Abstract
Persistent deficits in social behavior, motor behavior, and behavioral flexibility are among the major negative consequences associated with exposure to ethanol during prenatal development. Prior work from our laboratory has linked moderate prenatal alcohol exposure (PAE) in the rat to deficits in these behavioral domains, which depend upon the ventrolateral frontal cortex [20]. Manipulations of the social environment cause modifications of dendritic morphology and experience-dependent immediate early gene expression in ventrolateral frontal cortex [19], and may yield positive behavioral outcomes following PAE. In the present study we evaluated the effects of housing PAE rats with non-exposed control rats on adult behavior. Rats of both sexes were either paired with a partner from the same prenatal treatment condition (ethanol or saccharin) or from the opposite condition (mixed housing condition). At four months of age (~3 months after the housing manipulation commenced), social behavior, tongue protrusion, and behavioral flexibility in the Morris water task were measured as in [20]. The behavioral effects of moderate PAE were primarily limited to males and were not ameliorated by housing with a non-ethanol exposed partner. Unexpectedly, social behavior, motor behavior, and spatial flexibility were adversely affected in control rats housed with a PAE rat (i.e., in mixed housing), indicating that housing with a PAE rat has broad behavioral consequences beyond the social domain. These observations provide further evidence that moderate PAE negatively affects social behavior, and underscore the importance of considering potential negative effects of housing with PAE animals on the behavior of critical comparison groups.
Keywords: Fetal Alcohol Spectrum Disorders, Prefrontal Cortex, Morris water task, Spatial Navigation, Sex Differences, Enrichment
1. Introduction
Fetal Alcohol Spectrum Disorders (FASD) include Fetal Alcohol Syndrome (FAS), partial FAS (pFAS), and other disorders for which morphological or behavioral consequences are observed in the context of confirmed prenatal alcohol exposure (PAE) [9, 46, 57]. The negative consequences of FASD include, but are not limited to, deficits in social behavior, learning, memory, and cognition [1, 23, 34, 42, 43, 53, 57, 58, 62, 80]. FASD is a world-wide public health problem[59]. Estimates of FASD prevalence rates in the United States occur between 2%-5% [45, 48], with a large majority of FASD cases falling within the less severe range of the spectrum [47]. While high levels of PAE may lead to greater morphological, neurological, behavioral, and cognitive deficits, the consequences of moderate PAE lead to more subtle, yet persistent deficits in humans and non-human animals [11, 40, 64, 65, 73].
Deficits in social behavior, motor behavior, and spatial learning and memory have been repeatedly observed in children with FASD [23, 34, 43, 53, 54, 57, 80] and in non-human animal models of PAE [4, 12, 16, 17, 26, 31, 51, 52, 70, 72, 75, 77], though the detection of deficits with more moderate blood ethanol concentrations (BECs) (e.g., BECs of ~60–80mg/dl) [20, 66] or low exposure (e.g., BECs < ~40mg/dl) [13] may require more challenging tasks compared to heavy exposure (e.g., ~BECs > 200mg/dl). Prior work from our laboratory observed increased agonistic wrestling (in contrast to play behavior) in male, but not female, rats following moderate PAE [19]. In the same study, the influence of housing with novel partners on PAE-related social behavior was examined by pair-housing PAE or saccharin control rats with novel, untreated rats obtained from a vendor that were regularly changed (every 48 hours for 40 days). This manipulation increased wrestling in male and female PAE offspring, suggesting increased aggression toward novel partners. However, the interpretation of these results is complicated by the fact that moderate PAE has more commonly yielded null effects on social behavior in female rats under standard housing conditions [19], and the behavioral phenotype of rats provided by commercial vendors may differ from those of animals bred and reared on site [8]. In a recent study [20] we replicated the effect of moderate PAE on male social behavior and observed impaired motor behaviors (tongue protrusion [78, 79]), and decreased behavioral flexibility as measured by increases in perseverative errors in the Morris water task. This collection of deficits was of particular interest based on commonalities in the ventrolateral frontocortical circuitry required for these behaviors and reports of alterations in ventrolateral frontal cortex function following moderate PAE [6, 19, 22].
Currently, despite considerable need, there are no treatments for the array of behavioral and cognitive deficits associated with FASD. Achieving this goal will critically depend upon the use of animal models to identify mechanisms and evaluate treatment approaches. Alterations in social behavior have been observed in rat models of PAE across a variety of exposure durations, doses, and developmental timing, and are among the most common outcomes observed in FASD [15, 18, 39]. Thus, social behavior represents an important target for intervention. The social environment and experience in the social domain have been recognized as potential factors that could yield benefits for social behavior as well as other behavioral deficits observed following PAE [30]. For example, Middleton et al. [51] found that PAE on the twelfth day of gestation produced a socially avoidant phenotype that was normalized following housing with a pair of non-exposed control animals. Social manipulations may also, however, promote the expression of atypical social behaviors. Hamilton et al. [19] demonstrated that routinely changing the cage-mate (i.e., housing with novel partners every 48 h) from a cohort of non-exposed rats may actually enhance social behavioral alterations following moderate PAE. The goal of the present study was to evaluate the effects of prolonged social housing with a non-exposed cage-mate on long-term social behavioral consequences of moderate PAE, and to determine if the effects of this housing manipulation extend to deficits in motor behavior and behavioral flexibility we have previously reported in male rats following moderate PAE [20]. Because our prior report was limited to male rats, the present study also examined potential sex differences in the effects of moderate PAE and social experience on behavioral outcomes. Offspring of rat dams that voluntarily consumed moderate levels of alcohol throughout pregnancy [24] were pair-housed with a same-sex cage-mate from either the same prenatal treatment condition (PAE or saccharin) or from the opposite condition (mixed housing) until behavioral testing in adulthood. Social behavior, tongue protrusion (TP), and spatial response perseveration errors were quantified as described in [20].
2. Methods
2.1 Subjects
Subjects were 48 Long-Evans rats (24 male and 24 female) obtained from the University of New Mexico Health Sciences Center Animal Resource Facility (see breeding protocol below). All animals were generated in the same breeding round. After weaning, all animals were housed with a single animal from the same prenatal treatment or the other prenatal treatment condition in standard plastic cages with water and food available ad libitum. All cage-mate pairs were from different litters and matched for age and weight. All animals were at least 4 months of age prior to behavioral testing. Lights were maintained on a reverse 12h:12h light:dark cycle with lights off at 0900h. Approximately 3–4 weeks prior to behavioral testing all rats in this study underwent a single MRI scan under isoflurane anesthesia for ~45 min as part of a separate study. All procedures were approved by the Institutional Animal Care and Use Committee of either the main campus or Health Sciences Center at the University of New Mexico.
2.2 Materials and Procedures
2.2.1 Breeding and Voluntary Ethanol Consumption During Gestation
All breeding procedures were conducted in the University of New Mexico HSC Animal Resource Facility (ARF). Three to four-month-old Long-Evans rat breeders (Harlan Industries, Indianapolis, IN) were single-housed in plastic cages at 22°C and kept on a reverse 12-hour light/dark schedule (lights on from 2100 to 0900 hours) with Purina Breeder Block rat chow and tap water ad libitum. After at least one week of acclimation to the animal facility, all female rats were provided 0.066% saccharin in tap water for four hours each day from 1000 to 1400 hours. On Days 1 and 2, the saccharin water contained 0% ethanol, on Days 3 and 4, saccharin water contained 2.5% ethanol (v/v). On Day 5 and thereafter, saccharin water contained 5% ethanol (v/v). Daily four-hour consumption of ethanol was monitored for at least two weeks and mean daily ethanol consumption was determined for each female breeder. Following two weeks of daily ethanol consumption females that drank at levels more than one standard deviation from the mean of the entire group were removed from the study. The remainder of the females were assigned to either a saccharin control or 5% ethanol drinking group and matched such that the mean pre-pregnancy ethanol consumption by each group was comparable.
Subsequently, females were placed with proven male breeders until pregnant as evidenced by the presence of a vaginal plug. Female rats did not consume ethanol during the breeding procedure. Beginning on Gestational Day 1, rat dams were provided saccharin water containing either 0% or 5% ethanol for four hours a day, beginning precisely at 1000 hours (1 hour following the onset of the dark cycle). The volume of 0% ethanol saccharin water provided to the controls was matched to the mean volume of 5% ethanol in saccharin water consumed by the ethanol-drinking group, which has remained relatively consistent at about 16mL per four-hour drinking period over multiple breeding rounds. Food and tap water were available ad libitum during both the drinking and non-drinking periods. Maternal weight gain during pregnancy and offspring birth-weight did not differ based on prenatal treatment group [19, 74]. Daily four-hour ethanol consumption was recorded for each dam. Ethanol consumption was discontinued at birth, and all litters were weighed and culled to 10 pups. Offspring were weaned at 24 days of age and transferred from the HSC-ARF to the Department of Psychology ARF. To minimize potential litter effects only 1–2 animals per breeding pair were included in the present study.
2.2.2. Housing
At weaning all rats were pair-housed with a non-littermate of the same sex. The primary housing manipulation was the cage-mate’s prenatal treatment condition. Cage-mates were selected to ensure an equal number of pairs of cage-mates were from the same prenatal treatment condition (Same condition) or from different diet conditions (Mixed condition). Individual pairings were determined randomly with an additional constraint that they be matched for body weight (within 5g) at the time of assignment. Data from male animals in the same housing condition were previously reported [20] as part of a separate study conducted concurrently with the present study. Thus, data from the 12 male rats of the same housing condition do not represent an independent replication of behavioral effects of moderate PAE. 1
2.2.3. Social Behavior
The procedures for housing, data collection, and quantification of social behavior have been described elsewhere [19, 20, 24]. Cagemate pairs were placed into a box (95 cm × 47 cm × 43 cm) with a Plexiglass front, opaque sides, and a mirrored back wall for 30 minutes on each of three consecutive days to habituate them to the apparatus. At the end of the final habituation session the animals were housed in isolation for 24 h. At the end of the isolation period the animals were placed into the test apparatus together and videotaped for 12 minutes. Videos were digitized for behavioral quantification using software developed in our laboratory. The duration, frequency and latency to first occurrence of the following behaviors were quantified: wrestling (including pinning), anogenital sniffing, other sniffing of the partner’s body (body sniffing), and allogrooming (grooming of the partner). We have previously quantified boxing [19], however, this behavior was only observed briefly in one pair of animals and is, therefore, not included in the analyses reported here. The four behaviors quantified here were selected based on extant literature [2] to target partner-directed (e.g., wrestling, investigation) behavior that have been quantified in prior work in our laboratory with PAE rats [19]. Separate analyses of variance (ANOVAs) were conducted for each measure with prenatal treatment, sex, and housing as factors. Planned comparisons of prenatal treatment conditions within levels of the sex and housing factors, and housing effects within levels of the sex and prenatal treatment factors are also provided.
2.2.4. Tongue protrusion
Tongue protrusion measures were obtained in a single session in a room that was dimly lit by a red lamp. Rats were placed in a plastic hanging cage placed onto one side so that the wire mesh top was oriented vertically. To familiarize the animal with the food substrate used for measurement (chocolate syrup or creamy peanut butter) a small amount was placed inside the cage. Once it had been consumed an experimenter smeared a small amount of chocolate syrup or peanut butter onto one end of a thin piece of Plexiglass (2 mm X 5 cm X 7 cm). The end containing the food substrate was placed against the edge of the wire mesh. As the animal approached the food and attempted to lick, the Plexiglass was reoriented such that it was parallel to the animal’s mouth. After a lick attempt the Plexiglass was removed and an experimenter measured the length of the indentation left in the food substrate by the tongue. This was repeated until 5–12 measurements had been obtained for an individual animal in a 20 min session. All attempts for a single animal were averaged for analysis. An ANOVA was conducted with prenatal treatment, sex, and housing condition as factors. Planned comparisons were conducted to evaluate effects of prenatal treatment within each combination of sex and housing condition.
2.2.5. Morris water task
A circular pool, 1.5 m in diameter and 48 cm in height, was filled to a depth of 26 cm with room temperature water (23° C) made opaque with a small amount (~200g) of non-toxic powdered paint. An escape platform (15 cm × 15 cm) was submerged ~1 cm below the surface of the water. A single room adjacent to the animal colony was used for all water task testing. A variety of distinctive, conspicuous items (posters, cabinets, equipment, etc.) on the walls of the room served as visual cues. All behavior was recorded via an overhead camera and digital camcorder. Videos were transferred to a computer for tracking and analysis using custom software developed in our laboratory. All trials were conducted in blocks of four in which each of four release points around the perimeter of the pool (NE, NW, SW, SE) were utilized with the order determined by pseudorandom selection without replacement. A trial ended when the animal either reached the platform or after 60 s had elapsed in which case the rat was placed on the platform by the experimenter. Rats were removed from the platform after ~5 s and were placed in holding cages between trials. The intertrial interval was ~ 5 min. All testing was conducted ~1–2 hours after the onset of the dark cycle.
The water task was conducted in two phases. For both phases a total of three trial blocks were administered on each day of training. During Phase 1, rats were given 10 trial blocks; the first 9 blocks were distributed over days 1 through 3 and one additional block was conducted as the first block on day 4. Two possible platform locations were used (the center of the east or west quadrant), with each location used for half of the rats in each combination of prenatal treatment condition, housing condition, and sex. Initial training was conducted in this manner to establish strong responding to the initial location and to minimize the effects of between-day reductions in performance during the subsequent relocation trials. During Phase 2 the platform was repositioned to the other location (east or west) and 6 blocks of trials were conducted; the first 2 trial blocks were conducted on day 4 immediately following the last block of Phase 1, with 3 blocks on day 5 and one block at the beginning of day 6. A third phase was included during which the platform was returned to the original location, however to simplify presentation these results are not described. Mean latency and path length to reach the platform were measured for each trial block. For Phase 2, the number of trials per block that included a visit to the platform location used in Phase 1 phase was also quantified. Separate repeated measures ANOVAs with prenatal treatment, sex, and housing as between subjects factors and trial block as a within subjects factor were conducted for each phase. The multivariate approach to repeated measures ANOVA (i.e., MANOVA) was utilized for main effects of trial block and any interaction terms that included this factor. Based on our prior observation that increased visits to the previously trained platform location by PAE rats was limited to the final block of Phase 2 training [20], we conducted a planned analysis including prenatal treatment, sex, and housing condition as factors on this dependent measure.
2.2.6. Exploratory discriminant analysis
An additional, exploratory stepwise discriminant analysis [7] was conducted to evaluate which of 19 dependent variables from the three behavioral tasks provided the best discrimination between PAE rats and SAC control rats for each combination of sex and housing condition. The variables included tongue protrusion, number of visits to the initial platform locations during each of the 6 blocks of Phase 2 (platform relocation), and frequency, duration, and latency to first instance of wrestling, anogenital sniffing, body sniffing, and allogrooming.
3. Results
An alpha of p < 0.05 was adopted for all analyses. Effect sizes (partial eta squared, , which is equivalent to eta squared for oneway ANOVAs) are provided for all significant effects. Standard benchmarks for small (η2= 0.01), medium (η2= 0.06), and large (η2>= 0.14) effect sizes are based on the recommendations of Cohen [10] (see also refs. [38, 44]).
3.1. Maternal ethanol consumption
Female rats in the ethanol condition consistently drink an average of 2.1g/kg of ethanol per four-hour drinking session. Rat dams consume approximately one-half of the four-hour total during the first 15 to 30 minutes after the introduction of the drinking tubes, resulting in a peak maternal serum ethanol concentration of about 60 mg/dl, measured at the 45 min time point. Over the remaining 3.5 hours of the drinking period, they continue to consume 5% ethanol at a lower, but relatively stable rate of 0.4 g/kg body weight/hour. This level and pattern of voluntary ethanol consumption had no significant effects on maternal weight gain, offspring birth weight, litter size, maternal care, placental wet weight, offspring weight at behavioral testing, or whole brain, hippocampal or cerebellar wet weights (data not shown).
3.2. Social Behavior
Mean values for the frequency, duration and latency to first occurrence of each behavior quantified during the 12-minute social interaction session are shown in Table 1. For wrestling there were significant prenatal treatment main effects for frequency [PAE > SAC; F(1, 40) = 4.89, p = 0.033, ] and duration [PAE > SAC; F(1, 40) = 5.44, p = 0.025, ]. There were also significant sex main effects for the frequency [MALE > FEMALE; F(1, 40) = 41.70, p < 0.001, ], duration [MALE > FEMALE; F(1, 40) = 63.24, p < 0.001, ], and latency to first occurrence of wrestling [MALE < FEMALE; F(1, 40) = 24.33, p < 0.001, ]. There were no main effects of housing on dependent measures of wrestling [all ps > 0.11], however, there were several interactions involving the housing factor for these measures. These include Prenatal Treatment X Housing interactions for the frequency [F(1, 40) = 4.89, p = 0.033, ] and duration [F(1, 40) = 5.44, p = 0.025, ] of wrestling. Analysis of simple effects revealed prenatal treatment effects for animals in the same housing condition for frequency [PAE > SAC; F(1, 22) = 6.93, p = 0.015, ] and duration [PAE > SAC; F(1, 22) = 7.10, p = 0.014, ]. Because wrestling occurs for both members of a pair, PAE and SAC animals housed together (mixed condition) have identical values (i.e., no prenatal treatment effect can be measured in the mixed housing condition). There were also Sex X Housing interactions for the frequency [F(1, 40) = 14.70, p < 0.001, ] and duration [F(1, 40) = 6.27, p = 0.016, ] of wrestling. Analysis of simple effects indicated that the interaction for frequency of wrestling was due to distinct housing effects for males [MIXED > SAME; F(1, 22) = 6.91, p = 0.015, ] and females [MIXED < SAME; F(1, 22) = 4.57, p = 0.044, ]. The interaction for duration was primarily due to a similar pattern, although neither of the simple housing effects within levels of sex were significant [both ps > 0.06].
Table 1.
Mean (SEM) for each dependent measure (frequency, duration, and latency to first instance) for four behaviors (wrestling, anogenital sniffing, other body sniffing, and allogrooming) quantified during the 12-minute social interaction session. n = 6 for each combination of prenatal treatment (Saccharin or prenatal alcohol-exposed (PAE)), sex, and (partner) housing condition (same or mixed). Significant omnibus effects are indicated as subscripts [prenatal treatment (P), housing (H), and sex (S), with interactions indicated by combinations of P, H, and S]. Significant prenatal treatment effects (p < 0.05) within levels of the sex and housing factors are indicated by gray shading. Values in bold text indicate significant housing effects (p < 0.05) within levels of the sex and prenatal treatment factors.
| SACCHARIN - MALES | PAE - MALES | SACCHARIN - FEMALES | PAE - FEMALES | |||||
|---|---|---|---|---|---|---|---|---|
| 1a. Frequency | Same | Mixed | Same | Mixed | Same | Mixed | Same | Mixed |
| WrestlingP,S,PH,SH | 1.67 (0.21) | 5.67 (0.84) | 5.33 (0.42) | 5.67 (0.84) | 2.00 (0.63) | 0.83 (0.31) | 2.50 (1.15) | 0.83 (0.31) |
| Anogenital | 4.83 (0.87) | 4.50 (1.95) | 3.67 (1.33) | 3.50 (0.62) | 2.33 (0.49) | 2.83 (0.87) | 4.33 (0.49) | 3.50 (1.06) |
| Body Sniff | 15.17 (1.54) | 13.33 (1.50) | 15.67 (3.59) | 14.17 (1.54) | 16.67 (2.68) | 14.67 (0.67) | 14.83 (1.66) | 11.17 (1.70) |
| Allogr S,PSH | 5.00 (2.03) | 0.67 (0.49) | 1.33 (0.56) | 1.17 (0.60) | 0.33 (0.21) | 0.33 (0.21) | 0.00 (0.00) | 0.00 (0.00) |
| 1b. Duration (sec) | ||||||||
| Wrestling P,S,PH,SH | 16.21 (4.00) | 51.06 (9.55) | 50.49 (6.51) | 51.06 (9.55) | 6.76 (2.81) | 5.84 (2.79) | 12.83 (5.91) | 5.84 (2.79) |
| Anogenital PS | 9.59 (1.41) | 12.72 (4.57) | 5.29 (1.75) | 6.97 (1.18) | 5.56 (1.38) | 5.44 (1.73) | 10.14 (3.47) | 5.98 (1.56) |
| Body Sniff | 32.08 (3.55) | 25.68 (4.56) | 29.46 (6.95) | 30.02 (6.70) | 26.14 (5.23) | 28.43 (3.11) | 22.47 (2.56) | 23.91 (2.10) |
| Allogr P,S,H,PH,SH,PSH | 34.44 (10.81) | 2.79 (1.76) | 8.10 (4.06) | 4.10 (2.88) | 1.31 (1.18) | 0.74 (0.47) | 0.00 (0.00) | 0.00 (0.00) |
| 1c. Latency (sec) | ||||||||
| Wrestling S | 268.32 (32.15) | 199.64 (60.26) | 174.51 (17.25) | 199.64 (60.26) | 507.47 (102.61) | 472.00 (91.85) | 433.22 (93.21) | 472.00 (91.85) |
| Anogenital PSH | 64.83 (36.08) | 121.44 (46.97) | 181.62 (42.71) | 108.65 (28.98) | 158.80 (36.23) | 55.54 (15.31) | 66.82 (18.20) | 156.37 (54.51) |
| Body Sniff | 35.83 (13.64) | 20.26 (7.85) | 17.89 (4.78) | 31.49 (5.07) | 43.01 (15.73) | 23.24 (12.64) | 63.57 (25.27) | 48.52 (20.92) |
| Allogr S, PH | 261.24 (109.00) | 625.17 (80.49) | 549.10 (75.55) | 511.20 (100.90) | 636.65 (61.45) | 701.58 (16.00) | 720.00 (0.00) | 720.00 (0.00) |
Planned comparisons revealed prenatal treatment effects for male animals in the same housing condition for frequency [PAE > SAC; F(1, 10) = 60.50, p < 0.001, ], duration [PAE > SAC; F(1, 10) = 20.12, p = 0.001, ], and latency to first instance of wrestling [PAE > SAC; F(1, 10) = 6.61, p < 0.028, ] (previously reported in [20]). There were no effects of prenatal treatment on measures of wrestling in females in the same housing condition [all ps > 0.376]. Planned comparisons also revealed significant effects of housing in male SAC animals for frequency [MIXED > SAME; F(1, 10) = 21.17, p = 0.001, ] and duration of wrestling [MIXED > SAME; F(1, 10) = 11.33, p = 0.007, ]. None of the other housing effects for measures of wrestling within levels of sex and prenatal treatment were significant [all ps > 0.12].
For measures of anogenital sniffing (see Table 1), there was a significant Prenatal Treatment X Sex X Housing interaction for latency to first occurrence [F(1, 40) = 9.43, p = 0.004, ] and a Prenatal Treatment X Sex interaction for duration [F(1, 40) = 4.94, p = 0.032, ]. The three-way interaction can be understood by way of planned contrasts. SAC females were quicker to engage in anogenital sniffing when housed with a PAE female rather than another SAC female [F(1, 10) = 6.89, p = 0.025, ]. In contrast, PAE females displayed a comparable numerical preference to perform anogenital sniffing when in the same housing condition, however, this difference was not significant. PAE females were quicker to perform anogenital sniffing than SAC females in the same housing condition [F(1, 40) = 5.14, p = 0.047, ]. Male animals displayed opposite numerical trends to that observed in females, however, none of these comparisons were significant [all ps > 0.06]. The Prenatal Treatment X Sex interaction resulted from a significant sex effect for duration of anogenital sniffing in SAC animals [MALE > FEMALE; F(1, 22) = 4.91, p = 0.037, ] that was not observed for PAE animals [p = 0.386].
For allogrooming there were significant main effects of prenatal treatment for duration [PAE < SAC; F(1, 40) = 5.01, p < 0.031, ]. There was also a main effect housing for duration of allogrooming [MIXED < SAME; F(1, 40) = 8.96, p = 0.005, ], and the housing effects approached significance for frequency [MIXED < SAME; F(1, 40) = 3.94, p = 0.054, ] and latency to first occurrence of allogrooming [MIXED > SAME; F(1, 40) = 3.99, p = 0.053, ]. Main effects of sex were observed for frequency [MALE < FEMALE; F(1, 40) = 10.95, p = 0.002, ], duration [MALE < FEMALE; F(1, 40) = 15.34, p < 0.001, ], and latency to first occurrence of allogrooming [MALE > FEMALE; F(1, 40) = 18.06, p < 0.001, ]. Planned comparisons of allogrooming frequency for the housing factor within levels of sex and prenatal treatment and comparisons of prenatal treatment conditions within levels of sex and housing yielded no significant effects [all ps > 0.06]. For duration of allogrooming there were significant Prenatal Treatment X Housing [F(1, 40) = 5.45, p = 0.025, ], Sex X Housing [F(1, 40) = 8.40, p = 0.006, ], and Prenatal Treatment X Sex X Housing [F(1, 40) = 5.01, p = 0.031, ] interactions that can be explained by way of planned comparisons. There was a significant effect of prenatal treatment for male rats in the same housing condition [PAE < SAC; F(1, 10) = 5.21, p = 0.046, ] that was not observed for other combinations of sex and housing factors [all ps > 0.15]. There was a significant Prenatal Treatment X Housing interaction for latency to first occurrence of allogrooming [F(1, 40) = 5.69, p = 0.022, ]. This interaction was due to a significant housing effect for SAC animals [F(1, 22) = 4.93, p = 0.037, ] that was not observed for PAE animals [p = 0.47]. Planned comparisons of housing effects within levels of sex and prenatal treatment revealed that the main effect of housing in SAC animals was primarily driven by housing effects in male animals [MIXED > SAME; F(1, 10) = 8.25, p = 0.016, ]; no other planned comparisons for housing within levels of sex and prenatal treatment were significant [all ps > 0.44]. Planned comparisons of prenatal treatment within levels of sex and housing revealed no significant effects [all ps > 0.055].
For body sniffing there were no main effects or interactions [all ps > 0.09], and no simple of effects of prenatal treatment within levels of sex and housing [all ps > 0.08] or housing within levels of prenatal treatment and sex [all ps > 0.10].
3.3. Morris water task
Mean escape latency and path length for each trial block during Phases 1 and 2 for each combination of prenatal treatment, housing condition, and sex are shown in Figure 1.
Figure 1.
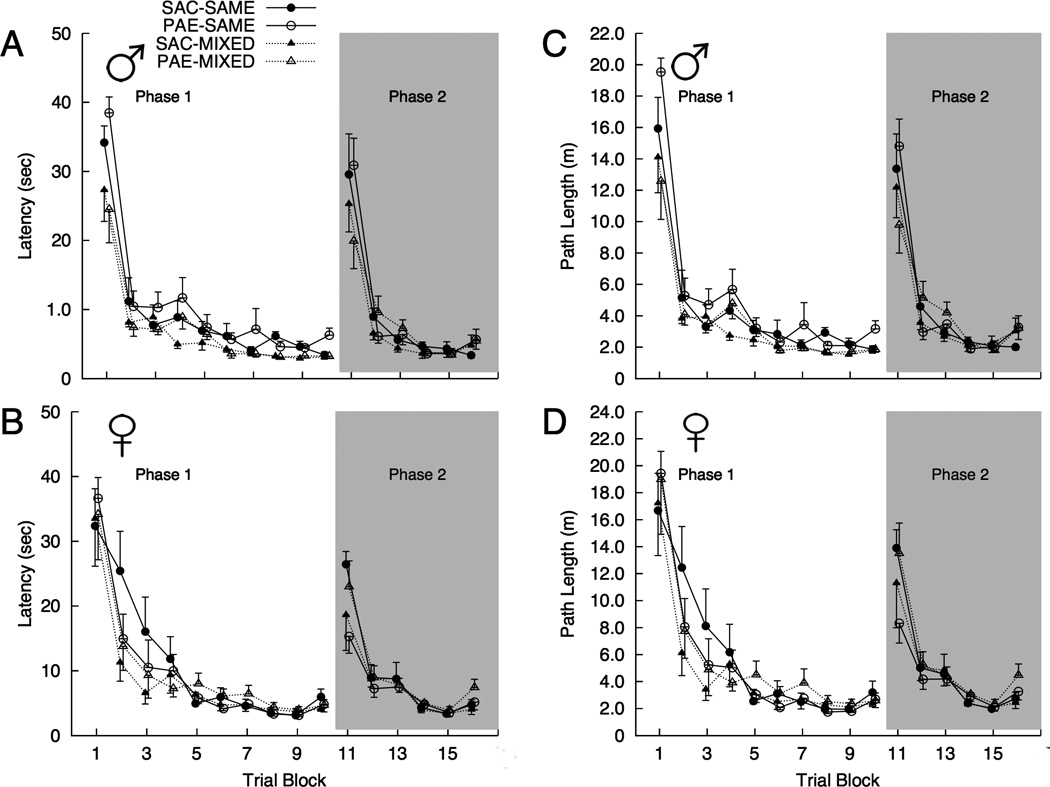
Mean (±SEM) latency (A–B) and path length (C–D) to navigate to the escape platform during each trial blocks of Phases 1–2 for each combination of prenatal treatment condition (SAC and PAE), sex, and housing (SAME and MIXED). [n = 6 per group].
Phase 1. Initial training
There were significant main effects of trial block for latency [Wilks’ Λ = 0.094, F(9, 32) = 34.33, p < 0.001, ] and path length [Wilks’ Λ = 0.102, F(9, 32) = 31.21, p < 0.001, ] which resulted from a decrease in both measures across trial blocks. There were significant main effects of sex for latency [MALE < FEMALE; F(1, 40) = 4.16, p = 0.048, ] and path length [MALE < FEMALE; F(1, 40) = 7.99, p = 0.007, ]. There was also a significant effect of housing for latency [MIXED < SAME; F(1, 40) = 9.15, p = 0.004, ], which approached significance for path length [MIXED < SAME; F(1, 40) = 4.04, p = 0.051, ]. The Trial Block X Prenatal Treatment interaction was significant for the path length measure [Wilks’ Λ = 0.593, F(9, 32) = 2.44, p = 0.03, ]. There were, however, no significant prenatal treatment effects for any trial block [all ps > 0.12]. Thus, to explore this multivariate interaction, Helmert contrasts were conducted on the trial block measure within prenatal treatment groups. Whereas SAC animals maintained asymptotic levels of performance from block 5 (i.e., Day 2, Block 2) on [all ps > 0.10], Helmert contrasts revealed that this was not the case for the PAE group. Specifically, several of the trial blocks beyond block 5 were significantly different from one another including the final two trial blocks [BLOCK 10 > BLOCK 9; F(1, 23) = 5.11, p = 0.033, ]. All other main effects and interactions were non-significant [all ps > 0.16].
Phase 2. Platform relocation
For the 6 trial blocks during which the platform was placed in a new location (see Figure 1) there was a significant Trial Block X Sex interaction for latency [Wilks’ Λ = 0.684, F(5, 36) = 3.33, p < 0.014, ]. This interaction was attributable to a pattern in which females had shorter latencies during block 1 of Phase 2, whereas males had shorter latencies in block 3 of Phase 2. There were also significant trial block main effects related to reductions in latency [Wilks’ Λ = 0.137, F(5, 36) = 45.21, p < 0.001, ] and path length [Wilks’ Λ = 0.15, F(5, 36) = 40.95, p < 0.001, ]. No other main effects or interactions were significant [all ps > 0.07].
The mean number of visits to the original platform location during each of the 6 trial blocks of Phase 2 are shown in Figure 2. There was a significant block effect [Wilks’ Λ = 0.07; F(5, 36) = 95.25, p < 0.001, ] which resulted from a decrease in visits to the Phase 1 platform location across trial blocks. There was also a significant Trial Block X Prenatal Treatment interaction [Wilks’ Λ = 0.07; F(5, 36) = 2.49, p = 0.017, ]; there were no other significant main effects or interactions [all ps > 0.13]. PAE rats continued to visit the Phase 1 location more frequently than saccharin-exposed rats during the later stages of training, particularly during the final block of Phase 2. Analysis of simple effects revealed that PAE rats visited the Phase 1 platform location significantly more than saccharin-exposed rats during the final block of Phase 2 [F(1, 46) = 7.041, p = 0.011, ], whereas no other prenatal treatment effects within levels of the trial block factor were significant [all ps > 0.26]. Planned comparisons yielded a significant prenatal treatment effect in male animals in the same housing condition for visits to the prior platform location during the final block of Phase 2 [PAE > SAC; F(1, 10) = 5.98, p = 0.035, ], and significant housing effects for male SAC animals [MIXED > SAME; F(1, 10) = 14.41, p = 0.004, ]. There was also a significant housing effect in male PAE rats for visits to the former platform location during the second block of Phase 2 [MIXED > SAME; F(1, 10) = 5.00, p = 0.049, ].
Figure 2.
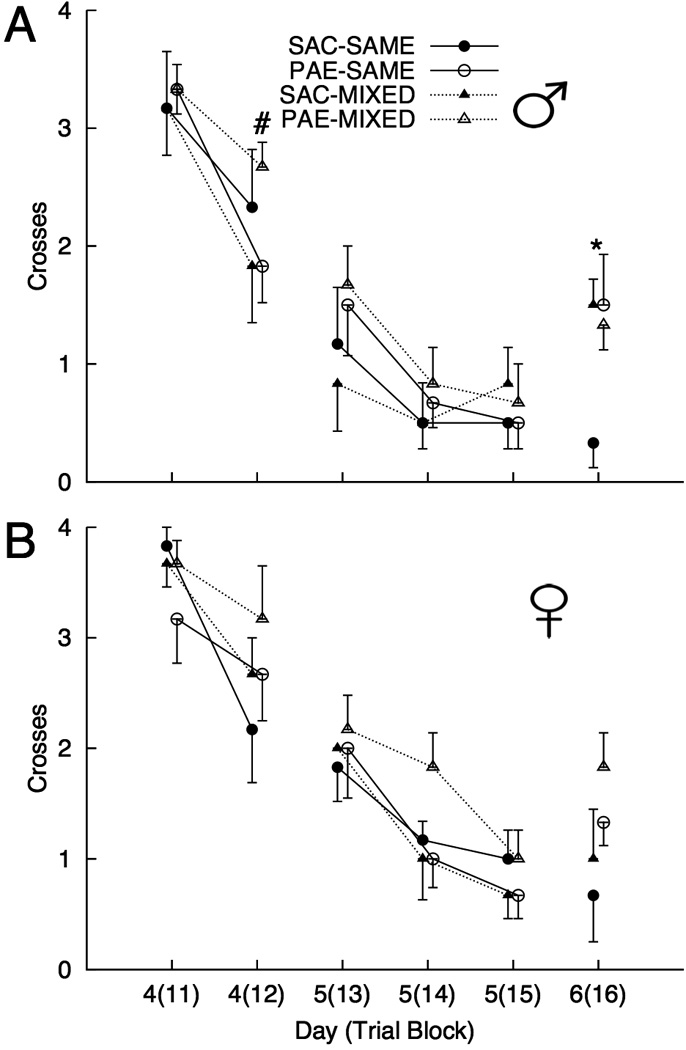
Mean (±SEM) number of visits (crosses) to the Phase 1 platform location during the 6 trial blocks of Phase 2 (blocks 11–16, conducted across days 4, 5, and 6) for males (A) and females (B) for each combination of prenatal treatment [saccharin (SAC) and prenatal alcohol-exposed (PAE)] and housing [Same and Mixed]. (n = 6 per group). [* indicates a significant effect of housing within SAC males and a significant prenatal treatment effect within males in the SAME housing condition, as well as a significant prenatal treatment effect collapsing across sex and housing at p < 0.05. # indicates a housing effect in PAE males at p < 0.05].
3.4. Tongue Protrusion
Mean TP values for each prenatal treatment, sex and housing group are shown in Figure 3. There were significant effects of sex [FEMALE > MALE; F(1, 40) = 49.67, p < 0.001, ], however, the main effect of prenatal treatment was not significant [PAE < SAC; F(1, 40) = 3.60, p = 0.065, ]. There was a significant Prenatal Treatment X Sex interaction [F(1, 40) = 12.30, p < 0.002, ] and a significant Housing X Sex interaction [F(1, 40) = 20.00, p < 0.001, ]. All other main effects and interactions for the omnibus ANOVA were non-significant [all ps > 0.487]. Analysis of simple effects indicated that the Prenatal Treatment X Sex interaction was attributable to a significant prenatal treatment effect in males [PAE < SAC; F(1, 20) = 12.79, p < 0.002, ] that was not present in females [F(1, 20) = 1.51, p = 0.233, ]. The Housing X Sex interaction resulted from opposite housing effects in males [MIXED < SAME; F(1, 20) = 12.79, p < 0.002, ] and females [SAME < MIXED; F(1, 20) = 9.67, p = 0.006, ]. Planned comparisons of PAE and SAC groups within each combination of sex and housing revealed a significant prenatal treatment effect in male rats housed with cage-mates from the same diet condition [PAE < SAC; F(1, 20) = 18.14, p < 0.003, ]. Although a similar difference between PAE and SAC rats was observed for males rats in the mixed housing condition, this effect was not significant [F(1, 20) = 3.89, p = 0.096, ].
Figure 3.
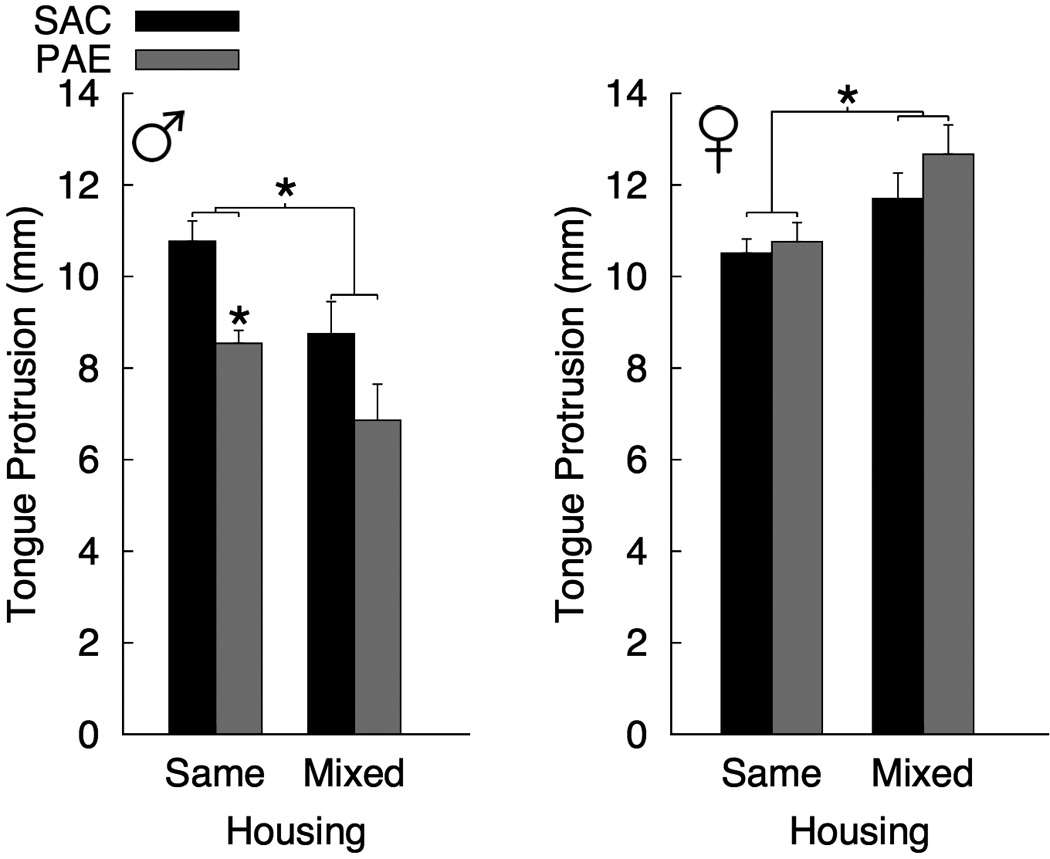
Mean (+SEM) tongue protrusion for each combination of prenatal treatment [saccharin (SAC) and prenatal alcohol-exposed (PAE)], housing [Same and Mixed], and sex (n = 6 per group) [* p < 0.05].
3.5. Exploratory discriminant analysis
Based on the pattern of findings in the omnibus analyses, a discriminant function analysis was performed separately for male rats and female rats in same and mixed housing conditions. Consistent with the omnibus findings, the discriminant function analysis for males in the mixed housing condition was not significant, as the behavior of PAE and SAC rats were not discriminable under these conditions [p = 0.193]. For males housed in the same housing condition, however, the discriminant function significantly differentiated PAE from SAC rats, explaining 100% of the variance [canonical R2 = 1.00, Λ = 0.001, X2(10) = 37.47, p = 0.000047]. The correlations between behavioral variables and the discriminant function revealed high loadings on the discriminant function primarily for social behaviors [Latency to Wrestle r = −0.48, Latency to Body Sniffing r = 0.46]. Also consistent with the omnibus findings are the discriminant function analysis findings for females. For females housed in the mixed housing condition, the discriminant function analysis significantly discriminated PAE from SAC rats, explaining 100% of the variance [canonical R2 = 0.998, Λ = 0.005, X2(10) = 26.55, p = 0.0031]. The correlations between behavioral variables and the discriminant function revealed high loadings on the discriminant function primarily for social behavior variables [Latency to Allogrooming r = −0.359, Duration of Body Sniffing r = −0.325]. The discriminant function analysis for females in the same housing condition was not significant [p = 0.137].
4. Discussion
4.1 General Discussion
The current study expanded upon previously published data from our laboratory examining the effects of moderate PAE on social, motor, and spatial behavior to include the evaluation of social housing effects and sex [20]. The present findings indicate that the effects of moderate PAE on social behavior, motor behavior (tongue protrusion), and spatial response preservation errors are primarily limited to male rats and are not ameliorated by prolonged housing (> 2 months) with a non-ethanol-exposed cage-mate. Somewhat unexpectedly, we observed a negative effect of housing with a PAE cage-mate on the behavior of male control animals. In contrast to the effects observed in males, female PAE animals displayed no evidence of impairment in these behavioral domains, and actually displayed enhanced social investigation. Further, prolonged housing with a partner from the opposite prenatal condition either had no effect, or benefited motor behavior in females from both prenatal treatment conditions.
In a previous study using the same PAE model reported here females displayed modest social behavior deficits compared to males [19], although females displayed comparable deficits in social-experience-dependent activity in ventrolateral frontal cortex. Thus, females may be more resilient to the negative effects of moderate PAE on neural circuits involved in social behavior. Moderate PAE in our model has also yielded robust long-term memory deficits in one-trial contextual fear conditioning in females [60]. Thus, females are not generally immune to the behavioral and cognitive effects of moderate PAE, however, considered with the present findings, the effects of moderate PAE on social behavior and other behaviors that depend upon ventrolateral frontal cortex appear to be considerably greater in males. We note that several other studies have also reported sexually dimorphic effects of developmental alcohol exposure on social behavior [12, 14, 29, 52, 75], several of which have reported more pronounced effects in males compared to relatively lower, opposing, or absent effects in females. Those studies have typically employed higher levels of ethanol than used here (e.g., > 125 mg/dl) and recent evidence indicates that low levels of ethanol exposure during gestation (~30mg/dl) alters social investigation in males but not females [12]. The less pronounced effects observed in females in the present study may, therefore, be primarily related to more moderate level of exposure. PAE in humans is clearly linked with deficits in adaptive behavior [35, 41]as well as deficits in social behavior and cognition [15, 18, 49, 50, 71, 76]. These deficits are present in both sexes and are not better explained by the presence of comorbidity (e.g., with ADHD)[76]. Though prominent sexual dimorphisms have not been characterized, we note that estimates of FASD prevalence are slightly higher in males [45] and males with a history of PAE display the highest rates of conduct disorder compared to non-exposed males and females[15]. Collectively these considerations along with the present findings underscore the importance of evaluating sex differences in the effects of prenatal alcohol exposure, which may yield important clues regarding variables that enhance or protect against the effects of ethanol exposure, In the sections that follow we provide a more detailed discussion of the principal outcomes and sex effects for each behavioral domain from the present study.
4.2 Social Behavior
Social-environment manipulations resulted in modified social behavior in both saccharin and PAE animals. Same-housed PAE males displayed increases in wrestling frequency and duration compared to same-housed saccharin males. Mixed-housed saccharin males displayed increases in the frequency and duration of wrestling behaviors compared to same-housed saccharin males. Wrestling frequency and duration measures in PAE males were not reduced by housing with a non-exposed control partner. Additionally, mixed-housed saccharin males and PAE males in both housing conditions displayed decreased time spent in allogrooming and longer latency to the first instance of allogrooming compared to same-housed saccharin males. Collectively, these outcomes and the discriminant analysis suggest that mixed housing resulted in a behavioral profile in control males similar to that of PAE rats.
The effects of the housing manipulation on social behaviors were sex specific. Mixed-housed saccharin females were significantly quicker to initiate partner investigation compared to same-housed saccharin females. Conversely, mixed-housed PAE females displayed a non-significant trend towards delayed engagement in social investigation behavior when compared to same-housed PAE females. Much like the observation of male controls, a more robust effect on social behavior was observed in the mixed-housed saccharin females, whereas mixed-housed PAE females did not benefit from enriched social environmental manipulations.
These observations are important when considering that rodents exposed to alcohol, generally, tend to benefit from experiential treatments [25]. Furthermore, among studies of PAE, experiential factors such as enriched housing and specific motor training have been shown to mitigate negative behavioral consequences even when associated neural plasticity deficits remain unaffected by such experience [5, 25, 51]. Indeed, Middleton and colleagues recently observed an amelioration of PAE-related social behavior deficits in male and female subjects housed in triads with two non-ethanol exposed animals [51]. In contrast to these studies, we observed a negative impact, especially in saccharin males, of housing with ethanol-exposed animals. The difference in outcomes of these studies might be related to the number of non-exposed partners, as housing with multiple partners may produce unique or more robust effects on social behavior [27]. Interestingly, the findings of Middleton and colleagues also included negative effects in the form of reduced social investigation and play fighting in non-ethanol exposed animals housed with untreated animals, possibly arising from the influence of housing with non-littermates. Because it is not possible to generate exposed and non-exposed animals from the same litter in our exposure paradigm, all animals were paired with non-littermates to avoid potential confounding of PAE with this factor. Unfortunately, this further complicates a direct comparison of the present study with that of Middleton et al. [51].
PAE is highly associated with persistent altered social behaviors reflected as increased rates of conduct disorder and aggression-related behaviors [15, 18, 39, 63]. Considering the results of Middleton and colleagues [51], we hypothesized that housing a PAE rat with a saccharin-exposed rat would improve social behavioral deficits found in PAE rats. However, our results suggest that housing manipulations are not beneficial to PAE rats and can negatively influence control rats. As a result, careful consideration of the housing environment is warranted when designing and evaluating experiments of this type.
One interpretation of our results is that PAE animals are less flexible than control animals with respect to adaptation in the social domain. As a result, saccharin animals could be more adept at modifying behaviors in response to conspecifics displaying abnormal social behaviors. Viewed in this way, the results provide further evidence that moderate PAE results in abnormal social behavior, as the social influence of moderate PAE rats contributes to abnormal social behavior in controls. Frontal cortex circuitry has been implicated in flexible learning (e.g., reversal learning) in various studies [21, 37] and increased play behavior is observed after early life ventrolateral frontal cortex lesions [55]. Prior work from our laboratory found that activity-dependent immediate early gene (IEG) expression in agranular insular cortex (AID) and lateral orbital (LO) elicited by social experience was reduced by moderate PAE [19, 22]. Thus, changes in wrestling, which is akin to play fighting, may be due to the effects of gestational ethanol exposure on the development of these frontal cortex regions.
4.3 Spatial Flexibility in the Morris Water Task
As expected, differences in latency or path length during initial acquisition of the MWT across treatment groups were not observed. Female rats had longer path lengths and increased latencies compared to male rats, although all rats performed to criteria by the beginning of day 2. Regardless of prenatal treatment or sex, all animals successfully learned to navigate to the hidden goal location. These results replicate the findings of multiple studies [20, 66, 74] and indicate that the behavioral effects of PAE observed after training are not due to inadequate training or baseline differences. Additionally, based on latency and path length all rats acquired the reversed location of the hidden platform at a similar rate across sexes, prenatal treatments, and housing conditions. Perseveration errors (visits to the initial goal location) during the last block of the reversal phase were increased in all PAE males and in mixed-housed control males compared to the same-housed control males. The outcomes in mixed-house PAE males were comparable to that same-housed PAE males, indicating no benefit of housing with a control partner. Further, control mixed-housed males displayed perseveration errors comparable to PAE rats, indicating that housing with a PAE rat results in decreased flexible behavior. All females displayed similar perseveration behaviors across treatment and housing conditions, thus, effects of PAE and housing are limited to males.
4.4 Tongue Protrusion
Whishaw and Kolb [78] determined that selective lesions to the ventrolateral frontal cortex lead to significant deficits in tongue protrusion, compared to lesions of the motor cortex, lateral hypothalamus, medial frontal cortex, and parietal cortex. These findings motivated our investigation of TP to determine if housing affects other behaviors associated with these neural circuits. Deficits in TP in PAE males were observed compared to control males, but no differences were observed in the females across treatment groups. There were different overall effects of housing in males and females. Mixed-housed males displayed deficits in TP compared to same-housed males, whereas mixed-housed females displayed increased TP compared to same-housed females. Similar to the findings for social behavior and spatial flexibility, mixed-housed saccharin males had almost identical TP measures as the same-housed PAE males, indicating that the negative effects of social housing with a PAE partner span several behavioral domains.
4.5 Limitations & Future Directions
The effects of moderate PAE on social behavior in males has been replicated by our laboratory several times [19, 20, 24]. It is, however, important to reiterate that the data from the 12 males in the same housing condition were previously reported as part of a separate, concurrent study on age effects and PAE [20]. Therefore, these data should not be considered an independent replication of the behavioral effects of moderate PAE reported in [20]. Importantly, the effects of PAE in the mixed-housed males were similar to that of same-housed males, and can be considered a replication of our previous results [20].
The present study focused on the effects of moderate PAE, sex, and social housing on social, motor, and flexible spatial behaviors in adulthood (~4 months of age). Future studies conducted in adolescence or additional stages of adulthood may help identify age-dependent effects of social housing manipulations on behavior. The potential causal factors by which social housing yields the diverse consequences described here also need to be explored. If deficits in social behavior following PAE are present early in development (e.g., soon after weaning and paired housing), then the accrued effects of prolonged experience in the mixed-housing condition may be an important factor to consider in future research. The work presented here did not include evaluation of social behavior in the home-cage over the course of the housing manipulation required to address this issue. Though longitudinal analyses of social interactions are challenging and rarely undertaken [67], such analyses can yield considerable insight into the accrued effects of social experience [61] and will be important for identifying potential experiential factors that contribute to the negative outcomes in social, motor, and spatial behavior observed following PAE or housing with a PAE cage-mate.
Importantly, the negative outcomes observed in control rats here were largely specific to behavioral outcomes that depend on the ventrolateral prefrontal cortex including increased spatial perseveration errors, impaired tongue protrusion, and alterations in some aspects social behavior. There were no effects on spatial learning in the water task or for some aspects of social behavior, as might be expected if the housing manipulations resulted in changes that affected behavior more broadly (e.g., stress). Studies targeting the experiential and neural bases of these effects are planned.
An additional variable that should be considered in future studies is the number of cage-mates utilized in the housing manipulation, as housing with multiple cage-mates might yield positive effects or otherwise amplify the effects of social housing. For example, the contrast between negative effects of paired-housing on ethanol-exposed and control rats in the present study and some positive effects of housing ethanol-exposed rats in triads with two control animals observed by Middleton et al. [51] suggests that the number of cage-mates may be a critical factor that should be explored in future studies. A number of studies have demonstrated that the presence of other rats can have a positive influence on physiological and behavioral responses to distress—a behavioral phenomenon known as social buffering [28, 32, 33, 56, 68, 69]. The present data suggest that adult PAE male rats do not benefit from social housing, however, whether PAE negatively impacts social buffering early in development and/or whether these acute manipulations of the social environment persist into adulthood has not been examined. Considering that social buffering is associated with positive outcomes in models of anxiety, stress and drug addiction, the investigation of social buffering represents an important line of future research on the consequences of moderate PAE. Because frontal cortex function has been implicated in altered perseveration errors [21], tongue protrusion [79], and social behavior [3, 19, 22, 36], future research should investigate distinct frontal circuits implicated in these behaviors, including the agranular insular cortex, lateral orbital frontal cortex, and prelimbic cortex to elucidate the functional contributions of these circuits to the behaviors in question.
4.6 Summary
The present findings indicate that the effects of moderate PAE on social behavior, motor behavior (tongue protrusion), and spatial response preservation errors are primarily limited to male rats and are not ameliorated by prolonged housing with a non-ethanol exposed animal. These findings are consistent with previous studies from our laboratory where we observed increased wrestling in PAE males and alterations in social sniffing in PAE animals [19]. When housed with a PAE animal, male control animals displayed deficits not only in social behavior, but also in motor behavior and spatial response preservation errors, indicating that the negative effect of prolonged housing with a PAE animal (i.e., mixed housing) has broad behavioral consequences beyond the social domain. These results indicate that control animals are either adversely affected, or, at least with respect to social behavior, adapt their behavior to that of PAE rats. Collectively, these findings provide further evidence that moderate PAE alters social behavior, and these effects may have negative consequences for social partners. Thus, experiential treatments that involve social experience must consider potential negative effects of housing with PAE animals on the behavior of critical comparison groups.
Highlights.
Prenatal alcohol exposure negatively affects social, motor, and spatial behavior
Robust behavioral effects of ethanol exposure were only observed in males
Housing with a non-exposed control rat did not benefit ethanol-exposed males
Housing with an ethanol-exposed partner negatively affected non-exposed males
Acknowledgments
SUPPORT: Support provided by grant AA019462 to DAH and AA019884 to DDS. BCF is supported by grants UL1TR001449 and KL2TR001448.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Because the data were collected by the same experimenters at the same time as those of the prior report, and the animals were obtained from the same litters and breeding rounds, there is no reason to expect differential effects other than those related to the variables that were directly manipulated in the present study.
References
- 1.Autti-Rämö I, Granström ML. The psychomotor development during the first year of life of infants exposed to intrauterine alcohol of various duration. Fetal alcohol exposure and development. Neuropediatrics. 1991;22:59–64. doi: 10.1055/s-2008-1071418. [DOI] [PubMed] [Google Scholar]
- 2.Barnett SA. A study in behaviour: Principles of ethology and behavioural physiology displayed mainly in the rat. London: 1963. [Google Scholar]
- 3.Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The Role of the Medial Prefrontal Cortex in the Play Fighting of Rats. Behav Neurosci. 2009;123:1158–1168. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- 4.Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Berman RF, Hannigan JH, Sperry MA, Zajac CS. Prenatal alcohol exposure and the effects of environmental enrichment on hippocampal dendritic spine density. Alcohol. 1996;13:209–216. doi: 10.1016/0741-8329(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 6.Bird CW, Candelaria-Cook FT, Magcalas CM, Davies S, Valenzuela CF, Savage DD, et al. Moderate Prenatal Alcohol Exposure Enhances GluN2B Containing NMDA Receptor Binding and Ifenprodil Sensitivity in Rat Agranular Insular Cortex. Plos One. 2015:10. doi: 10.1371/journal.pone.0118721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns R, Burns R. Business Research Methods and Statistics using SPSS. London: Sage Publications Ltd; 2008. [Google Scholar]
- 8.Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent Rearing Conditions Influence the Relationship Between Initial Anxiety-Like Behavior and Ethanol Drinking in Male Long Evans Rats. Alcohol Clin Exp Res. 2013;37:E394–E403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chasnoff IJ, Wells AM, Telford E, Schmidt C, Messer G. Neurodevelopmental functioning in children with FAS, pFAS, and ARND. Journal of developmental and behavioral pediatrics : JDBP. 2010;31:192–201. doi: 10.1097/DBP.0b013e3181d5a4e2. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic; 1988. [Google Scholar]
- 11.Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcoholism, clinical and experimental research. 1990;14:650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 12.Cullen CL, Burne THJ, Lavidis NA, Moritz KM. Low Dose Prenatal Ethanol Exposure Induces Anxiety-Like Behaviour and Alters Dendritic Morphology in the Basolateral Amygdala of Rat Offspring. Plos One. 2013:8. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullen CL, Burne THJ, Lavidis NA, Moritz KM. Low Dose Prenatal Alcohol Exposure Does Not Impair Spatial Learning and Memory in Two Tests in Adult and Aged Rats. Plos One. 2014:9. doi: 10.1371/journal.pone.0101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz MR, Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol on gestational day 12 elicits opposing deficits in social behaviors and anxiety-like behaviors in Sprague Dawley rats. Behav Brain Res. 2016;310:11–19. doi: 10.1016/j.bbr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disney ER, Iacono W, McGue M, Tully E, Legrand L. Strengthening the case: Prenatal alcohol exposure is associated with increased risk for conduct disorder. Pediatrics. 2008;122:E1225–E1230. doi: 10.1542/peds.2008-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianoulakis C. Rats exposed prenatally to alcohol exhibit impairment in spatial navigation test. Behav Brain Res. 1990;36:217–228. doi: 10.1016/0166-4328(90)90060-r. [DOI] [PubMed] [Google Scholar]
- 17.Goodlett CR, Kelly SJ, West JR. Early postnatal alcohol exposure that produces high blood-alcohol levels impairs development of spatial navigation learning. Psychobiology. 1987;15:64–74. [Google Scholar]
- 18.Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J. Social Cognitive and Emotion Processing Abilities of Children With Fetal Alcohol Spectrum Disorders: A Comparison With Attention Deficit Hyperactivity Disorder. Alcohol Clin Exp Res. 2009;33:1656–1670. doi: 10.1111/j.1530-0277.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, et al. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behavioural brain research. 2014;269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton DA, Brigman JL. Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes Brain and Behavior. 2015;14:4–21. doi: 10.1111/gbb.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, et al. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav Brain Res. 2010;214:66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton DA, Magcalas CM, Barto D, Bird CW, Rodriguez CI, Fink BC, et al. Moderate Prenatal Alcohol Exposure and Quantification of Social Behavior in Adult Rats. Journal of Visualized Experiments. 2014:94. doi: 10.3791/52407. article number e52407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannigan JH, O'Leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neuroscience and biobehavioral reviews. 2007;31:202–211. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Heck DH, Roy S, Xie N, Waters RS. Prenatal alcohol exposure delays acquisition and use of skilled reaching movements in juvenile rats. Physiol Behav. 2008;94:540–544. doi: 10.1016/j.physbeh.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Himmler SM, Himmler BT, Pellis VC, Pellis SM. Play, variation in play and the development of socially competent rats. Behaviour. 2016 in press. [Google Scholar]
- 28.Hodges TE, Green MR, Simone JJ, McCormick CM. Effects of social context on endocrine function and Zif268 expression in response to an acute stressor in adolescent and adult rats. Int J Dev Neurosci. 2014;35:25–34. doi: 10.1016/j.ijdevneu.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Kelly SJ, Dillingham RR. Sexually dimorphic effects of perinatal alcohol exposure on social interactions and amygdala DNA and DOPAC concentrations. Neurotoxicol Teratol. 1994;16:377–384. doi: 10.1016/0892-0362(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 30.Kelly SJ, Goodlett CR, Hannigan JH. Animal models of fetal alcohol spectrum disorders: Impact of the social environment. Dev Disabil Res Rev. 2009;15:200–208. doi: 10.1002/ddrr.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol Teratol. 1997;19:383–389. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 32.Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiyokawa Y, Hiroshima S, Takeuchi Y, Mori Y. Social buffering reduces male rats' behavioral and corticosterone responses to a conditioned stimulus. Horm Behav. 2014;65:114–118. doi: 10.1016/j.yhbeh.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neuroscience and biobehavioral reviews. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Kodituwakku PW, May PA, Clericuzio CL, Weers D. Emotion-related learning in individuals prenatally exposed to alcohol: an investigation of the relation between set shifting, extinction of responses, and behavior. Neuropsychologia. 2001;39:699–708. doi: 10.1016/s0028-3932(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 36.Kolb B. Social-behavior of rats with chronic prefrontal lesions. Journal of Comparative and Physiological Psychology. 1974;87:466–474. doi: 10.1037/h0036969. [DOI] [PubMed] [Google Scholar]
- 37.Kolb B, Sutherland RJ, Whishaw IQ. A comparison of the contribution of frontal and parietal association cortex to spatial localization in rats. Behav Neurosci. 1983;97:13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013:4. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larkby CA, Goldschmidt L, Hanusa BH, Day NL. Prenatal Alcohol Exposure Is Associated With Conduct Disorder in Adolescence: Findings From a Birth Cohort. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:262–271. doi: 10.1016/j.jaac.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquardt K, Brigman JL. The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models. Alcohol. 2016;51:1–15. doi: 10.1016/j.alcohol.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattson SN, Crocker N, Tanya TN. Fetal Alcohol Spectrum Disorders: Neuropsychological and Behavioral Features. Neuropsychology Review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- 43.Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, et al. Further development of a neurobehavioral profile of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2013;37:517–528. doi: 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maxwell SE, Delaney HD. Designing experiments and analyzing data : a model comparison perspective. 2nd. Mahwah, N.J.: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- 45.May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcoholism, clinical and experimental research. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.May PA, Fiorentino D, Coriale G, Kalberg WO, Hoyme HE, Aragon AS, et al. Prevalence of children with severe fetal alcohol spectrum disorders in communities near Rome, Italy: new estimated rates are higher than previous estimates. International journal of environmental research and public health. 2011;8:2331–2351. doi: 10.3390/ijerph8062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental disabilities research reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- 49.McGee CL, Bjorkquist OA, Price JM, Mattson SN, Riley EP. Social Information Processing Skills in Children with Histories of Heavy Prenatal Alcohol Exposure. Journal of Abnormal Child Psychology. 2009;37:817–830. doi: 10.1007/s10802-009-9313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGee CL, Fryer SL, Bjorkquist OA, Mattson SN, Riley EP. Deficits in social problem solving in adolescents with prenatal exposure to alcohol. Am J Drug Alcohol Abuse. 2008;34:423–431. doi: 10.1080/00952990802122630. [DOI] [PubMed] [Google Scholar]
- 51.Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Dev Neurosci. 2012;34:115–128. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: Effects of age, sex, and timing of exposure. Behav Brain Res. 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen TT, Ashrafi A, Thomas JD, Riley EP, Simmons RW. Children with heavy prenatal alcohol exposure have different frequency domain signal characteristics when producing isometric force. Neurotoxicol Teratol. 2013;35:14–20. doi: 10.1016/j.ntt.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen TT, Levy SS, Riley EP, Thomas JD, Simmons RW. Children with Heavy Prenatal Alcohol Exposure Experience Reduced Control of Isotonic Force. Alcohol Clin Exp Res. 2013;37:315–324. doi: 10.1111/j.1530-0277.2012.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pellis SM, Hastings E, Shimizu T, Kamitakahara H, Komorowska J, Forgie ML, et al. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and nonplayful social contexts. Behav Neurosci. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- 56.Pentkowski NS, Painter MR, Thiel KJ, Peartree NA, Cheung TH, Deviche P, et al. Nicotine-induced plasma corticosterone is attenuated by social interactions in male and female adolescent rats. Pharmacol Biochem Behav. 2011;100:1–7. doi: 10.1016/j.pbb.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley EP, Infante MA, Warren KR. Fetal Alcohol Spectrum Disorders: An Overview. Neuropsychology Review. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roebuck-Spencer TM, Mattson SN, Marion SD, Brown WS, Riley EP. Bimanual coordination in alcohol-exposed children: role of the corpus callosum. Journal of the International Neuropsychological Society : JINS. 2004;10:536–548. doi: 10.1017/S1355617704104116. [DOI] [PubMed] [Google Scholar]
- 59.Roozen S, Peters G-JY, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res. 2016;40:18–32. doi: 10.1111/acer.12939. [DOI] [PubMed] [Google Scholar]
- 60.Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, et al. Effects of a Novel Cognition-Enhancing Agent on Fetal Ethanol-Induced Learning Deficits. Alcohol Clin Exp Res. 2010;34:1793–1802. doi: 10.1111/j.1530-0277.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider P, Patz M, Spanagel R, Schneider M. Adolescent social rejection alters pain processing in a CB1 receptor dependent manner. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2016;26:1201–1212. doi: 10.1016/j.euroneuro.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Simmons RW, Thomas JD, Levy SS, Riley EP. Motor response programming and movement time in children with heavy prenatal alcohol exposure. Alcohol. 2010;44:371–378. doi: 10.1016/j.alcohol.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose-response effect. Pediatrics. 2001;108 doi: 10.1542/peds.108.2.e34. art. no.-e34. [DOI] [PubMed] [Google Scholar]
- 64.Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. Jama. 1991;265:1961–1967. [PubMed] [Google Scholar]
- 65.Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcoholism, clinical and experimental research. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 66.Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on learning and memory in adult offspring. Psychobiology. 2000;28:532–539. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 67.Taborsky B. Opening the Black Box of Developmental Experiments: Behavioural Mechanisms Underlying Long-Term Effects of Early Social Experience. Ethology. 2016;122:267–283. [Google Scholar]
- 68.Terranova ML, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology. 1999;24:639–656. doi: 10.1016/s0306-4530(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 69.Thiel KJ, Painter MR, Pentkowski NS, Mitroi D, Crawford CA, Neisewander JL. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 2012;17:365–377. doi: 10.1111/j.1369-1600.2011.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcoholism: Clinical and Experimental Research. 1998;22:528–533. [PubMed] [Google Scholar]
- 72.Tunc-Ozcan E, Ullmann TM, Shukla PK, Redei EE. Low-dose thyroxine attenuates autism-associated adverse affects of fetal alcohol in male offspring's social behavior and hippocampal gene expression. Alcohol Clin Exp Res. 2013;37:1986–1995. doi: 10.1111/acer.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends in neurosciences. 2012;35:284–292. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varaschin RK, Akers KG, Rosenberg MJ, Hamilton DA, Savage DD. Effects of the Cognition-Enhancing Agent ABT-239 on Fetal Ethanol-Induced Deficits in Dentate Gyrus Synaptic Plasticity. J Pharmacol Exp Ther. 2010;334:191–198. doi: 10.1124/jpet.109.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varlinskaya EI, Mooney SM. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behav Brain Res. 2014;261:106–109. doi: 10.1016/j.bbr.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ware AL, O'Brien JW, Crocker N, Deweese BN, Roesch SC, Coles CD, et al. The Effects of Prenatal Alcohol Exposure and Attention-Deficit/Hyperactivity Disorder on Psychopathology and Behavior. Alcohol Clin Exp Res. 2013;37:507–516. doi: 10.1111/j.1530-0277.2012.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wellmann KA, George F, Brnouti F, Mooney SM. Docosahexaenoic acid partially ameliorates deficits in social behavior and ultrasonic vocalizations caused by prenatal ethanol exposure. Behavioural Brain Research. 2015;286:201–211. doi: 10.1016/j.bbr.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whishaw IQ, Kolb B. Stick out your tongue: Tongue protrusion in neocortex and hypothalamic damaged rats. Physiology and Behavior. 1983;30:471–480. doi: 10.1016/0031-9384(83)90154-3. [DOI] [PubMed] [Google Scholar]
- 79.Whishaw IQ, Kolb B. Tongue protrusion mediated by spared anterior ventrolateral neocortex in neonatally decorticate rats : Behavioral support for the neurogenetic hypothesis. Behav Brain Res. 1989;32:101–113. doi: 10.1016/s0166-4328(89)80078-6. [DOI] [PubMed] [Google Scholar]
- 80.Williams L, Jackson CPT, Choe N, Pelland L, Scott SH, Reynolds JN. Sensory-motor deficits in children with Fetal Alcohol Spectrum Disorder assessed using a robotic virtual reality platform. Alcohol Clin Exp Res. 2014;38:116–125. doi: 10.1111/acer.12225. [DOI] [PubMed] [Google Scholar]


