Abstract
Introduction
Severe burns trigger a hyperdynamic state, necessitating accurate measurement of cardiac output (CO) for cardiovascular observation and guiding fluid resuscitation. However, it’s unknown whether, in burned children, the increasingly popular transthoracic echocardiography (TTE) method of CO measurement is as accurate as the widely used transpulmonary thermodilution (TPTD) method.
Materials and Methods
We retrospectively compared near-simultaneously performed CO measurements in severely burned children using TPTD with the PiCCO (Pulse index Continuous Cardiac Output) system or TTE. Outcomes were compared using t-tests, multiple linear regression, and a Bland-Altman plot.
Results
Fifty-four children (9 ± 5 years) with 68 ± 18% total body surface area burns were studied. An analysis of 105 data pairs revealed that PiCCO yielded higher CO measurements than TTE (190 ± 39% vs. 150 ± 50% predicted values; p < 0.01). PiCCO- and TTE-derived CO measurements correlated moderately well (R2 = 0.54, p < 0.01). A Bland-Altman plot showed a mean bias of 1.53 L/minute with a 95% prediction interval of 4.31 L/minute.
Conclusions
TTE-derived estimates of CO may underestimate severity of the hyperdynamic state in severely burned children. We propose using the PiCCO system for objective cardiovascular monitoring and to guide goal-directed fluid resuscitation in this population.
Keywords: cardiac output, cardiac index, burns, transthoracic echocardiography, transpulmonary thermodilution, hypermetabolism
Introduction
Severe burn injuries exceeding 30% of the total body surface area (TBSA) are marked by an acute inflammatory response occurring alongside with catabolism and hypermetabolism. Fluid shifts and a vast increase in proinflammatory cytokine output lead to hemodynamic instability and decreases in systemic vascular resistance (1). Generalized edema and transdermal fluid loss further exacerbate the systemic response to burn injury. Improvements in intensive care such as adequate fluid resuscitation, real-time monitoring, and early excision of the burn eschar followed by immediate wound coverage have greatly reduced mortality in burned children (2,3). Close monitoring of hemodynamic status during burn shock resuscitation (4) is needed to prevent alterations in afterload and myocardial depression (5–7). Cardiac output (CO) shifts from a depressed state in the early phase (24 to 28 hours postburn) to an elevated state (two- to three-fold increase) in the hyperdynamic phase (>72 hours postburn) (1,4). Furthermore, our earlier investigations have shown that the hyperdynamic state continues throughout acute hospitalization in children with severe burn injury (8). We have also demonstrated that the hypermetabolic response persists for up to 2 years after the initial injury (9).
Hemodynamic monitoring via pulmonary artery catheters (PACs) has become a standard practice in critical care over the past decades (10). PACs are used for continuous measurement of oxygen delivery and consumption, central venous pressure (CVP), CO (and by extension, cardiac index [CI]), systemic vascular resistance index (SVRI), pulmonary artery occlusion pressure (PAOP), and pulmonary capillary wedge pressure (PCWP). However, placement of a PAC into the right heart is an invasive procedure and can cause cardiopulmonary complications (10–13). Introduction of the less-invasive transpulmonary thermodilution (TPTD) method using the Pulse Index Cardiac Output system (PiCCO, Pulsion Medical Systems, Munich, Germany) has achieved new standards in the hemodynamic observation of critically ill patients (4,14–17). The PiCCO system provides beat-to-beat measurements of mean arterial pressure (MAP), CO/CI, and SVRI. During each thermal injection measurement, the PiCCO system provides a new derived transpulmonary thermodilution CO that approximates global end-diastolic volume (GEDV; reflects cardiac preload). This system also provides extravascular lung water index (EVLW), a marker for pulmonary edema (18). Our group previously used the PiCCO system to document that hyperdynamic circulation begins one week following a severe burn and lasts throughout the acute hospitalization period (8). Due to the fact that TPTD is less-invasive than PAC, and the adaptability of the various measurable parameters, the PiCCO system has been established as standard-of-care for hemodynamic observation in severely burned pediatric patients (> 30% TBSA burned) at our burn center.
Medical imaging and new developments in the field of ultrasonography have allowed physicians to implement transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) methods in order to determine whether hemodynamic instability is attributable to cardiac or non-cardiac causes (19). Several studies have shown that echocardiography is a reliable noninvasive method that can be used to guide resuscitation efforts and assess cardiac function in critically ill burn patients (15,19–21). Echocardiography is becoming a standard-of-care in many intensive care centers, and more clinicians are learning how to perform this technique and interpret echocardiographic images. Therefore, the discussion of which hemodynamic monitoring system should be used is more pertinent than ever (22). To date, TTE and TPTD methods of cardiovascular monitoring have not been compared in severely burned children. Here we assessed differences between these two widely used methods for CO measurement during the acute ICU stay in pediatric patients with severe burns.
Methods
Study design and patients
Over a two year period we retrospectively studied a cohort of pediatric patients admitted to Shriners Hospitals for Children®—Galveston. The Institutional Review Board at the University of Texas Medical Branch (Galveston, TX) approved this study (protocol #15-0074). Inclusion criteria were defined as follows: age < 18 years, TBSA burn size of ≥ 40%, and a burn to admission time of ≤ 72 hours. Patients were excluded from the study if they had any prior history of cardiopulmonary illness (e.g., ventricular or atrial septal defect, aortic or pulmonic stenosis, or atrioventricular canal defect).
The standard protocol of care at our burn hospital includes determining weight at admission and calculating all indexed values derived from percent TBSA burned at admission and individual body surface area (BSA). Sedation, analgesia, and mechanical ventilation were performed according to our institutional guidelines. Early total burn wound excision of the necrotized tissue and skin grafting were performed between 48 and 72 hours after injury. Further skin grafting procedures were performed until all wounds were covered with autograft or homograft in weekly intervals.
Length of cumulative hospital stay, type of burn, BSA (m2), percent TBSA burned, percent TBSA with third-degree (full-thickness) burns, number of TTE and PiCCO (TPTD) measurements, CO (L/min), age-predicted CO, CO expressed as a percent above age-predicted CO, and CI (L/min/m2) were recorded. Weight was measured at hospital admission and release with monthly-calibrated, standard clinical scales.
Cardiac output measurements
At admission, central venous lines (inferior or superior vena cava) and arterial lines (brachial, radial, or femoral artery) were placed in all patients. A Pulsiocath 3- or 4-French thermistor-tipped catheter (Pulsion Medical Systems, Munich, Germany) was used to collect TPTD (PiCCO) measurements. Near simultaneously performed TTE and TPTD measurements (± 15 minutes between each cardiac output measurement) were included in the current analysis.
To measure CI and CO with the PiCCO system, we cooled 10 mL of saline solution to 0°C to 6°C and administered it into the central venous catheter. Saline was manually injected, without regard to the respiratory cycle. PiCCO measurements were repeated three times in a row within 10 minutes. Final analysis was performed on the mean of the three values. Heart rate (HR), mean arterial pressure (MAP), and CVP were recorded directly via hardware at each point of injection. All recorded PiCCO data were exported to a computer that was connected to the Pulsion PICCOPlus device (PC 8100 software version V6.0; Pulsion Medical Systems, Munich, Germany) and loaded with PICCO-VoLEF-WIN software (version 4.0; Pulsion Medical Systems).
Transthoracic echocardiography was performed by a certified echocardiographer. Patients studied, were age < 18 years old, did not have pre-existing cardiac disease e.g., ventricular septal defect and generally lean (BMI<25), which allowed for good imaging for two and four-chamber apical view. A 3.5 MHz transducer probe and ultrasound system (SonoSite Titan ultrasound, Fujifilm, Tokyo, Japan) was used to determine end-diastolic volume (EDV) and end-systolic volume (ESV). Specific measurements were performed in the parasternal LV long axis view. The left ventricular (LV) area and length were traced during end diastole and end systole. The modified Simpson’s rule was applied for calculating EDV, ESV, stroke volume (SV) as, EDV – ESV and Ejection Fraction (EF%) as, (EDV-ESV)/EDV. Several cardiac cycles (over 3-5 seconds) were digitally sampled and measurements were obtained at end-expiration. CO and CI were then calculated as follows:
Statistical analysis
Patients’ specific measurements were collected in an encrypted spreadsheet in Excel (Microsoft, Richmond, VA, USA). Statistical analyses were performed using SigmaSat Version 4.0 and SigmaPlot 11 (Systat Software Inc., San Jose, CA, USA). CO and CI values from both devices were compared using a Student’s t-test for matched pair samples. Multiple linear regression analysis and Bland-Altman plots were also used as appropriate. Significance was accepted at p < 0.05.
Results
We included 54 severely burned children into our cohort study. Demographics of all included patients are summarized in Table 1.
Table 1.
Patient Characteristics
| Parameter | N = 54 |
|---|---|
| Age, years | 9 ± 5 |
| Sex, males (%) | 41 (76) |
| Burn type, n (%) | |
| Electrical | 4 (7) |
| Flame | 41 (76) |
| Scald | 9 (17 |
| BSA, m2 | 1.1 ± 0.4 |
| TBSA burn, % | 68 ± 18 |
| TBSA third, % | 56 ± 24 |
| LOS (Survivors), d | 35 ± 22 |
Data presented as mean ± standard deviation or count (percentage). BSA: Body Surface Area, TBSA: Total Body Surface Area, LOS: Length of Stay
CO was measured during two separate encounters for each patient. In the complete cohort, a total of 105 paired CO measurements were collected using each cardiovascular monitoring method— TTE and the PiCCO system (Table 2). Thus, a total of 210 measurements were performed using both methods. The mean CO measured with the PiCCO system was 1.5 L/min above the CO measured with TTE (p < 0.01). Furthermore, CI measured with the PiCCO was, on average, 1.4 L/min/m2 higher than measurements obtained with TTE (p < 0.01). Compared to the age predicted CO (20), mean values derived from both methods were elevated throughout the cohort. CI values in this study group were significantly higher than age-predicted values for nonburned, healthy children.
Table 2.
Cardiovascular Parameters Measured Using TTE and PiCCO (TPTD)
| Cardiovascular Parameter | PiCCO | TTE | p value |
|---|---|---|---|
| Measurements, n | 105 | 105 | -- |
| CO, L/m | 7.6 ± 3.0 | 6.1 ± 2.7 | < 0.001 |
| Coefficient of Variation | 0.4 | 0.5 | -- |
| Age-Predicted CO (L/m) | 4.0 ± 1.4 | -- | |
| % Above Predicted CO | 88 ± 39 | 50 ± 50 | < 0.001 |
| CI, L/min/m2 | 7.1 ± 1.4 | 5.7 ± 1.7 | < 0.001 |
| Normal Value of CI, L/min/m2 | 3.5 ± 0.7 | -- |
Data presented as mean ± standard deviation. CO: Cardiac Output, CI: Cardiac Index
As shown in Figure 1, an adjusted R2 of 0.54 showed moderate-to-good correlation between the two cardiovascular-monitoring methods. Multiple linear regression analysis showed that the dependent variable “Cardiac Output PiCCO” could be predicted from a linear combination of the independent variables “Cardiac Output TTE” (p = 0.006) and “Age” (p < 0.01), while the dependent variable “Burn Size” did not contribute to the prediction (p = 0.73). The regression equation is as follows:
The plot showed an improved correlation (R2 of 0.7538 when the age-corrected value for TTE was used (Figure 2).
Figure 1.
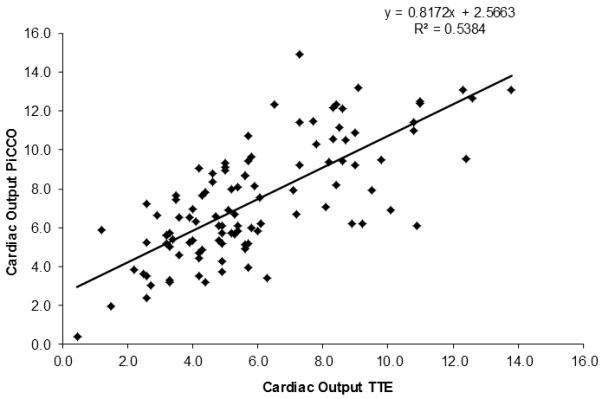
CO measured with PiCCO (y-axis) and TTE (x-axis) show moderate-to-good correlation.
Figure 2.
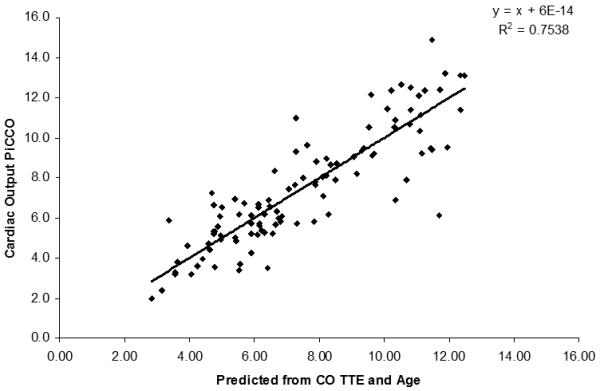
Predicted CO values from TTE (x-axis) and CO measured with PiCCO (y-axis) show an improved correlation.
Bland-Altman plots with mean PiCCO CO minus CO TTE on the x-axis and CO PiCCO minus CO TTE on the y-axis showed a mean bias of 1.53 L/min with a 95% prediction interval of 4.31 L/min (Figure 3).
Figure 3.
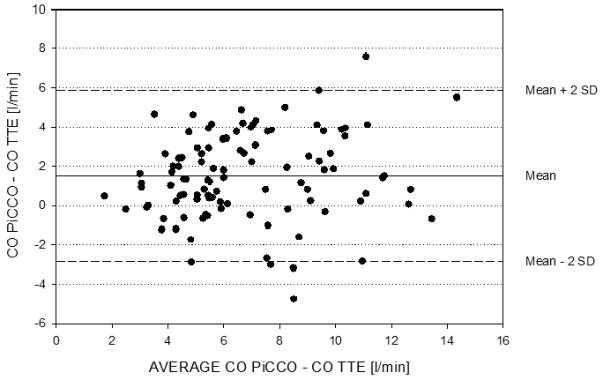
Bland-Altman plot of the two measurement methods shows a mean bias of 1.53 L/min with a 95% prediction interval of 4.31 /minute.
Discussion
Over the past several decades, methods of monitoring critically ill patients have ranged from invasive (pulmonary catheterization), partially invasive (transpulmonary thermodilution), and non-invasive (echocardiography) (22). In 2011, Branski and colleagues (8) showed that close monitoring of the hyperdynamic pediatric burn patient is crucial for improving morbidity and that accurate measurement of CO is beneficial for assessing cardiovascular status. Therefore, our study focused on comparing the accuracy of the non-invasive TTE with that of the partially-invasive PiCCO system in assessing cardiovascular parameters in critically ill pediatric burn patients. This study clearly showed that, in the pediatric burn setting, marked differences exist in CO and CI measurements derived from the TTE and the PiCCO system.
Modern medical imaging, such as TTE, has become one of the most common procedures for bedside cardiac evaluation of critically ill patients. Newer technologies have provided higher-resolution and more user-friendly ultrasonography devices, increasing the popularity of these products for routine clinical care. Ventricular size, wall thickness, and size of great vessels are the main parameters incorporated using an ultrasound machine. Furthermore, evaluation of heart valvular disease and calculation of ventricular function (e.g., stroke volume, CO, and CI) are possible. Ultrasonography measurement of the pediatric heart can be a challenge, even for skilled and experienced technicians. This is attributable to poorly demarcated heart borders (particularly during 2D axis measurement) and to the fact that cardiac shape is different in children than in adults. Our findings showed that, although TTE is generally a helpful tool for assessing cardiac status, it can lead to inaccurate calculated and estimated values. This may be due to lack of experience by the user and morphological differences in the studied population. Indeed, this study was limited by the fact that the ultrasound instrument that was not of the highest quality and we relied upon trained ultrasonographers to perform and interpret exams without experienced oversight, as has been done in other studies (21). An additional limitation was that we could view only a single cardiac cycle measured by a 2D picture; this did not provide an ideal image. Typically, three cardiac cycles are used and averaged to determine EDV and ESV measurements and calculate CO. Because monitoring hemodynamic status is crucial to detecting early burn-associated cardiac heart failure (8,21,23,24), a subjective method such as TTE may not be suitable for assessing myocardial function.
Since its introduction by the German Ulrich Pfeiffer in the 1980s, the PiCCO system has become a well-established tool for hemodynamic observation in critically ill adults (25) and children (15,26,27). The PiCCO system is based on the principals of TPTD. Briefly, patients receive a bolus of cooled saline in a central venous catheter, and a thermistor-tipped arterial catheter detects the change in blood temperature (18). In light of the fact that all of the patients in our burns ICU have both venous and arterial access, patients undergoing PiCCO examination have comparable risk to those who do not receive PiCCO examination. In fact, no PiCCO-related complications were observed in this study. Our assessment of correlation between PiCCO and TTE measurements revealed a mean difference of 1.53 L/min, with higher CO being observed with the PiCCO system. This result could be due to the objective nature of TPTD measurements. TPTD measurements are user independent and do not require expertise once arterial and venous access has been obtained and the system has been calibrated. Specifically, ancillary staff can easily perform TPTD bolus injections. In contrast, TTE measurements are more subjective and are highly dependent on the ultrasound technique and/or parameters such as inadequate scan angle (e.g., foreshortened chamber reducing volumetric calculations).
Measuring ongoing hemodynamic changes accurately is of the utmost importance, as it prevents over- and under-resuscitation during acute burn shock resuscitation. Previous studies have shown that the TPTD system can be beneficial in this regard (4,16). For fluid resuscitation within the acute phase of the burn trauma (24 up to 48 hours), many formulae and estimation methods haven been described and critically discussed (28–30). Nevertheless, the needs of the individual patient should be met by assessing the urinary output (1.5 mL/kg/h for burn victims weighing less than 30 kg (31)). As an alternative to urinary output, volumetric measurements can be determined through cardiovascular monitoring and serve as pivotal determinants of appropriate resuscitation (4,16). A fine balance must be struck between under-resuscitation leading to hypovolemia and tissue hypoxia, and over-resuscitation leading to “fluid creep”(32), massive edema, and organ failure. In a randomized clinical trial of 50 burn patients, Holm and colleagues compared traditional resuscitation using the Baxter formula (4 mL/kg body weight/% BSA burn) to resuscitation guided by TPTD system according to a preload endpoint (33). The Baxter formula resulted in significant under-resuscitation compared to goal-directed therapy using TPTD. Thus, all burn fluid resuscitation formulas should be considered as recommended guidelines, while the actual volume of fluid used during resuscitation must be based on individual needs and physiologic parameters, such as TPTD-derived CO (31,34). TPTD has also been shown to be particularly helpful in avoiding “fluid creep.” In a prospective trial, Sanchez et al.(4) showed that the initial hypovolemia during early burn shock resuscitation can be detected with TPTD, whereas hourly urinary output and MAP can mask ongoing hemodynamic states.
The greatest limitation of the current analysis is the retrospective design of the study. The retrospective analysis allowed us to compare near simultaneously measured CO values using TTE and PiCCO. Thus, future aims for studies are to determine the accuracy of PiCCO and TTE values to PAC values. At our institution, both the PiCCO system and TTE are used, and we compared CO measurements because both studied techniques provide this value. The PiCCO system allows the observation of continuous CO trends in severely burn children. However, continuous PiCCO measurements must be seen as trends rather than actual values. This was the reason why we compared PiCCO values during thermodilution to the non-continuous TTE measurements. Unfortunately, TTE does not provide continuous trends for CO measurements, so the compared values have to be seen as two CO assessments, with two techniques, in an intra-patient comparison. We are interested in both the accuracy of the measured values as well as the trends over time, so another future direction would be the comparison of non-invasive CO assessment devices to currently used modalities. Nevertheless, it remains unknown if CO measured with the PiCCO system is an underestimate or if TTE overestimates the patients’ hemodynamic status, and further studies are need to determine the most accurate hemodynamic monitoring tool for ICU patients (22) as well as severely burned children.
A secondary finding of this study was that both TTE and PiCCO revealed an overall increase in CO compared to age-predicted values, highlighting the hyperdynamic cardiac response to severe burn injury. These results corroborate findings from Branski et al.(8), who reported that CO in children with severe burn injury is elevated throughout the whole ICU stay. Elevated CO is a predictor of hyperdynamic circulation caused by general inflammation and the hypermetabolic state induced by severe burns. These findings call for close and accurate observation of the hyperdynamic cardiovascular response in the management of severe burn injury in children.
Conclusions
CO and CI measurements with the PiCCO system match the previously reported results and confirm the ongoing hyperdynamic state of severely burned children. TPTD may provide a more objective way for observing cardiovascular and hyperdynamic states in critically ill pediatric patients and can be performed by inexperienced clinical staff. We propose that this semi-invasive device be used for goal-directed resuscitation in children with severe burn injury.
Acknowledgements
The authors thank the nursing staff at the Pediatric Burns ICU at Shriners Hospitals for Children®—Galveston for their support during the clinical trial. They also thank Dr. Kasie Cole for editing and proofreading of the manuscript.
Funding: This study was supported by grants from Shriners Hospitals for Children (71008, 80100, 84080, 84291), National Institutes of Health (P50-GM060338, R01-GM056687, R01-HD049471 and T32-GM008256), the National Institute of Disability, Independent Living, and Rehabilitation Research (H133A020102 and H133A70019), and the American Surgical Association Foundation.
Abbreviations
- BSA
body surface area
- CI
cardiac index
- CO
cardiac output
- CVP
central venous pressure
- ED
end-diastolic
- ES
end-systolic
- EVLW
extravascular lung water index
- GEDV
global end-diastolic volume
- HR
heart rate
- ICU
intensive care unit
- ITBVI
intrathoracic blood volume index
- MAP
mean arterial pressure
- PAC
pulmonary artery catheter
- PAOP
pulmonary artery occlusion pressure
- PCWP
pulmonary capillary wedge pressure
- PiCCO
pulse index cardiac output
- SVRI
systemic vascular resistance index
- TBSA
total body surface area
- TEE
transesophageal echocardiography
- TPTD
transpulmonary thermodilution
- TTE
transthoracic echocardiography
Footnotes
Authors’ contributions
All authors made substantial contributions to the conception or design of the work (AA, CCF, FJB, LKB, LPK, MGJ, MPK, PW, WBN) or to the acquisition, analysis, or interpretation of data for the work (PW, LKB, GH, MGJ, FNW, DNH) and the drafting of the work or revision it critically for important intellectual content (All authors).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bittner EA, Shank E, Woodson L, Martyn JAJ. Acute and Perioperative Care of the Burn-injured Patient. Anesthesiology. 2015;122(2):448–64. doi: 10.1097/ALN.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan RL, Remensnyder JP, Schnitzer JJ, Schulz JT, Ryan CM, Tompkins RG. Current expectations for survival in pediatric burns. Arch Pediatr Adolesc Med. 2000;154(3):245–9. doi: 10.1001/archpedi.154.3.245. [DOI] [PubMed] [Google Scholar]
- 3.Tompkins RG. Survival from burns in the new millennium: 70 years’ experience from a single institution. Ann Surg. 2015;261(2):263–8. doi: 10.1097/SLA.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez M, García-de-Lorenzo A, Herrero E, Lopez T, Galvan B, Asensio MJ, Cachafeiro L, Casado C. A protocol for resuscitation of severe burn patients guided by transpulmonary thermodilution and lactate levels: a 3-year prospective cohort study. Crit Care. 2013;17(4):R176. doi: 10.1186/cc12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxter CR. Fluid volume and electrolyte changes of the early postburn period. Clin Plast Surg. 1974;1(4):693–703. [PubMed] [Google Scholar]
- 6.Hilton JG, Marullo DS. Effects of thermal trauma on cardiac force of contraction. Burns. 1986;12(3):167–71. doi: 10.1016/0305-4179(86)90154-3. [DOI] [PubMed] [Google Scholar]
- 7.Herndon DN, Barrow RE, Linares HA, Rutan RL, Prien T, Traber LD, Traber DL. Inhalation injury in burned patients: effects and treatment. Burns. 1988;14(5):349–56. doi: 10.1016/0305-4179(88)90002-2. [DOI] [PubMed] [Google Scholar]
- 8.Branski LK, Herndon DN, Byrd JF, Kinsky MP, Lee JO, Fagan SP, Jeschke MG. Transpulmonary thermodilution for hemodynamic measurements in severely burned children. Crit Care. 2011;15(2):R118. doi: 10.1186/cc10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistance of the pathophysiologic response to severe burn injury. PloS One. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993-2004. JAMA. 2007;298(4):423–9. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 11.Connors AF, Speroff T, Dawson NV, Thomas C, Harrell FE, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276(11):889–97. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- 12.Bowdle TA. Complications of invasive monitoring. Anesthesiol Clin N Am. 2002;20(3):571–88. doi: 10.1016/s0889-8537(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 13.Bowdle A. Vascular complications of central venous catheter placement: evidence-based methods for prevention and treatment. J Cardiothorac Vasc Anesth. 2014;28(2):358–68. doi: 10.1053/j.jvca.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Fakler U, Pauli C, Balling G, Lorenz HP, Eicken A, Hennig M, Hess J. Cardiac index monitoring by pulse contour analysis and thermodilution after pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2007;133(1):224–8. doi: 10.1016/j.jtcvs.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Lemson J, de Boode WP, Hopman JCW, Singh SK, van der Hoeven JG. Validation of transpulmonary thermodilution cardiac output measurement in a pediatric animal model. Pediatr Crit Care Med. 2008;9(3):313–9. doi: 10.1097/PCC.0b013e31816c6fa1. [DOI] [PubMed] [Google Scholar]
- 16.Kraft R, Herndon DN, Branski LK, Finnerty CC, Leonard KR, Jeschke MG. Optimized fluid management improves outcomes of pediatric burn patients. J Surg Res. 2013;1(1):121–8. doi: 10.1016/j.jss.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aboelatta Y, Abdelsalam A. Volume overload of fluid resuscitation in acutely burned patients using transpulmonary thermodilution technique. J Burn Care Res. 2013;34(3):349–54. doi: 10.1097/BCR.0b013e3182642b32. [DOI] [PubMed] [Google Scholar]
- 18.PiCCO Technology [Internet] [cited 2015 Feb 20];PULSION Medical Systems. Available from: http://www.pulsion.com/international-english/critical-care/picco-technology/
- 19.Poth JM, Beck DR, Bartels K. Ultrasonography for haemodynamic monitoring. Best Pract Res Clin Anaesthesiol. 2014;28(4):337–51. doi: 10.1016/j.bpa.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Barratt-Boyes BG, Wood EH. Cardiac output and related measurements and pressure values in the right heart and associated vessels, together with an analysis of the hemo-dynamic response to the inhalation of high oxygen mixtures in healthy subjects. J Lab Clin Med. 1958;51(1):72–90. [PubMed] [Google Scholar]
- 21.Howard TS, Hermann DG, McQuitty AL, Woodson LC, Kramer GC, Herndon DN, Ford PM, Kinsky MP. Burn-induced cardiac dysfunction increases length of stay in pediatric burn patients. J Burn Care Res. 2013;34(4):413–9. doi: 10.1097/BCR.0b013e3182685e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsingh D, Alexander B, Cannesson M. Clinical review: Does it matter which hemodynamic monitoring system is used? Crit Care. 2013;17(2):208. doi: 10.1186/cc11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams FN, Herndon DN, Suman OE, Lee JO, Norbury WB, Branski LK, Mlcak RP, Jeschke MG. Changes in cardiac physiology after severe burn injury. J Burn Care Res. 2011;32(2):269–74. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C-Y, Wu C-K, Yeong E-K, Lin H-H, Huang Y-T, Lee J-K, Lin Y-H, Chiang F-T, Tang Y-B, Tsai C-T. Prognostic significance of left ventricular diastolic function in burn patients. Shock. 2012;37(5):457–62. doi: 10.1097/SHK.0b013e31824caa72. [DOI] [PubMed] [Google Scholar]
- 25.Reuter DA, Huang C, Edrich T, Shernan SK, Eltzschig HK. Cardiac output monitoring using indicator-dilution techniques: basics, limits, and perspectives. Anesth Analg. 2010;110(3):799–811. doi: 10.1213/ANE.0b013e3181cc885a. [DOI] [PubMed] [Google Scholar]
- 26.Lemson J, Nusmeier A, van der Hoeven JG. Advanced hemodynamic monitoring in critically ill children. Pediatrics. 2011;128(3):560–71. doi: 10.1542/peds.2010-2920. [DOI] [PubMed] [Google Scholar]
- 27.Proulx F, Lemson J, Choker G, Tibby SM. Hemodynamic monitoring by transpulmonary thermodilution and pulse contour analysis in critically ill children. Pediatr Crit Care Med. 2011;12(4):459–66. doi: 10.1097/PCC.0b013e3182070959. [DOI] [PubMed] [Google Scholar]
- 28.Yowler CJ, Fratianne RB. Current status of burn resuscitation. Clin Plast Surg. 2000;27(1):1–10. [PubMed] [Google Scholar]
- 29.Faraklas I, Cochran A, Saffle J. Review of a fluid resuscitation protocol: “fluid creep” is not due to nursing error. J Burn Care Res. 2012;33(1):74–83. doi: 10.1097/BCR.0b013e318234d949. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell KB, Khalil E, Brennan A, Shao H, Rabbitts A, Leahy NE, Yurt RW, Gallagher JJ. New management strategy for fluid resuscitation: quantifying volume in the first 48 hours after burn injury. J Burn Care Res. 2013;34(1):196–202. doi: 10.1097/BCR.0b013e3182700965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein MB, Hayden D, Elson C, Nathens AB, Gamelli RL, Gibran NS, Herndon DN, Arnoldo B, Silver G, Schoenfeld D, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007;245(4):622–8. doi: 10.1097/01.sla.0000252572.50684.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruitt BA. Fluid resuscitation of burn patients: does clinical “success” necessitate “excess”. South Med J. 1976;69(11):1399. doi: 10.1097/00007611-197611000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Holm C, Mayr M, Tegeler J, Hörbrand F, Henckel von Donnersmarck G, Mühlbauer W, Pfeiffer UJ. A clinical randomized study on the effects of invasive monitoring on burn shock resuscitation. Burns. 2004;30(8):798–807. doi: 10.1016/j.burns.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Marinov Z, Kvalténi K, Koller J. Fluid resuscitation in thermally injured pediatric patients. Acta Chir Plast. 1997;39(1):28–32. [PubMed] [Google Scholar]


