Abstract
Objective
Patients in intensive care units are susceptible to subacute, potentially catastrophic illnesses such as respiratory failure, sepsis, and hemorrhage that present as severe derangements of vital signs. More subtle physiologic signatures may be present before clinical deterioration, when treatment might be more effective. We performed multivariate statistical analyses of bedside physiologic monitoring data to identify such early, subclinical signatures of incipient life-threatening illness.
Design
We report a study of model development and validation of a retrospective observational cohort using resampling (TRIPOD Type 1b internal validation), and a study of model validation using separate data (Type 2b internal/external validation).
Setting
University of Virginia Health System (Charlottesville), a tertiary-care, academic medical center.
Patients
Critically ill patients consecutively admitted between January 2009 and June 2015 to either the neonatal, surgical/trauma/burn, or medical intensive care units with available physiologic monitoring data.
Interventions
None.
Measurements and Main Results
We analyzed 146 patient-years of vital sign and electrocardiography waveform time series from the bedside monitors of 9,232 ICU admissions. Calculations from 30-minute windows of the physiologic monitoring data were made every 15 minutes. Clinicians identified 1,206 episodes of respiratory failure leading to urgent, unplanned intubation, sepsis, or hemorrhage leading to multi-unit transfusions from systematic, individual chart reviews. Multivariate models to predict events up to 24 hours prior had internally-validated C-statistics of 0.61 to 0.88. In adults, physiologic signatures of respiratory failure and hemorrhage were distinct from each other but externally consistent across ICUs. Sepsis, on the other hand, demonstrated less distinct and inconsistent signatures. Physiologic signatures of all neonatal illnesses were similar.
Conclusions
Subacute, potentially catastrophic illnesses in 3 diverse ICU populations have physiologic signatures that are detectable in the hours preceding clinical detection and intervention. Detection of such signatures can draw attention to patients at highest risk, potentially enabling earlier intervention and better outcomes.
Keywords: critical care, physiologic monitoring, hemorrhage, sepsis, respiratory insufficiency, statistical models
Introduction
Intensive care units (ICU) continue to see growth in number of patients and in their illness acuity (1-3). The severity of the original insult often determines prognosis, and the risk of death is further increased by events that occur during the stay, such as acute respiratory failure, sepsis, and hemorrhage. These new insults in critically ill patients, though not necessarily common, are potentially catastrophic (4).
These syndromes may begin with more innocuous conditions – volume overload, pneumonia, or a bleeding ulcer– that are tractable early in their course, and averting deterioration by prompt diagnosis and therapy may improve patient outcomes (5). Our hypothesis is that illnesses present signatures in the physiologic data prior to clinical suspicion (6-9).
To test this hypothesis we analyzed data from a large number of highly diverse ICU patients for whom events had been meticulously identified. By doing so, we hoped to understand how broadly our concepts might apply. The promise of Big Data predictive analytics and information technology in improving patient safety has been recognized, though implementation has been hindered by a fragmented healthcare informatics ecosystem (10-12). Notwithstanding, the bedside physiologic monitor is a ubiquitous and continuous source of information, and we have analyzed how physiologic measurements change in the hours before clinicians suspected that illness might be developing. Here, we tested the hypothesis that subacute, potentially catastrophic illnesses have detectable signatures of deranged physiology in the monitoring data prior to clinical diagnosis across a broad spectrum of ICU patients and events.
Materials and Methods
Study design
We conducted a retrospective study from January 23, 2009 to June 25, 2015 at the University of Virginia (UVa) Health System, an academic, tertiary-care center. We studied admissions to the Neonatal ICU (NICU), Surgical/Trauma/Burn ICU (SICU), and Medical ICU (MICU) comprised of 45, 15, and 28 beds respectively, each with continuous physiologic monitoring systems and an electronic data warehouse that archived the complete medical record.
Primary end points were the times of intubation for respiratory failure, blood culture order for the suspicion of hospital-acquired sepsis, and first transfusion order of packed red blood cells (PRBC) for hemorrhage. Through systematic review of individual medical records, clinicians identified instances of sepsis and respiratory failure leading to urgent, unplanned intubation and entered the details into a database form. The institutional blood bank prospectively collected the date, time, and relevant circumstances of PRBC transfusions. We abstained from using secondary administrative data such as International Classification of Diseases (ICD) diagnosis codes.
We have used the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement checklist(13) in analyzing and reporting this study. The UVa Institutional Review Board approved this study with a waiver of informed consent.
Study populations and outcome definitions
To identify events of respiratory failure leading to urgent unplanned intubation, we searched records for documentation of the intubation procedure (7, 8).
To identify severe sepsis in adults, we used the Surviving Sepsis Campaign guidelines, which require clinical suspicion of infection, as suggested by an order for blood cultures, at least 2 of 4 systemic inflammatory response syndrome (SIRS) criteria, and specific evidence of end-organ dysfunction or damage (see Supplemental Methods, Supplemental Digital Content 1) (14). The time of the event was the instance of the first order of a blood culture for suspicion of infection. Some prolonged admissions were complicated by multiple episodes; we analyzed only the first.
In neonates, septicemia was defined as clinical deterioration at 3 or more days of age associated with a positive blood culture (excluding Corynebacterium and diphtheroids) and at least 5 days of antibiotics. Subsequent episodes of septicemia were included if they occurred more than 7 days after the previous episode or, if less than 7 days from the prior episode, were due to a different organism.
For hemorrhage in adults, the time of the event was the initiation of the first of a 3 or more units transfusion of PRBC within 24 hours (9). For infants, this definition was modified to 2 transfusions within 24 hours.
We studied only those patients who were at risk for each particular subacute, potentially catastrophic illness. Thus, for the study of urgent intubations we excluded intubations performed in the emergency department, operating room, and those performed electively, and we excluded observations occurring during periods of mechanical ventilation, after “Do Not Intubate” orders, or in patients who had a functional tracheostomy. For septicemia in the NICU, we included only very low birth weight preterm infants. For the study of sepsis, we excluded admissions with the diagnosis of sepsis present on arrival to the ICU. For the study of hemorrhage, we excluded events with PRBC transfusion in the preceding 24 hours.
Physiologic data acquisition and predictors
Measurements reported by the bedside monitor every 2 seconds included heart rate (HR), respiratory rate (Resp), pulse oximeter saturation (SpO2), and both invasive and noninvasive blood pressure (see Supplemental Methods, Supplemental Digital Content 1). In adults, every 15 minutes, we calculated the means and standard deviations of vital signs (HR, Resp, SpO2) for the prior 30 minutes, resulting in 96 measurements per day. In neonates, we used the hourly average of every 5-minute calculations from the preceding 10 minutes, consistent with previously published results (7). The cross-correlation coefficient of HR-Resp, Resp-SpO2, and HR-SpO2 pairs were also calculated (maximum value for lags between plus or minus 20 seconds). Given the collinearity of blood pressure measurements and the postulated differences in pulse pressure responses to vasodilatory and vasoconstrictive pathophysiologic states as found in septic and hemorrhagic shock respectively, we chose to only include diastolic blood pressure (DBP) and pulse pressure (PP), calculated as the difference between systolic and diastolic BP measurements. From the ECG waveform we performed QRS detection to identify the RR interbeat intervals and then calculated linear and nonlinear dynamical measures of HR designed to detect arrhythmia and ectopy; these include standard deviation of the RR intervals or HR variability (HRV), detrended fluctuation analysis (DFA), and the coefficient of sample entropy (COSEn) (9, 15-17). Admissions without archived physiologic monitoring data due to technical complications were excluded.
Statistical analysis
Missing data (mean 7.4%; range: <0.5 – 39.2%) were multiply imputed under the fully conditional specification with chained equations using predictive mean matching (see Supplemental Methods, Supplemental Digital Content 1) (18). We developed binary logistic regression models labeling observations during the time window prior to the event as ‘one’, and observations outside this timeframe as ‘zero’. In the NICU, optimization of the models based on C-statistics resulted in lookback windows of 8, 24, and 24 hours for intubation, sepsis, and transfusion respectively. In the MICU, windows of 4, 6, and 8 hours led to optimal models for intubation, severe sepsis, and hemorrhage respectively. In the SICU, the optimal windows for intubation, severe sepsis, and hemorrhage were 6, 4, and 4 hours. In sensitivity analyses, adjusting the lookback window in 2-hour increments backwards beyond these times did not greatly alter the performance of any model. We allowed our pre-specified candidate predictors to have non-monotonic and non-linear associations by modeling them with restricted cubic spline transformations (see Supplemental Methods, Supplemental Digital Content 1) (19). We adjusted for the repeated and correlated observations within individual patients with procedures that modify the variance-covariance matrix, and we quantified predictive accuracy using a concordance index (C-statistic) (20).
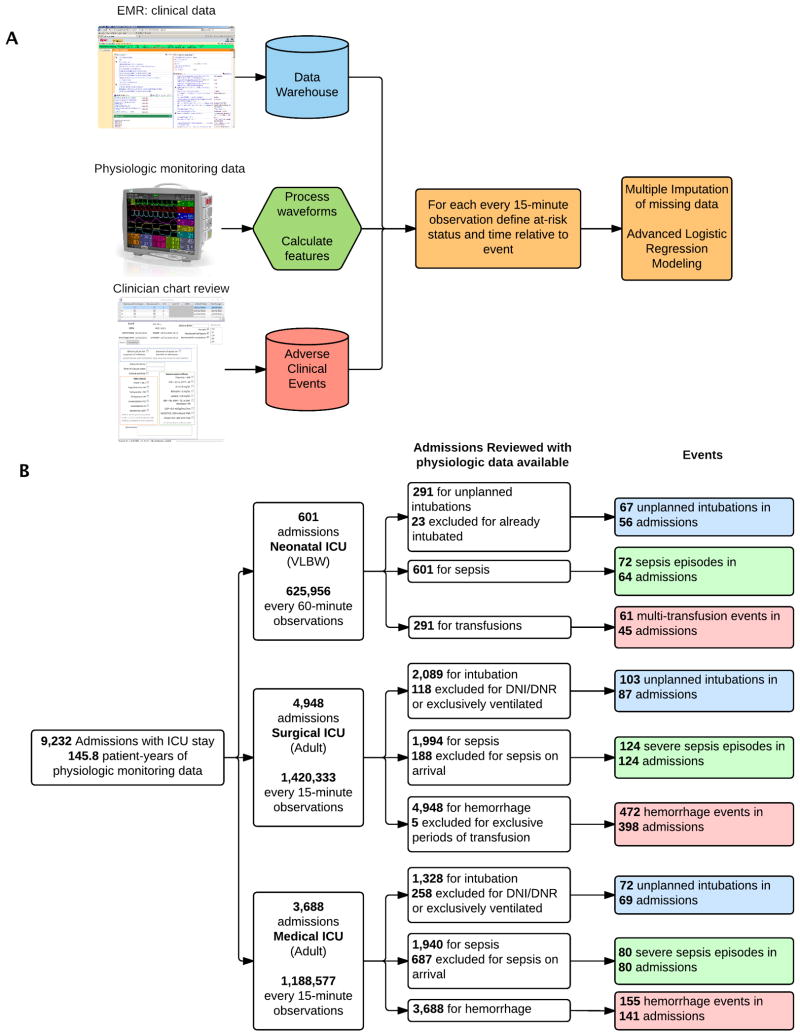
We validated these models internally using bootstrap resampling (TRIPOD Type 1b model study) to estimate the performance on a new sample of observations from the same patient population. We justify bootstrapping methodology over split-sample or cross-validation techniques by noting that the estimates generated by these alternative strategies are less stable (different splits lead to different results) and exhibit greater bias (samples that vary only by chance will probably show similar performance as in the development set) (21, 22). We additionally evaluated each model on separate data (TRIPOD Type 2b model study) in all other ICU-illness cohorts (23). To facilitate subsequent comparisons between models, we constrained all models to have identical pre-specified terms. All statistical analyses were performed in R (18, 20, 24). Figure 1A shows the flow of the data extraction, processing, and subsequent statistical analysis steps.
Figure 1. Methods Flowchart and Admissions Reviewed.
(A) Flowchart depiction of data collection, processing, and analysis. (B): Flowchart depiction of patient admissions reviewed, time series data analyzed, and events identified. ICU: intensive care unit
Results
Study patients
We reviewed the charts of 10,559 hospital admissions to the NICU, SICU, and MICU. Of these, 9,232 admissions had 145.8 patient-years (3.2 million observations) of physiologic time series measurement data available for analysis. Table 1 shows their baseline characteristics, and Figure 1B shows their flow through the study. We identified 1,206 episodes of either incident respiratory failure leading to urgent, unplanned intubation; severe sepsis; or multi-unit transfusions of PRBC.
Table 1. Baseline Characteristics.
| Characteristic | NICU | SICU | MICU |
|---|---|---|---|
| Number of patients reviewed with available data | 601 | 4,948 | 3,688 |
| Number at risk for: | |||
| Unplanned intubation | 268 | 1,971 | 1,070 |
| Sepsis | 601 | 1,806 | 1,253 |
| Multi-unit transfusion | 291 | 4,943 | 3,688 |
| Age (years) or Estimated Gestational Age (weeks) | |||
| median | 27 | 58.2 | 60.7 |
| IQR | 25-29 | 45.9-70.1 | 49.7-71.6 |
| Male gender | 298 (49.6%) | 2,946 (59.6%) | 1,936 (52.5%) |
| Number of observations analyzed | 625,956 | 1,374,180 | 1,171,048 |
| ICU length of stay (days) | |||
| median | 59.0 | 1.8 | 2.4 |
| IQR | 34.0-94.3 | 1.0-3.8 | 1.1-4.8 |
| In-hospital mortality | 65 (10.8%) | 311 (6.3%) | 662 (18.0%) |
Values are n (percentage) unless otherwise specified. NICU: neonatal intensive care unit, SICU: surgical intensive care unit, MICU: medical intensive care unit, IQR: interquartile range, ICU: intensive care unit.
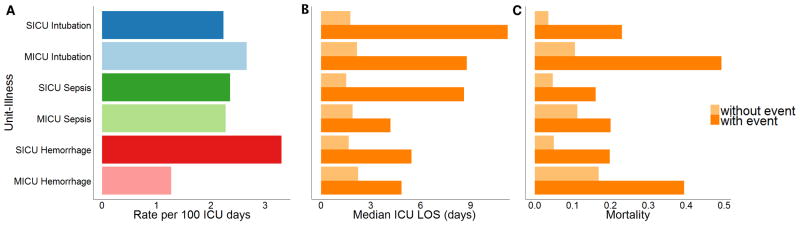
The incident rates of events in the NICU were 0.78, 0.27, and 0.55 per 100 ICU days for unplanned intubation, sepsis, and multiple transfusions respectively. Figure 2A compares the rates of each adult illness specific to each ICU, which ranged from 1.3 to 3.3 events per 100 patient ICU days. In the SICU, the aggregate incidence rate was 7.9 per 100 ICU days, which, in this 15-bed ICU, equates to 1.2 events per day—13.8% of all SICU admissions experienced at least one illness event. Hemorrhage commonly occurred within the first 12 hours of SICU admissions (40% of all hemorrhage events), while intubations (14%) and sepsis (9%) were less likely to occur early in the ICU course.
Figure 2. Incidence and Associated Outcomes.
Incidence (A) and impact of each adult ICU-illness event on median ICU length of stay in days (B) and in-hospital mortality (C). MICU: medical intensive care unit; SICU: surgical/trauma intensive care unit; ICU: intensive care unit; LOS: length of stay
Outcomes
In adults, we examined the association of illness on ICU length of stay (LOS) and in-hospital mortality. The association of each illness on the median ICU LOS was dramatic with an absolute difference ranging from 2.3 to 9.5 additional ICU days (Fig. 2B). There was a 1.8 to 6.4-fold increase in in-hospital mortality—ranging from an absolute increase of 8.7% to 38.7% for admissions with events when compared to admissions without events (Fig. 2C).
Signatures of illness in physiologic monitoring time series: model development
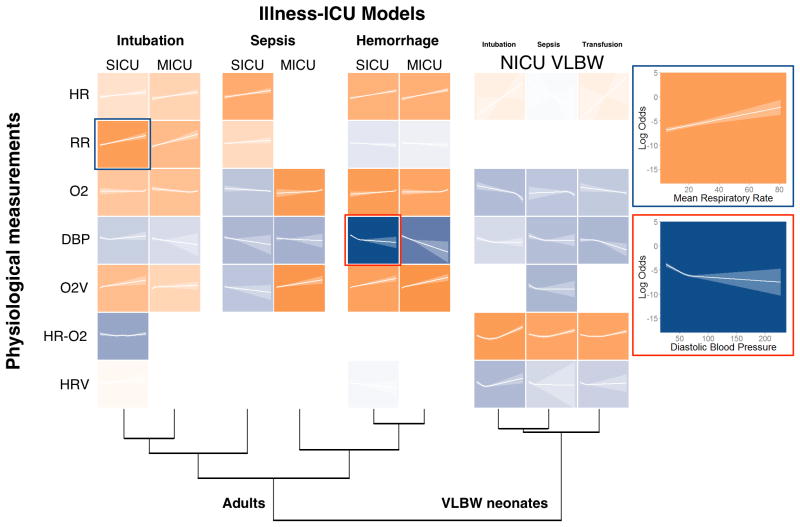
We identically analyzed the time series data regardless of its source. We calculated the same parameters and used multivariable logistic regression analysis, adjusting for repeated measures, to test the hypothesis that illness signatures were present prior to clinical diagnosis. For each parameter, we determined its relationship to the outcome, that is to say, whether the value changed significantly as the diagnosis approached, and whether the value rose or fell. These model development results are presented in Figure 3. The rows present the calculated parameters and the columns present the ICU and the diagnoses. Each colored tile represents the quantitative relationship of the calculated parameter to the ICU-specific diagnosis. The color saturation reflects the degree of statistical significance, and darker hues mean stronger association. The color orange signifies that the measured parameter rose prior to the diagnosis; the color blue signifies that it fell. The lines plot the log odds as calculated from the regression model as a function of the calculated value of the parameter, and the ribbons show the 95% confidence intervals. Two panels on the right further explain the process. The orange tile highlighted in blue shows that rising respiratory rate is highly significantly associated with the outcome of emergency intubation in the SICU; the blue tile highlighted in red signifies that falling DBP is highly significantly associated with the outcome of hemorrhage in the SICU.
Figure 3. Predictor Effects.
Heatmap depiction of statistical significance and effect associations of predictors in each illness-ICU model. Each tile represents the predictor's effect and is a plot of the log-odds of the event of interest as a function of the predictor across its range. The translucent ribbon represents the 95% confidence interval. Saturation depicts the statistical significance.and hue represents the direction of association, with orange representing positive association and blue representing negative association. Non-monotonic associations were categorized based on the polarity of association in the range of the greatest density of data. For example, examining the respiration rate in the SICU, an observation of 20 breaths per minute when all other measurements are at the median yields a log-odds of -6.0. Dendrogram clustering was performed on a matrix of C-statistics generated by the evaluation of each model on itself and all other illness-ICU cohorts. MICU: medical intensive care unit; SICU: surgical/trauma intensive care unit; HR: heart rate; RR: respiratory rate; O2: oxygen saturation; DBP: diastolic blood pressure; O2V: oxygen saturation variability; HR-O2: cross-correlation coefficient of heart rate and oxygen saturation; HRV: heart rate variability. SICU: surgical intensive care unit; MICU: medical intensive care unit; NICU: neonatal intensive care unit. For complete representation including all predictors, see Supplemental Fig. 1 (Supplemental Digital Content 1).
The major findings are that the signatures of respiratory failure leading to intubation (first and second columns) and hemorrhage leading to multi-unit transfusion (fifth and sixth) are consistent between the SICU and MICU, as demonstrated by the similarity of the saturations and colors of the tiles. Signatures of sepsis in the adult ICUs, on the other hand, differ. Finally, all illnesses in the NICU lead to the same physiologic signature, one that does not present in adults. The dendrograms at the bottom of the figure further emphasize the clustering of the intubation, hemorrhage and NICU results. Sepsis, however, clusters separately – in the SICU, it clusters with respiratory failure; in the MICU, it clusters with hemorrhage.
Signatures of illness in physiologic monitoring time series: model validation
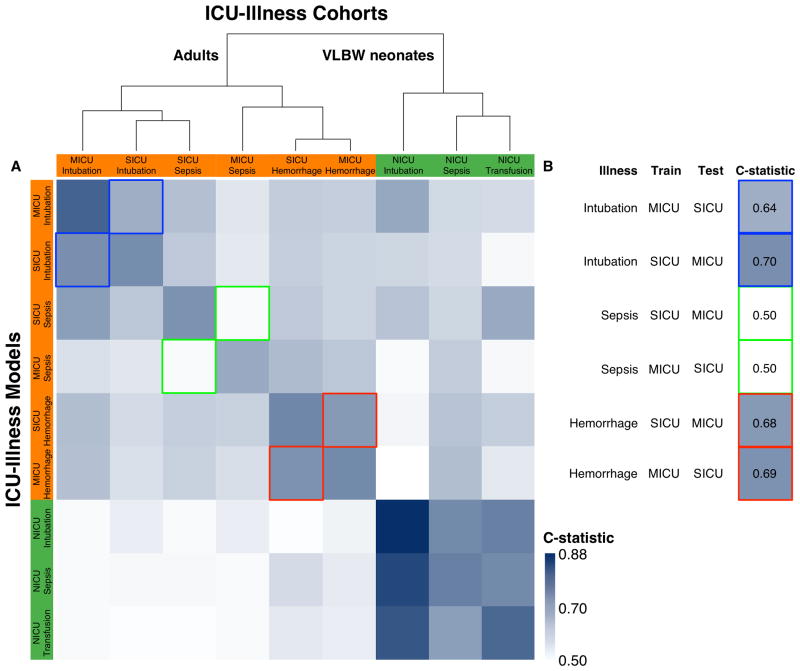
We sought to characterize the performance of the statistical models in independent data sets not used for model development. Since we tested all models on each ICU population, this is a form of external validation; however, as all the ICUs are in the same institution, this exercise is an intermediary internal / external validation (TRIPOD type 2b). Figure 4 is a matrix of blue tiles in which the saturation reflects the C-statistic, as shown by the color legend on the lower right, and darker tiles represent better performance. The rows are the ICU-specific models; the columns are the ICU populations. The diagonal represents the C-statistic for the model as it was developed. Other tiles represent the testing of a model on an independent participant population not used in the development. The important findings are the tiles outlined in blue, green and red – these are performance of statistical models developed in one adult ICU and tested in the other. They are excerpted and arranged vertically on the right. Models to predict respiratory failure (outlined in blue) and hemorrhage (outlined in red) had C-statistics from 0.64 to 0.69 when tested in the opposite ICU. We interpret this as reasonable performance. Models to detect sepsis (outlined in green), on the other hand, had no predictive capability in the alternate ICU.
Figure 4. Comparison of ICU-Illness Models.
(A) Heatmap depiction of the relative C-statistics for each model evaluated on itself as well as all other datasets. Intensity of saturation depicts higher C-statistics. Dendrogram clustering was performed on a matrix of C-statistics generated by the evaluation of each model on itself and all other ICU-illness cohorts. The largest distinction is between adults and very low birth weight neonates. Within the adult cluster, the next distinction is between hemorrhage and intubation with sepsis across adult ICUs not clustering. (B) Subset of (A), further demonstrating concordance of models trained and tested on the other adult ICUs. Sepsis, unlike intubation and hemorrhage has discrepant physiologic signatures between the adult ICUs. MICU: medical intensive care unit; SICU: surgical/trauma intensive care unit.
Very low birth weight neonates
We identified 67, 72, and 61 cases of intubation for respiratory failure, septicemia, and multiple transfusions in VLBW neonates from 291, 601, and 268 at-risk NICU admissions that had physiologic monitoring data available and did not meet exclusion criteria. The models had internally validated C-statistics of 0.88, 0.71, and 0.74 (optimism: 0.02, 0.04, 0.05 respectively) for intubation, sepsis, and transfusion. All three models had similar physiologic signatures in terms of the direction and significance of the component variables—marked by increased risk associated with falling DBP, falling SpO2 from the normal range, and elevated HR-SpO2 cross correlation. The final three columns of Figure 3 demonstrates this relative homogeneity for the VLBW NICU models where the predictor rows have both similar directions of association and statistical significance as indicated by the hues and saturations (Supplemental Fig. 1, Supplemental Digital Content 1). The cluster of dark tiles in the lower right corner of Figure 4 reflects the similarity of models in the NICU, suggesting that a model to detect any of the diagnoses serves about equally well in detecting the other diagnoses.
Urgent, unplanned intubations in adults
We identified 103 and 72 episodes of respiratory failure leading to urgent, unplanned intubations in 1,971, and 1,070 admissions to the SICU and MICU respectively. The models were both characterized by a signature of rising respiratory rate, rising HR, and rising SpO2 variability (Fig. 3 and Supplemental Fig. 1, Supplemental Digital Content 1). The models had internally validated C-statistics of 0.68 and 0.77 (optimism: 0.03 and 0.02 respectively) for the SICU and MICU. The illnesses and models had similar characteristics and clustered together when evaluated on all other ICU-illness combinations (Fig. 4) suggesting that respiratory failure in adult critical care has a distinct physiologic signature that is consistent across adult ICUs.
Severe sepsis in adults
We identified 124 and 80 episodes of incident severe sepsis from 1,806 and 1,253 admissions to the SICU and MICU respectively. The models had internally validated C-statistics of 0.68 and 0.61 (optimism: 0.03 and 0.05 respectively) for the SICU and MICU. Of all the ICU-illness models studied, the physiologic signature of severe sepsis in the MICU was most dissimilar from that seen in the SICU. The MICU sepsis model clustered with the adult hemorrhage models whereas the SICU sepsis model clustered with the adult intubation models (Fig. 3 and Supplemental Fig. 1, Supplemental Digital Content 1). Evaluating the models on all other ICU-illness cohorts led to similar disparate clustering of the two adult sepsis models (Fig. 4).
Hemorrhage in adults
We identified 472 and 155 hemorrhage events requiring transfusion of at least 3 units of PRBC within a 24-hour period without any PRBC transfusion in the preceding 24 hours in 4,943 and 3,688 admissions to the SICU and MICU respectively. Bleeding of this magnitude was more likely to occur in the SICU when compared to the MICU (3.3 vs 1.3 per 100 patient ICU days; Fig. 2A). The models had internally validated C-statistics of 0.71 and 0.70 (optimism: 0.01 and 0.02 respectively) for the SICU and MICU. The physiologic signatures were characterized by falling DBP, rising HR, rising SpO2, and rising SpO2 variability (Fig. 3 and Supplemental Fig. 1, Supplemental Digital Content 1). The illnesses and models had similar characteristics and clustered together when evaluated on all other ICU-illness combinations (Fig. 4) suggesting that hemorrhage in adult critical care has a similar and distinct physiologic signature.
Dynamic performance of predictive models
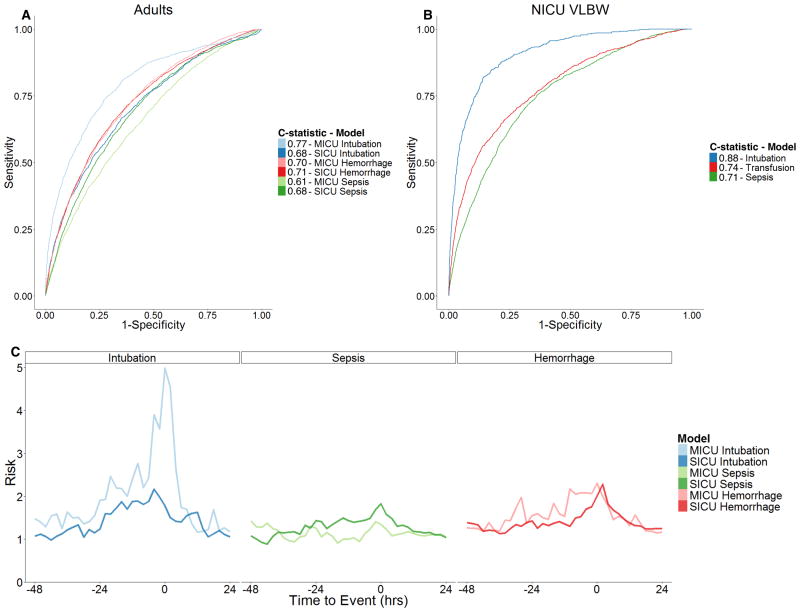
Evaluating the predictive model outputs in the several hours leading up to the time of clinical recognition or intervention, Figure 5C depicts the mean fold-increase in risk of event that dynamically increases prior to events and decreases afterwards in the context of clinical recognition and treatment. Respiratory failure presented the largest and most prolonged changes several hours before events. Hemorrhage and sepsis had more moderate changes in predictions accompanied by shorter prodromes.
Figure 5. Discriminatory Power and Dynamic Performance of ICU-Illness Models.
(A) Receiver Operator Characteristic curves with biased estimates of area under the curve (C-statistic) for each adult model. MICU: medical intensive care unit; SICU: surgical/trauma intensive care unit. (B) Receiver Operator Characteristic curves with biased estimates of area under the curve (C-statistics) for each infant model. NICU: neonatal intensive care unit; VLBW: very low birth weight infants. (C) Mean predicted fold increase in risk as a function of the time relative to the event for all patients suffering an event and for whom physiologic monitoring data was available within the time period beginning 48 hours before and ending 24 hours after. Panels are arranged by illness type. Line color represents each ICU-illness model respectively. For example, the statistical model for intubation in the MICU predicted patients, on average, to be at twice the baseline risk of the event at 24 hours prior and the estimated risk continued to increase in the remaining hours preceding urgent intubations. MICU: medical intensive care unit; SICU: surgical/trauma intensive care unit; hrs: hours.
Discussion
We applied Big Data analytics to the study of physiologic signatures of subacute, potentially catastrophic illnesses – respiratory failure leading to urgent, unplanned intubation, incident sepsis, and hemorrhage leading to multi-unit transfusion – in neonatal and adult ICU patients. We identified events through a time-consuming, individual chart inspection process, a substantially more accurate method than database queries on ICD diagnosis codes (25-27).
We found that the physiologic signatures for respiratory failure and hemorrhage were both distinct and consistent across different adult ICUs. The major elements – rising respiratory rate in the former, and rising HR and falling blood pressure in the latter – are consistent with existing clinical knowledge, affirming the validity of the statistical modeling.
We found that severe sepsis, on the other hand, had less distinct physiologic signatures thatdiffered between medical and surgical ICU patients. These findings underscore the clinical experience that manifestations of sepsis in adult ICUs patients are variable and may reflect different sources of sepsis between these populations (28).
Unlike adults, premature infants have a common physiologic signature for all three conditions. This is likely because premature infants with septicemia commonly present with respiratory failure requiring intubation, and commonly receive red blood cell transfusions for anemia and low blood pressure when they develop signs of sepsis. A prominent feature in neonatal illness is the correlation of HR and SpO2. This metric captures the concomitant bradycardia and SpO2 desaturation that accompanies severe neonatal apnea, and corresponds to clinical experience that increasing apnea can be an early sign of neonatal distress. Not captured in this analysis is the phenomenon of abnormal HR characteristics of reduced variability and transient decelerations that we have previously described (6). These abnormal heart rate characteristics are not known to occur in adults. In this work, we sought to analyze all of the time series data in the same way and thus excluded HR metrics optimized solely for detection of neonatal sepsis. The surprisingly limited repertoire of neonatal cardiorespiratory responses to illness compared to the adult is not explained but may be related to prematurity.
Model development and validation
We have followed the TRIPOD statement recommendations for internal validation by resampling rather than sample splitting. Further, we measured performance of statistical models across ICUs; for example, we developed a model for hemorrhage in the MICU and then tested it in the SICU. This kind of nonrandom split-sample development and validation, named Type 2b by the TRIPOD Group, is intermediary between internal and external validation(22). The major findings in adults were that respiratory failure and hemorrhage had consistent signatures in independent populations, but sepsis did not.
We note that the range of C-statistics from 0.61 (sepsis in the MICU) to 0.88 (respiratory failure in the NICU) is similar to other well-established clinical risk scores, such as CHADS2, CHA2DS2-VASc, and the ASCVD pooled cohort risk equations (29, 30). The C-statistic for heart rate characteristics monitoring, which improved NICU survival(31) is only 0.70 (6).
Limitations
These results are based on a single-center observational study. We previously analyzed subsets of these study populations and reported optimized, parsimonious models that did not include all of methodologies or measurements presented in this study (7-9). Our aims in this study did not include optimizing models for early detection using all available data, but rather hypothesis testing about the presence and distinguishing features of physiologic signatures that might allow for early detection and treatment. As a result, we omitted a number of well-recognized metrics such as lab results, nursing assessments, and charted comorbidities. We justify their omission based on the fact that the models here presented could be implemented using only the streaming data from bedside monitors, without the encumbrance of integrating with proprietary electronic medical records or relying on the accuracy of codes used for administrative and billing purposes. Moreover, we used the Surviving Sepsis Campaign Guidelines, but note that the definition and diagnosis of sepsis and septic shock, particularly as acquired in the ICU, remain controversial (32).
Predictive analytics at the bedside
We have used multivariable statistical tools to test the hypothesis that there are physiologic signatures in ICU patients preceding clinical deteriorations, and we fail to reject it based on the statistical significance of the regression models. We note that these tools might also serve as risk estimators for imminent deteriorations at the bedside of future patients.
Several prediction models have been developed for the ICU, most commonly to predict mortality or quantify illness severity for the purpose of comparing study cohorts. APACHE, perhaps the most popular, is representative and is calculated only at a fixed point in time using only the worst objective and semi-quantitative values (33). The scores thus remain static, are prone to interobserver variation, and fail to adapt to a patient's subsequent ICU course.
An advantage of models based on physiologic monitoring is that they provide continuously updated estimates of risk within much shorter horizons (the next few hours) based on the most recent measurements. A test of the dynamicity of our models is the observation of rising risk estimates leading up to an event as shown in Figure 5C. The risk estimates could improve by including additional data such as laboratory tests and clinical signs(34-36). We recognize the need for clinical trials of predictive monitoring to test for safety and efficacy as well as to validate our findings at additional sites. The penalty for predictive monitoring might be additional testing such as chest x-rays and blood tests for arterial gases, cell counts, and cultures. We note that heart rate characteristics monitoring for neonatal sepsis led to a 10% increase in blood cultures but no increase in antibiotic use.
Conclusions
Signatures of deranged physiology were present up to 24-hours in advance of clinical detection and intervention of subacute, potentially catastrophic illnesses in a large patient population from 3 diverse ICUs. We propose that multivariable techniques such as the statistical tools described here may find a home in the care of the critically ill adult.
Supplementary Material
Acknowledgments
We thank G. Huband, and J. Huband for design and operation of infrastructure to collect, process, and archive physiologic monitoring data; T. Hope for assistance with data warehouse queries; A. David, C. Elliott, E. Holland, C. Horton, N. Ivanick, R. Kronfol, A. Politano, and C. Ruminski for assistance with data collection; M. Clark, A. Politano for their instrumental role in prior analyses in subsets of these cohorts.
Sources of Funding: Dr. Moorman holds equity and is the chief medical officer of Advanced Medical Predictive Devices, Diagnostics, and Displays in Charlottesville, VA, which has licensed technologies from the University of Virginia Licensing and Ventures Group. Dr. Delos shares a patent for apnea detection software. For the remaining authors none were declared. Supported in part by the UVA Health System, NIH GO Grant 1RC2HD064488, and the Wallace H. Coulter Foundation.
Copyright form disclosures: Dr. Moss disclosed other support (Dr. Moorman holds equity and is the chief medical officer of Advanced Medical Predictive Devices, Diagnostics, and Displays in Charlottesville, VA, which has licensed technologies from the University of Virginia Licensing and Ventures Group. Dr. Delos shares a patent for apnea detection software) and received support for article research from the National Institutes of Health (NIH). His institution received funding from the University of Virginia Health System, the NIH, and Wallace H. Coulter Foundation. Dr. Delos received support for article research from the NIH and disclosed other support (Group has submitted grant proposals, they are not yet approved, and no money was paid). His institution received funding from the NIH. Dr. Fairchild received support for article research from the NIH. Her institution received grant support from the NICHD. Dr. Moorman received support for article research from the NIH and the Wallace Coulter Foundation and received funding from the Medical Predictive Science Corporation, Charlottesville, VA and from the Advanced Medical Predictive Diagnostics, Devices and Displays, Charlottesville, VA. His institution received funding from the NICHD and Wallace Coulter Foundation.
Footnotes
The study was performed at the University of Virginia Health System.
No reprints requested.
Conflicts of Interest: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Wallace DJ, Angus DC, Seymour CW, et al. Critical care bed growth in the united states. A comparison of regional and national trends Am J Respir Crit Care Med. 2015;191:410–416. doi: 10.1164/rccm.201409-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpern NA, Pastores SM. Critical care medicine in the united states 2000-2005: An analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for united states intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17:R81. doi: 10.1186/cc12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakeam E, Hyder JA, Jiang W, et al. Risk and patterns of secondary complications in surgical inpatients. JAMA Surg. 2014 doi: 10.1001/jamasurg.2014.1795. [DOI] [PubMed] [Google Scholar]
- 5.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 6.Griffin MP, O'Shea TM, Bissonette EA, et al. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res. 2003;53:920–926. doi: 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- 7.Clark MT, Vergales BD, Paget-Brown AO, et al. Predictive monitoring for respiratory decompensation leading to urgent unplanned intubation in the neonatal intensive care unit. Pediatr Res. 2013;73:104–110. doi: 10.1038/pr.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Politano AD, Riccio LM, Lake DE, et al. Predicting the need for urgent intubation in a surgical/trauma intensive care unit. Surgery. 2013;154:1110–1116. doi: 10.1016/j.surg.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss TJ, Clark MT, Lake DE, et al. Heart rate dynamics preceding hemorrhage in the intensive care unit. J Electrocardiol. 2015 doi: 10.1016/j.jelectrocard.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Bates DW, Saria S, Ohno-Machado L, et al. Big data in health care: Using analytics to identify and manage high-risk and high-cost patients. Health Aff (Millwood) 2014;33:1123–1131. doi: 10.1377/hlthaff.2014.0041. [DOI] [PubMed] [Google Scholar]
- 11.Bates DW, Zimlichman E. Finding patients before they crash: The next major opportunity to improve patient safety. BMJ Qual Saf. 2015;24:1–3. doi: 10.1136/bmjqs-2014-003499. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz HM. Big data and new knowledge in medicine: The thinking, training, and tools needed for a learning health system. Health Aff (Millwood) 2014;33:1163–1170. doi: 10.1377/hlthaff.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lake DE, Moorman JR. Accurate estimation of entropy in very short physiological time series: The problem of atrial fibrillation detection in implanted ventricular devices. Am J Physiol Heart Circ Physiol. 2011;300:H319–25. doi: 10.1152/ajpheart.00561.2010. [DOI] [PubMed] [Google Scholar]
- 16.Carrara M, Carozzi L, Moss TJ, et al. Heart rate dynamics distinguish among atrial fibrillation, normal sinus rhythm and sinus rhythm with frequent ectopy. Physiol Meas. 2015;36:1873–1888. doi: 10.1088/0967-3334/36/9/1873. [DOI] [PubMed] [Google Scholar]
- 17.Moss TJ, Lake DE, Moorman JR. Local dynamics of heart rate: Detection and prognostic implications. Physiol Meas. 2014;35:1929–1942. doi: 10.1088/0967-3334/35/10/1929. [DOI] [PubMed] [Google Scholar]
- 18.van Buuren S, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. 2011 [Google Scholar]
- 19.Harrell FE., Jr . Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 20.Harrell FE., Jr Rms: Regression modeling strategies. 2015 [Google Scholar]
- 21.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, et al. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 22.Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): Explanation and elaboration. Ann Intern Med. 2015;162:W1–73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 23.Steyerberg EW, Harrell FE., Jr Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2015 doi: 10.1016/j.jclinepi.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. R: A language and environment for statistical computing. 2015 [Google Scholar]
- 25.Odden AJ, Rohde JM, Bonham C, et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588–2334. doi: 10.1186/1471-2334-13-588. 13-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanathan R, Leavell P, Stockslager G, et al. Validity of international classification of diseases, ninth revision, clinical modification (ICD-9-CM) screening for sepsis in surgical mortalities. Surg Infect (Larchmt) 2014;15:513–516. doi: 10.1089/sur.2013.089. [DOI] [PubMed] [Google Scholar]
- 27.van Mourik MS, van Duijn PJ, Moons KG, et al. Accuracy of administrative data for surveillance of healthcare-associated infections: A systematic review. BMJ Open. 2015;5:e008424–2015. doi: 10.1136/bmjopen-2015-008424. 008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 29.van den Ham HA, Klungel OH, Singer DE, et al. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: Results from a national primary care database. J Am Coll Cardiol. 2015;66:1851–1859. doi: 10.1016/j.jacc.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: A randomized trial. J Pediatr. 2011;159:900–6. doi: 10.1016/j.jpeds.2011.06.044. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman JE, Kramer AA, McNair DS, et al. Acute physiology and chronic health evaluation (APACHE) IV: Hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 34.Griffin MP, Lake DE, Bissonette EA, et al. Heart rate characteristics: Novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116:1070–1074. doi: 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- 35.Griffin MP, Lake DE, Moorman JR. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics. 2005;115:937–941. doi: 10.1542/peds.2004-1393. [DOI] [PubMed] [Google Scholar]
- 36.Griffin MP, Lake DE, O'Shea TM, et al. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res. 2007;61:222–227. doi: 10.1203/01.pdr.0000252438.65759.af. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.