Abstract
Myelination of axons in the central nervous system results from the remarkable ability of oligodendrocytes to wrap multiple axons with highly specialized membrane. Because myelin membrane grows as it ensheaths axons, cytoskeletal rearrangements that enable ensheathment must be coordinated with myelin production. Because the myelin sheaths of a single oligodendrocyte can differ in thickness and length, mechanisms that coordinate axon ensheathment with myelin growth likely operate within individual oligodendrocyte processes. Recent studies have revealed new information about how assembly and disassembly of actin filaments helps drive the leading edge of nascent myelin membrane around and along axons. Concurrently, other investigations have begun to uncover evidence of communication between axons and oligodendrocytes that can regulate myelin formation.
Oligodendrocyte precursors and their distribution
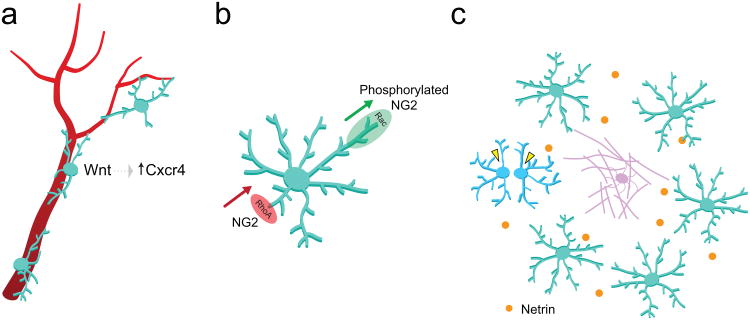
In the central nervous system (CNS), oligodendrocytes ensheath multiple neuronal axons and form myelin, a concentrically layered membrane structure intimately associated with the axon. Myelin aids in fast synaptic transmission, reduces neuronal energetic costs, and provides metabolic support to axons. During development, myelinating oligodendrocytes are generated from oligodendrocyte precursor cells (OPCs), which are specified in germinal zones in the cortex and spinal cord and subsequently undergo substantial migration and proliferation to populate the entire nervous system. While the mechanisms regulating OPC migration have been studied extensively, recent findings continue to add to our understanding of this process. One study shows OPC migration during embryonic development is guided by interactions with endothelial cells via Wnt signaling and the chemokine receptor Cxcr4 as OPCs migrate along brain vasculature to distribute throughout the developing forebrain [1] (Figure 1a). After reaching their final position, OPCs extend multiple processes bearing motile fine protrusions, which constantly survey the brain parenchyma [2] (Figure 1b). Another recent study shows that the polarity and directional migration of these motile precursors is regulated by chondroitin sulfate proteoglycan NG2 (CSPG4), which modulates RhoA and Rac signaling via polarity complexes [3] (Figure 1b).
Figure 1. Recent findings underlying oligodendrocyte precursor cell (OPCs) migration, motility, and distribution.
a) Embryonic migration along blood vessels is mediated by OPC-endothelial interactions via Wnt -Cxcr4 signaling [6]. b) The modulation of Rho GTPases by chondroitin proteoglycan, NG2, regulates OPC polarity and directional migration [8]. c) Local proliferation (yellow arrowheads) regulates OPC density in response to OPC turnover (differentiating OPC, purple) [11]; recent work indicates netrin-1 signaling may underlie this spatial homeostasis [13].
OPCs retain the capacity for migration and motility in the adult CNS, where they continue to generate new oligodendrocytes under normal conditions [4,5] and following demyelinating injuries [6]. In spite of ongoing turnover due to differentiation and cell death, OPCs remain evenly distributed throughout the adult CNS by tightly regulating their density through local proliferation and contact-mediated inhibition of process growth [2,7] (Figure 1c). While it is unknown whether OPC distribution is regulated by similar molecular mechanisms that govern neuronal spacing, recent work examining an experimentally-induced OPC ablation model has implicated a role for netrin signaling in the repopulation and distribution of OPCs in the adult CNS [8] (Figure 1c). Why is the density and distribution of oligodendrocyte precursors robustly maintained across the entire adult CNS? One hypothesis suggests that OPCs may play “non-precursor” roles as shown by recent studies where OPCs play direct roles in responding to injury [7] and modulating angiogenesis [9]. Alternatively, this distribution could provide a constant source for new oligodendrocytes in the adult CNS, which may be required for acquiring novel motor skills [10]**.
Axon ensheathment and myelin wrapping
During OPC differentiation and myelination, OPC processes transform from fine membrane extensions to multi-layered, tube-like structures ensheathing axons. Although this transformation is known to be accompanied by a profound rearrangement of the cytoskeleton, the details of axon ensheathment have been obscure, mostly because of limitations of optical imaging methods. One of the first ideas was that the nascent myelin membrane extends lengthwise to form a sheet along the axon before encircling it, much like rolling up a piece of carpet [11]. However, electron microscopy (EM) indicated that the thickness of a newly forming myelin sheath differs along an axon [12], raising the possibility that growth of myelin is non-uniform across the length of the internode. Subsequent analysis of proteins localized within nascent myelin membrane revealed the presence of coiled turns, suggesting that myelin spirals around the axon as it grows in length [13,14]. More recently, a combination of state-of-the art techniques provided a highly detailed description of axon ensheathment, leading to three particularly important new insights [15]**. First, using live imaging in zebrafish, Snaidero et al. showed that the fluorescence intensity of reporters tethered to myelin membrane decreased in quantal steps away from the point of contact of the oligodendrocyte process on the axon at early stages of sheath formation, but that fluorescence intensity became more uniform as the sheaths reached their full length. This suggests that the oligodendrocyte process begins to encircle the axon followed by lateral growth of newly formed layers, consistent with observations of axon ensheathment in vitro [14]. Second, three-dimensional reconstructions of images obtained by serial section EM analysis of mouse optic nerve indicated that the inner tongue, or leading edge, of myelin membrane advances underneath earlier-formed layers, while at the same time each layer extends laterally, producing the effect of a step-wise change in myelin thickness along the length of the growing myelin segment. Because the inner tongue turns around the axon more frequently than the outer tongue, myelin must wrap the axon by localized growth at the leading edge, rather than being spooled onto the axon from the oligodendrocyte process like thread on a bobbin. Third, tracking localization of glycoprotein G expressed by vesicular stomatitis virus combined with EM analysis provided evidence of transient cytoplasmic channels from oligodendrocyte processes to the leading edge of myelin membrane, forming conduits for polarized molecular transport, which presumably supports membrane growth at the leading edge. Earlier EM studies found evidence that, in Schwann cells, labeled phospholipids and glycoproteins appeared first in outer myelin layers and later at inner layers, suggesting that they move within the membrane from outside to inside [16, 17]. Whether Schwann cells and oligodendrocytes form myelin membrane differently or if the cytoplasmic channels of oligodendrocytes transport only a portion of myelin components are questions for future investigations.
What are the forces that propel axon wrapping by myelin membrane? In migrating mesenchymal cells, membrane protrusions are anchored to an extracellular substrate by transmembrane receptors that also link to the actin cytoskeleton, thereby providing traction to drive the cell forward. By contrast, in ameboid cell migration, fluctuations in cortical actomyosin contractility create membrane blebs that propel movement in the absence of adhesive forces [18,19]. Could myelin membrane wrap axons using one or the other of these mechanisms? Two recent publications provide significant advances toward answering that question. Using cultured cell experiments, Nawaz et al. learned that whereas OPC processes made adhesive contacts to the substrate, the motile edge of myelin membrane made by differentiating oligodendrocytes was non-adhesive [20]**, indicating that the transition from membrane process extension to axon ensheathment is accompanied by a change in the mechanism by which the membrane moves forward. They also noted that, in both zebrafish and cell culture, actin filaments were closely associated with the leading edge of myelin membrane during sheath formation, but absent from the membrane sheets, raising the possibility that coordinated assembly and disassembly of actin filaments helps drive myelin sheath formation. Consistent with this, pharmacologically-driven disassembly of actin filaments resulted in myelin membrane spreading. Notably, membrane spreading in vitro was coupled with reduced membrane surface tension, raising the possibility that myelin membrane moves in a manner distinct from blebbing-based motility driven by increasing cortical tension. Drawing on the analogy of liquid droplet spreading, Nawaz et al. proposed that myelin sheath growth occurs in a two-step process. First, assembly of an actin network inflates the leading edge, clamping it between the axon and the overlying oligodendrocyte membrane layer. Second, actin filament disassembly permits myelin membrane spreading, advancing it around and along the axon. Whether or not a spreading mechanism is sufficient to move the leading edge of myelin membrane forward within the tight space between an axon and overlying myelin remains an open question.
What are the mechanisms that coordinate actin dynamics within the myelin membrane? Noting that myelin basic protein (MBP), an important structural component of CNS myelin, is necessary for myelin wrapping [21,22], Zuchero et al. [23]** investigated the relationship between actin filaments and MBP localization in nascent and mature myelin membranes and found that, although they were closely approximate, they mostly did not overlap. Furthermore, Shiverer mutant mice, which lack MBP, retained high levels of actin filaments in spinal cord regions that are normally myelinated. Could MBP formation trigger actin filament disassembly and, if so, how? Actin is disassembled by cofilin and gelsolin family proteins, which are activated by their release from membrane PI(4,5)P2. Remarkably, MBP also binds PI(4,5)P2 [24]. Using PI(4,5)P2-coated beads, Zuchero et al. showed that MBP can compete effectively for binding with cofilin and gelsolin, suggesting that MBP can promote axon wrapping by releasing cofilin and gelsolin from the membrane, thereby stimulating actin disassembly. These observations provide an elegant explanation for how the oligodendrocyte differentiation program might be coupled with axon wrapping.
Signals regulating myelination
Given that oligodendrocyte membrane is in close contact with axons during ensheathment, it seems plausible that axonal signals direct the myelination program. However, oligodendrocytes cultured in the absence of axons express myelin genes and oligodendrocytes can myelinate fixed axons and synthetic fibers [25–27]. Additionally, oligodendrocytes obtained from spinal cord make longer sheaths on synthetic fibers than those obtained from cortex [27]. These observations indicate that oligodendrocytes have an intrinsic program that can promote myelination in the absence of axonal cues, raising the possibility that axons are a passive substrate benefitting from the propensity of glial cells to ensheath other objects. However, not all axons are myelinated and some axons are myelinated intermittently with variable myelin coverage [28]*. Consequently, even if oligodendrocytes are programmed for myelination, the program might be adaptable to extrinsic signals, including those from axons.
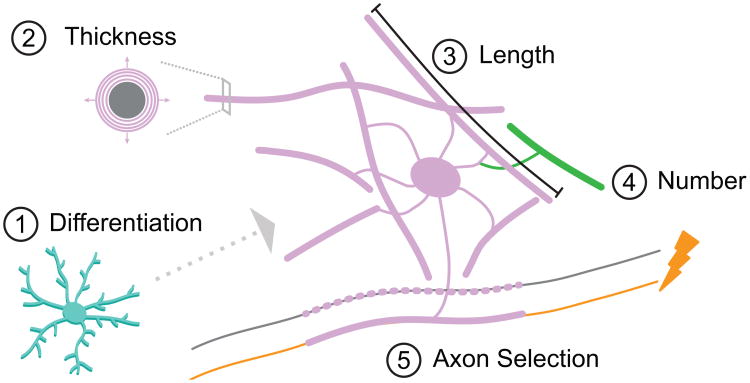
What sorts of signals could influence myelination? One of the most intriguing ideas is that myelination can be modified by experience, and thereby affect brain function [29]. For example, myelination of specific brain regions correlates with cognitive ability in children [30] and mastery of new tasks [31–33] whereas social isolation can result in myelin deficits [34–37]. Thus, specific neural circuits engaged in learning could modify their myelin profiles via activity-dependent communication between axons and oligodendrocytes. In principle, several distinct features of oligodendrocytes could respond to such signals (Figure 2). First, activity might stimulate OPCs to produce more oligodendrocytes. Consistent with this possibility, mice learning to run on a complex wheel made more oligodendrocytes, and new oligodendrocyte production was necessary for the mice to learn the task [10]*. Second, oligodendrocytes might produce thicker myelin on axons in response to activity. Indeed, optogenetic stimulation of the premotor cortex of mice increased myelin thickness, as well as OPC proliferation and oligodendrocyte differentiation [38]*. Third, activity could modulate the number of processes and myelinating sheaths formed by individual oligodendrocytes. Live imaging of zebrafish larvae revealed that blocking electrical activity or vesicle release reduced sheath number whereas pharmacological stimulation of activity increased sheath number [39]*. Fourth, oligodendrocytes might modulate the length of myelinating sheaths in response to activity. In the avian and mammalian auditory brainstem, axons differ in axon diameter and myelinating sheath lengths permitting the precise regulation of conduction timing essential for calculating interaural time differences for sound localization [40]. Fifth, activity might determine whether or not a particular axon is selected for myelination. In vitro experiments showed that secretion-competent axons were favored for myelination over those in which vesicle secretion was blocked [41]. Additionally, blocking vesicle secretion from individual phox2b+ locomotor axons in zebrafish larvae reduced the frequency with which they were wrapped by myelin membrane and also shortened the sheaths, resulting in a decrease in myelin coverage [42]*. Importantly, time-lapse imaging revealed that blocking vesicle release did not change the frequency of sheath initiation on phox2b+ axons but instead caused newly formed sheaths to be shorter and retract from axons more often. Thus, in this instance, activity-evoked vesicle release appears to promote sheath growth and stability on select axons.
Figure 2. Features of oligodendrocytes amenable to neuronal activity-induced plasticity. 1).
Generation of new oligodendrocytes: optogenetic stimulation or motor learning increases differentiation of oligodendrocyte precursor cells (OPCs) [15,48]. 2) Myelin sheath thickness: life experience or optogenetic stimulation increases myelin thickness relative to axonal diameter (g ratio) [44,48]. 3) Myelin sheath length: axon conduction times are regulated by differential axon diameter and myelin sheath length [50, (Ford,Grothe 15), 81]. 4) Myelin sheath number: electrical activity of axons or life experience modulates the number of myelin sheaths [47,49]. 5) Selection of axons to myelinate: activity-evoked vesicle release increases the stability and growth of nascent myelin sheaths [54].
Nonmyelinated axons secrete numerous neurotransmitters and growth factors extrasynaptically and OPCs express many of the corresponding receptors [43]. Could these factors relay axonal activity information to oligodendrocytes to promote myelination? One candidate signal is glutamate. Electrical activity stimulated vesicle release of glutamate from cultured neurons and increased the number of myelin sheaths formed on axons, an effect that could be blocked by inhibiting axon vesicle secretion and glutamate receptors [44]**. Consistent with these results, NMDA receptor function promoted oligodendrocyte differentiation in vitro [45]. However, mice lacking oligodendrocyte lineage cell-specific function of NMDA receptors had apparently normal myelin [46,47]. Although these in vivo studies indicate that NMDA receptor-mediated glutamate signaling is not a major driver of myelination, they do not eliminate the possibility that glutamate modulates subtle changes in myelin profiles on specific axons. Other possible myelin-promoting factors released from axon vesicles include growth factors, particularly BDNF and NrgI. Activity evoked secretion of BDNF is well established [48] and mice deficient for BDNF or its receptor TrkB are hypomyelinated [49–51]. Similarly, manipulation of NrgI or its ErbB receptors modulate CNS myelination [37,52]. Furthermore, social isolation of mice resulted in decreased NrgI expression levels accompanied by hypomyelination [37], revealing a potential link between experience, growth factor expression and myelination.
Collectively, these studies suggest that oligodendrocytes engage an intrinsic myelination program that can be modified by extrinsic cues, such as activity-evoked secretion of axonal factors. Intriguingly, NrgI and BDNF applied to neuron-glial co-cultures switched oligodendrocytes from activity-independent to activity-dependent myelination, and in so doing increased the amount of myelin formation, by stimulating NMDA receptor currents [53]*. Thus, neurotransmitters and growth factors, secreted from axons in response to electrical impulses, might act coordinately to relay axonal activity information to nascent myelin sheaths.
Local control of myelin membrane growth
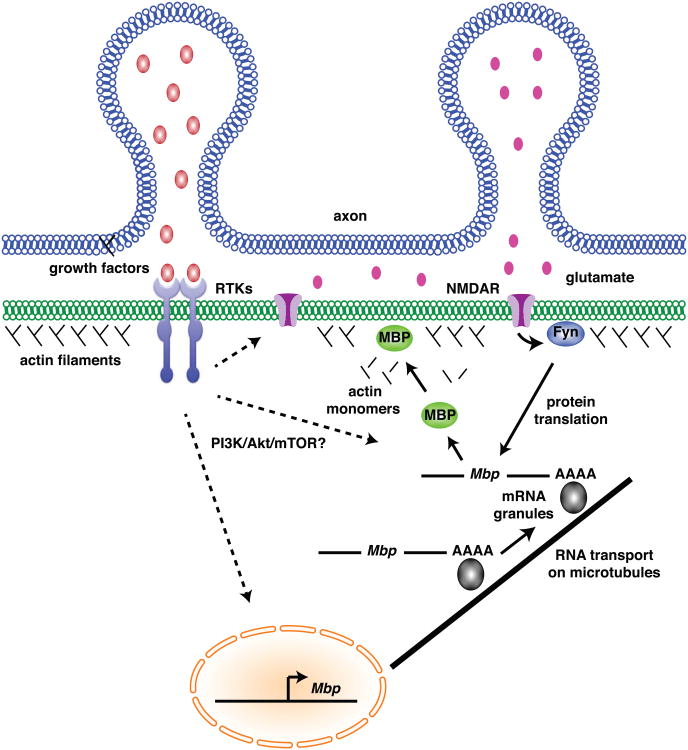
Oligodendrocytes wrap multiple axons, and the thickness and lengths of individual myelin sheaths formed by a single oligodendrocyte can vary considerably. Therefore, whereas the intrinsic myelination program is likely controlled primarily in the nucleus, the activity-dependent myelination program is probably controlled locally, within the nascent myelin sheath. Consistent with this idea, transcripts encoding some myelin proteins, notably MBP, are transported through oligodendrocyte processes and translated near the point of axon contact [54]. Consequently, mRNA localization, stability and translation could be regulated by mechanisms that transduce myelin-promoting signals from axons (Figure 3). Accordingly, membrane localization of transferrin receptor, which clusters in cholesterol-rich microdomains, was stimulated in cultured oligodendrocytes by electrical activity [44]. Cholesterol-rich microdomains are assembly points for signaling molecules [55] and oligodendrocytes require high levels of cholesterol to express myelin genes and make myelin [56,57]. Fyn kinase localized to lipid-rich membrane microdomains of oligodendrocytes [58] and enhanced translation of an MBP reporter [59]. Additionally, experiments performed by the Fields lab indicated that Fyn kinase and MBP translation could be stimulated by activity-dependent vesicle release of glutamate [44] and that an MBP reporter was preferentially translated in oligodendrocyte processes that contacted electrically active, vesicle secretion competent axons [41]. When coupled with the observation that MBP promotes axon wrapping via actin filament disassembly, evidence that MBP is translated within nascent myelin sheaths in response to neuronal activity provides an intriguing model for the control of sheath formation.
Figure 3. Potential roles of axon secreted factors in driving myelin sheath formation.
In response to electrical activity, axon vesicles release growth factors, such as NrgI and BDNF, and glutamate. Receptor tyrosine kinase signaling, activated by growth factors, sensitizes NMDA receptors to glutamate and possibly initiates signal transduction that promotes myelin gene transcription and local myelin protein translation, possibly via PI3K/Akt/mTOR signaling (dashed arrows). NMDA receptor signaling leads to Fyn kinase activity, promoting MBP translation from transcripts that are transported into oligodendrocyte processes on microtubules. MBP production near the point of axon contact initiates disassembly of actin filaments, enabling axon wrapping via membrane spreading.
Summary and outlook
The development of new experimental models and approaches coupled with powerful new imaging methods has helped elevate the status of myelin from a simple “insulator” to that of a dynamic, exquisitely regulated modulator of brain function. This new status is accompanied by a wealth of new questions. For instance, are there distinct kinds of oligodendrocyte lineage cells and, if so, how many and how do they differ in function? Though OPCs arise from several distinct regions of the embryonic CNS, ablation studies suggest that these sites produce functionally similar populations of OPCs [60]. In contrast, transplant studies showed that OPCs in the white matter have a greater intrinsic drive towards differentiation than OPCs in the gray matter [61]. Whether these differences arise from influence of the local environment or represent inherently distinct OPC subpopulations remains unclear. Large-scale single-cell RNA sequencing has begun to classify the stages of oligodendrocyte lineage maturation and even suggested diversity of post mitotic oligodendrocytes [62]. While these studies hint at considerable diversity, a full appreciation of molecular, cellular and functional distinctions will require more extensive sequencing analyses, manipulation of differentially expressed genes and careful analysis to separate population heterogeneity from lineage progression. Following on the question of cellular diversity, how many modes of myelination are there? Recent studies imply that an intrinsic, “hard-wired” myelin program can be modified by extrinsic factors. Could there be numerous extrinsic regulatory mechanisms, perhaps functioning to determine distinct myelin profiles for specific axons and neural circuits? If so, what are the signal transduction mechanisms that function within nascent myelin sheaths that promote myelin membrane growth in response to extrinsic cues? The PI3K/Akt/mTOR and ERK/MAPK pathways are potent drivers of myelin gene expression, myelin thickness and myelin sheath length [63–66] but whether or not it controls myelin production within oligodendrocyte processes in response to axonal signals is not yet known. Ultimately, finding answers to these questions will require focused investigation of specific neurons to correlate their electrical activity characteristics, their expression of factors that promote or inhibit myelination, signal transduction mechanisms that operate in oligodendrocytes and myelin coverage. Finally, how is myelin production coordinated with the cytoskeletal dynamics necessary for axon wrapping? Given the unusual characteristics of membrane growth during axon ensheathment, mechanisms that rapidly assemble and disassemble actin filaments in concert with myelin membrane production must be tightly regulated. New experimental tools to observe and manipulate cytoskeletal dynamics in vivo should provide exciting insights to this unique feature of oligodendrocyte behavior.
Highlights.
High resolution imaging studies show that the leading edge, or inner tongue, of myelin membrane winds around the axon while layers of myelin membrane also spread laterally.
Actin filaments, which are closely associated with the leading edge of myelin membrane during initial ensheathment, are disassembled to promote axon wrapping.
Synthesis of MBP triggers actin filament disassembly and axon wrapping.
Axon factors might modulate myelin production within individual sheaths, thereby modulating sheath thickness and length.
Acknowledgments
We thank Brad Zuchero for very helpful comments on the manuscript. B.A. is supported by National Institutes of Health grants NS04668 and NS095679 and the Gates Frontiers Fund.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsai H, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien A, Kuo CJ, Chan JR, Daneman R, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351:379–384. doi: 10.1126/science.aad3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- 3.Binamé F, Sakry D, Dimou L, Jolivel V, Trotter J. NG2 regulates directional migration of oligodendrocyte precursor cells via Rho GTPases and polarity complex proteins. J Neurosci. 2013;33:10858–74. doi: 10.1523/JNEUROSCI.5010-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeung MSY, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, et al. Dynamics of Oligodendrocyte Generation and Myelination in the Human Brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte Dynamics in the Healthy Adult CNS: Evidence for Myelin Remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–90. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birey F, Aguirre A. Age-Dependent Netrin-1 Signaling Regulates NG2+ Glial Cell Spatial Homeostasis in Normal Adult Gray Matter. J Neurosci. 2015;35:6946–51. doi: 10.1523/JNEUROSCI.0356-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–96. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.McKenzie Ia, Ohayon D, Li H, Paes de Faria J, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. This manuscript showed that mice learning to run on a wheel with a complex rung pattern made new oligodendrocytes and that learning to run effectively required new oligodendrocyte formation. Thus, formation of new oligodendrocytes and myelin facilitates mastery of a new motor task. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunge M, Bunge R, Physiology C, York N. Ultrastructural Study of in an Experimental Lesion in Adult Cat Spinal Cord Explanation of Scale Marks. J Biophys Biochem Cytol. 1961;10:67–94. doi: 10.1083/jcb.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knobler RL, Stempak JG, Laurencin M. Nonuniformity of the oligodendroglial ensheathment of axons during myelination in the developing rat central nervous system. A serial section electron microscopical study. J Ultrastruct Res. 1976;55:417–32. doi: 10.1016/s0022-5320(76)80097-4. [DOI] [PubMed] [Google Scholar]
- 13.Pedraza CE, Monk R, Lei J, Hao Q, Macklin WB. Production, characterization, and efficient transfection of highly pure oligodendrocyte precursor cultures from mouse embryonic neural progenitors. Glia. 2008;56:1339–1352. doi: 10.1002/glia.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N. CNS live imaging reveals a new mechanism of myelination: The liquid croissant model. Glia. 2011;59:1841–1849. doi: 10.1002/glia.21228. [DOI] [PubMed] [Google Scholar]
- 15**.Snaidero N, Möbius W, Czopka T, Hekking LHP, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, et al. Myelin Membrane Wrapping of CNS Axons by PI(3,4,5)P3-Dependent Polarized Growth at the Inner Tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. By employing a powerful and cutting-edge combination of imaging techniques, this manuscript provides the most complete description of axon wrapping. The work provides evidence that myelin sheath growth occurs at the membrane leading edge and that transient channels extend through the nascent myelin sheath to the leading edge. These channels might serve as conduits for trafficking of molecules necessary for myelin growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould RM. Incorporation of Glycoproteins into Peripheral Nerve Myelin. J Cell Biol. 1977;75:326–338. doi: 10.1083/jcb.75.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould RM, Dawson RMC. Incorporation of newly formed lecithin into peripheral nerve myelin. J Cell Biol. 1976;68:480–496. doi: 10.1083/jcb.68.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruprecht V, Wieser S, Callan-Jones A, Smutny M, Morita H, Sako K, Barone V, Ritsch-Marte M, Sixt M, Voituriez R, et al. Cortical Contractility Triggers a Stochastic Switch to Fast Amoeboid Cell Motility. Cell. 2015;160:673–685. doi: 10.1016/j.cell.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YJ, Le Berre M, Lautenschlaeger F, Maiuri P, Callan-Jones A, Heuzé M, Takaki T, Voituriez R, Piel M. Confinement and Low Adhesion Induce Fast Amoeboid Migration of Slow Mesenchymal Cells. Cell. 2015;160:659–672. doi: 10.1016/j.cell.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 20**.Nawaz S, Sánchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, Brückner BR, Alexopoulos I, Czopka T, Jung SY, et al. Actin Filament Turnover Drives Leading Edge Growth during Myelin Sheath Formation in the Central Nervous System. Dev Cell. 2015;34:139–151. doi: 10.1016/j.devcel.2015.05.013. This work attempts to distinguish between distinct mechanisms that might drive ensheathment of axons by myelin membrane. The data indicate that, instead of being powered by adhesive contacts and cytoskeletal forces, myelin membrane spreads like a liquid following disassembly of actin filaments at the membrane edge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbluth J. Central myelin in the mouse mutant shiverer. J Comp Neurol. 1980;194:639–48. doi: 10.1002/cne.901940310. [DOI] [PubMed] [Google Scholar]
- 22.Shine HD, Readhead C, Popko B, Hood L, Sidman RL. Morphometric analysis of normal, mutant, and transgenic CNS: correlation of myelin basic protein expression to myelinogenesis. J Neurochem. 1992;58:342–9. doi: 10.1111/j.1471-4159.1992.tb09316.x. [DOI] [PubMed] [Google Scholar]
- 23**.Zuchero JB, Fu M, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, et al. CNS Myelin Wrapping Is Driven by Actin Disassembly. Dev Cell. 2015;34:152–167. doi: 10.1016/j.devcel.2015.06.011. Similarly to work described in Ref. [20], this manuscript provides evidence that actin filament disassembly is necessary for axon wrapping. The authors argue that disassembly is initiated by MBP, an important compenent of myelin membrane. This potentially serves as a mechanism to coordinate myelin wrapping and growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nawaz S, Kippert A, Saab AS, Werner HB, Lang T, Nave KA, Simons M. Phosphatidylinositol 4,5-Bisphosphate-Dependent Interaction of Myelin Basic Protein with the Plasma Membrane in Oligodendroglial Cells and Its Rapid Perturbation by Elevated Calcium. J Neurosci. 2009;29:4794–4807. doi: 10.1523/JNEUROSCI.3955-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:14662–7. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Leach MK, Redmond SA, Chong SYC, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9:917–22. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bechler ME, Byrne L, Ffrench-Constant C. CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr Biol. 2015;25:2411–2416. doi: 10.1016/j.cub.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli a, Seung HS, Lichtman JW, Arlotta P. Distinct Profiles of Myelin Distribution Along Single Axons of Pyramidal Neurons in the Neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. Reconstructions of serial electron micrographs of mouse neocortex revealed variable myelin profiles on distinct axons. Surprisingly, some axons were intermittantly myelinated along their lengths. Intermittant myelination might be an important feature of myelin plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deoni SCL, O'Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Dirks H, Piryatinsky I, Dean DC, Jumbe NL. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B, Park JY, Jung WH, Kim HS, Oh JS, Choi CH, Jang JH, Kang DH, Kwon JS. White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. Neuroimage. 2010;52:9–19. doi: 10.1016/j.neuroimage.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–50. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 33.Scholz J, Klein MC, Behrens TEJ, Johansen-berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Chugani DC, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1624. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–60. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science. 2014;344 doi: 10.1126/science.1252304. This study used optogenetic stimulation of premotor cortex neurons in mice to investigate the effect of neuronal activity on myelination. Experimental animals produced more oligodendrocytes via elevated OPC proliferation and formed more myelin. Additionally, optogenetic stimulation improved performance in a limb motor task. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons Da. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:6–10. doi: 10.1038/nn.3991. Using genetic and pharmacological manipulations combined with live imaging in zebrafish, these investigators found that the number of myelinating processes formed by individual oligodendrocytes can be positively regulated by activity-evoked vesicle secretion by axons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun. 2015;6:8073. doi: 10.1038/ncomms9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat Commun. 2015;6:7844. doi: 10.1038/ncomms8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. This study is complementary to the study reported in Ref. [39]. By using time-lapse imaging to directly observe myelination of single locomotor axons in zebrafish, these authors found that activity-evoked vesicle secretion from axons promotes the growth and stability of nascent myelin sheaths following excessive initiation of axon ensheathement. This study implies that mechanisms that promote myelin growth in response to axonal signals are important for the selection of axons for myelination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larson VA, Zhang Y, Bergles DE. Electrophysiological properties of NG2+ cells: Matching physiological studies with gene expression profiles. Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–51. doi: 10.1126/science.1206998. Using co-cultures of neurons and oligodendrocytes, these authors found that electrical activity stimulates vesicle release of glutamate from axons and that glutamate can stimulate calcium signaling in oligodendrocytes. Additionally, electrical activity stimulated translation of a MBP reporter in oligodendrocyte processes, dependent on glutamate receptor and Fyn kinase functions. Collectively, the data described in this paper point to a mechanism for the local control of myelination in reponse to axonal signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia. 2013;61:732–49. doi: 10.1002/glia.22469. [DOI] [PubMed] [Google Scholar]
- 46.Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, Soulika A, Miers L, Burns T, Itoh T, Shen H, et al. Disruption of NMDA receptors in oligodendroglial lineage cells does not alter their susceptibility to experimental autoimmune encephalomyelitis or their normal development. J Neurosci. 2012;32:639–45. doi: 10.1523/JNEUROSCI.4073-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi Pa, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 2011;31:12650–62. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leßmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci Res. 2009;65:11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J Neurosci. 2013;33:4947–57. doi: 10.1523/JNEUROSCI.3990-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- 51.Cellerino A, Carroll P, Thoenen H, Barde Y. Reduced Size of Retinal Ganglion Cell Axons. Mol Cell Neurosci. 1997;408:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- 52.Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III Neuregulin-1 Promotes Oligodendrocyte Myelination. Glia. 2008;293:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- 53*.Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans Ka, Swire M, Volbracht K, Gautier HOB, Franklin RJM, Ffrench-Constant C, et al. Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. PLoS Biol. 2013;11:e1001743. doi: 10.1371/journal.pbio.1001743. Myelination appears to be regulated by activity-dependent and activity-independent mechanisms. Furthermore, both glutamate and growth factors have been implicated as myelin-promoting signals. This work takes an important step toward integrating these distinct features of myelinating control by showing that growth factors can change how oligodendrocytes respond to glutamate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ainger K, Avossa D, Morgan F, Hill SJ, Barry C, Barbarese E, Carson JH. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993;123:431–41. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 56.Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8:468–75. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- 57.Mathews ES, Mawdsley DJ, Walker M, Hines JH, Pozzoli M, Appel B. Mutation of 3-Hydroxy-3-Methylglutaryl CoA Synthase I Reveals Requirements for Isoprenoid and Cholesterol Synthesis in Oligodendrocyte Migration Arrest, Axon Wrapping, and Myelin Gene Expression. J Neurosci. 2014;34:3402–3412. doi: 10.1523/JNEUROSCI.4587-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krämer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274:29042–9. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- 59.White R, Gonsior C, Krämer-Albers EM, Stöhr N, Hüttelmaier S, Trotter J. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J Cell Biol. 2008;181:579–586. doi: 10.1083/jcb.200706164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viganò F, Möbius W, Götz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16:1370–2. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- 62.Zeisel A, Manchado ABM, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–42. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 63.Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, Macklin WB. Conditional Ablation of Raptor or Rictor Has Differential Impact on Oligodendrocyte Differentiation and CNS Myelination. J Neurosci. 2014;34:4466–4480. doi: 10.1523/JNEUROSCI.4314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fyffe-Maricich SL, Schott A, Karl M, Krasno J, Miller RH. Signaling through ERK1/2 controls myelin thickness during myelin repair in the adult central nervous system. J Neurosci. 2013;33:18402–8. doi: 10.1523/JNEUROSCI.2381-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kearns CA, Ravanelli AM, Cooper K, Appel B. Fbxw7 Limits Myelination by Inhibiting mTOR Signaling. J Neurosci. 2015;35:14861–14871. doi: 10.1523/JNEUROSCI.4968-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL. Mammalian Target of Rapamycin Promotes Oligodendrocyte Differentiation, Initiation and Extent of CNS Myelination. J Neurosci. 2014;34:4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]