pareja JA. Myofascial trigger points, neck mobility, and forward head posture in episodic tension-type headache. Headache. 2007;47:662–72. [DOI] [PubMed] [Google Scholar]
- 15.Hong CZ. Treatment of myofascial pain syndrome. Curr Pain Headache Rep. 2006;10:345–9. [DOI] [PubMed] [Google Scholar]
- 16.Mayoral del Moral O. Dry needling treatments for myofascial trigger points. J Muscoskel Pain. 2010;18:411–6. [Google Scholar]
- 17.Dommerholt J, Mayoral O, Gröbli C. Trigger point dry needling. J Man Manip Ther. 2006;14:E70–E87. [Google Scholar]
- 18.APTA Description of dry needling in clinical practice: an educational resource paper. Alexandria, VA, USA: APTA Public Policy, Practice, and Professional Affairs Unit; 2013. [Google Scholar]
- 19.Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil. 2001;82:986–92. [DOI] [PubMed] [Google Scholar]
- 20.Tough EA, White AR, Cummings TM, Richards SH, Campbell JL. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. Eur J Pain. 2009;13:3–10. [DOI] [PubMed] [Google Scholar]
- 21.Kietrys DM, Palombaro KM, Azzaretto E, Hubler R, Schaller B, Schlussel JM, et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43:620–34. [DOI] [PubMed] [Google Scholar]
- 22.France S, Bown J, Nowosilskyj M, Mott M, Rand S, Walters J. Evidence for the use of dry needling and physiotherapy in the management of cervicogenic or tension-type headache: a systematic review. Cephalalgia. 2014;34:994–1003. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YL, Kao MJ, Kuan TS, Chen SM, Chen JT, Hong CZ. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil. 2007;86:397–403. [DOI] [PubMed] [Google Scholar]
- 24.Tsai CT, Hsieh LF, Kuan TS, Kao MJ, Chou LW, Hong CZ. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil. 2010;89:133–40. [DOI] [PubMed] [Google Scholar]
- 25.Mayoral O, Salvat I, Martín MT, Martín S, Santiago J, Cotarelo J, et al. Efficacy of myofascial trigger point dry needling in the prevention of pain after total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. J Evid Based Complementary Altern Med. 2013;2013:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spanish Government Real Decreto 488/1997, de 14 de abril. BOE n° 97, de 23 de abril. Evaluación y prevención de los riesgos relativos a la utilización de equipos con Pantallas de Visualización. 1997.
- 27.Baldry PE.. Acupuncture, trigger points and musculoskeletal pain. London: Elsevier-Churchill-Livingstone; 2005. [Google Scholar]
- 28.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- 29.Fischer AA. Algometry in diagnosis of musculoskeletal pain and evaluation of treatment outcome: an update. J Muscoskel Pain. 1998;6:5–32. [Google Scholar]
- 30.Persson AL, Brogardh C, Sjolund BH. Tender or not tender: test-retest repeatability of pressure pain thresholds in the trapezius and deltoid muscles of healthy women. J Rehabil Med. 2004;36:17–27. [DOI] [PubMed] [Google Scholar]
- 31.Edwards J, Knowles N. Superficial dry needling and active stretching in the treatment of myofascial pain – a randomised controlled trial. Acupunct Med. 2003;21:80–6. [DOI] [PubMed] [Google Scholar]
- 32.Youdas JW, Carey JR, Garrett TR. Reliability of measurements of cervical spine range of motion – comparison of three methods. Phys Ther. 1991;71:98–104, [discussion 105–6]. [DOI] [PubMed] [Google Scholar]
- 33.Colgrove YS, Sharma N, Kluding P, Potter D, Imming K. Effect of yoga on motor function in people with Parkinson's disease a randomized, controlled pilot study. J Yoga Phys Ther. 2012;12:112. [Google Scholar]
- 34.Ga H, Choi JH, Park CH, Yoon HJ. Dry needling of trigger points with and without paraspinal needling in myofascial pain syndromes in elderly patients. J Altern Complement Med. 2007;13:617–24. [DOI] [PubMed] [Google Scholar]
- 35.Hong C-Z. Considerations and recommendations of myofascial trigger points injection. J Muscoskel Pain. 1994;2:29–59. [Google Scholar]
- 36.Brady S, McEvoy J, Dommerholt J, Doody C. Adverse events following trigger point dry needling: a prospective survey of chartered physiotherapists. J Man Manip Ther. 2014;22:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas KR, Polus BI, Rich PA. Latent myofascial trigger points: their effects on muscle activation and movement efficiency. J Bodyw Mov Ther. 2004;8:160–6. [Google Scholar]
- 38.Irnich D, Behrens N, Gleditsch JM, Stor W, Schreiber MA, Schops P, et al. Immediate effects of dry needling and acupuncture at distant points in chronic neck pain: results of a randomized, double-blind, sham-controlled crossover trial. Pain. 2002;99:83–9. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-de-las-Penas C, Alonso-Blanco C, Miangolarra JC. Myofascial trigger points in subjects presenting with mechanical neck pain: a blinded, controlled study. Man Ther. 2007;12:29–33. [DOI] [PubMed] [Google Scholar]
- 40.Sari H, Akarirmak U, Uludag M. Active myofascial trigger points might be more frequent in patients with cervical radiculopathy. Eur J Phys Rehabil Med. 2012;48:237–44. [PubMed] [Google Scholar]
- 41.Ma C, Wu S, Li G, Xiao X, Mai M, Yan T. Comparison of miniscalpel-needle release, acupuncture needling, and stretching exercise to trigger point in myofascial pain syndrome. Clin J Pain. 2010;26:251–7. [DOI] [PubMed] [Google Scholar]
- 42.Domingo A, Mayoral O, Monterde S, Santafé M. Neuromuscular damage and repair after dry needling in mice. J Evid Based Complementary Altern Med. 2013;2013:260806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong C-Z. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil. 1994;73:256–63. [DOI] [PubMed] [Google Scholar]
- 44.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–84. [DOI] [PubMed] [Google Scholar]
- 45.Iwama H, Akama Y. The superiority of water-diluted 0.25% to neat 1% lidocaine for trigger-point injections in myofascial pain syndrome: a prospective, randomized, double-blinded trial. Anesth Analg. 2000;91:408–9. [DOI] [PubMed] [Google Scholar]
- 46.Chiu TT, Lam TH, Hedley AJ. Maximal isometric muscle strength of the cervical spine in healthy volunteers. Clin Rehabil. 2002;16:772–9. [DOI] [PubMed] [Google Scholar]
- 47.Jordan A, Mehlsen J, Bulow PM, Ostergaard K, Danneskiold-Samsoe B. Maximal isometric strength of the cervical musculature in 100 healthy volunteers. Spine (Phila Pa 1976). 1999;24:1343–8. [DOI] [PubMed] [Google Scholar]
- 48.Vasavada AN, Li S, Delp SL. Three-dimensional isometric strength of neck muscles in humans. Spine (Phila Pa 1976). 2001;26:1904–9. [DOI] [PubMed] [Google Scholar]
- 49.Ylinen J, Salo P, Nykänen M, Kautiainen H, Häkkinen A. Decreased isometric neck strength in women with chronic neck pain and the repeatability of neck strength measurements. Arch Phys Med Rehabil. 2004;85:1303–8. [DOI] [PubMed] [Google Scholar]
- 50.Berg HE, Berggren G, Tesch PA. Dynamic neck strength training effect on pain and function. Arch Phys Med Rehabil. 1994;75:661–5. [DOI] [PubMed] [Google Scholar]
- 51.Highland TR, Dreisinger TE, Vie LL, Russell GS. Changes in isometric strength and range of motion of the isolated cervical spine after eight weeks of clinical rehabilitation. Spine (Phila Pa 1976). 1992;17:S77–S82. [DOI] [PubMed] [Google Scholar]
- 52.Falla D, Jull G, Rainoldi A, Merletti R. Neck flexor muscle fatigue is side specific in patients with unilateral neck pain. Eur J Pain. 2004;8:71–7. [DOI] [PubMed] [Google Scholar]
- 53.Pollock ML, Graves JE, Bamman MM. Frequency and volume of resistance training: effect on cervical extension strength. Arch Phys Med Rehabil. 1993;74:1080–6. [DOI] [PubMed] [Google Scholar]
- 54.Vasavada AN, Li S, Delp SL. Influence of muscle morphometry and moment arms on the moment-generating capacity of human neck muscles. Spine (Phila Pa 1976). 1998;23:412–22. [DOI] [PubMed] [Google Scholar]
- 55.Huang YT, Lin SY, Neoh CA, Wang KY, Jean YH, Shi HY. Dry needling for myofascial pain: prognostic factors. J Altern Complement Med. 2011;17:755–62. [DOI] [PubMed] [Google Scholar]
- 56.Chu J. Does EMG (dry needling) reduce myofascial pain symptoms due to cervical nerve root irritation? Electromyogr Clin Neurophysiol. 1997;37:259–72. [PubMed] [Google Scholar]
- 57.Maghera A, Kahlke P, Lau A, Zeng Y, Hoskins C, Corbett T, et al. You are how you recruit: a cohort and randomized controlled trial of recruitment strategies. BMC Med Res Methodol. 2014;14(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]