Abstract
Objective
Chronic viral infections, HCV and HIV, are characterized by systemic inflammation. Yet the relative levels, drivers and correlates of inflammation in these settings are not well defined.
Methods
We studied seventy-nine HIV-infected patients who had been receiving antiretroviral therapy (ART) for more than two years and had suppressed plasma HIV levels (<50 copies/ml). Two patient groups: HCV+/HIV+, HCV−/HIV+, and a control group comprised of healthy volunteers (n=20) were examined. Markers of systemic inflammation (IL-6, IP-10, sTNF-RI, and sTNF-RII), monocyte/macrophage activation (sCD163, sCD14, and neopterin), intestinal epithelial barrier loss (I-FABP and LPS), and coagulation (D-dimers) were analyzed. CD4+ naïve T cells and CD4+ recent thymic emigrants (RTE) were enumerated.
Results
Plasma levels of IP-10, neopterin, and sCD163 were higher in HCV/HIV co-infection than in HIV monoinfection and were positively correlated with indices of hepatic damage (AST, ALT, and APRI). Levels of I-FABP were comparably increased in both HIV monoinfection and HIV/HCV co-infection but LPS concentrations were highest in HCV/HIV co-infection suggesting impaired hepatic clearance of LPS. Plasma HCV levels were related to no inflammatory indices but for sCD163. In co-infected subjects, a previously recognized relationship of CD4+ naïve T cell and RTE counts to hepatocellular injury was defined more mechanistically by an inverse relationship to sCD163.
Conclusion
Hepatocellular injury in HCV/HIV co-infection is linked to elevated levels of certain inflammatory cytokines and an apparent failure to clear systemically translocated microbial products. A related decrease in CD4+ naïve T cells and recent thymic emigrants also merits further exploration.
Keywords: Antigens, CD31, Antiretroviral Therapy, Highly Active, Hepatitis C, HIV Infections, Inflammation Mediators
Introduction
An estimated 10–15% of the 35 million people living with HIV-infection worldwide are also infected with hepatitis C virus (HCV) (1). These two viral diseases can adversely influence each other. HIV speeds the course of hepatitis C infection, accelerating liver fibrosis and cirrhosis, and promoting liver cancer (2, 3). In turn, HCV co-infection has been linked to CD4+ and CD8+ T cell activation (4, 5), increased CD4+ T cell apoptosis (6, 7), and in some studies, has been associated with diminished CD4+ T lymphocyte restoration with antiretroviral therapy (ART) (8).
Indices of systemic inflammation and coagulation are now recognized as important predictors of morbidity and mortality in treated HIV infection (9–11). Here we ask if HIV infected patients with suppressed viremia on combination antiretroviral therapy have different systemic levels of inflammation or coagulation than HCV co-infected and if so, are these levels related to indices of hepatic damage.
Patients and methods
This work was approved by the Institutional Review Board of Perm Regional Center for Protection against AIDS and Infectious Diseases (IRB00008964). All patients provided their written informed consent.
Seventy-nine HIV-infected patients receiving ART for more than two years and twenty healthy controls participated. All patients had a confirmed diagnosis of HIV-infection, were adherent to their ART regimen, and had plasma HIV RNA levels <50 copies/ml. ART regimens included 2 nucleoside reverse transcriptase inhibitors (NRTIs) together with a ritonavir-boosted protease inhibitor or a non-nucleoside reverse transcriptase inhibitor. Hepatitis C virus co-infection was confirmed by the demonstration of HCV RNA in plasma by a PCR-based assay (“Quantitative RT-Gepatogen C” kit; DNA-Technology, Russia); HCV uninfected subjects each had a negative test for serum antibodies to HCV. Patients who had been exposed to interferon/ribavirin treatment were excluded from the study. HIV-infection duration was timed from the date of the first positive western blot analysis. HCV-infection duration was calculated from when the first positive ELISA was received. A report describing lymphocyte phenotype in these subjects has been published previously (12).
We studied three groups:
HIV/HCV co-infected patients (n=42);
HIV monoinfected patients (n=37);
Uninfected volunteers (n=20).
The two infected groups had no differences in nadir CD4+ T cell count (table) or prior AIDS defining conditions. No information on the alcohol consumption and smoking was provided.
Table.
Clinical characteristics of HIV/HCV co-infected and HIV mono infected patients
| Characteristics | HIV/HCV co-infected | HIV monoinfected | Uninfected |
|---|---|---|---|
|
| |||
| 1 | 2 | 3 | |
| Examined subjects (n) | 42 | 37 | 20 |
| Age (years) | 33 (32/37)• | 34 (31/41) | 31 (26/35) |
| Male | 26 (61.9%) | 8 (21.6%) | 8 (40.0%) |
| HIV transmission route | |||
| Intravenous | 36 (85.7%) | 1 (2.7%) | – |
| Sexual | 6 (14.3%) | 36 (97.3%) | – |
| Homosexuals | 0 | 0 | 0 |
| Sex workers | 0 | 0 | 0 |
| Active drug users | 0 | 0 | 0 |
| HIV infection characteristics | |||
| Infection duration (years) | 11 (9/12) P1-2<0.001 |
8 (6/10) | – |
| HAART duration (years) | 3.5 (2/5) P1-2>0.05 |
4 (3/5) | – |
| Nadir CD4+ T cell count (μl−1) | 140 (100/170) P1-2>0.05 |
150 (106/170) | – |
| CD4+ T cells at the study (μl−1) | 350 (260/450) P1-2>0.05 |
410 (290/570) P2-3<0.001 |
1050 (660/1280) P1-3<0.001 |
| HIV viral load (copies/ml) | < 50 | < 50 | – |
| HCV infection characteristics | |||
| Infection duration (years) | 11 (8/12) | – | – |
| HCV viral load (log10 copies/ml) | 6.21 (2.88/6.59) | < 2,88 | < 2,88 |
| AST (U/l) | 47 (29/75) P1-2<0.001 |
19 (17/23) P2-3>0.05 |
19 (15/24) P1-3<0.001 |
| ALT (U/l) | 59 (28/112) P1-2<0.001 |
18 (14/23) P2-3>0.05 |
19 (15/26) P1-3<0.001 |
| γ-GT (U/l) | 71 (35/122) P1-2<0.001 |
30 (23/45) P2-3>0.05 |
27 (21/34) P1-3<0.001 |
| albumin (g/l) | 41.7 (40.9/42.5) P1-2>0.05 |
41.3 (40.4/43.5) P2-3>0.05 |
41.8 (40.8/42.6) P1-3>0.05 |
| platelets (109/l) | 202 (167/244) P1-2>0.05 |
234 (177/276) | – |
| APRI | 0.6 (0.4/1.2) P1-2<0.001 |
0.2 (0.2/0.3) | – |
AST – aspartate aminotransferase; ALT – alanine aminotransferase; γ-GT – γ-glutamyl transpeptidase; APRI – AST-to-platelet ratio index.
Median with interquartile range (25th/75th%); statistics was done by Mann-Whitney method.
HIV and HCV levels in plasma
Plasma levels of HIV RNA were assessed using a Versant 440 amplifier (Siemens) and «Versant HIV 1 RNA 3.0 assay b» kits (Bayer, Germany). HCV RNA levels in plasma were measured using an iCycler IQ5 (Bio-Rad, USA) and real-time PCR «Quantitative RT-Gepatogen C» kits (DNA-Technology; Russia).
Blood samples for T cell phenotyping
Approximately 30 ml of blood was taken from each participant in Vacutainer tubes containing EDTA (Becton Dickinson). CD4+ T cell numbers were counted in real time using the IMK-Lymphocyte Kit (San Jose, CA) and a BD FACSCalibur flow cytometer. Peripheral blood mononuclear cells (PBMC) were isolated using Diacoll-1077 (Dia-M; Russia) density sedimentation. PBMC were cryopreserved in fetal calf serum and dimethyl sulfoxide, and then stored at −196°C.
Monoclonal antibodies
Fluorochrome tagged monoclonal antibodies (anti-CD3-PerCP, anti-CD4-AF700, anti-CD27-APC-Cy7, anti-CD45RA-APC, anti-CCR7-PE-Cy7, and anti-CD31-FITC) and isotype control antibodies were obtained from Becton Dickinson (San Jose, CA). LIVE/DEAD® Fixable Yellow Dead Cell Stain Kit was obtained from Life Technologies (Grand Island, NY).
Flow cytometry
PBMC were thawed and stained and viable cells were enumerated using a Becton Dickinson LSR II Flow Cytometer. Naïve CD4+ T cells were identified as CD3+CD4+CD27+CD45RA+CCR7+. Naïve CD31+ T lymphocytes were considered to be recent thymic emigrants. At least 100,000 events in the lymphocyte gate were collected for each sample. Relative values were determined from the cytometer data. Absolute lymphocyte subpopulations were calculated based on CD4+ T cell numbers detected in fresh blood.
ELISA
ELISA kits for the detection of interleukin-6 (IL-6), interferon gamma-induced protein 10 (IP-10), soluble CD163 (sCD163), soluble CD14 (sCD14), soluble tumor necrosis factor receptor-I (sTNF-RI), soluble tumor necrosis factor receptor-II (sTNF-RII), and intestinal fatty acid binding protein (I-FABP) were purchased from R&D Systems (Minneapolis, MN). D-dimer kits were purchased from Diagnostica Stago (Asnieres, France). Neopterin competitive ELISA kits were purchased from IBL International (Hamburg, Germany). Lipopolysaccharide (LPS) levels were assessed using a Hycult Biotech Limulus amebocyte lysate chromogenic endpoint assay kit (Uden, Netherlands). Assays were performed according to kit instructions. Plasma samples were diluted as needed to assure that results were within the linear range of the assay.
Statistical analysis
Data were reported as medians and interquartile ranges. Groups were compared by the Mann-Whitney test. Multiple regression analysis was used to control for the effects of possible confounding factors. Correlation analysis was performed using the Spearman method. All statistical analyses were done using “STATISTICA 6.0” software.
Results
Clinical characteristics
The ages of the HIV infected patient groups and healthy controls were comparable (table). The median age was 33 years in HIV/HCV co-infected, 34 years in HIV monoinfected and 31 years in the uninfected group. Men were overrepresented (61.9%) among HIV/HCV co-infected patients reflective of the epidemic features of HCV infection in Russia (13). In contrast, the HIV monoinfected patients in this study were predominantly (78.4%) women. The difference in the gender ratio was significant (P<0.001). In the healthy control group 40% were men. The known duration of infection was longer in “dually” infected subjects than in HIV monoinfected subjects (11 vs. 8 years, respectively). In the two infected groups, there were no differences in CD4+ T cell numbers before or after ART initiation. In HIV/HCV co-infected subjects median HCV RNA levels exceeded 1,000,000 copies/ml and liver enzymes were elevated compared with the levels in HIV monoinfected patients, while albumin levels and platelet counts in co-infected and monoinfected persons were not significantly different. Aspartate transaminase (AST) to platelet ratio index (APRI) for predicting fibrosis and cirrhosis (14) was higher in the HIV/HCV co-infected group than in the HIV monoinfected group.
Systemic inflammation indices are elevated in HCV/HIV co-infection
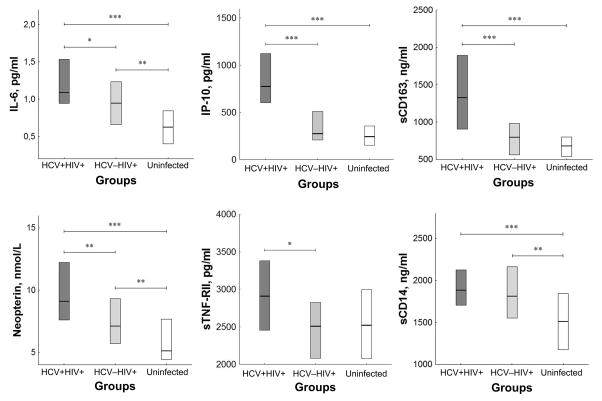
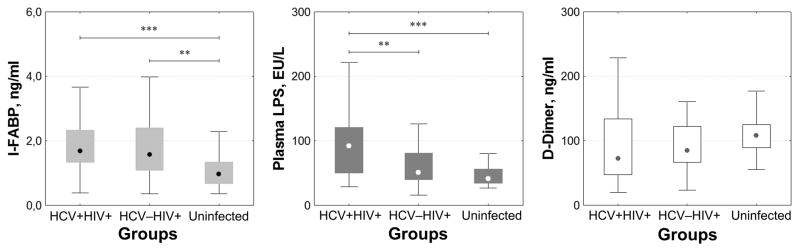
Plasma levels of the inflammatory cytokines IL-6, IP-10, and monocyte/macrophage markers (neopterin and sCD163), and sTNF-RII were higher in HIV/HCV co-infected patients than in patients who were singly HIV infected and but for sTNF-RII, were also higher than among healthy controls (fig. 1). As the two infected groups differed in duration of HIV infection and gender composition, we asked if these factors might have confounded our results. After adjustment for these factors the difference between the two HIV positive groups in the levels of IL-6 and TNF-RII lost statistical significance. With correction for gender and duration of HIV infection, levels of IP-10, sCD163, and neopterin remained significantly higher in HCV/HIV co-infected subjects than in HIV monoinfection. Median levels of IP-10, sCD163, and sTNF-RII in singly HIV infected subjects were not different from those in healthy controls, but IL-6, neopterin and sCD14 levels were higher in singly HIV infected patients than in healthy subjects. These differences in inflammatory markers may be associated with intestinal epithelium damage, as plasma I-FABP levels in both groups of HIV infected patients were also significantly higher (P<0.01) than those in the control group, and plasma LPS concentrations were higher in HCV/HIV co-infected subjects than among HIV monoinfected patients and uninfected controls (fig 2). In contrast, plasma D-dimer levels, reflecting coagulation and fibrinolysis, were similar among the three groups.
Fig. 1. Plasma indicators of systemic inflammation in HCV/HIV co-infected patients.
Plasma concentrations of IL-6, IP-10, sCD163, neopterin, sTNF-RII and sCD14 are shown in three patient groups: co-infected with HCV/HIV, HIV monoinfected, and healthy volunteers without HIV or HCV infection. Columns with horizontal lines show medians with interquartile ranges. * – P<0.05; ** – P<0.01; *** – P<0.001 (Mann-Whitney test).
Fig. 2. Plasma concentrations of I-FABP, LPS and D-dimers in HCV/HIV coinfected and HIV monoinfected patients.
Three patient groups are shown: HCV/HIV co-infected, HIV monoinfected, and healthy uninfected volunteers. Medians, interquartile ranges, upper and lower ranges are presented. ** – P <0.01; *** – P <0.001 (Mann-Whitney test).
To explore the possibility that HCV co-infection increases monocyte/macrophage activation (sCD163 and neopterin levels) and stimulates IFN-dependent production of the chemokine IP-10, we assessed the relationship of these markers to indices of HCV replication and hepatic injury.
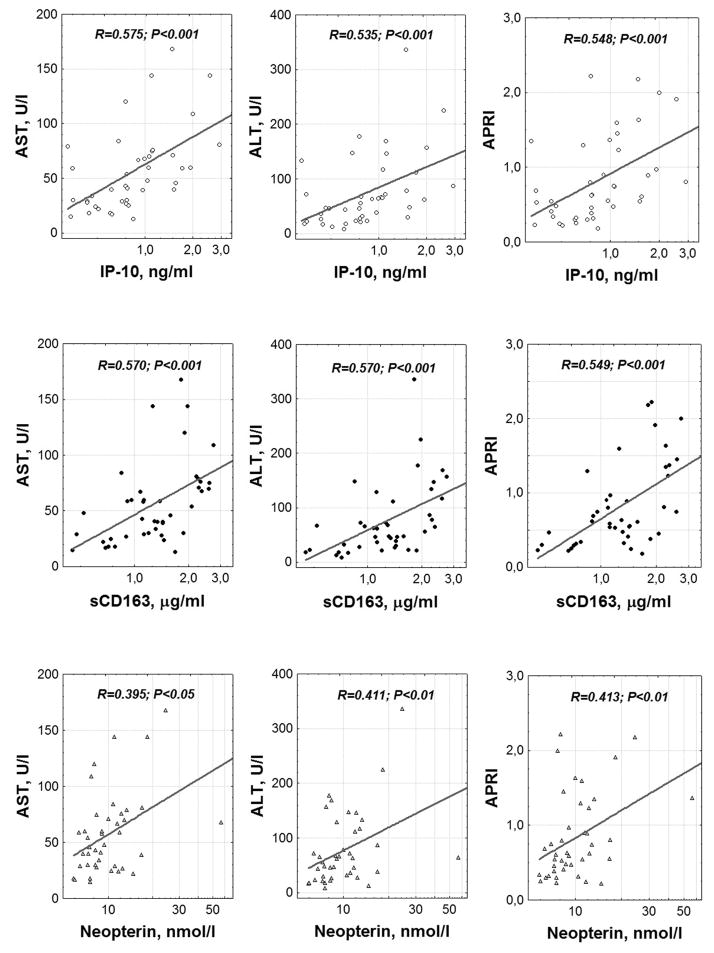
In the group of HCV/HIV co-infected patients we found highly significant and consistent correlations between indices of hepatic damage (AST, alanine transaminase (ALT), and APRI) and plasma IP-10, sCD163 and neopterin (fig. 3). Correlations with levels of sCD14, I-FABP, sTNF-RI, and sTNF-RII were not significant. AST levels correlated with D-dimers, albeit weakly (R = 0.326; P<0.05). HCV levels in plasma were also associated with liver enzyme elevations: RAST-HCV = 0.527 (P<0.001), RALT-HCV = 0.483 (P<0.01), RAPRI-HCV = 0.361 (P<0.05), but among all the markers of systemic inflammation, correlated only with sCD163 concentration (RsCD163-HCV = 0.316; P<0.05).
Fig. 3. Relationship between hepatocellular damage and indices of systemic inflammation.
Correlation analysis was performed using the Spearman method.
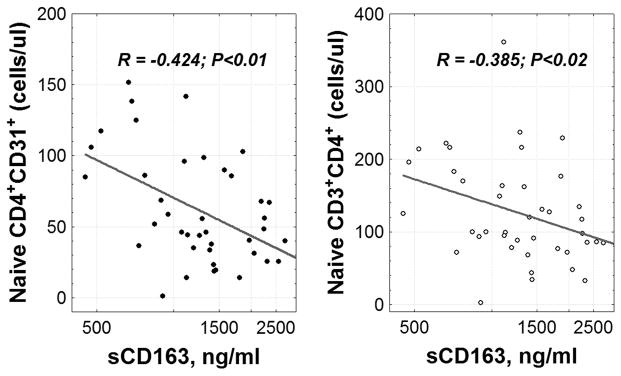
In an earlier report in this cohort, we found inverse significant relationships between the magnitude of hepatic damage (ALT, AST, and APRI) and absolute numbers of circulating CD4+ recent thymic emigrants (12). Having found that indices of hepatocellular injury are linked to inflammatory markers in HIV/HCV co-infection, we examined here the relationship between these inflammatory markers and CD4+ RTE and found that higher levels of sCD163 were associated with fewer circulating CD4+ RTE and with fewer CD4+ naïve T cells (fig. 4).
Fig. 4. Relationship between numbers of CD4+ recent thymic emigrants, naïve CD4+ T cell counts and plasma sCD163 levels in HCV/HIV co-infected subjects.
Correlation analysis was performed using Spearman method.
Discussion
HIV and HCV infections are each characterized by increases of various inflammatory marker levels in blood (15–17). With suppressive antiretroviral therapy, plasma concentrations of inflammatory markers tend to decrease but do not always normalize (18). Here, we compared plasma levels of inflammatory and coagulation markers in HIV infected and HIV/HCV co-infected patients who are receiving suppressive antiretroviral therapy. In both groups, HIV levels in plasma were suppressed while HCV replication was uncontrolled – providing a window by which to explore the effects of HCV replication in the setting of chronic HIV infection while attenuating the direct effects of HIV replication. Plasma concentrations of IL-6, IP-10, sCD163, neopterin, and sTNF-RII were significantly higher in co-infected subjects than in HIV infected patients not infected with HCV. As the patient groups were not comparable in gender composition and recognized duration of HIV infection, adjustment for these two factors left only differences in IP-10, sCD163, and neopterin remaining significantly and independently higher in HCV/HIV co-infected patients than among HIV infected subjects not infected with HCV. Although a contribution of ART-induced hepatotoxicity in the setting of HCV+HIV+ co-infection cannot be excluded, a simpler and more plausible explanation is that the observed effects are related to HCV-HCV mediated liver damage (19, 20).
Whereas there are numerous sources of IP-10, in HCV infection, IP-10 is synthesized by liver sinusoidal lining cells (21) and is induced by interferons and co-stimulated by TNF-α and IL-1 (22, 23). The primary role of the chemokine IP-10 is to recruit CD4+ Th1 cells, CD8+ cytotoxic T cells, and NK cells through interaction with CXCR3 to provide a proinflammatory antiviral immune response (24–27).
During chronic HCV infection an increase in the number of macrophage-like Kupffer cells is observed (28, 29). These cells acquire an activation phenotype (30), and express greater levels of CD33 and CD163 (31, 32). Blood levels of sCD163 may reflect systemic macrophage activation. Higher levels of sCD163 are seen in subjects with HCV/HIV co-infection than in HIV monoinfected patients (33) and in HCV infection are linked to the development of cirrhosis (34).
While effective antiretroviral therapy does not reliably result in complete suppression of immune activation and inflammatory responses in HIV infected patients (35, 36), we find here that plasma levels of IL-6, neopterin and sCD14 in subjects not co-infected with HCV remain higher than among controls while levels of IP-10, sCD163 and TNF-RII are similar to control levels. The drivers of persistent inflammation in treated HIV infection and HIV/HCV co-infection are not entirely clear but in these settings, damage to the gut epithelial barrier has been implicated in promoting translocation of microbial products from the gut lumen into the systemic circulation (37). Elevated plasma levels of I-FABP are regarded as a marker of this intestinal damage (38, 19). Interestingly, we found increased plasma levels of LPS in HIV infection and even higher levels in HIV/HCV co-infection, while in both HIV monoinfection and HIV/HCV co-infection the elevated levels of I-FABP and the LPS co-receptor sCD14 were comparable. These data suggest that in both HIV infected/HCV uninfected and HIV/HCV co-infected individuals the gut barrier defect may be comparable, allowing bacterial products access to the portal vein. However, impaired hepatic clearance of LPS in HCV co-infection may result in even higher levels of LPS in peripheral blood of HIV/HCV co-infected compared with HIV+ patients not infected with HCV. It is also possible that the impaired clearance of LPS and other microbial products not measured here may contribute to the profound increases in other inflammatory mediators that we found in HCV/HIV co-infection when compared to levels in HIV infected patients negative for HCV.
While sCD14 levels were elevated in both HIV infection and HCV/HIV co-infection sCD14 levels did not distinguish between HIV monoinfection and HIV/HCV co-infection. In contrast, Sandler et al. found that sCD14 level was correlated with markers of hepatic destruction (AST) and abnormal liver function (γ-glutamyl transpeptidase, alkaline phosphatase, and α-fetoprotein) (19), and French et al. showed that levels of sCD14 were higher in HIV/HCV co-infected women during periods of liver disease progression than during intervals when minimal or no progression occurred (39). We did not find a relationship between sCD14 and any indices of hepatocellular damage in this cohort where APRI scores are consistent with a low probability of advanced liver damage. On the other hand, we found here that plasma levels of two other markers of macrophage activation, sCD163 and neopterin and levels of the interferon-inducible protein IP-10 were significantly higher in HCV/HIV co-infection than in HIV+ patients not infected with HCV and while only sCD163 levels were correlated with plasma levels of HCV, each was correlated with three indices of hepatic damage (AST, ALT, and APRI).
In earlier works, we and others had found an inverse relationship between indices of hepatocellular damage and the frequency of circulating CD4+ T cell recent thymic emigrants as determined by expression of CD31 (12, 40). In the current work we find that circulating levels of sCD163 that are linked to indices of hepatocellular inflammation are also correlated inversely with the numbers of circulating naïve CD4 T cells and CD4+ recent thymic emigrants. The relationship among these indices remains incompletely understood but it is possible that processes taking place in the liver may play a role in alteration of CD4+ T cell recovery during ART in the setting of HCV/HIV co-infection.
Acknowledgments
Funding
This work was supported by: National Institute of Allergy and Infectious Diseases [AI 36219]; the Center for AIDS Research at Case Western Reserve University; Civilian Research and Development Foundation l; Russian Foundation for Basic Research; and Presidium of the Ural Branch of the Russian Academy of Sciences.
Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of CRDF Global, NIAID or the National Science Foundation.
Footnotes
Conflicts of interest
The authors have no conflict of interests.
References
- 1.Barreiro P, Fernandez-Montero JV, de Mendoza C, Labarga P, Soriano V. Towards hepatitis C eradication from the HIV-infected population. Antiviral Res. 2014;105:1–7. doi: 10.1016/j.antiviral.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Bourcier V, Winnock M, Ait Ahmed M, Sogni P, Pambrun E, Poizot-Martin I, et al. Primary liver cancer is more aggressive in HIV-HCV coinfection than in HCV infection. A prospective study (ANRS CO13 Hepavih and CO12 Cirvir) Clin Res Hepatol Gastroenterol. 2012;36:214–221. doi: 10.1016/j.clinre.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Curry MP. HIV and hepatitis C virus: special concerns for patients with cirrhosis. J Infect Dis. 2013;207(Suppl1):S40–44. doi: 10.1093/infdis/jis763. [DOI] [PubMed] [Google Scholar]
- 4.Feuth T, Arends JE, Fransen JH, Nanlohy NM, van Erpecum KJ, Siersema PD, et al. Complementary role of HCV and HIV in T-cell activation and exhaustion in HIV/HCV coinfection. PLoS One. 2013;8:e59302. doi: 10.1371/journal.pone.0059302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacs A, Karim R, Mack WJ, Xu J, Chen Z, Operskalski E, et al. Activation of CD8 T cells predicts progression of HIV infection in women coinfected with hepatitis C virus. J Infect Dis. 2010;201:823–834. doi: 10.1086/650997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Körner C, Tolksdorf F, Riesner K, Krämer B, Schulte D, Nattermann J, et al. Hepatitis C coinfection enhances sensitization of CD4(+) T-cells towards Fas-induced apoptosis in viraemic and HAART-controlled HIV-1-positive patients. Antivir Ther. 2011;16:1047–1055. doi: 10.3851/IMP1882. [DOI] [PubMed] [Google Scholar]
- 7.Núñez M, Soriano V, López M, Ballesteros C, Cascajero A, González-Lahoz J, et al. Coinfection with hepatitis C virus increases lymphocyte apoptosis in HIV-infected patients. Clin Infect Dis. 2006;43:1209–1212. doi: 10.1086/508355. [DOI] [PubMed] [Google Scholar]
- 8.Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV 1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 9.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shmagel KV, Saidakova EV, Korolevskaya LB, Shmagel NG, Chereshnev VA, Anthony DD, et al. Influence of hepatitis C virus coinfection on CD4+ T cells of HIV-infected patients receiving HAART. AIDS. 2014;28:2381–2388. doi: 10.1097/QAD.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 13.Kozlov AP, Shaboltas AV, Toussova OV, Verevochkin SV, Masse BR, Perdue T, et al. HIV incidence and factors associated with HIV acquisition among injection drug users in St Petersburg, Russia. AIDS. 2006;20:901–906. doi: 10.1097/01.aids.0000218555.36661.9c. [DOI] [PubMed] [Google Scholar]
- 14.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 15.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ipp H, Zemlin AE, Erasmus RT, Glashoff RH. Role of inflammation in HIV-1 disease progression and prognosis. Crit Rev Clin Lab Sci. 2014;51:98–111. doi: 10.3109/10408363.2013.865702. [DOI] [PubMed] [Google Scholar]
- 17.Leeansyah E, Malone DF, Anthony DD, Sandberg JK. Soluble biomarkers of HIV transmission, disease progression and comorbidities. Curr Opin HIV AIDS. 2013;8:117–124. doi: 10.1097/COH.0b013e32835c7134. [DOI] [PubMed] [Google Scholar]
- 18.Funderburg NT, Andrade A, Chan ES, Rosenkranz SL, Lu D, Clagett B, et al. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naïve patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One. 2013;8(12):e83514. doi: 10.1371/journal.pone.0083514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, et al. Host response to translocated microbial products predicts outcomes of patients with HBV and HCV infection. Gastroenterology. 2011;141:1220–1230. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlatzer DM, Sugalski JM, Chen Y, Barnholtz-Sloan J, Davitkov P, Hazlett FE, et al. Plasma proteome analysis reveals overlapping, yet distinct mechanisms of immune activation in chronic HCV and HIV infections. J Acquir Immune Defic Syndr. 2013;63(5):563–571. doi: 10.1097/QAI.0b013e3182909847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihm S, Schweyer S, Ramadori G. Expression of the chemokine IP-10 correlates with the accumulation of hepatic IFN-gamma and IL-18 mRNA in chronic hepatitis C but not in hepatitis B. J Med Virol. 2003;70:562–570. doi: 10.1002/jmv.10431. [DOI] [PubMed] [Google Scholar]
- 22.Apolinario A, Majano PL, Lorente R, Núñez O, Clemente G, García-Monzón C. Gene expression profile of T-cell-specific chemokines in human hepatocyte-derived cells: evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat. 2005;12:27–37. doi: 10.1111/j.1365-2893.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 23.Hu S, Ghabril M, Amet T, Hu N, Byrd D, Yang K, et al. HIV-1 coinfection profoundly alters intrahepatic chemokine but not inflammatory cytokine profiles in HCV-infected subjects. PLoS One. 2014;9:e86964. doi: 10.1371/journal.pone.0086964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 25.Wang J, Holmes TH, Cheung R, Greenberg HB, He XS. Expression of chemokine receptors on intrahepatic and peripheral lymphocytes in chronic hepatitis C infection: its relationship to liver inflammation. J Infect Dis. 2004;190:989–997. doi: 10.1086/423283. [DOI] [PubMed] [Google Scholar]
- 26.Larrubia JR, Calvino M, Benito S, Sanz-de-Villalobos E, Perna C, Pérez-Hornedo J, et al. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632–641. doi: 10.1016/j.jhep.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48(5):1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khakoo SI, Soni PN, Savage K, Brown D, Dhillon AP, Poulter LW, et al. Lymphocyte and macrophage phenotypes in chronic hepatitis C infection. Correlation with disease activity. Am J Pathol. 1997;150:963–970. [PMC free article] [PubMed] [Google Scholar]
- 29.McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260–269. doi: 10.1136/gut.46.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgio VL, Ballardini G, Artini M, Caratozzolo M, Bianchi FB, Levrero M. Expression of costimulatory molecules by kupffer cells in chronic hepatitis of hepatitis C virus etiology. Hepatology. 1998;27:1600–1606. doi: 10.1002/hep.510270620. [DOI] [PubMed] [Google Scholar]
- 31.Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraoka A, Horiike N, Akbar SM, Michitaka K, Matsuyama T, Onji M. Expression of CD163 in the liver of patients with viral hepatitis. Pathol Res Pract. 2005;201:379–384. doi: 10.1016/j.prp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Beltrán LM, Muñoz Hernández R, de Pablo Bernal RS, García Morillo JS, Egido J, Noval ML, et al. Reduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infection. PLoS One. 2014;9:e90541. doi: 10.1371/journal.pone.0090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen ES, Rødgaard-Hansen S, Moessner B, Christensen PB, Møller HJ, Weis N. Macrophage-related serum biomarkers soluble CD163 (sCD163) and soluble mannose receptor (sMR) to differentiate mild liver fibrosis from cirrhosis in patients with chronic hepatitis C: a pilot study. Eur J Clin Microbiol Infect Dis. 2014;33:117–122. doi: 10.1007/s10096-013-1936-3. [DOI] [PubMed] [Google Scholar]
- 35.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis. 2013;208:249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen KK, Pedersen M, Gaardbo JC, Ronit A, Hartling HJ, Bruunsgaard H, et al. Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long-term treatment with combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63:272–279. doi: 10.1097/QAI.0b013e318289bced. [DOI] [PubMed] [Google Scholar]
- 37.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 38.Derikx JP, Vreugdenhil AC, Van den Neucker AM, Grootjans J, van Bijnen AA, Damoiseaux JG, et al. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol. 2009;43(8):727–733. doi: 10.1097/MCG.0b013e31819194b0. [DOI] [PubMed] [Google Scholar]
- 39.French AL, Evans CT, Agniel DM, Cohen MH, Peters M, Landay AL, et al. Microbial translocation and liver disease progression in women coinfected with HIV and hepatitis C virus. J Infect Dis. 2013;208(4):679–689. doi: 10.1093/infdis/jit225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yonkers NL, Sieg S, Rodriguez B, Anthony DD. Reduced naive CD4 T cell numbers and impaired induction of CD27 in response to T cell receptor stimulation reflect a state of immune activation in chronic hepatitis C virus infection. J Infect Dis. 2011;203(5):635–645. doi: 10.1093/infdis/jiq101. [DOI] [PMC free article] [PubMed] [Google Scholar]