Abstract
Individuals with sickle cell anemia (SCA) have increased susceptibility to infections, secondary to impairment of immune function. Besides the described dysfunction in innate immunity, including impaired opsonization and phagocytosis of bacteria, evidence of dysfunction of T and B lymphocytes in SCA has also been reported. This includes reduction in the proportion of circulating CD4+ and CD8+ T cells, reduction of CD4+ helper : CD8+ suppressor T cell ratio, aberrant activation and dysfunction of regulatory T cells (Treg), skewing of CD4+ T cells towards Th2 response and loss of IgM-secreting CD27+IgMhighIgDlow memory B cells. These changes occur on the background of immune activation characterized by predominance of memory CD4+ T cell phenotypes, increased Th17 signaling and elevated levels of C-reactive protein and pro-inflammatory cytokines IL-6 and TNF-α, which may affect the immunogenicity and protective efficacy of vaccines available to prevent infections in SCA. Thus, in order to optimize the use of vaccines in SCA, a thorough understanding of T and B lymphocyte functions and vaccine reactivity among individuals with SCA is needed. Studies should be encouraged of different SCA populations, including sub-Saharan Africa where the burden of SCA is highest. This article summarizes our current understanding of lymphocyte biology in SCA, and highlights areas that warrant future research.
Keywords: T cells, B cells, phenotype, function, vaccine, sickle cell anemia
INTRODUCTION
Individuals with Sickle Cell Anemia (SCA), the homozygous (HbSS) sickle cell disease (SCD), are at an increased risk of invasive bacterial infections1–3. Particularly, the risk is high for infection with encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae2–4. Pneumococcal pneumonia, septicemia and meningitis in particular are important causes of morbidity and mortality in individuals with SCA, especially at younger age3, 5, 6. The use of penicillin prophylaxis has reduced the mortality attributable to invasive pneumococcal disease in children with SCA7–9. However, for more sustainable control of infections, engagement of the immune system via vaccination is believed to be the most durable and cost effective approach10, 11. To this effect, pneumococcal vaccines have been the standard of care for individuals with SCA in developed countries since their introduction8, 12, 13. In sub-Saharan Africa, where the burden of SCA is highest, pneumococcal vaccines are currently being introduced. Besides bacterial infections, children with SCA in sub-Saharan Africa are also at risk of mortality from malaria which is endemic in the region14–16. Thus, besides vaccines against bacterial infections, introduction of a malaria vaccine could have enormous impact on mortality in children with SCA in sub-Saharan Africa17.
The current recommendations for usage of vaccines in children with SCA are based on efficacy trials done mostly in individuals without SCA12, 18–20. Limited studies conducted thus far have indicated adaptive immune abnormalities in individuals with SCA21–29. These abnormalities, including reduction in the proportion of circulating CD4+ and CD8+ T cells, dysfunction of regulatory T cells and impaired B cell IgM secretion23, 25–27, occur concurrent with increased immune activation25, 30–40 and may affect vaccine reactivity in individuals with SCA. Thus, immune abnormalities in SCA may potentially explain incidences of impaired magnitude or duration of response to vaccines reported in individuals with SCA26, 41–45. Of note, critical studies reporting adaptive immune changes in SCA were done among SCA populations in the United States and Europe23, 25–27. It is however possible that geographic differences in lymphocyte function may also occur between SCA populations in the West compared to those in sub-Saharan Africa due to differences in environmental exposures, including malaria and other infections which are endemic in sub-Saharan Africa15, and have an impact on the immune system46, 47. Indeed, regional differences in responsiveness to Bacille Calmette-Guerin (BCG) between recipients in the West and those in sub-Saharan Africa have previously been observed48–50. Specific regional consideration is therefore essential to effective vaccine planning, since the sub-Saharan region faces a double challenge—the rates of both SCA and endemic infectious disease, including malaria, are the highest in the world15, and both factors could directly influence vaccine reactivity.
Currently, there is a critical shortage of insight on the immune status, especially adaptive immunity, in SCA. This shortage of insight limits our understanding of the potential for vaccine reactivity in SCA. It is precisely this acute gap in knowledge that calls for a detailed review of what is currently known about the immune function in SCA. This review summarizes our current understanding of the immune phenotype and function in SCA, and highlights areas that warrant future research. Thorough understanding of the immune function in SCA may lead to optimization of care, including delivery of vaccines in individuals with SCA, aiming at overcoming the burden of infections in SCA.
INNATE IMMUNE DYSFUNCTION IN SCA
Impairment of the innate immunity is the most well described immune dysfunction in individuals with SCA. Part of the dysfunction has been shown to include increased peripheral blood neutrophil count (granulocytosis), which often accounts for leukocytosis in SCA.37, 51, 52. However, the increased neutrophils are mostly dysfunctional due to impaired chemotaxis, migration and killing ability28, 52, 53. Impairment of the alternate pathway of complement activation through qualitative and quantitative deficiencies of factors B and C3 has also been reported54, 55, although this observation has not been consistent between studies27, 56.
Spleen and Innate Immune Dysfunction in SCA
Most of the innate immune changes in SCA are a manifestation of reduced splenic function (hyposplenism), which can occur in the context of atrophied or enlarged spleen53, 57, 58. This loss of splenic function in SCA has been attributed primarily to repeated sickling in the spleen with eventual destruction of the architecture and function of the spleen57, 58. The BABY HUG study demonstrated that loss of splenic function starts early on during infancy in individuals with SCA59. Splenic filtration function is often compromised as well in this setting, leading to decreased ability for trapping and removing bacteria from the circulation58, 60. Overall, SCA patients with hyposplenism show reduced opsonophagocytic activity and ability to clear bacteria from the blood28, 52, 57, 58. The loss of splenic function and ensuing reduction in opsonophagocytic activity is the most well accepted cause for the increased risk of infection with encapsulated bacteria in SCA58. In the absence of interventions, including timely vaccinations and proper coverage with prophylactic antibiotics, immune impairment due to hyposplenism in SCA can results in severe and life-threatening infections58, 61.
ADAPTIVE IMMUNE DYSFUNCTION IN SCA
High rates of alloimmunization, connective tissue diseases and transplant rejections62–66, as well as incidences of aberrant vaccine reactivity26, 41–45, have bought to surface adaptive immune abnormalities in SCA. Currently, however, little has been done to characterize T and B lymphocyte phenotype, function and contribution to chronic inflammatory diseases in SCA. Limited studies done indicate that abnormalities in both T and B cells occur in SCA21–29. These abnormalities may be induced by SCA disease itself, or may arise as a result of complications of its treatment with repeated blood transfusions. A focus on adaptive immune abnormalities in SCA will refresh our outlook towards SCA as an immune disease, and may open up novel research on immunity in SCA as well as lead to development of newer approaches in combating the immune derangement, inflammatory diseases and improving vaccine outcomes in individuals with SCA.
Disease-Induced Changes in T and B Lymphocytes
The number of circulating T cells was found to be highly variable between individuals with SCA at steady state21–23, 29. However, most studies have reported a reduction in the proportion of circulating CD4+ and CD8+ T cells in SCA21, 23, 27, 29, with normal or increased absolute CD4+ and CD8+ T cell count22, 27,37, particularly of the memory phenotype37. The reduction in the proportion of circulating CD4+ and CD8+ T cells was shown to be more profound in the presence of splenic defects and vaso-occlusive crises, where patients also show reduced CD4+ helper : CD8+ suppressor T cell ratios22, 29. In vaso-occlusive crises, there is also an increase in interleukin-4 (IL-4) secretion, suggesting a shift of CD4+ T cell response towards a T helper 2 (Th2) phenotype29. This polarization is further supported by reduced expression of the Th1 transcriptional factor T-bet and corresponding Th1 cytokines gamma interferon (INFγ) and IL-2 by CD4+ T cells in SCA24, 39. The impairment of antiviral Th1 response may explain the increased risk of hospitalization from influenza infection among children with SCA compared to those without SCA67. The frequency of regulatory T cells (Treg) in patients with SCA was found to be variable between studies25, 37, 68. A recent study by Vingert, B. et al compared Treg phenotype in individuals with and without SCA, showing that Treg in SCA were more activated and expressed high levels of CTLA-425. Similarly high CTLA-4-expressing Treg were shown to mediate suppression of pneumococcal-specific CD4+ T cells acquired through nasal carriage69, suggesting that the activated and partially dysfunctional Treg in SCA may modulate host immunity and susceptibility to invasive pneumococcal disease. Whether these Treg also impact the vaccine-induced immunity to pneumococcus and other pathogens is yet to be studied. Building on the emerging insights, further characterization of these CD4+ T cell subsets, especially from SCA populations in sub-Saharan Africa, is much needed. This will help elucidate the functional status and role of the critical CD4+ T cells in the immune defense against infections in SCA.
Unlike T cells, the proportions of circulating B cells in SCA are generally unaltered23, 26, 27, although modest increases in B cells have been observed21, 37. However, functional abnormalities in humoral immunity have been reported. These include reduction in antigen-specific B cell proliferative response and IgM secretion, with preservation of other classes of immunoglobulins26, 27. The selective loss of IgM secretion is thought to occur due to loss of the non-T cell dependent CD27+IgMhighIgDlow “IgM memory B cells” that are normally resident in the marginal zone of the spleen57, 58, 70. Loss of splenic function is thought to be the main driver of the impairment in IgM secretion since individuals without SCA who have undergone splenectomy show similar immunological alteration27. Impairment in B cell response in individuals with SCA has been shown to result in loss of IgM response to an influenza vaccine41 and significant reduction in the number of antigen-specific immunoglobulin secreting cells following vaccination with a pneumococcal polysaccharide vaccine26. Beside IgM memory B cells, more insight is also needed regarding other B cell populations, namely the naïve, pre-germinal center, germinal center, plasma cells and other B cell memory subtypes, to establish their contribution to immunity against infections in SCA71.
Among individuals with SCA, the observed immunological abnormalities vary depending on severity of disease. Unlike individuals with severe disease, those with mild SCA may have normal immunological parameters, with the exception of serum opsonization activity which is often impaired in severe as well as mild disease28. This variability is consistent with the considerable heterogeneity in the genetic makeup of individuals with SCA that impacts disease severity72–75.
Spleen and Adaptive Immune Dysfunction in SCA
As is the case with innate immune dysfunction, most disease-induced changes in adaptive immunity in SCA are attributed to hyposplenism22, 26, 27, 57, 58, 61, 76. Since loss of splenic function begins early on during infancy59, it is possible that adaptive immune dysfunction begins early on in children with SCA. Because critical IgM memory B cells require functioning spleen, patients with functional asplenia and hyposplenism, or those who have undergone splenectomy, experience loss of the non-class switched CD27+IgMhighIgDlow IgM memory B cells27, 57, 58, 76. The loss of splenic tissue has been associated with impaired IgM response following immunization with an influenza vaccine in individuals with SCA41. Similarly, the spectrum of T cell derangements in SCA, including reduction in the proportion of circulating CD4+ and CD8+ T cells, is highly associated with asplenia, hyposplenism or splenectomy22. The persistence of immune derangement following splenectomy in patients with SCA is in contrast to other blood disorders such as hereditary spherocytosis. In the latter, most hematological and immunological parameters, with the exception of reduced IgM and soluble CD8 (sCD8) secretion, are usually corrected 3 to 6 months following splenectomy27, 77, 78. More studies are needed to fully characterize the impact of hyposplenism and splenectomy on lymphocyte phenotype and function in SCA. Areas of interest include specific T and B cell subsets alteration, and kinetics of infection- and vaccine-elicited cellular and humoral immune responses during hyposplenism and following splenectomy in individuals with mild as well as severe SCA.
Transfusion-Induced Changes in T and B Lymphocytes
Patients with SCA who are recipients of multiple blood transfusions for the treatment of severe anemia or primary stroke prevention have an additional risk for immune derangements79–81. These derangements have been observed in the presence or absence of alloimmunization. Regardless of alloimunization status, patients with SCA recipients of multiple blood transfusions were found to have increased proportion of central memory CD4+ T cells39, reduced CD4+ helper : CD8+ suppressor T cell ratios and impaired natural killer (NK) cell activity82. The reduction of CD4+ helper : CD8+ suppressor T cell ratio likely represents normal immune response to multiple blood transfusions as it has also been observed in individuals with other blood disorders necessitating repeated blood transfusions82–84. The immunomodulation brought about by repeated blood transfusions was linked to reduced responsiveness of children with SCA to H1N1 vaccine45, and is hypothesized to contribute to increased infection risk81, 85, 86.
Repeated blood transfusions can result in alloimmunization in individuals with SCA. In routinely cross-matched blood, alloimmunization normally occurs via sensitization to non-ABO and non-Rhesus D antigens, including Rhesus antigens C and E, Kidd antigen Jkb, Duffy antigen Fya and MNS antigen S on red blood cell (RBC) surfaces39, 63. A number of lymphocyte abnormalities have been observed in individuals with SCA who are alloimmunized, although it is not clear whether they are a cause or effect of alloimmunization. The predominant immunological alterations observed in alloimmunized SCA individuals is the skewing of CD4+ T response towards Th2 and Th17 phenotypes with increased IL-4 and IL-17 expression, respectively, and reduction of Treg activity30, 39, 87, 88. The increase in Th2 signaling with elevated IL-4 secretion is similar to that observed in individuals with SCA during vaso-occlusive crises29. Alloimmunization has also been linked with an accentuated increase in the proportions of the central and effector memory CD4+ T cells in patients with SCA36.
To date, little has been done to characterize follicular helper CD4+ T cells (Tfh) in individuals with SCA. These cells express inducible T cell co-stimulator (ICOS) and CD40 ligand (CD40L), and secrete IL-21 and IL-4 which are potent stimulators of B cell differentiation and immunoglobulin secretion89–91. Tfh cells were shown to be play a pivotal role in responsiveness to HIV, hepatitis B, influenza and malaria vaccines92–95. Different transitional stages of Tfh cells are currently recognized based on their surface expression of the chemokine receptors CXCR5 and CCR7, as well as programmed death-1 (PD-1)91. Increased frequency of PD1+CXCR5+ Tfh cells has been observed in individuals with SCA who were not alloimmunized39. Among individuals with SCA who were alloimmunized, an increase in IL-21 secretion by PD1+CXCR5+ Tfh cells and novel “T-cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory domains” positive (TIGIT+) Tfh cells has been observed39, 96. Given their central role in modulating immune responses, it is imperative to fully characterize the phenotype and function of the different Tfh subsets in individuals with SCA.
The major consequence of alloimmunization in SCA is the increase in risk for delayed hemolytic transfusion reactions (DHTR) upon subsequent blood transfusions63, 97, which is an important cause of morbidity and mortality in individuals with SCA97. Management of alloimmunized individuals with SCA may also be complicated by difficulties in finding compatible blood which may cause delays in receiving transfusions63. Besides DHTR, alloimmunized individuals with SCA recipients of hematopoietic stem cell (HPSC) transplants were also found to have high rates of graft rejection64, 65. This is thought to be caused by sensitization to donor minor histocompatibility antigens during prior blood transfusions64, 65, 98. Overall, alloimmunization appears to significantly reduce survival in patients with SCA99.
In mitigating the risk of alloimmunization brought about by repeated blood transfusions, several approaches including extended screening for rare antigens and leukoreduction of blood for transfusion have been proposed as effective measures63, 81. Immune therapy has also shown promise in treating complications of alloimmunization in individuals with SCA. Rituximab, an anti-CD20 antibody, has been successfully used to treat and prevent severe DHTR in patients with SCA100, 101. Antithymocyte globulin (ATG) has also been used with impressive results in reducing the incidence of HPSC transplant rejection among patients with SCA102, 103. It is imperative to continue to optimize these approaches, as well as advance elucidation of immune and non-immune mechanisms that protect select SCA patients against alloimmunization.
IMMUNE ACTIVATION IN SCA
SCA is increasingly recognized as a chronic inflammatory disease characterized by considerable immune activation104, 105. Immune activation is thought to contribute to the pathogenesis of alloimmunization, transplant rejection and other inflammatory diseases in individuals with SCA.
The Spectrum and Sequelae of Immune Activation in SCA
The scope of immune activation in SCA is broad, involving cellular as well as humoral mechanisms. Besides neutrophil, monocyte, natural killer cell, platelet, mast cell and endothelial cell activation106–111, evidence of lymphocyte activation has also been reported in SCA. Markers of lymphocyte activation reported in SCA include increased number of memory T cells36, 37, 39, increased CD4+ and CD8+ T cell expression of Ki6737 and increased Treg expression of CTLA-425. Augmentation of the pro-inflammatory Th17 response has also been observed in SCA68, more so among recipients of multiple blood transfusions30, 39 (Figure 1). Furthermore, patients with SCA have increased levels of C-reactive protein31, 33, 112 and pro-inflammatory cytokines IL-6 and TNF-α31, 32, 34, 35, and increased signaling through the pro-inflammatory Toll-like receptor 4, 7 and 8 (TLR4, TLR7 and TLR8) as well as through the inflammasome complex pathways38, 40, 113, 114.
Figure 1.
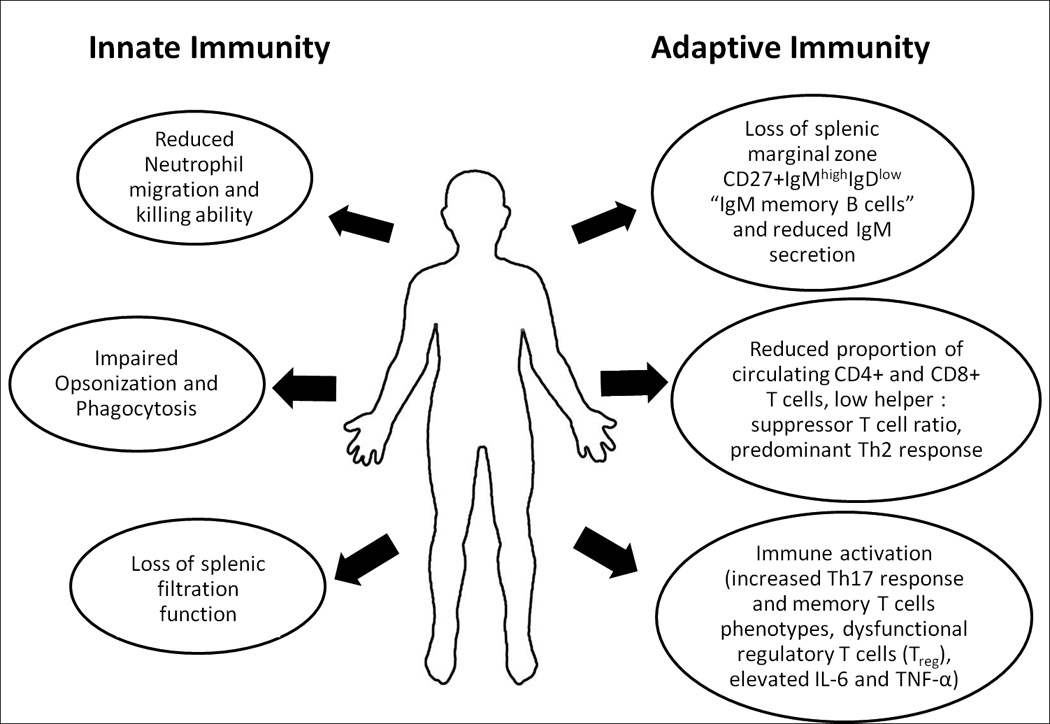
Changes in innate and adaptive immune functions in sickle cell anemia.
The state of immune activation is hypothesized to contribute to the pathophysiology of RBC alloimmunization62, 63, and may also modulate immune responses to vaccines115. The risk for alloimmunization among SCA patients receiving blood transfusions is however is not uniform63, suggesting role of genetic, immunologic and other factors in modification of alloimmunization risk30, 39, 63, 96, 116, 117. Most studies implicate a tipped balance towards increased inflammation through either impaired regulatory T cell (Treg) and B cell (Breg) activity, or enhanced pro-inflammatory environment with increased Th17 response and higher circulating levels of IL-6 and TNF-α as the underlying mechanisms that promote sensitization to allo-antigens in individuals with SCA30, 39, 63, 116, 117. Furthermore, TLR signaling was shown to enhance sensitization to allogeneic RBC antigens in mice118, 119, although its contribution to RBC alloimmunization in individuals with SCA is yet to be determined. However, protracted immune activation has been associated with T cell exhaustion and impaired immune responses120, 121, suggesting a balance between the degree and duration of immune activation in modulation of immunization risk. This delicate balance may explain the conundrum where both alloimmunization63–65 and impaired responsiveness to vaccines22, 41–43, 45 have been observed in individuals with SCA. Indeed, the risk of alloimmunization was found to be higher among SCA patients in the presence of inflammatory events such as acute chest syndrome, vaso-occlusive crisis and acute febrile illness at the time of transfusion62, where circulating levels of the pro-inflammatory cytokines are high34, 63. On the other hand, impaired responsiveness to a yellow fever vaccine has been reported in an African population in the presence of immune activation115, potentially due to immune cell exhaustion115. Infection with malaria has also been associated with lymphocyte activation46, 47, and may thus augment inflammation among individuals with SCA living in malaria-endemic areas in sub-Saharan Africa15. Given the burden of infections in SCA, it is imperative to evaluate the impact of immune activation on vaccine responsiveness among individuals with SCA.
The incidence of systemic lupus erythromatosus (SLE) was found to be higher in individuals with SCA compared to that of the general population66, concordant with an increase in titers of anti-nuclear antibodies (ANA)66. Cases of rheumatoid arthritis have also been reported in SCA122, 123, although no clear association of their occurrence with the level of immune activation in SCA has thus far been provided122. Thus, detailed elucidation of the pathogenesis of these inflammatory disorders in SCA is currently lacking. Part of the difficulties in studying these diseases in the setting of SCA is the salient similarity of their clinical presentations to that of the underlying SCA which may result in cases of SLE or rheumatoid arthritis being under-reported123–125. Whether development of SLE, rheumatoid arthritis and other inflammatory diseases in SCA is also linked to situational increases in levels of inflammation as observed with RBC alloimmunization62 will be of interest to determine. Future studies should seek to further elucidate contribution of immune activation to the risk of inflammatory diseases in SCA.
Mitigating Immune Activation in SCA: Role of Hydroxyurea
Several approaches are currently being advanced as measures to mitigate the level of immune activation in SCA. Besides reducing the transfusion-induced alloimmunization risk via extended antigen screening and leukoreduction of blood for transfusion63, 81, pharmacological agents have also shown benefits in reducing immune activation in patients with SCA. A notable example is hydroxycarbamide (hydroxyurea), which has been shown to confer beneficial reduction of immune activation in SCA. Specifically, the use of hydroxyurea has been shown to significantly overturn increased lymphocyte count, particularly circulating memory T cells, in individuals with SCA37, 126. This is postulated to occur via hydroxyurea-induced delay in G1 – S phase transition of naïve to memory T cell phenotypes through inhibition of ribonucleaotide reductase126. Hydroxyurea has also been shown to reduce TNF-α expression by lymphocytes and other immune cells34, 35, 127, and is thought to reduce mast cell activation in individuals with SCA108. Importantly, hydroxyurea was generally found to be safe and well tolerated in children with SCA128, 129, and did not increase risk of infections129 or impair responsiveness to a pneumococcal vaccine126, although a delay in responsiveness to a measles vaccine was observed126. Overall, immune changes due to hydroxyurea usage are thought to lead to beneficial normalization of the immune function and reversal of inflammation in SCA34, 35, 37, 126, 127. Indeed, in a randomized trial of hydroxyurea usage among patients with HIV on antiretroviral medication, the use of hydroxyurea resulted in the reduction of immune activation and improvement of HIV-specific CD4+ and CD8+ T cell responses130. As the usage of hydroxyurea is increasing in sub-Saharan Africa, more studies will be needed to assess its impact on immune function and vaccine reactivity in individuals with SCA.
Other inflammation-lowering strategies currently under investigation include the use of intravenous gammaglobulins, platelet adenosine diphosphate (ADP)-receptor blockers, adenosine A2A receptor agonists and triterpenoids that have shown promise in reducing neutrophil activation, platelet activation, invariant natural killer T (iNKT) cell activation and oxidative stress, respectively, in patients with SCA106, 107, 111, 131. Further investigation of the benefits of these and other agents in managing immune activation in patients with SCA is warranted.
VACCINATION IN SCA
The goal of immunization in SCA is to elicit durable memory T and B cell responses that will help fight against infections, particularly due to encapsulated bacteria, especially during childhood. Currently, a number of vaccines have been licensed for use in individuals with SCA. These include vaccines against Streptococcus pneumoniae, Haemophilus influenzae type B, Neisseria meningitides, hepatitis B and influenza57, 132. Since malaria in SCA is associated with high risk of mortality, the advent of a malaria vaccine will also help prevent deaths of children with SCA in malaria-endemic areas14, 15, 17.
Studies on safety and immunogenicity indicate that most of the available vaccines, including pneumococcal, Haemophilus influenzae type B and influenza vaccines, are generally safe and immunogenic in children with SCA133–141. Indeed, the use of pneumococcal conjugate vaccines has substantially reduced the incidence of invasive pneumococcal disease in children with SCA13, 142. Nonetheless, incidences of impaired magnitude or duration of response to vaccines have been reported in individuals with SCA26, 41–45. In a study by Hord J et al, additional booster dose of the vaccine was required to optimize responsiveness to hepatitis B vaccine among individuals with SCA43. These observations potentially reflect the unique immune environment in individuals with SCA that may influence vaccine reactivity. To date, however, few randomized controlled trials of vaccines have been done among individuals with SCA, including only one trial of pneumococcal vaccine and none of Haemophilus influenzae type B vaccine12, 20, 44. Thus, recommendations for the use of these vaccines in SCA are based on efficacy trials done mostly in individuals without SCA12, 18–20. Although observational studies of the impact of pneumococcal conjugate vaccines in SCA have reported substantial reduction in invasive pneumococcal disease risk13, 142, room still exists to improve vaccine outcomes even further to meet protection levels conferred among individuals without SCA142. Randomized controlled trials of these vaccines in SCA are therefore much needed, especially in sub-Saharan Africa where the burden of SCA is highest. Insights from these trials will inform optimization of vaccination and booster regimen required to improve the magnitude and duration of vaccine protection against infection in individuals with SCA12.
Besides safety, immunogenicity and protective efficacy, coverage of pneumococcal vaccines against pneumococcal disease in SCA may be a challenge in sub-Saharan Africa. This is because of the highly diverse circulating pneumococcal serotypes in the region143, 144. Thus, although majority of the prevalent pneumococcal serotypes among children in sub-Saharan Africa are contained in the currently available pneumococcal conjugate vaccine - 10 and 13 (PCV-10, PCV-13)61 and pneumococcal polysaccharide vaccine-23 (PPSV-23)61, some prevalent serotypes including pneumococcal serotype 13 are not covered in the three vaccines143, 144. In settings where the proportion of circulating non-vaccine pneumococcal serotypes is high143, dual usage of both the pneumococcal vaccines and penicillin prophylaxis is recommended and is considered complementary in individuals with SCA9, 61, 145, 146. A recent Cochrane review could not conclude when is safe to withdraw penicillin prophylaxis in children with SCA9, but the American Academy of Pediatricians recommends continued use of penicillin prophylaxis at least until the age of 5 years145, 146. Prolonged use of penicillin prophylaxis beyond the age of 5 years may be recommended, depending on the perceived risk of pneumococcal infection61. Penicillin resistance is varied, but may be high is select locations in sub-Saharan Africa144, 147–149, and this may pose a challenge on the choice of regionally appropriate antibiotic to complement pneumococcal vaccination. Future studies should address both discovery of next generation pneumococcal conjugate vaccines with increased serotype coverage and identification of regionally appropriate antibiotics to complement pneumococcal vaccines in high penicillin resistance areas.
The schedules for the delivery of vaccines in individuals with SCA take into account the age of vaccine recipients and pathogen-specific infection risk in particular age groups57, 58, 132. For instance, the protein-conjugated PCV-10 and PCV-13, which elicit primarily T-cell dependent memory responses, are usually given during the first 2 years of life58, 61. The non-protein conjugated pneumococcal polysaccharide vaccine-23 (PPSV-23), which elicits T cell independent IgM memory B cell responses, is given to older individuals with mature spleen57, 58, 61. However, since splenic function is lost with age in individuals with SCA, this schedule should be modified in line with the recent recommendations for vaccination of individuals with hyposplenism61. Adaptation of the modified vaccine regimen must however be based on assessment of splenic function58–61, 150, the capacity of which is currently lacking in most settings in sub-Saharan Africa. In view of the reported perturbations of lymphocyte phenotypes and functions in SCA, and accounts of impaired vaccine responsiveness in individuals with SCA, it is possible that differences in vaccine reactivity exists between patients with SCA compared to that in individuals without SCA. Furthermore, because of paucity of randomized controlled trials of vaccines in individuals with SCA, it is imperative to compare vaccines outcomes between individuals with and without SCA. The capacity to screen for hyposplenism should also be enhanced in resource limited settings, concurrent with increased research on its immune impact, in order to go hand in hand with the proposed recommendations for immunization of individuals with hyposplenism. Important areas for future research are summarized in Table 1 (Supplementary online information). Insights from these studies will inform optimization of the delivery of vaccines in individuals with SCA, including optimization of vaccination and booster schedules as may be needed, aiming at eliciting optimal and durable responses that will lead to reduction of the burden of morbidity and mortality due to infections in SCA.
CONCLUSIONS
It is clear that innate immune dysfunction is commonplace in SCA. Limited available evidence indicates that T and B cell functions are also compromised in individuals with SCA. Derangements reported include reduction in the proportion of circulating CD4+ and CD8+ T cells, reduction of CD4+ helper : CD8+ suppressor T cell ratio, a skewed Th2 response, dysfunction of regulatory T cells and loss of IgM-secreting CD27+IgMhighIgDlow memory B cells, all occurring at the background of increased immune activation characterized by predominance of memory CD4+ T cell phenotypes, increased Th17 signaling and elevated levels of C-reactive protein and pro-inflammatory cytokines IL-6 and TNF-α. These abnormalities, and others uncovered, may affect vaccine reactivity in individuals with SCA. Since T and B lymphocytes are the primary cells for induction of vaccine-mediated immunity, it is imperative that their phenotypes and functions are characterized in individuals with SCA. A comprehensive immunological and molecular analysis of T and B cells in SCA is therefore warranted, concurrent with detailed evaluation of the immunogenicity and protective efficacy of vaccines in individuals with SCA. This should include expanded analysis of responses of the different T and B cell subsets to infection and vaccination in SCA, together with assessment of their modulation by hyposplenism, splenectomy, genetic polymorphisms, alloimmunization, malaria endemicity and hydroxyurea usage. Studies should be encouraged of different SCA populations, including sub-Saharan Africa where the burden of SCA is highest. Insights from these studies will inform optimization of vaccination and booster schedules in order to overcome the burden of infections due to encapsulated bacteria and other pathogens in individuals SCA.
Supplementary Material
Acknowledgments
We acknowledge support by NIH Research Training Grant # R25 TW009343 funded by the Fogarty International Center and the National Health, Lung and Blood Institute, as well as the University of California Global Health Institute (UCGHI).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84:500–508. [PubMed] [Google Scholar]
- 2.Makani J, Mgaya J, Balandya E, Msami K, Soka D, Cox SE, Komba AN, Rwezaula S, Meda E, Muturi D, Kitundu J, Fegan G, Kirkham FJ, Newton CR, Snow RW, Lowe B. Bacteraemia in sickle cell anaemia is associated with low haemoglobin: a report of 890 admissions to a tertiary hospital in Tanzania. British journal of haematology. 2015 doi: 10.1111/bjh.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan M, Moisi JC, Klugman KP, Iglesias JM, Grant LR, Mpoudi-Etame M, Levine OS. Increased risk of invasive bacterial infections in African people with sickle-cell disease: a systematic review and meta-analysis. The Lancet Infectious diseases. 2010;10:329–337. doi: 10.1016/S1473-3099(10)70055-4. [DOI] [PubMed] [Google Scholar]
- 4.Gill FM, Sleeper LA, Weiner SJ, Brown AK, Bellevue R, Grover R, Pegelow CH, Vichinsky E. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- 5.Thomas AN, Pattison C, Serjeant GR. Causes of death in sickle-cell disease in Jamaica. Br Med J (Clin Res Ed) 1982;285:633–635. doi: 10.1136/bmj.285.6342.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarkowsky HS, Gallagher D, Gill FM, Wang WC, Falletta JM, Lande WM, Levy PS, Verter JI, Wethers D. Bacteremia in sickle hemoglobinopathies. The Journal of pediatrics. 1986;109:579–585. doi: 10.1016/s0022-3476(86)80216-5. [DOI] [PubMed] [Google Scholar]
- 7.Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, Zarkowsky H, Vichinsky E, Iyer R, Lobel JS, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. The New England journal of medicine. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 8.Knight-Madden J, Serjeant GR. Invasive pneumococcal disease in homozygous sickle cell disease: Jamaican experience 1973–1997. The Journal of pediatrics. 2001;138:65–70. doi: 10.1067/mpd.2001.109709. [DOI] [PubMed] [Google Scholar]
- 9.Hirst C, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. The Cochrane database of systematic reviews. 2014;11:CD003427. doi: 10.1002/14651858.CD003427.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Murray J, Agocs M, Serhan F, Singh S, Deloria-Knoll M, O'Brien K, Mwenda JM, Mihigo R, Oliveira L, Teleb N, Ahmed H, Wasley A, Videbaek D, Wijesinghe P, Thapa AB, Fox K, Paladin FJ, Hajjeh R, Schwartz S, Van Beneden C, Hyde T, Broome C, Cherian T. Global invasive bacterial vaccine-preventable diseases surveillance-2008–2014. MMWR Morbidity and mortality weekly report. 2014;63:1159–1162. [PMC free article] [PubMed] [Google Scholar]
- 11.Brenzel L, Wolfson LJ, Fox-Rushby J, Miller M, Halsey NA. In: Vaccine-preventable Diseases. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Washington (DC): 2006. [PubMed] [Google Scholar]
- 12.Davies EG, Riddington C, Lottenberg R, Dower N. Pneumococcal vaccines for sickle cell disease. The Cochrane database of systematic reviews. 2004:CD003885. doi: 10.1002/14651858.CD003885.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Halasa NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, Schaffner W, Craig AS, Griffin MR. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44:1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 14.Makani J, Komba AN, Cox SE, Oruo J, Mwamtemi K, Kitundu J, Magesa P, Rwezaula S, Meda E, Mgaya J, Pallangyo K, Okiro E, Muturi D, Newton CR, Fegan G, Marsh K, Williams TN. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115:215–220. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Williams TN, Weatherall DJ, Hay SI. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nature communications. 2010;1:104. doi: 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aneni EC, Hamer DH, Gill CJ. Systematic review of current and emerging strategies for reducing morbidity from malaria in sickle cell disease. Tropical medicine & international health : TM & IH. 2013;18:313–327. doi: 10.1111/tmi.12056. [DOI] [PubMed] [Google Scholar]
- 17.Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, Lyke KE, Mordmuller B, Alonso P, Duffy PE, Doumbo OK, Sauerwein RW, Tanner M, Abdulla S, Kremsner PG, Seder RA, Hoffman SL. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33:7452–7461. doi: 10.1016/j.vaccine.2015.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. The Pediatric infectious disease journal. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. The New England journal of medicine. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 20.Allali S, Chalumeau M, Launay O, Ballas SK, de Montalembert M. Conjugate Haemophilus influenzae type b vaccines for sickle cell disease. The Cochrane database of systematic reviews. 2016;2:CD011199. doi: 10.1002/14651858.CD011199.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Kaaba SA, al-Harbi SA. Reduced levels of CD2+ cells and T-cell subsets in patients with sickle cell anaemia. Immunology letters. 1993;37:77–81. doi: 10.1016/0165-2478(93)90135-o. [DOI] [PubMed] [Google Scholar]
- 22.Koffi KG, Sawadogo D, Meite M, Nanho DC, Tanoh ES, Attia AK, Sanogo I, Sangare A. Reduced levels of T-cell subsets CD4+ and CD8+ in homozygous sickle cell anaemia patients with splenic defects. The hematology journal : the official journal of the European Haematology Association / EHA. 2003;4:363–365. doi: 10.1038/sj.thj.6200310. [DOI] [PubMed] [Google Scholar]
- 23.Sanhadji K, Chout R, Gessain A, Sasco AJ, Yoyo M, Mezard F, de The G, Touraine JL. Cell-mediated immunity in patients with sickle cell anaemia. Thymus. 1988;12:203–213. [PubMed] [Google Scholar]
- 24.Taylor SC, Shacks SJ, Villicana SM, Olivares J, Dinkins GA. Interferon production in sickle cell disease. Lymphokine research. 1990;9:415–423. [PubMed] [Google Scholar]
- 25.Vingert B, Tamagne M, Desmarets M, Pakdaman S, Elayeb R, Habibi A, Bernaudin F, Galacteros F, Bierling P, Noizat-Pirenne F, Cohen JL. Partial dysfunction of Treg activation in sickle cell disease. American journal of hematology. 2014;89:261–266. doi: 10.1002/ajh.23629. [DOI] [PubMed] [Google Scholar]
- 26.Rautonen N, Martin NL, Rautonen J, Rooks Y, Mentzer WC, Wara DW. Low number of antibody producing cells in patients with sickle cell anemia. Immunology letters. 1992;34:207–211. doi: 10.1016/0165-2478(92)90215-a. [DOI] [PubMed] [Google Scholar]
- 27.Wang WC, Herrod HG, Valenski WR, Wyatt RJ. Lymphocyte and complement abnormalities in splenectomized patients with hematologic disorders. American journal of hematology. 1988;28:239–245. doi: 10.1002/ajh.2830280406. [DOI] [PubMed] [Google Scholar]
- 28.Cetiner S, Akoglu TF, Kilinc Y, Akoglu E, Kumi M. Immunological studies in sickle cell disease: comparison of homozygote mild and severe variants. Clinical immunology and immunopathology. 1989;53:32–39. doi: 10.1016/0090-1229(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 29.Musa BO, Onyemelukwe GC, Hambolu JO, Mamman AI, Isa AH. Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis. Clinical and vaccine immunology : CVI. 2010;17:602–608. doi: 10.1128/CVI.00145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao W, Zhong H, Li X, Lee MT, Schwartz J, Sheth S, Yazdanbakhsh K. Immune regulation in chronically transfused allo-antibody responder and nonresponder patients with sickle cell disease and beta-thalassemia major. American journal of hematology. 2011;86:1001–1006. doi: 10.1002/ajh.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A. Acute phase proteins and interleukins in steady state sickle cell disease. European journal of haematology. 1998;61:49–54. doi: 10.1111/j.1600-0609.1998.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 32.Dworkis DA, Klings ES, Solovieff N, Li G, Milton JN, Hartley SW, Melista E, Parente J, Sebastiani P, Steinberg MH, Baldwin CT. Severe sickle cell anemia is associated with increased plasma levels of TNF-R1 and VCAM-1. American journal of hematology. 2011;86:220–223. doi: 10.1002/ajh.21928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hibbert JM, Hsu LL, Bhathena SJ, Irune I, Sarfo B, Creary MS, Gee BE, Mohamed AI, Buchanan ID, Al-Mahmoud A, Stiles JK. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med (Maywood) 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keikhaei B, Mohseni AR, Norouzirad R, Alinejadi M, Ghanbari S, Shiravi F, Solgi G. Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition. European cytokine network. 2013;24:45–52. doi: 10.1684/ecn.2013.0328. [DOI] [PubMed] [Google Scholar]
- 35.Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. Journal of leukocyte biology. 2009;85:235–242. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- 36.Nickel RS, Horan JT, Fasano RM, Meyer E, Josephson CD, Winkler AM, Yee ME, Kean LS, Hendrickson JE. Immunophenotypic parameters and RBC alloimmunization in children with sickle cell disease on chronic transfusion. American journal of hematology. 2015;90:1135–1141. doi: 10.1002/ajh.24188. [DOI] [PubMed] [Google Scholar]
- 37.Nickel RS, Osunkwo I, Garrett A, Robertson J, Archer DR, Promislow DE, Horan JT, Hendrickson JE, Kean LS. Immune parameter analysis of children with sickle cell disease on hydroxycarbamide or chronic transfusion therapy. British journal of haematology. 2015;169:574–583. doi: 10.1111/bjh.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Beers EJ, Yang Y, Raghavachari N, Tian X, Allen DT, Nichols JS, Mendelsohn L, Nekhai S, Gordeuk VR, Taylor JGt, Kato GJ. Iron, inflammation, and early death in adults with sickle cell disease. Circulation research. 2015;116:298–306. doi: 10.1161/CIRCRESAHA.116.304577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vingert B, Tamagne M, Habibi A, Pakdaman S, Ripa J, Elayeb R, Galacteros F, Bierling P, Ansart-Pirenne H, Bartolucci P, Noizat-Pirenne F. Phenotypic differences of CD4(+) T cells in response to red blood cell immunization in transfused sickle cell disease patients. European journal of immunology. 2015;45:1868–1879. doi: 10.1002/eji.201445187. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Wandersee NJ, Guo Y, Jones DW, Holzhauer SL, Hanson MS, Machogu E, Brousseau DC, Hogg N, Densmore JC, Kaul S, Hillery CA, Pritchard KA., Jr Sickle cell disease increases high mobility group box 1: a novel mechanism of inflammation. Blood. 2014;124:3978–3981. doi: 10.1182/blood-2014-04-560813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballester OF, Abdallah JM, Prasad AS. Impaired IgM antibody responses to an influenza virus vaccine in adults with sickle cell anemia. American journal of hematology. 1985;20:409–412. doi: 10.1002/ajh.2830200413. [DOI] [PubMed] [Google Scholar]
- 42.Disu EA, Akodu SO, Arinola OG, Diaku-Akinwumi IN, Adedokun B, Olopade CO, Njokanma OF. Pneumococcal-specific IgG levels after 13-valent pneumococcal conjugate vaccination in Nigerian children with sickle cell disease. Paediatrics and international child health. 2016:1–4. doi: 10.1080/20469047.2015.1106076. [DOI] [PubMed] [Google Scholar]
- 43.Hord J, Windsor B, Koehler M, Blatt J, Janosky J, Mirro J. Diminished antibody response to hepatitis B immunization in children with sickle cell disease. Journal of pediatric hematology/oncology. 2002;24:548–549. doi: 10.1097/00043426-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 44.John AB, Ramlal A, Jackson H, Maude GH, Sharma AW, Serjeant GR. Prevention of pneumococcal infection in children with homozygous sickle cell disease. Br Med J (Clin Res Ed) 1984;288:1567–1570. doi: 10.1136/bmj.288.6430.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purohit S, Alvarez O, O'Brien R, Andreansky S. Durable immune response to inactivated H1N1 vaccine is less likely in children with sickle cell anemia receiving chronic transfusions. Pediatric blood & cancer. 2012;59:1280–1283. doi: 10.1002/pbc.24206. [DOI] [PubMed] [Google Scholar]
- 46.Mandala WL, Msefula CL, Gondwe EN, Gilchrist JJ, Graham SM, Pensulo P, Mwimaniwa G, Banda M, Taylor TE, Molyneux EE, Drayson MT, Ward SA, Molyneux ME, MacLennan CA. Lymphocyte Perturbations in Malawian Children with Severe and Uncomplicated Malaria. Clinical and vaccine immunology : CVI. 2015 doi: 10.1128/CVI.00564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roetynck S, Olotu A, Simam J, Marsh K, Stockinger B, Urban B, Langhorne J. Phenotypic and functional profiling of CD4 T cell compartment in distinct populations of healthy adults with different antigenic exposure. PloS one. 2013;8:e55195. doi: 10.1371/journal.pone.0055195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lalor MK, Ben-Smith A, Gorak-Stolinska P, Weir RE, Floyd S, Blitz R, Mvula H, Newport MJ, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. Population differences in immune responses to Bacille Calmette-Guerin vaccination in infancy. The Journal of infectious diseases. 2009;199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. The Journal of infectious diseases. 2011;204:1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kollmann TR. Variation between Populations in the Innate Immune Response to Vaccine Adjuvants. Frontiers in immunology. 2013;4:81. doi: 10.3389/fimmu.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong WY, Zhou Y, Operskalski EA, Hassett J, Powars DR, Mosley JW. Hematologic profile and lymphocyte subpopulations in hemoglobin SC disease: comparison with hemoglobin SS and black controls. The Transfusion Safety Study Group. American journal of hematology. 1996;52:150–154. doi: 10.1002/1096-8652(199607)52:3<150::aid-ajh2830520302>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Anyaegbu CC, Okpala IE, Akren'Ova YA, Salimonu LS. Peripheral blood neutrophil count and candidacidal activity correlate with the clinical severity of sickle cell anaemia (SCA) European journal of haematology. 1998;60:267–268. doi: 10.1111/j.1600-0609.1998.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 53.Humbert JR, Winsor EL, Githens JM, Schmitz JB. Neutrophil dysfunctions in sickle cell disease. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 1990;44:153–158. doi: 10.1016/0753-3322(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 54.Dieye TN, Ndiaye O, Ndiaye AB, Thiam D, Fall-Seck K, Diop S, Diop BM, Fall M, Diakhate L. Complement and serum immunoglobulins in homozygous and heterozygous sickle cell anemia in Senegal. Dakar medical. 1999;44:175–179. [PubMed] [Google Scholar]
- 55.Larcher VF, Wyke RJ, Davis LR, Stroud CE, Williams R. Defective yeast opsonisation and functional deficiency of complement in sickle cell disease. Archives of disease in childhood. 1982;57:343–346. doi: 10.1136/adc.57.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anyaegbu CC, Okpala IE, Aken'ova AY, Salimonu LS. Complement haemolytic activity, circulating immune complexes and the morbidity of sickle cell anaemia. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 1999;107:699–702. doi: 10.1111/j.1699-0463.1999.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 57.Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2010;14:e2–e12. doi: 10.1016/j.ijid.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. British journal of haematology. 2014;166:165–176. doi: 10.1111/bjh.12950. [DOI] [PubMed] [Google Scholar]
- 59.Rogers ZR, Wang WC, Luo Z, Iyer RV, Shalaby-Rana E, Dertinger SD, Shulkin BL, Miller JH, Files B, Lane PA, Thompson BW, Miller ST, Ware RE. Biomarkers of splenic function in infants with sickle cell anemia: baseline data from the BABY HUG Trial. Blood. 2011;117:2614–2617. doi: 10.1182/blood-2010-04-278747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lammers AJ, de Porto AP, Bennink RJ, van Leeuwen EM, Biemond BJ, Goslings JC, van Marle J, ten Berge IJ, Speelman P, Hoekstra JB. Hyposplenism: comparison of different methods for determining splenic function. American journal of hematology. 2012;87:484–489. doi: 10.1002/ajh.23154. [DOI] [PubMed] [Google Scholar]
- 61.Kuchar E, Miskiewicz K, Karlikowska M. A review of guidance on immunization in persons with defective or deficient splenic function. British journal of haematology. 2015 doi: 10.1111/bjh.13660. [DOI] [PubMed] [Google Scholar]
- 62.Fasano RM, Booth GS, Miles M, Du L, Koyama T, Meier ER, Luban NL. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. British journal of haematology. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 63.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–537. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horan JT, Liesveld JL, Fenton P, Blumberg N, Walters MC. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone marrow transplantation. 2005;35:171–177. doi: 10.1038/sj.bmt.1704745. [DOI] [PubMed] [Google Scholar]
- 65.Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A, Amylon M, Sullivan KM, Storb RF, Walters MC. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9:519–528. doi: 10.1016/s1083-8791(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 66.Alkindi S, Al-Maini M, Pathare A. Clinical and laboratory characteristics of patients with sickle-cell and autoimmune/connective tissue diseases. Rheumatology international. 2012;32:373–378. doi: 10.1007/s00296-010-1632-x. [DOI] [PubMed] [Google Scholar]
- 67.Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125:234–243. doi: 10.1542/peds.2009-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olenscki Gilli SC, Pericole FV, Benites BD, Sippert EA, Castilho LM, Addas-Carvalho M, Olalla Saad ST. Cytokine polymorphisms in sickle cell disease and the relationship with cytokine expression. Experimental hematology. 2016 doi: 10.1016/j.exphem.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 69.Pido-Lopez J, Kwok WW, Mitchell TJ, Heyderman RS, Williams NA. Acquisition of pneumococci specific effector and regulatory Cd4+ T cells localising within human upper respiratory-tract mucosal lymphoid tissue. PLoS pathogens. 2011;7:e1002396. doi: 10.1371/journal.ppat.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackson SM, Wilson PC, James JA, Capra JD. Human B cell subsets. Advances in immunology. 2008;98:151–224. doi: 10.1016/S0065-2776(08)00405-7. [DOI] [PubMed] [Google Scholar]
- 72.Makani J, Menzel S, Nkya S, Cox SE, Drasar E, Soka D, Komba AN, Mgaya J, Rooks H, Vasavda N, Fegan G, Newton CR, Farrall M, Thein SL. Genetics of fetal hemoglobin in Tanzanian and British patients with sickle cell anemia. Blood. 2011;117:1390–1392. doi: 10.1182/blood-2010-08-302703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mtatiro SN, Makani J, Mmbando B, Thein SL, Menzel S, Cox SE. Genetic variants at HbF-modifier loci moderate anemia and leukocytosis in sickle cell disease in Tanzania. American journal of hematology. 2015;90:E1–E4. doi: 10.1002/ajh.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mtatiro SN, Mgaya J, Singh T, Mariki H, Rooks H, Soka D, Mmbando B, Thein SL, Barrett JC, Makani J, Cox SE, Menzel S. Genetic association of fetal-hemoglobin levels in individuals with sickle cell disease in Tanzania maps to conserved regulatory elements within the MYB core enhancer. BMC medical genetics. 2015;16:4. doi: 10.1186/s12881-015-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mtatiro SN, Singh T, Rooks H, Mgaya J, Mariki H, Soka D, Mmbando B, Msaki E, Kolder I, Thein SL, Menzel S, Cox SE, Makani J, Barrett JC. Genome wide association study of fetal hemoglobin in sickle cell anemia in Tanzania. PloS one. 2014;9:e111464. doi: 10.1371/journal.pone.0111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cameron PU, Jones P, Gorniak M, Dunster K, Paul E, Lewin S, Woolley I, Spelman D. Splenectomy associated changes in IgM memory B cells in an adult spleen registry cohort. PloS one. 2011;6:e23164. doi: 10.1371/journal.pone.0023164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss C, Wulf G, Ho AD, Hunstein W. Decrease in soluble CD8 antigen levels in splenectomized patients as an index for reduced suppressor/cytotoxic cell activity. Immunology letters. 1991;30:113–118. doi: 10.1016/0165-2478(91)90098-u. [DOI] [PubMed] [Google Scholar]
- 78.Englum BR, Rothman J, Leonard S, Reiter A, Thornburg C, Brindle M, Wright N, Heeney MM, Jason Smithers C, Brown RL, Kalfa T, Langer JC, Cada M, Oldham KT, Scott JP, Peter SD, Sharma M, Davidoff AM, Nottage K, Bernabe K, Wilson DB, Dutta S, Glader B, Crary SE, Dassinger MS, Dunbar L, Islam S, Kumar M, Rescorla F, Bruch S, Campbell A, Austin M, Sidonio R, Blakely ML, Rice HE. Hematologic outcomes after total splenectomy and partial splenectomy for congenital hemolytic anemia. Journal of pediatric surgery. 2015 doi: 10.1016/j.jpedsurg.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee MT, Piomelli S, Granger S, Miller ST, Harkness S, Brambilla DJ, Adams RJ. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108:847–852. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wahl S, Quirolo KC. Current issues in blood transfusion for sickle cell disease. Current opinion in pediatrics. 2009;21:15–21. doi: 10.1097/MOP.0b013e328321882e. [DOI] [PubMed] [Google Scholar]
- 81.Lannan KL, Sahler J, Spinelli SL, Phipps RP, Blumberg N. Transfusion immunomodulation--the case for leukoreduced and (perhaps) washed transfusions. Blood cells, molecules & diseases. 2013;50:61–68. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308–310. [PubMed] [Google Scholar]
- 83.Grady RW, Akbar AN, Giardina PJ, Hilgartner MW, de Sousa M. Disproportionate lymphoid cell subsets in thalassaemia major: the relative contributions of transfusion and splenectomy. British journal of haematology. 1985;59:713–724. doi: 10.1111/j.1365-2141.1985.tb07367.x. [DOI] [PubMed] [Google Scholar]
- 84.Pahwa S, Sia C, Harper R, Pahwa R. T lymphocyte subpopulations in high-risk infants: influence of age and blood transfusions. Pediatrics. 1985;76:914–917. [PubMed] [Google Scholar]
- 85.Blumberg N, Heal JM. Blood transfusion immunomodulation: the silent epidemic. Archives of pathology & laboratory medicine. 1998;122:117–119. [PubMed] [Google Scholar]
- 86.Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. The American journal of medicine. 1996;101:299–308. doi: 10.1016/S0002-9343(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 87.Blumberg N, Heal JM. The transfusion immunomodulation theory: the Th1/Th2 paradigm and an analogy with pregnancy as a unifying mechanism. Seminars in hematology. 1996;33:329–340. [PubMed] [Google Scholar]
- 88.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood reviews. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 90.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annual review of immunology. 2016 doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 92.Hessell AJ, Malherbe DC, Pissani F, McBurney S, Krebs SJ, Gomes M, Pandey S, Sutton WF, Burwitz BJ, Gray M, Robins H, Park BS, Sacha JB, LaBranche CC, Fuller DH, Montefiori DC, Stamatatos L, Sather DN, Haigwood NL. Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens. J Immunol. 2016;196:3064–3078. doi: 10.4049/jimmunol.1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duan Z, Chen X, Liang Z, Zeng Y, Zhu F, Long L, McCrae MA, Zhuang H, Shen T, Lu F. Genetic polymorphisms of CXCR5 and CXCL13 are associated with non-responsiveness to the hepatitis B vaccine. Vaccine. 2014;32:5316–5322. doi: 10.1016/j.vaccine.2014.07.064. [DOI] [PubMed] [Google Scholar]
- 94.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, Pascual V, Banchereau J, Garcia-Sastre A, Palucka AK, Ramilo O, Ueno H. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Science translational medicine. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Godefroy E, Zhong H, Pham P, Friedman D, Yazdanbakhsh K. TIGIT-positive circulating follicular helper T cells display robust B-cell help functions: potential role in sickle cell alloimmunization. Haematologica. 2015;100:1415–1425. doi: 10.3324/haematol.2015.132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nickel RS, Hendrickson JE, Fasano RM, Meyer EK, Winkler AM, Yee MM, Lane PA, Jones YA, Pashankar FD, New T, Josephson CD, Stowell SR. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016;56:107–114. doi: 10.1111/trf.13379. [DOI] [PubMed] [Google Scholar]
- 98.Zimring JC, Hair GA, Deshpande SS, Horan JT. Immunization to minor histocompatibility antigens on transfused RBCs through crosspriming into recipient MHC class I pathways. Blood. 2006;107:187–189. doi: 10.1182/blood-2005-07-3059. [DOI] [PubMed] [Google Scholar]
- 99.Telen MJ, Afenyi-Annan A, Garrett ME, Combs MR, Orringer EP, Ashley-Koch AE. Alloimmunization in sickle cell disease: changing antibody specificities and association with chronic pain and decreased survival. Transfusion. 2015;55:1378–1387. doi: 10.1111/trf.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bachmeyer C, Maury J, Parrot A, Bachir D, Stankovic K, Girot R, Lionnet F. Rituximab as an effective treatment of hyperhemolysis syndrome in sickle cell anemia. American journal of hematology. 2010;85:91–92. doi: 10.1002/ajh.21578. [DOI] [PubMed] [Google Scholar]
- 101.Noizat-Pirenne F, Habibi A, Mekontso-Dessap A, Razazi K, Chadebech P, Mahevas M, Vingert B, Bierling P, Galacteros F, Bartolucci P, Michel M. The use of rituximab to prevent severe delayed haemolytic transfusion reaction in immunized patients with sickle cell disease. Vox sanguinis. 2015;108:262–267. doi: 10.1111/vox.12217. [DOI] [PubMed] [Google Scholar]
- 102.Fitzhugh CD, Abraham AA, Tisdale JF, Hsieh MM. Hematopoietic stem cell transplantation for patients with sickle cell disease: progress and future directions. Hematology/oncology clinics of North America. 2014;28:1171–1185. doi: 10.1016/j.hoc.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, Vannier JP, Yakouben K, Thuret I, Bordigoni P, Fischer A, Lutz P, Stephan JL, Dhedin N, Plouvier E, Margueritte G, Bories D, Verlhac S, Esperou H, Coic L, Vernant JP, Gluckman E. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110:2749–2756. doi: 10.1182/blood-2007-03-079665. [DOI] [PubMed] [Google Scholar]
- 104.Platt OS. Sickle cell anemia as an inflammatory disease. The Journal of clinical investigation. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Holtzclaw JD, Jack D, Aguayo SM, Eckman JR, Roman J, Hsu LL. Enhanced pulmonary and systemic response to endotoxin in transgenic sickle mice. American journal of respiratory and critical care medicine. 2004;169:687–695. doi: 10.1164/rccm.200302-224OC. [DOI] [PubMed] [Google Scholar]
- 106.Manwani D, Chen G, Carullo V, Serban S, Olowokure O, Jang J, Huggins M, Cohen HW, Billett H, Atweh GF, Frenette PS, Shi PA. Single-dose intravenous gammaglobulin can stabilize neutrophil Mac-1 activation in sickle cell pain crisis. American journal of hematology. 2015;90:381–385. doi: 10.1002/ajh.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jakubowski JA, Zhou C, Winters KJ, Lachno DR, Howard J, Payne CD, Mant T, Jurcevic S, Frelinger AL., 3rd The effect of prasugrel on ADP-stimulated markers of platelet activation in patients with sickle cell disease. Platelets. 2015;26:474–479. doi: 10.3109/09537104.2014.940887. [DOI] [PubMed] [Google Scholar]
- 108.Afrin LB. Mast cell activation syndrome as a significant comorbidity in sickle cell disease. The American journal of the medical sciences. 2014;348:460–464. doi: 10.1097/MAJ.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hatzipantelis ES, Pana ZD, Gombakis N, Taparkou A, Tzimouli V, Kleta D, Zafeiriou DJ, Garipidou V, Kanakoudi F, Athanassiou M. Endothelial activation and inflammation biomarkers in children and adolescents with sickle cell disease. International journal of hematology. 2013;98:158–163. doi: 10.1007/s12185-013-1392-y. [DOI] [PubMed] [Google Scholar]
- 110.Wun T, Cordoba M, Rangaswami A, Cheung AW, Paglieroni T. Activated monocytes and platelet-monocyte aggregates in patients with sickle cell disease. Clinical and laboratory haematology. 2002;24:81–88. doi: 10.1046/j.1365-2257.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 111.Field JJ, Lin G, Okam MM, Majerus E, Keefer J, Onyekwere O, Ross A, Campigotto F, Neuberg D, Linden J, Nathan DG. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood. 2013;121:3329–3334. doi: 10.1182/blood-2012-11-465963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]
- 113.Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pitanga TN, Oliveira RR, Zanette DL, Guarda CC, Santiago RP, Santana SS, Nascimento VM, Lima JB, Carvalho GQ, Maffili VV, Carvalho MO, Alcantara LC, Borges VM, Goncalves MS. Sickle red cells as danger signals on proinflammatory gene expression, leukotriene B4 and interleukin-1 beta production in peripheral blood mononuclear cell. Cytokine. 2016;83:75–84. doi: 10.1016/j.cyto.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 115.Muyanja E, Ssemaganda A, Ngauv P, Cubas R, Perrin H, Srinivasan D, Canderan G, Lawson B, Kopycinski J, Graham AS, Rowe DK, Smith MJ, Isern S, Michael S, Silvestri G, Vanderford TH, Castro E, Pantaleo G, Singer J, Gillmour J, Kiwanuka N, Nanvubya A, Schmidt C, Birungi J, Cox J, Haddad EK, Kaleebu P, Fast P, Sekaly RP, Trautmann L, Gaucher D. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. The Journal of clinical investigation. 2014;124:3147–3158. doi: 10.1172/JCI75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bao W, Zhong H, Manwani D, Vasovic L, Uehlinger J, Lee MT, Sheth S, Shi P, Yazdanbakhsh K. Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease. American journal of hematology. 2013;88:736–740. doi: 10.1002/ajh.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhong H, Bao W, Friedman D, Yazdanbakhsh K. Hemin controls T cell polarization in sickle cell alloimmunization. J Immunol. 2014;193:102–110. doi: 10.4049/jimmunol.1400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu J, Heck S, Yazdanbakhsh K. Prevention of red cell alloimmunization by CD25 regulatory T cells in mouse models. American journal of hematology. 2007;82:691–696. doi: 10.1002/ajh.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hendrickson JE, Saakadze N, Cadwell CM, Upton JW, Mocarski ES, Hillyer CD, Zimring JC. The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells. Transfusion. 2009;49:1678–1684. doi: 10.1111/j.1537-2995.2009.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Valiathan R, Asthana D. Increase in frequencies of circulating Th-17 cells correlates with microbial translocation, immune activation and exhaustion in HIV-1 infected patients with poor CD4 T-cell reconstitution. Immunobiology. 2016;221:670–678. doi: 10.1016/j.imbio.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 121.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. The Journal of experimental medicine. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toly-Ndour C, Rouquette AM, Obadia S, M'Bappe P, Lionnet F, Hagege I, Boussa-Khettab F, Tshilolo L, Girot R. High titers of autoantibodies in patients with sickle-cell disease. The Journal of rheumatology. 2011;38:302–309. doi: 10.3899/jrheum.100667. [DOI] [PubMed] [Google Scholar]
- 123.Nistala K, Murray KJ. Co-existent sickle cell disease and juvenile rheumatoid arthritis. Two cases with delayed diagnosis and severe destructive arthropathy. The Journal of rheumatology. 2001;28:2125–2128. [PubMed] [Google Scholar]
- 124.Katsanis E, Hsu E, Luke KH, McKee JA. Systemic lupus erythematosus and sickle hemoglobinopathies: a report of two cases and review of the literature. American journal of hematology. 1987;25:211–214. doi: 10.1002/ajh.2830250211. [DOI] [PubMed] [Google Scholar]
- 125.Maamar M, Tazi-Mezalek Z, Harmouche H, Mounfaloti W, Adnaoui M, Aouni M. Systemic lupus erythematosus associated with sickle-cell disease: a case report and literature review. Journal of medical case reports. 2012;6:366. doi: 10.1186/1752-1947-6-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lederman HM, Connolly MA, Kalpatthi R, Ware RE, Wang WC, Luchtman-Jones L, Waclawiw M, Goldsmith JC, Swift A, Casella JF. Immunologic effects of hydroxyurea in sickle cell anemia. Pediatrics. 2014;134:686–695. doi: 10.1542/peds.2014-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Castilhos LG, Doleski PH, Bertoldo TM, Passos DF, Bertoncheli Cde M, Rezer JF, Schlemmer JB, Leal DB. Sickle cell anemia induces changes in peripheral lymphocytes E-NTPDase/E-ADA activities and cytokines secretion in patients under treatment. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2015;73:102–108. doi: 10.1016/j.biopha.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 128.Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik SA, Howard TH, Wynn LW, Kutlar A, Armstrong FD, Files BA, Goldsmith JC, Waclawiw MA, Huang X, Thompson BW. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thornburg CD, Files BA, Luo Z, Miller ST, Kalpatthi R, Iyer R, Seaman P, Lebensburger J, Alvarez O, Thompson B, Ware RE, Wang WC. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120:4304–4310. doi: 10.1182/blood-2012-03-419879. quiz 4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lopez M, Benito JM, Lozano S, Barreiro P, Martinez P, Gonzalez-Lahoz J, Soriano V. Enhanced HIV-specific immune responses in chronically HIV-infected patients receiving didanosine plus hydroxyurea. AIDS. 2004;18:1251–1261. doi: 10.1097/00002030-200406180-00003. [DOI] [PubMed] [Google Scholar]
- 131.Owusu-Ansah A, Choi SH, Petrosiute A, Letterio JJ, Huang AY. Triterpenoid inducers of Nrf2 signaling as potential therapeutic agents in sickle cell disease: a review. Frontiers of medicine. 2015;9:46–56. doi: 10.1007/s11684-015-0375-1. [DOI] [PubMed] [Google Scholar]
- 132.Gorham MW, Smith CR, Smith SK, Wong L, Kreze O. Vaccinations in sickle cell disease: An audit of vaccination uptake in sickle cell patients attending Newham University Hospital. Vaccine. 2015;33:5005–5011. doi: 10.1016/j.vaccine.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 133.De Montalembert M, Abboud MR, Fiquet A, Inati A, Lebensburger JD, Kaddah N, Mokhtar G, Piga A, Halasa N, Inusa B, Rees DC, Heath PT, Telfer P, Driscoll C, Al Hajjar S, Tozzi A, Jiang Q, Emini EA, Gruber WC, Gurtman A, Scott DA. 13-valent pneumococcal conjugate vaccine (PCV13) is immunogenic and safe in children 6–17 years of age with sickle cell disease previously vaccinated with 23-valent pneumococcal polysaccharide vaccine (PPSV23): Results of a phase 3 study. Pediatric blood & cancer. 2015 doi: 10.1002/pbc.25502. [DOI] [PubMed] [Google Scholar]
- 134.de Montalembert M, Begue P, Fritzell B, Houmeau P. Immunogenicity and tolerability of Haemophilus b-tetanus protein conjugate (PRP-T) in children with sickle cell anemia. Archives francaises de pediatrie. 1993;50:863–866. [PubMed] [Google Scholar]
- 135.Goldblatt D, Johnson M, Evans J. Antibody responses to Haemophilus influenzae type b conjugate vaccine in sickle cell disease. Archives of disease in childhood. 1996;75:159–161. doi: 10.1136/adc.75.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaplan SL, Duckett T, Mahoney DH, Jr, Kennedy LL, Dukes CM, Schaffer DM, Mason EO., Jr Immunogenicity of Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine in children with sickle hemoglobinopathy or malignancies, and after systemic Haemophilus influenzae type b infection. The Journal of pediatrics. 1992;120:367–370. doi: 10.1016/s0022-3476(05)80898-4. [DOI] [PubMed] [Google Scholar]
- 137.Newcomer W, Santosham M, Bengston S, Panny S, Dover G. Immunogenicity of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer membrane protein complex conjugate vaccine in infants and children with sickle cell disease. The Pediatric infectious disease journal. 1993;12:1026–1027. doi: 10.1097/00006454-199312000-00014. [DOI] [PubMed] [Google Scholar]
- 138.Rubin LG, Voulalas D, Carmody L. Immunogenicity of Haemophilus influenzae type b conjugate vaccine in children with sickle cell disease. Am J Dis Child. 1992;146:340–342. doi: 10.1001/archpedi.1992.02160150080026. [DOI] [PubMed] [Google Scholar]
- 139.Vernacchio L, Romero-Steiner S, Martinez JE, MacDonald K, Barnard S, Pilishvili T, Carlone GM, Ambrosino DM, Molrine DC. Comparison of an opsonophagocytic assay and IgG ELISA to assess responses to pneumococcal polysaccharide and pneumococcal conjugate vaccines in children and young adults with sickle cell disease. The Journal of infectious diseases. 2000;181:1162–1166. doi: 10.1086/315307. [DOI] [PubMed] [Google Scholar]
- 140.Souza AR, Braga JA, de Paiva TM, Loggetto SR, Azevedo RS, Weckx LY. Immunogenicity and tolerability of a virosome influenza vaccine compared to split influenza vaccine in patients with sickle cell anemia. Vaccine. 2010;28:1117–1120. doi: 10.1016/j.vaccine.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 141.Hakim H, Allison KJ, Van De Velde LA, Li Y, Flynn PM, McCullers JA. Immunogenicity and safety of inactivated monovalent 2009 H1N1 influenza A vaccine in immunocompromised children and young adults. Vaccine. 2012;30:879–885. doi: 10.1016/j.vaccine.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 142.Payne AB, Link-Gelles R, Azonobi I, Hooper WC, Beall BW, Jorgensen JH, Juni B, Moore M. Invasive pneumococcal disease among children with and without sickle cell disease in the United States, 1998 to 2009. The Pediatric infectious disease journal. 2013;32:1308–1312. doi: 10.1097/INF.0b013e3182a11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kinabo GD, van der Ven A, Msuya LJ, Shayo AM, Schimana W, Ndaro A, van Asten HA, Dolmans WM, Warris A, Hermans PW. Dynamics of nasopharyngeal bacterial colonisation in HIV-exposed young infants in Tanzania. Tropical medicine & international health : TM & IH. 2013;18:286–295. doi: 10.1111/tmi.12057. [DOI] [PubMed] [Google Scholar]
- 144.Moyo SJ, Steinbakk M, Aboud S, Mkopi N, Kasubi M, Blomberg B, Manji K, Lyamuya EF, Maselle SY, Langeland N. Penicillin resistance and serotype distribution of Streptococcus pneumoniae in nasopharyngeal carrier children under 5 years of age in Dar es Salaam Tanzania. Journal of medical microbiology. 2012;61:952–959. doi: 10.1099/jmm.0.042598-0. [DOI] [PubMed] [Google Scholar]
- 145.American Academy of Pediatrics. Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106:362–366. doi: 10.1542/peds.106.2.362. [DOI] [PubMed] [Google Scholar]
- 146.Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526–535. doi: 10.1542/peds.109.3.526. [DOI] [PubMed] [Google Scholar]
- 147.Lliyasu G, Habib AG, Mohammad AB. Antimicrobial Susceptibility Pattern of Invasive Pneumococcal Isolates in North West Nigeria. Journal of global infectious diseases. 2015;7:70–74. doi: 10.4103/0974-777X.154440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mudhune S, Wamae M. Report on invasive disease and meningitis due to Haemophilus influenzae and Streptococcus pneumonia from the Network for Surveillance of Pneumococcal Disease in the East African Region. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(Suppl 2):S147–S152. doi: 10.1086/596494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bles P, de Mast Q, van der Gaast-de Jongh CE, Kinabo GD, Kibiki G, van de Ven A, de Jonge MI. Antibiotic resistance of Streptococcus pneumoniae colonising the nasopharynx of HIV-exposed Tanzanian infants. Tropical medicine & international health : TM & IH. 2015;20:1559–1563. doi: 10.1111/tmi.12582. [DOI] [PubMed] [Google Scholar]
- 150.William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology. 2007;12:1–13. doi: 10.1080/10245330600938422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


