Abstract
Nuclear expression of the calcium-binding protein S100A4 is a biomarker of increased invasiveness in cholangiocarcinoma (CCA), a primary liver cancer with scarce treatment opportunities and dismal prognosis. In this study, we provide evidence that targeting S100A4 nuclear import by low dose paclitaxel (PTX), a microtubule stabilizing agent, inhibits CCA invasiveness and metastatic spread. Administration of low dose PTX to established (EGI-1) and primary (CCA-TV3) CCA cell lines expressing nuclear S100A4 triggered a marked reduction in nuclear expression of S100A4 without modifying its cytoplasmic levels, an effect associated with a significant decrease in cell migration and invasiveness. While low dose PTX did not affect cellular proliferation, apoptosis or cytoskeletal integrity, it significantly reduced SUMOylation of S100A4, a critical posttranslational modification that directs its trafficking to the nucleus. This effect of lose dose PTX was reproduced by ginkolic acid, a specific SUMOylation inhibitor. Downregulation of nuclear S100A4 by low dose PTX was associated with a strong reduction in RhoA and Cdc42 GTPase activity, MT1-MMP expression and MMP-9 secretion. In a SCID mouse xenograft model, low dose metronomic PTX treatment decreased lung dissemination of EGI-1 cells without significantly affecting their local tumor growth. In the tumor mass, nuclear S100A4 expression by CCA cells was significantly reduced, whereas rates of proliferation and apoptosis were unchanged. Overall, our findings highlight nuclear S100A4 as a candidate therapeutic target in CCA and establish a mechanistic rationale for the use of low dose PTX in blocking metastatic progression of cholangiocarcinoma.
Introduction
Cholangiocarcinoma (CCA), a malignancy arising from either the intrahepatic or the extrahepatic bile ducts, still carries a severe prognosis. CCA is responsible for the 10–20% of deaths related to primary liver tumors (1). In the Western countries, its incidence is steadily increasing in the last decades (1,2), but, unfortunately, the prognosis of CCA has not changed, with less than 5% of patients surviving up to 5 years from diagnosis (1). At the time of diagnosis, less than 30% of patients are eligible for surgical resection or liver transplantation, the only potentially curative strategies. Thus, in 70% of patients the stage is advanced, because of the tumor invasiveness and early extrahepatic dissemination. Furthermore, success of curative treatments is hindered by the high rate of recurrence, with a 5-year survival after resection around 20–40%(3). Combined cisplatin and gemcitabine therapy, the current standard of care for advanced CCA, increases patient’s overall survival by less than four months with respect to gemcitabine alone (4). The lack of effective treatments reflects the deep gap in knowledge on the molecular mechanisms underlying CCA invasiveness. Better understanding of these mechanisms is needed to predict the invasiveness of the individual tumor and to devise molecular targeted therapy (5).
Among the biomarkers of increased tumour invasiveness, S100A4 has drawn particular attention in the last few years. S100A4, a low molecular weight, cytoskeleton-associated calcium-binding protein, is normally expressed by mesenchymal (mostly fibroblasts and macrophages), but not by epithelial cells. S100A4 may handle different functions depending upon its cellular localization. When localized in the cytoplasm, it may interact with cytoskeleton and plasma membrane proteins (including actin, non-muscle myosin-IIA and -IIB, p53, liprin-β1, methionine aminopeptidase-2)(6), thereby contributing to the regulation of cell proliferation, survival, differentiation, as well as cell reshaping and cytoskeletal rearrangement. When translocated to the nucleus, S100A4 may act as transcription factor for several genes, including those encoding adherence junction proteins, thus controlling cell motility (7). A number of studies have shown that S100A4 is a marker of poor prognosis in breast and colon cancers (7,8). We have shown that in CCA patients undergoing surgical resection, nuclear expression of S100A4 in tumor cells is a strong, independent prognostic marker of poor outcome in terms of both metastasization and tumor-related death (9). Furthermore, S100A4 lentiviral silencing significantly reduced motility and invasive capabilities of CCA cells (9), suggesting that nuclear expression of S100A4 is not merely a marker of cancer invasiveness, but is a key determinant of the metastatic phenotype of CCA.
Aim of this study was to understand the mechanisms by which nuclear S100A4 induces an invasive phenotype in CCA and the mechanism regulating nuclear translocation of S100A4. Unfortunately, mechanisms governing S100A4 expression in the nucleus remain elusive, and there are no strategies to selectively target S100A4 nuclear import. In planning our experiments, we came across studies from the early ‘90s showing that paclitaxel (PTX) was able to reduce the expression of S100A4 in the B16 murine melanoma cells (10,11).
PTX is a semisynthetic derivative of taxol, a natural diterpene alkaloid, isolated from the bark of Taxus brevifolia. Because of its anti-proliferative and pro-apoptotic effects, PTX is currently used in chemotherapy protocols for the treatment of ovary, lung, thyroid and breast carcinomas (12). Our study shows that low dose PTX inhibits tumor invasiveness and hematogenous metastases by blocking SUMOylation-dependent S100A4 nuclear import in CCA.
Materials and methods
Human established and primary CCA cell lines
The S100A4-expressing established CCA cell lines, EGI-1 (both in the nucleus and in the cytoplasm) and TFK-1 (only in the cytoplasm), both obtained from extrahepatic CCA (9), were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Germany). The primary CCA cell line CCA-TV3 was isolated from a human sample derived from surgical resection of an intrahepatic mass-forming CCA, histologically categorized as cholangiocellular carcinoma (grading G3), performed in Treviso Regional Hospital (MM, TS) as described (13). Local regional ethical committee approval was obtained for tissue collection and cell preparation. Cultured cells were grown in RPMI1680 supplemented with 10% FBS and 1% penicillin at 37°C in a 5% CO2 atmosphere, and then frozen at low passages (<5). After any resuscitation, cell authentication was performed by checking morphology and by evaluating their immunophenotype as characterized by our previous studies (9,13), including cytokeratin (K)-7, K19, EpCAM (clone HEA125), E-cadherin, β-catenin and S100A4. Following experiments were run in cultured cells with <20 passages. Mycoplasma contamination was excluded using a specific biochemical test (Lonza).
Treatment with PTX
In all experiments, cultured CCA cells were seeded and grown for 24h (otherwise differently indicated) before exposure for 24h to PTX at low doses (1.5 and 15nM, diluted in DMSO, Sigma), except for assessment of cell proliferation, viability, apoptosis and cytoskeletal integrity, where high doses (150 and 1500nM) were further tested. Untreated CCA cells served as controls.
Expression of S100A4
Differential expression of S100A4 was evaluated in cytoplasmic and nuclear cell fractions by Western blotting (WB) using the same primary antibody (DAKO, 1:2000) and the NePer Kit (Pierce) as detection system, as already performed by us (9,13).
Membrane-type 1 (MT1) matrix metalloproteinase (MMP) expression
MT1-MMP expression was evaluated by WB in total lysate of EGI-1 before and after PTX treatment using an anti-MT1-MMP monoclonal antibody (Millipore, 1:500). The membrane expression levels of MT1-MMP were then evaluated by assessing the fluorescence intensity profile on cultured cells in five random fields for each experiment (14). See supplemental-online section for details.
Cell proliferation and cell viability
were evaluated by BRDU (GE healthcare) and MTS (Promega) respectively, while cell apoptosis was assessed by immunofluorescence for cleaved caspase-3 (Cell Signaling). See supplemental on-line section for details.
Cytoskeletal integrity assessment
In PTX-treated CCA cells, actin filaments were stained by Alexa Fluor 488-conjugated phalloidin (Invitrogen), and then the percentage of cells showing a damaged cytoskeleton on the total cultured cells, was evaluated (15). In additional experiments, we assessed the expression of β-tubulin by WB in EGI-1 cells before and after exposure to PTX at different doses, in microtubule fractions purified by ultracentrifugation (Cytoskeleton Inc).
Cell migration (wound healing) assay
See supplemental on-line materials for details.
Cell invasion (Boyden chamber) assay
Rho-A, Rac-1 and Cdc-42 GTP levels
See supplemental on-line section for details.
MMP-9 secretion
Since ELISA assessed both pro and active MMP-9 forms (RayBiotech), we performed gelatin zymography in EGI-1 with and without PTX treatment to see whether MMP-9 was actually active. See on-line supplement for details.
SUMOylation assay
CCA cells were seeded in a 6-well plate and let to grow until confluence, before exposure to PTX. Cell lysates were prepared using Cellytic (Sigma) and 200µl (1mg/ml) were loaded into column coated with VIVAbind SUMO matrix (VivaBioscience), to capture SUMOylated proteins. SUMOylated protein fraction was then eluted and analyzed by WB (1:1000). Amount of SUMOylated S100A4 was then related to that of total S100A4 (flow through) and compared with controls.
SUMO E1, E2, and E3 enzyme expression
In CCA cells cultured and exposed to PTX as before, expression levels of the three enzymatic subunits, E1 (activating enzyme), E2 (conjugating enzyme) and E3 (ligase), involved in SUMO modification, were measured by Real-Time PCR using specific probes (LifeTechnologies) and compared to untreated cells.
Effects of ginkolic acid (GA) on S100A4 nuclearization, and on CCA cell viability and migration
Selected effects of GA, a well-established SUMOylation inhibitor, on nuclearization of S100A4 (by WB), cell viability (by MTS assay) and migration (by wound healing assay) of CCA cells were studied and compared with PTX. Given the toxicity of GA, a preliminary dose-response experiment of cell viability was run treating EGI-1 with increasing concentrations of GA (1, 10, 100µM) for 72h (16).
Xenotransplantation experiments in severe combined immune-deficient (SCID) mice
EGI-1 (500.000 cells suspended in PBS 100µl) were injected into the spleen of SCID mice (6–8 weeks old; Charles River Laboratories), after transduction with a lentiviral vector encoding the firefly luciferase gene to enable detection of tumor engraftment by in vivo bioluminescence imaging. Tumor engraftment was checked at weekly intervals using the Living Image® software (Xenogen), and considered positive when reaching an average of at least 103 p/sec/cm2/sr, according to our previous studies (9,13). CCA cells injected intrasplenically are delivered straight into the liver through the portal venous axis, giving rise to orthotopic CCAs. Procedures involving animals and their care were conform to the institutional guidelines that comply with national and international laws and policies (EEC Council Directive 86/609, OJ L 358, December 12, 1987), and approved by the Ethical Committee of the University of Padua.
Low dose metronomic (LDM) PTX treatment of SCID mice xenotransplanted with human EGI-1 cells
Once tumor engraftment was confirmed by bioluminescence imaging (time 0), we started metronomic infusion of PTX (diluted in a 50%/50% solution of Cremophor EL (BASF) and ethanol (Carlo Erba) (vehicle)) for 2 weeks at the dosage of 2.6mg/kg/die by i.p. injection using micro-osmotic pumps (Alzet 1004, Durec). Mice were randomly divided into 2 experimental groups: a) controls (vehicle only, n=14); b) LDM PTX (n=10). After the first week of treatment, mice were checked by bioluminescence imaging (time 1) to detect metastatic spread, as previously performed (9,13). At the end of treatment (time 2), after a further bioluminescence analysis, mice were anesthetized and sacrificed for necroscopic examination and sample harvesting from spleen (to evaluate the tumor mass at the site of injection) and lungs (to evaluate hematogenous metastases). Tissue samples were fixed in buffered formalin and embedded in paraffin for immunohistochemical analysis. The xenograft model is illustrated in Supplementary Fig. 1A.
Assessment of the tumor growth in the site of engraftment
The tumor growth in the site of injection was evaluated in paraffin-embedded sections obtained from the tumor-bearing spleen, collected at the time of sacrifice. The tumor mass area was measured using an electronic caliper and expressed in mm2.
Metastasis analysis
Serial sections from 10 different cutting plans at a 200µm-interval were taken from lungs of sacrificed mice and stained by H&E (Supplementary Fig. 1B). In the same section, immunohistochemistry for human mitochondria (1:100, Millipore) was performed to detect two different types of metastatic invasion, isolated tumor cells (ITC), represented by single cells or small clumps up to 5 cells, and micro metastases (MM), larger clusters containing more than 5 cells (17–19). Immunohistochemistry for human mitochondria provides a useful tool to improve human cancer cell detection in xenograft models. The metastases were expressed as total number of human mitochondria-expressing cells in the 10 cutting plans.
Immunohistochemistry for S100A4, p-Hist3 and CC3 in SCID mice spleen specimens
See supplemental on-line section for details.
Statistical analysis
In vitro experiments
Results were shown as the mean ± standard deviation (SD). Statistical comparisons were made using Student’s t-test. Statistical analyses were performed using SPSS 20.0 software (IBM Corp.). A 2-tailed p value <0.05 was considered significant.
In vivo experiments
Continuous data were shown as mean±SD, and categorical data as counts and percentages. Distributions of tumor size, number of MM and ITC in lung samples were graphically displayed by box plots and scatter dot plots comparing the two groups (PTX vs vehicle). One-sample Kolmogorov-Smirnov test was used to check distributions for normality, while Levene's statistic for homogeneity of variances. Mean differences between the two groups were analyzed with the two independent samples t-test or Welch's test, according to distributions characteristics. Welch's t-test is an adaptation of Student's t-test and is more reliable when the two samples have unequal variances and unequal sample sizes. Data were collected and reviewed in Microsoft Excel, and statistical analysis was performed using SPSS 20.0 software. All 2-tailed p<0.05 were considered statistically significant.
Results
Low dose PTX decreased expression of S100A4 in the nucleus but not in the cytoplasm of EGI-1 and primary CCA cell lines
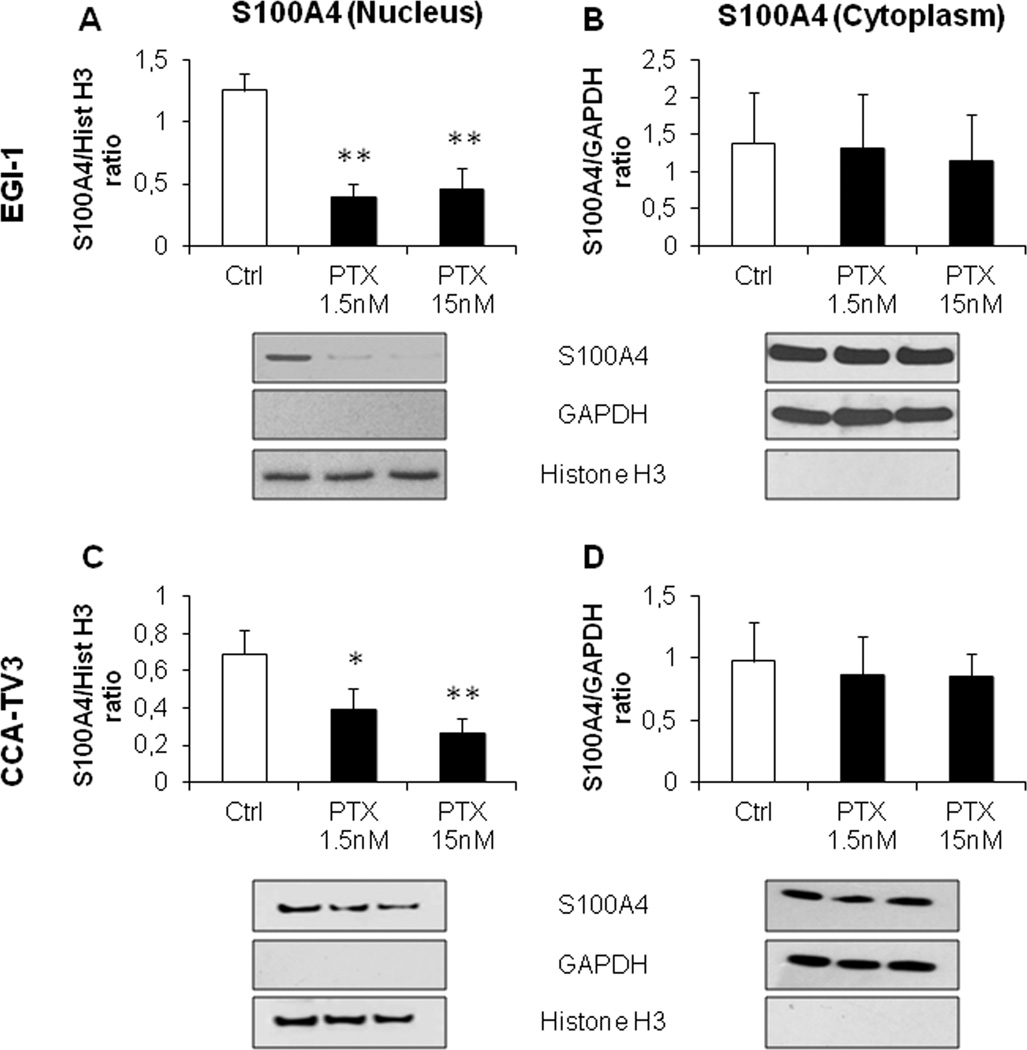
As previously shown (9), EGI-1 cells constitutively expressed S100A4 in the nucleus, and therefore represent a good model to study the effects of its nuclear down-modulation. Treatment with low dose PTX (1.5 and 15nM) induced a significant and marked reduction in S100A4 nuclear expression (of about 60 and 80%, with respect to controls, respectively) (Fig. 1A), without modifying its cytoplasmic expression (Fig. 1B). This finding was further confirmed in primary CCA cell lines (CCA-TV3) obtained from a surgical sample similarly expressing S100A4 in the nucleus; PTX induced a dose-dependent reduction in the nuclear levels of S100A4 of 38% (1.5nM) and 62% (15nM) as compared with controls, again without affecting the cytoplasmic fraction (Fig. 1C,D).
Figure 1. Low dose PTX reduced nuclear but not cytoplasmic S100A4 expression in established (EGI-1) and primary (CCA-TV3) CCA cell lines.
A–D. Treatment with PTX at low doses (1.5, 15nM) induced a significant reduction in the nuclear (A,C) but not in the cytoplasmic S100A4 content (B,D), with respect to controls, in both cell lines. Below each column plot, representative blots of S100A4 together with histone H3 and GAPDH (markers of nuclear and cytoplasmic fractions, respectively), are shown (n=5 for EGI-1; n=3 for CCA-TV3). *p<0.05 vs Ctrl, **p<0.01 vs Ctrl.
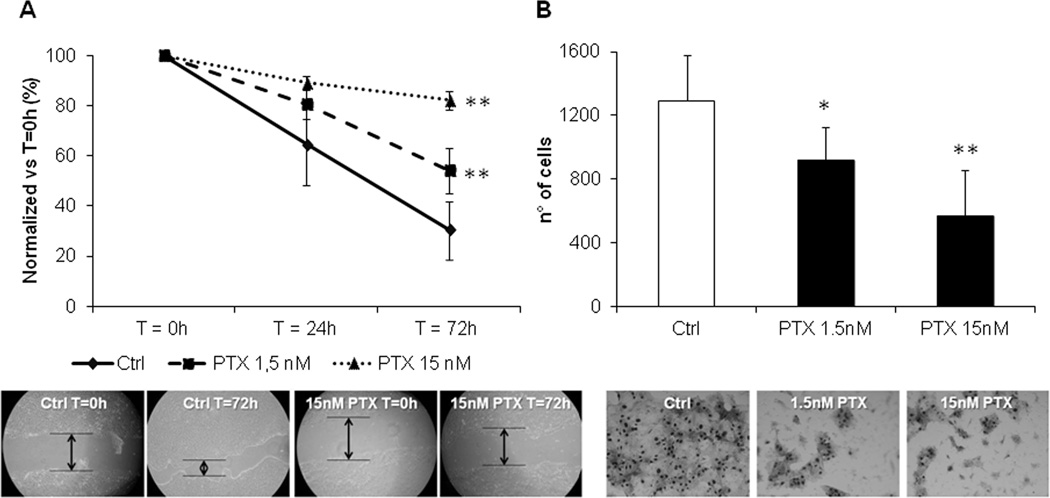
Low dose PTX reduced cell motility and invasiveness of EGI-1 and primary CCA cell lines, without affecting cell proliferation, cell viability and apoptosis
Following exposure to PTX at 1.5 and 15nM, both EGI-1 (Fig. 2A) and CCA-TV3 cells (Supplementary Fig. 2A) showed a significant dose-dependent reduction in cell motility, compared with controls. Cell motility and cell invasiveness of EGI-1 and CCA-TV3 were also significantly reduced in a dose-dependent manner by low dose PTX (Fig. 2B and Supplementary Fig. 2B, respectively). Interestingly, by comparing PTX 1.5 and 15nM effects on CCA cells, we found that the degree of cell motility inhibition paralleled the extent of S100A4 nuclear reduction (Fig. 2A–B).
Figure 2. Low dose PTX reduced motility and invasiveness of EGI-1 cells.
A. In the wound healing assay, cell motility of EGI-1 significantly decreased in a dose-dependent fashion, following PTX 1.5 (dotted line) and 15nM (dashed line) exposure, compared with controls (continue line) (n=12). B. In Boyden chambers coated with Matrigel, the same PTX dose regimens significantly attenuated the invasive properties of EGI-1, with respect to controls (n=6). Representative images of scratch and transwell filter are shown below their respective plot. *p<0,05 vs Ctrl; **p<0.01 vs Ctrl.
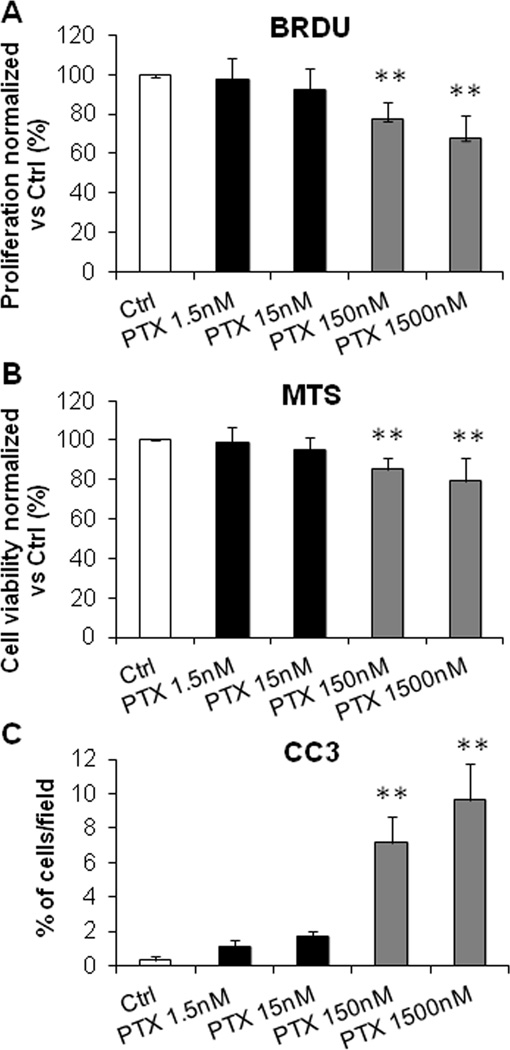
In contrast, low dose PTX did not induce significant change in cell proliferation (Fig. 3A for EGI-1, Supplementary Fig. 3A for CCA-TV3), cell viability (Fig. 3B for EGI-1, Supplementary Fig. 3B for CCA-TV3) and apoptosis (Fig. 3C for EGI-1, Supplementary Fig. 3C for CCA-TV3). Cell proliferation, viability and apoptosis were instead strongly affected by high dose PTX (Fig. 3A–C, Supplementary Fig. 3A–C) in both CCA cell lines. These data indicate that in CCA cells, nuclear expression of S100A4 exerts clear pro-motile and pro-invasive effects, without influencing the proliferation/apoptosis balance.
Figure 3. In contrast with high doses, low dose PTX did not affect cell proliferation, viability, and apoptosis of EGI-1 cells.
A–C. Effects of low dose PTX (1.5, 15nM) on cell proliferation (BRDU incorporation, A), viability (MTS assay, B), and apoptosis (CC3 immunofluorescence, C) were evaluated in EGI-1 and compared with effects of higher doses (150, 1500nM) and with untreated cells. In contrast with the highest doses, these cell activities were not affected by low dose PTX (n=6 in all experiments). **p<0.01 vs Ctrl.
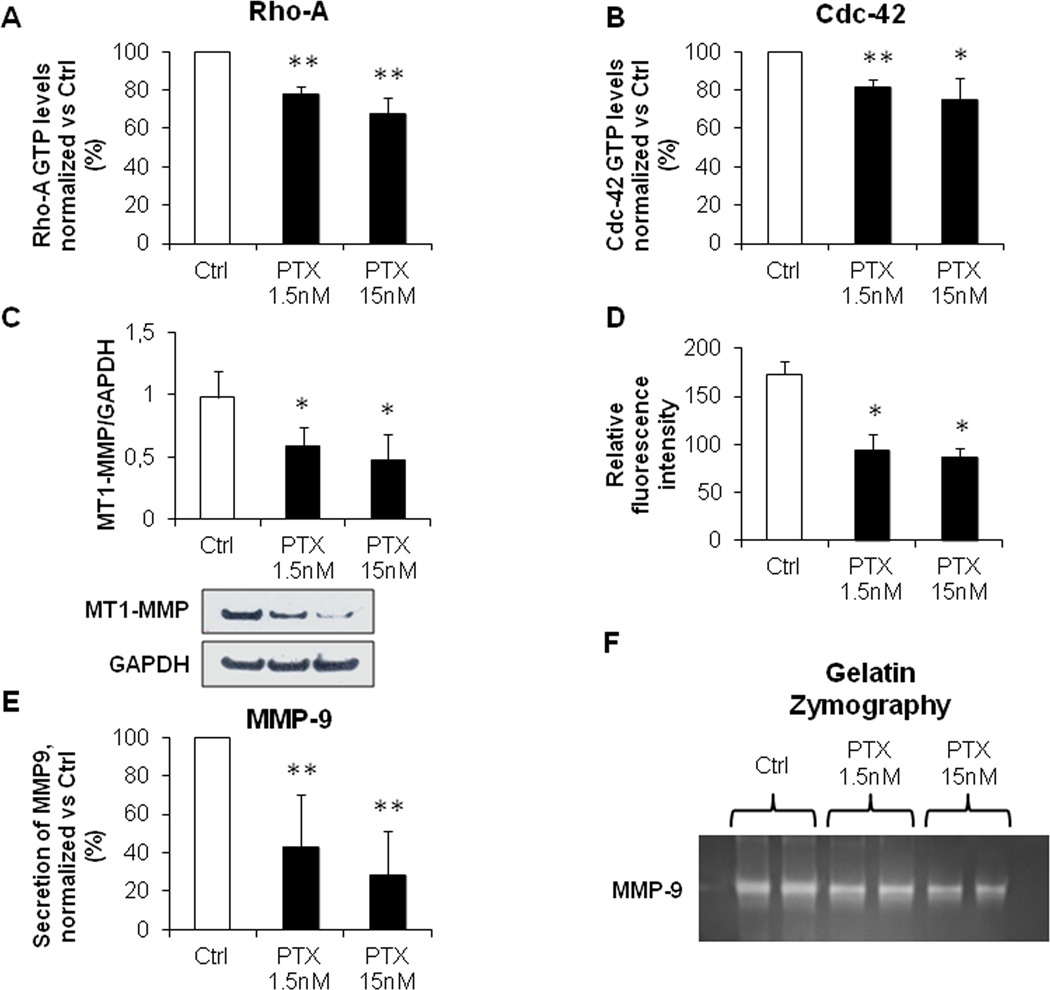
Low dose PTX reduced Rho-A and Cdc-42 activation and MMP-9 secretion in EGI-1 cells
To better understand the mechanisms promoting cell motility and invasiveness dependent upon S100A4 nuclearization, we turned to study the effects of low dose PTX on the activity of small Rho GTPases, the expression of MT1–MMP and the secretion of MMP-9. Small Rho GTPases are key effectors of cell motility by inducing the formation of stress fibers (Rho-A), lamellipodia (Rac-1) and filopodia (Cdc-42)(20). MMP-9 on the other hand, is a proteolytic enzyme secreted by many cancer cell types: it potently stimulates matrix degradation, facilitating the invasive migration of tumoral cells from the primary site of growth (21). Its activation depends upon the expression of membrane-anchored MMP, particularly of MT1-MMP, whose expression at the surface of cancer cells is critical for breaking the basement membrane (22). Since EGI-1 phenocopied the behavior of primary CCA-TV3 cell line, the following experiments were performed in EGI-1 only. As compared with controls, EGI-1 treated with low dose PTX showed a significant reduction in Rho-A (Fig. 4A) and Cdc-42 (Fig. 4B) GTP levels, but not in Rac-1 (Supplementary Fig. 4). As evaluated by WB and immunofluorescence on cultured EGI-1, MT1-MMP expression significantly decreased after PTX treatment (inhibition of 40 and 46% with 1.5, and of 50 and 49% with 15nM, for WB and immunofluorescence, respectively) (Fig. 4C–D). Similarly, MMP-9 secretion and activation were inhibited by challenging CCA cell cultures with PTX, as shown by ELISA (inhibition of 57% with 1.5, and of 72% with 15nM) and gel zymography (Fig. 4E–F).
Figure 4. Low dose PTX inhibited Rho-A and Cdc-42 GTP levels, together with MT1-MMP expression and MMP-9 secretion.
A, B. By G-LISA assay, low dose PTX (1.5, 15nM) significantly reduced Rho-A (A) and Cdc-42 (B) GTP levels in EGI-1, with respect to controls (n=3, in duplicate). C, D. Low dose PTX strongly inhibited MT1-MMP expression (WB, C), specifically on its membrane localization (fluorescence intensity profile, D) (n=3, for both experiments). E, F. Low dose PTX blunted both secretion (ELISA, E) (n=5, in duplicate) and activation (gelatin zymography, F) of MMP-9. *p<0.05 vs Ctrl, **p<0.01 vs Ctrl.
Low dose PTX did not induce cytoskeletal damage in EGI-1 cells
S100A4 is normally associated to cytoskeletal fibers. To study if low dose PTX altered cytoskeletal integrity, we performed phalloidin fluorescence in dose-dependent experiments, ranging from 1.5–15nM to 150–1500nM. In contrast with high dose PTX, which induced actin fiber changes (shortening, thickening, fragmentation often leading to dense coiling, accumulating in the perinuclear area) in 16% (150nM) and 32% (1500nM) of cultured EGI-1, more than 90% of cells treated with low dose PTX showed preserved cytoskeletal structure (Supplementary Fig. 5). These data indicate that effects of low dose PTX on cell motility and invasiveness are not due to a cytoskeletal damage. Next, we sought to understand the mechanisms regulating the nuclear import of S100A4.
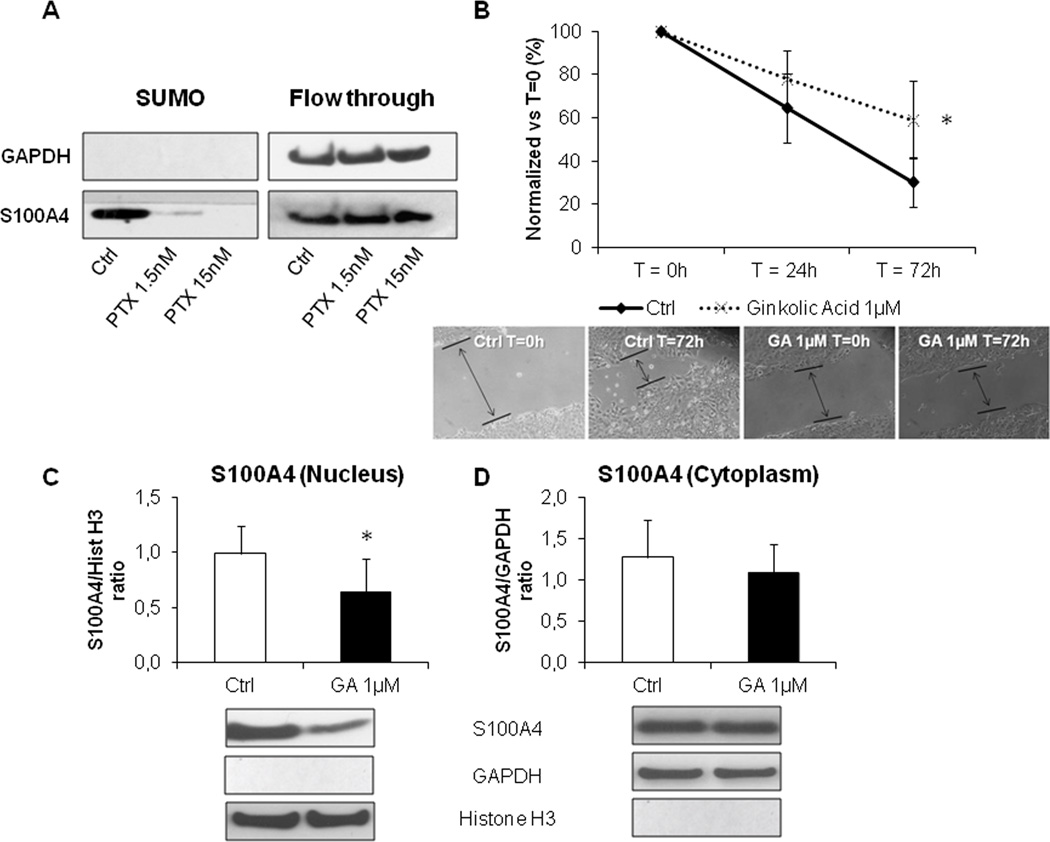
PTX selectively reduced the SUMOylation fraction of S100A4, a critical mechanism for cell invasiveness of EGI-1 cells
Post-translational modification by small ubiquitin-like modifier (SUMO) of target proteins is an important mechanism directing their intracellular shuttling. We showed that EGI-1 contained much higher amounts of SUMOylated S100A4 than TFK-1, a CCA cell line expressing S100A4 only in the cytoplasm, where instead un-SUMOylated S100A4 was detected (Supplementary Fig. 6A). Furthermore, after treatment with low dose PTX, EGI-1 showed a marked reduction selectively in the SUMOylated fraction of S100A4 as compared with controls, whereas the S100A4 unSUMOylated levels remained unchanged (Fig. 5A). SUMO inhibition by PTX was not associated with decreased mRNA expression levels of the three SUMO E components of the SUMOylating complex (Supplementary Fig. 6B–D). To study if inhibition of SUMOylation halted cell invasive capabilities, EGI-1 were treated with GA, a specific natural inhibitor of the E1 subunit. Preliminary dose-response experiments to assess toxicity levels of GA on EGI-1 cells identified 1µM as the dose devoid of effects on cell viability (not shown), and therefore used onwards. Consistent with our hypothesis, GA significantly reduced cell motility of EGI-1 with respect to controls (Fig. 5B), an effect associated with a significant reduction (37%) in the nuclear expression of S100A4 of an extent comparable to PTX 1.5nM, without affecting the cytoplasmic levels of S100A4 (Fig. 5C,D).
Figure 5. SUMOylation of S100A4 was modulated by low dose PTX and led motility and S100A4 nuclear import in EGI-1 cells.
A. By SUMOylation assay, EGI-1 exposed to PTX 1.5 and 15nM showed a stark reduction in the amount of SUMOylated S100A4 compared with controls, without changes in the total amount of un-SUMOylated S100A4 protein (flow through). B–D. PTX effects were reproduced by ginkolic acid (GA, 1µM), a specific SUMOylation inhibitor, which significantly reduced EGI-1 cell migration (B, dotted line, n=12). This effect was associated with a significant decrease in the nuclear S100A4 (C) compared with controls, whilst the cytoplasmic fraction was unaffected (D). Below each column plot, representative blots of S100A4 together with histone H3 (nuclear marker) and GAPDH (cytoplasmic marker), are shown (n=6). *p<0.05 vs Ctrl.
PTX treatment reduced lung metastasization but not the tumor growth at the site of injection in the experimental model of CCA
Altogether, the in vitro data suggest that the SUMOylation-dependent nuclear import of S100A4 is indeed a mechanistic determinant of the invasive phenotype of CCA cells, which can be inhibited by PTX at nM doses without altering the cytoskeletal integrity, as well as the proliferation and/or apoptosis activities. To test in vivo whether targeting nuclear S100A4 by low dose PTX is therapeutically relevant to reduce CCA invasiveness, we moved to the experimental model of CCA generated by EGI-1 cell xenotransplantation in the SCID mouse (9,13).
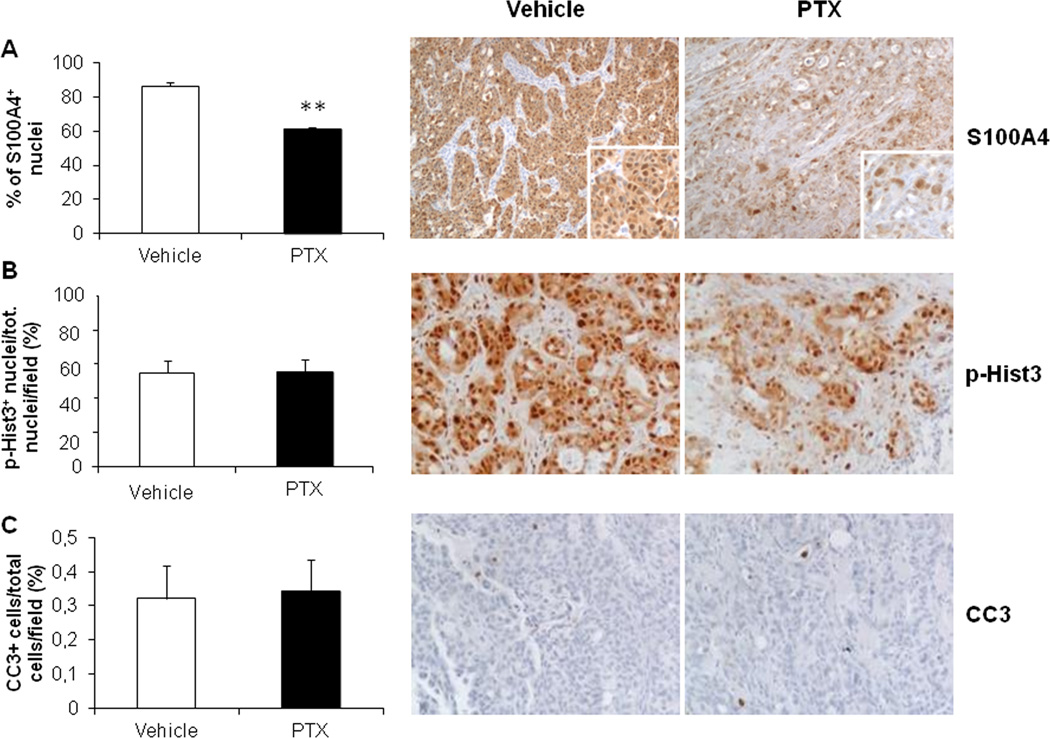
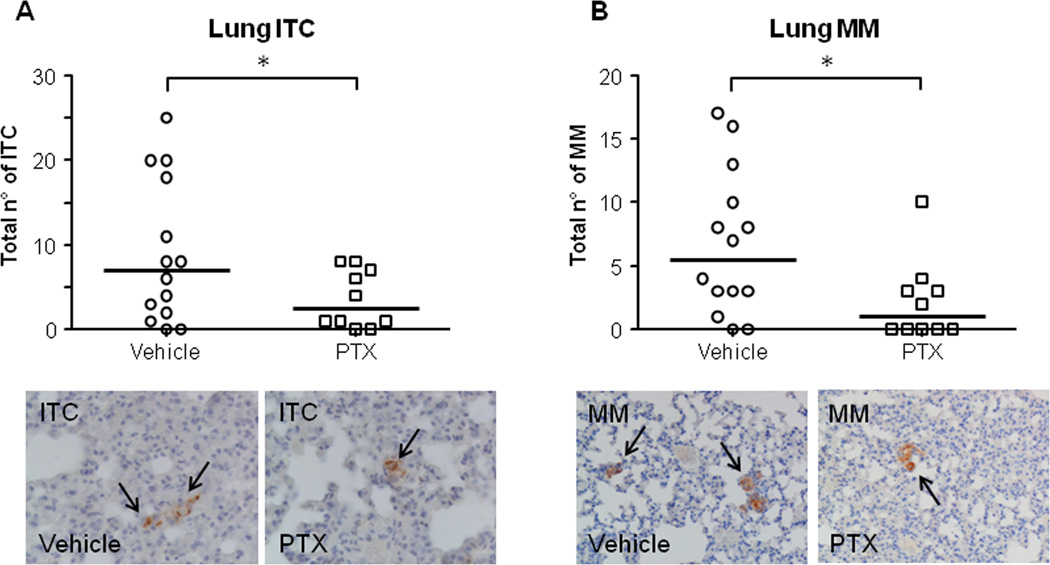
To reproduce the small nM doses of PTX able to hamper S100A4 nuclear entry in CCA cells in vitro, a low dose metronomic regimen was chosen for drug infusion in xenografted mice (LDM PTX). To assess specific effects on cancer invasiveness, the treatment was started upon successful EGI-1 cell engraftment as confirmed by bioluminescence imaging (on average 29.75±4.53 days after intrasplenic injection). Notably, before starting LDM PTX, the levels of photon emission were comparable between the two groups. With respect to control animals, LDM PTX did not significantly reduce neither photon emission from the spleen through the treatment time (Supplementary Fig. 7A,B), nor the size of the splenic tumor mass at the time of sacrifice (Supplementary Fig. 7C). Immunohistochemistry in tissue sections obtained from the tumor mass confirmed that LDM PTX was effective in decreasing S100A4 expression in the nucleus of engrafted EGI-1 cells compared with controls (Fig. 6A), however, the expression of p-Hist3 (proliferation marker) and CC3 (apoptosis marker) did not significantly differ between LDM PTX-treated and control mice (Fig. 6B,C). These findings indicate that LDM PTX reproduces the down-modulating effects on S100A4 nuclear expression by EGI-1 shown in vitro, without causing a dysregulation in the proliferation/apoptosis balance of CCA cells. In contrast, LDM PTX significantly halted metastatic dissemination. Immunohistochemistry for human mitochondria revealed that both ITC and MM in the lung were significantly reduced by PTX treatment (Fig. 7A,B). These data further prove the functional impact of S100A4 nuclear expression on CCA biology as mechanism specifically driving hematogenous metastasization, without stimulating tumorigenesis.
Figure 6. LDM PTX reduced nuclear S100A4 expression without affecting proliferation and apoptosis of xenotransplanted EGI-1 cells, in vivo.
A. Nuclear immunoreactivity for S100A4 was significantly reduced in the tumor mass at the site of injection (spleen) of LDM PTX-treated mice (n=10) with respect to vehicle-treated controls (n=14). B–C. Conversely, LDM PTX did not modify neither cell proliferation (immunohistochemistry for p-Hist3, B) nor apoptosis (immunohistochemistry for CC3, C) in the splenic mass compared with controls. Representative micrographs of spleen sections immunostained for S100A4, p-Hist3 and CC3 are shown in the right side of the plot (immunoperoxidase; A, M=100×; B, C, M=200×; insets, M=200×). **p<0.01 vs Ctrl.
Figure 7. LDM PTX inhibited lung metastatic colonization of EGI-1 cells, in vivo.
A–B. A significant reduction in the number of both ITC (A) and MM (B) was found in treated mice (n=10) with respect to controls (n=14). Representative micrographs of ITC and MM (black arrows) derived from EGI-1 cell dissemination to the lung parenchyma after xenograft in SCID mice undergoing LDM PTX and in controls, identified by the specific immunoreactivity for human mitochondria, are shown below their respective dot plot. *p<0.05 vs Ctrl; Immunoperoxidase; original magnification, M=400× (ITC), M=200× (MM).
Discussion
An unmet need in CCA, as in other malignancies whose dismal prognosis relates to limited therapeutic approaches, is the development of biomarkers able to identify patients most likely to take advantage of curative treatments. Biomarkers may also represent disease-relevant targets for therapeutic interventions (9). Our previous studies showed that nuclear expression of S100A4 in cancer cells of resected CCA (nearly a half) identified a more invasive clinical phenotype, characterized by increased metastasization and reduced survival after surgery (9). Notably, a worse prognosis after surgery was still observed even when S100A4 nuclear expression was scattered, limited to less than 30% of the neoplastic ducts (9). The aim of the present study was to understand the mechanisms by which nuclear expression of S100A4 promotes cancer invasiveness, and to elucidate the mechanism regulating nuclear translocation of S100A4. We also wanted to understand if these mechanisms are putative target for therapeutic intervention.
We initially found that PTX given in vitro at low doses (1.5 and 15nM) was able to effectively and selectively down-regulate S100A4 expression in the nucleus of CCA cells, leaving cytoplasmic expression unaffected. Then, by combining in vitro and in vivo techniques, we showed that: a) PTX-induced down-regulation of nuclear S100A4 was associated with a reduction in motility and invasiveness of CCA cells, in activity of Rho-A and Cdc-42, and in secretion of MMP-9; b) at the doses able to down-regulate nuclear expression of S100A4, PTX did not affect cell proliferation, apoptosis, and cytoskeletal architecture; c) the nuclear translocation of S100A4 was regulated by SUMOylation, a post-translational mechanism that was affected by PTX; d) in SCID mice xenografted with human nuclear S100A4-expressing CCA cells, down-regulation of nuclear S100A4 by LDM PTX was associated with a reduction in hematogenous metastasization.
The biological functions of S100A4 are largely unknown. They depend on its interacting partners, which are mainly located in the cytoplasm, where S100A4 is commonly expressed. In the nucleus, interacting partners have not been characterized yet, opening the possibility that nuclear S100A4 may act independently as transcription factor (7).
Earlier studies showed that in mouse melanoma cells, taxol at conventional doses, reduced the total amount of S100A, an effect associated with maintenance in the G0 phase of the cells and increased expression of p53(10,11). We initially found that in CCA cells, low doses of PTX (1.5–15nM) induced a marked reduction in S100A4 selectively in the nucleus, without altering its cytoplasmic expression. This effect, obtained in an established CCA cell line (EGI-1) was reproduced in a primary CCA cell line derived from a patient undergoing surgical resection. The nuclear down-regulation of S100A4 was biologically relevant since it associated with a strong inhibition of the motile and invasive properties displayed by CCA cells in culture. Interestingly, at the same small doses, PTX did not exert anti-proliferative and pro-apoptotic functions, nor it affected cell viability or the integrity of the actin cytoskeletal filaments of cultured CCA cells. All these cytotoxic effects were instead induced by PTX at higher doses (150–1500nM), coupled with pronounced anti-proliferative and pro-apoptotic activities, in line with the mechanism of action supporting the current indications of PTX for the chemotherapeutic treatment of several aggressive carcinomas, from ovary, to breast and thyroid cancer (23,24). The lack of pro-proliferative stimuli when S100A4 translocates into the nucleus has been recently shown also in colorectal cancer cells (25). In this study, although S100A4 translocated into the nucleus in a cell cycle-dependent fashion, being most prominent in the G2/M phase, lentiviral silencing of nuclear S100A4 did not induce changes in cell proliferation. Our findings are consistent with these observations, and confirm that nuclear S100A4 confers specific pro-invasive functions.
Among putative molecular players mediating the effects of nuclear S100A4 on cell motility and invasiveness, we focused on the small G proteins (GTPases) belonging to the Rho family and on matrix metalloproteinases (MMPs). The small Rho GTPases are recognized as key effectors able to activate invasion and metastasis programs (20,26). In EGI-1 cells, inhibition of S100A4 nuclear import by low dose PTX significantly reduced the activation of Rho-A and Cdc-42. In cancer cells, Cdc-42 is one of the factors involved in the formation of specialized plasma-membrane actin-based microdomains combining adhesive properties with matrix degrading activities, called invadopodia, which support cancer invasion by dismantling the basement membrane and then by invading the stromal environment mostly composed of fibrillar type I collagen. A main component of invadopodia is the transmembrane metalloproteinase MT1-MMP, whose function is essential for the in situ to invasive carcinoma transition in breast cancer (14). MT1-MMP promotes the activation of several soluble MMPs, such as MMP-2 and MMP-9. Our data indicate that EGI-1 cells constitutively expressed MT1-MMP and MMP-9, but not MMP-2, and when exposed to low dose PTX, they showed a significant dose-dependent reduction in the surface expression of MT1-MMP along with the ability to secrete MMP-9. Interestingly, the interplay between Rho-A and Cdc-42 regulates the delivery and accumulation of MMPs at the invading surface (27). Furthermore, since MMP-9 secretion can be also modulated by the activation of the Rho-A/ROCK signaling (21), and the membrane translocation of MT1-MMP is directly regulated by Cdc-42(28), we suggest the cooperation of Rho-A/MMP-9 and Cdc-42/MT1-MMP pathways to form invadopodia activated by nuclear S100A4.
Cytoskeletal damages induced by PTX, a well-established depolymerizing agent (29), could in theory, influence the nuclear translocation of S100A4. From this point of view, it is important to underline that structural cytoskeleton alterations were absent with low dose PTX, in contrast with what coherently observed with high dose PTX.
The mechanisms regulating S100A4 entry into the nucleus remain enigmatic. Notably, S100A4 does not possess nuclear docking sites or nuclear import sequences. In human chondrocytes, nuclearization of S100A4 was induced by IL-1β through a SUMOylation-dependent mechanism (30). SUMOylation is a post-translational mechanism similar to ubiquitination, operated by SUMO proteins, and involved in several biological functions, including protein stability, DNA repair, cell cycle regulation, apoptosis, nuclear transport and gene transcription (31). The SUMOylation processes are catalyzed by three enzymes, SUMO E1, E2 and E3, acting as pacemaker of the cascade reaction leading to the modification of their substrate protein (32). Components of the SUMOylation machinery have been found deregulated in several human cancers, and are emerging as relevant players in tumour invasiveness and in epithelial-mesenchymal transition (33). We found that EGI-1 (expressing S100A4 the nucleus) contained higher amounts of SUMOylated S100A4 with respect to TFK-1 (expressing S100A4 only in the cytoplasm). Then, in EGI-1 treated with low dose PTX, down-regulation of nuclear S100A4 was associated with a significant, dose-dependent reduction in the S100A4 SUMOylated fraction, without changes in the un-SUMOylated subset. These effects occurred without affecting the expression levels of E1, E2, E3 subunits of the SUMOylating complex, thus suggesting a functional inhibitory mechanism, similar to GA. GA is a natural compound derived from Gingko biloba that specifically inhibits the first step of the SUMOylation reaction by directly binding E1 and inhibiting the formation of the E1-SUMO intermediate (16). Of note, also the reduction in nuclear levels of S100A4 induced by GA significantly inhibited EGI-1 cell motility, to an extent comparable to PTX. This observation confirms the relevance of SUMOylation in mediating the pro-oncogenic functions of S100A4 when translocated into the nucleus.
To translate the results of the in vitro experiments, we turned to an experimental model of CCA generated by xenotransplantation of human EGI-1 cells into the spleen of SCID mice, as we performed in previous studies (9,13). Because of its short half-life (34), PTX was administered to xenografted SCID mice with a LDM infusion. Continuous delivery of PTX by LDM ensured the achievement of constant low concentrations of PTX comparable with the small doses used for the in vitro experiments (34). To evaluate specific effects on tumor invasion, LDM PTX was started only after human CCA cell engraftment was confirmed by bioluminescence imaging. At the end of treatment, histological evaluation of the splenic tumor showed in the LDM PTX-treated group, a significant reduction in the amount of nuclear S100A4-expressing EGI-1 cells. In line with what observed in vitro, down-regulation of S100A4 was not associated with reduced tumour cell proliferation (p-Hist3) or with increased apoptosis (CC3) of CCA cells. Therefore, our experimental model is ideal to study if pharmacologic targeting of nuclear S100A4 is a useful strategy to inhibit invasiveness and metastasization. We found that LDM PTX did not affect the growth of the tumor at the site of injection, but caused a significant reduction in both ITC and MM in the lungs, where EGI-1 cells metastatise following a hematogenous route through the portal vein and the hepatic veins. This finding is of great clinical value, because the lung is the site most frequently involved in the extrahepatic progression of CCA, fostered by a specific mechanism of vascular encasement by tumor cells (5).
It is important to underline that PTX may exert additional functions not related to modulation of S100A4 nuclear transport that potentially may contribute to its anti-invasive effects. These are largely dependent upon PTX ability to promote microtubule polymerization and stabilization, which inhibits mitosis and leads to apoptosis. While these mechanisms of action are well evident at the conventional doses, at the much lower doses used in the current study, they do not seem to occur. Recent studies performed in ovarian carcinoma cells indicate that alternatively, PTX may inhibit the expression of other critical molecular factors of tumor progression, such as hypoxia-inducible factor-1α and vascular endothelial growth factor (35).
In conclusion, this study unveils a specific role of SUMOylation-dependent nuclear import of S100A4 in CCA on hematogenous metastasization. The small GTPases Cdc-42 and Rho-A, in concert with MT1-MMP and MMP-9, are the molecular effectors mediating the pro-invasive functions promoted by S100A4 nuclearization. Bearing in mind the potential toxic effects of PTX in patients with overt cirrhosis and cholestasis (36), these mechanisms represents a promising therapeutic target aimed at preventing metastatic dissemination after detection of the tumor.
Supplementary Material
Acknowledgments
Financial Support: Progetto di Ricerca Ateneo 2011 (Grant #CPD113799/11) to L. Fabris and M. Cadamuro. Grant Associazione Chirurgica Tarvisium to M. Massani, T. Stecca and N. Bassi. Grant Associazione Italiana Ricerca sul Cancro (AIRC) #IG14295 to S. Indraccolo. Projects CARIPLO 2011-0470 and PRIN 2009ARYX4T_005 to M. Strazzabosco. NIH Grants DK079005 to M. Strazzabosco, RO1DK 101528 to C. Spirli, and DK034989 Silvio O. Conte Digestive Diseases Research Core Centers to M. Strazzabosco and C. Spirli.
Footnotes
Disclosure: All authors have no conflict of interest regarding this study to declare.
References
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 3.Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;31:49–60. doi: 10.1055/s-0031-1272839. [DOI] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 5.Fabris L, Alvaro D. The prognosis of peri-hilar cholangiocarcinoma after radical treatments. Hepatology. 2012;56:800–802. doi: 10.1002/hep.25808. [DOI] [PubMed] [Google Scholar]
- 6.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci U S A. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gongoll S, Peters G, Mengel M, Piso P, Klempnauer J, Kreipe H, et al. Prognostic significance of calcium-binding protein S100A4 in colorectal cancer. Gastroenterology. 2002;123:1478–1484. doi: 10.1053/gast.2002.36606. [DOI] [PubMed] [Google Scholar]
- 9.Fabris L, Cadamuro M, Moserle L, Dziura J, Cong X, Sambado L, et al. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology. 2011;54:890–899. doi: 10.1002/hep.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshmi MS, Parker C, Sherbet GV. Metastasis associated MTS1 and NM23 genes affect tubulin polymerisation in B16 melanomas: a possible mechanism of their regulation of metastatic behaviour of tumours. Anticancer Res. 1993;13:299–303. [PubMed] [Google Scholar]
- 11.Parker C, Lakshmi MS, Piura B, Sherbet GV. Metastasis-associated mts1 gene expression correlates with increased p53 detection in the B16 murine melanoma. DNA Cell Biol. 1994;13:343–351. doi: 10.1089/dna.1994.13.343. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5:S3–S6. [PubMed] [Google Scholar]
- 13.Cadamuro M, Nardo G, Indraccolo S, Dall'olmo L, Sambado L, Moserle L, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58:1042–1053. doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodillinsky C, Infante E, Guichard A, Chaligné R, Fuhrmann L, Cyrta J, et al. p63/MT1-MMP axis is required for in situ to invasive transition in basal-like breast cancer. Oncogene. 2016;35:344–357. doi: 10.1038/onc.2015.87. [DOI] [PubMed] [Google Scholar]
- 15.Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda I, Ito A, Hirai G, Nishimura S, Kawasaki H, Saitoh H, et al. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol. 2009;16:133–140. doi: 10.1016/j.chembiol.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 18.Amin BD, Hoda SA. Minimal metastatic disease in sentinel lymph nodes in breast carcinoma: some modest proposals to refine criteria for "isolated tumor cells". Adv Anat Pathol. 2006;13:185–189. doi: 10.1097/00125480-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZY, Ge HY. Micrometastasis in gastric cancer. Cancer Lett. 2013;336:34–45. doi: 10.1016/j.canlet.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Developmental Biology. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Turner NA, O'Regan DJ, Ball SG, Porter KE. Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. FASEB J. 2005;19:804–806. doi: 10.1096/fj.04-2852fje. [DOI] [PubMed] [Google Scholar]
- 22.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 23.Cresta S, Sessa C, Catapano CV, Gallerani E, Passalacqua D, Rinaldi A, et al. Phase I study of bortezomib with weekly paclitaxel in patients with advanced solid tumours. Eur J Cancer. 2008;44:1829–1834. doi: 10.1016/j.ejca.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Ain KB, Egorin MJ, DeSimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. 2000;10:587–594. doi: 10.1089/thy.2000.10.587. [DOI] [PubMed] [Google Scholar]
- 25.Egeland EV, Boye K, Pettersen SJ, Haugen MH, Øyjord T, Malerød L, et al. Enrichment of nuclear S100A4 during G2/M in colorectal cancer cells: possible association with cyclin B1 and centrosomes. Clin Exp Metastasis. 2015;32:755–767. doi: 10.1007/s10585-015-9742-1. [DOI] [PubMed] [Google Scholar]
- 26.Franco-Barraza J, Valdivia-Silva JE, Zamudio-Meza H, Castillo A, García-Zepeda, Benítez-Bribiesca L, et al. Actin Cytoskeleton Participation in the Onset of IL-1β Induction of an Invasive Mesenchymal-like Phenotype in Epithelial MCF-7 Cells. Archives of Medical Research. 2010;41:170–181. doi: 10.1016/j.arcmed.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Spuul P, Ciufici P, Veillat V, Leclercq A, Daubon T, Kramer IJ, et al. Importance of RhoGTPases in formation, characteristics, and functions of invadosomes. Small GTPases. 2014;5:e28195. doi: 10.4161/sgtp.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ispanovic E, Serio D, Haas TL. Cdc42 and RhoA have opposing roles in regulating membrane type 1-matrix metalloproteinase localization and matrix metalloproteinase-2 activation. Am J Physiol Cell Physiol. 2008;295:C600–C610. doi: 10.1152/ajpcell.00460.2007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Zang H, Zang F, Liu S, Wang R, Sun Y, et al. Folate-targeted paclitaxel-conjugated polymeric micelles inhibits pulmonary metastatic hepatoma in experimental murine H22 metastasis model. Int J Nanomed. 2014;9:2019–2030. doi: 10.2147/IJN.S57744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda KJ, Loeser RF, Yammani RR. SumoylSUMOylation and nuclear translocation of S100A4 regulate IL-1beta-mediated production of matrix metalloproteinase-13. J Biol Chem. 2010;285:31517–31524. doi: 10.1074/jbc.M110.125898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichler A, Melchior F. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic. 2002;3:381–387. doi: 10.1034/j.1600-0854.2002.30601.x. [DOI] [PubMed] [Google Scholar]
- 32.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 33.Bogachek MV, De Andrade JP, Weigel RJ. Regulation of epithelial-mesenchymal transition through SUMOylation of transcription factors. Cancer Res. 2015;75:11–15. doi: 10.1158/0008-5472.CAN-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stearns ME. Taxol reduces circulating tumor cells to prevent bone metastases in SCID mice. Invasion Metastasis. 1995;15:232–241. [PubMed] [Google Scholar]
- 35.Kim BR, Yoon K, Byun HJ, Seo SH, Lee SH, Rho SB. The anti-tumor activator sMEK1 and paclitaxel additively decrease expression of HIF-1α and VEGF via mTORC1-S6K/4E-BP-dependent signaling pathways. Oncotarget. 2014;5:6540–6551. doi: 10.18632/oncotarget.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie JD, Huang Y, Chen DT, Pan JH, Bi BT, Feng KY, et al. Fentanyl Enhances Hepatotoxicity of Paclitaxel via Inhibition of CYP3A4 and ABCB1 Transport Activity in Mice. PLoS One. 2015;10:e0143701. doi: 10.1371/journal.pone.0143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.