Abstract
Cholinergic input to the neocortex, dorsal hippocampus (dHipp), and basolateral amygdala (BLA) is critical for neural function and synaptic plasticity in these brain regions. Synaptic plasticity in the neocortex, dHipp, ventral Hipp (vHipp), and BLA has also been implicated in fear and extinction memory. This finding raises the possibility that basal forebrain (BF) cholinergic neurons, the predominant source of acetylcholine in these brain regions, have an important role in mediating fear and extinction memory. While empirical studies support this hypothesis, there are interesting inconsistencies among these studies that raise questions about how best to define the role of BF cholinergic neurons in fear and extinction memory. Nucleus basalis magnocellularis (NBM) cholinergic neurons that project to the BLA are critical for fear memory and contextual fear extinction memory. NBM cholinergic neurons that project to the neocortex are critical for cued and contextual fear conditioned suppression, but are not critical for fear memory in other behavioral paradigms and in the inhibitory avoidance paradigm may even inhibit contextual fear memory formation. Medial septum and diagonal band of Broca cholinergic neurons are critical for contextual fear memory and acquisition of cued fear extinction. Thus, even though the results of previous studies suggest BF cholinergic neurons modulate fear and extinction memory, inconsistent findings among these studies necessitates more research to better define the neural circuits and molecular processes through which BF cholinergic neurons modulate fear and extinction memory. Furthermore, studies determining if BF cholinergic neurons can be manipulated in such a manner so as to treat excessive fear in anxiety disorders are needed.
Keywords: nucleus basalis, magnocellular, fear conditioning, contextual conditioning, inhibitory avoidance, fear extinction, extinction recall
1. Introduction
Decades of research have demonstrated that cholinergic neurons in the basal forebrain (BF) serve as an extra-thalamic relay of sensory information (Jones, 2003), contribute to arousal (Fournier, Materi, Semba, and Rasmusson, 2004; Jones, 2003; Manns, Alonso, and Jones, 2000a; 2000b; 2003), attentional processing (Baxter and Chiba, 1999; Chiba, Bucci, Holland, and Gallagher, 1995; Kozak, Bruno, and Sarter, 2006; McGaughy, Kaiser, and Sarter, 1996; St Peters, Demeter, Lustig, Bruno, and Sarter, 2011), cortical sensory plasticity (Ashe, McKenna, and Weinberger, 1989; Conner, Culberson, Packowski, Chiba, and Tuszynski, 2003; Ramanathan, Tuszynski, and Conner, 2009), place field re-mapping in the dorsal hippocampus (dHipp) (Ikonen, McMahan, Gallagher, Eichenbaum, and Tanila, 2002), and spatial learning and memory (McGaughy, Koene, Eichenbaum, and Hasselmo, 2005; Winters and Bussey, 2005). These findings demonstrate that BF cholinergic input to the neocortex and dHipp modulate neural plasticity in these brain regions and are critical for mediating psychological function that are dependent on these brain regions.
The medial prefrontal cortex (mPFC), dHipp, ventral Hipp (vHipp) and basolateral complex of the amygdala (BLA) are critical for fear and extinction memory (see below). Given that BF cholinergic neurons project to these brain regions (Mesulam, Mufson, Levey, and Wainer, 1983a; Mesulam, Mufson, Wainer, and Levey, 1983b; Woolf and Butcher, 1982; Woolf, Eckenstein, and Butcher, 1983; 1984; Zaborszky, Pang, Somogyi, Nadasdy, and Kallo, 1999), one would expect BF cholinergic neurons to have an important role in modulating fear and extinction memory. Indeed, BF cholinergic neurons were included in early neural circuits believed to be critical for fear memory (LeDoux, 1992; Weinberger, 1998; Weinberger and Bakin, 1998). While studies have revealed a role for BF cholinergic neurons in fear and extinction memory, a number of other studies have reported that manipulation of cholinergic input to the neocortex, Hipp, and the BLA have no effect on fear memory. Possibly, these inconsistent findings have dampened enthusiasm for a thorough investigation of the role BF cholinergic neurons in mediating fear and extinction memory.
In this article I review studies that have examined the role of BF cholinergic neurons in fear and extinction memory. First, I define fear and extinction memory and then, briefly, the BF cholinergic system. I then review studies that have examined the effects of selective BF cholinergic manipulation on fear and extinction memory using a variety of dependent variables (e.g. startle, freezing, conditioned suppression) and experimental methods (e.g. selective lesion, pharmacological manipulation, optogenetics). Together, these studies demonstrate that different clusters of BF cholinergic neurons have different roles in mediating fear and extinction memory. However, the variable effects BF cholinergic manipulations have on fear and extinction memory, rather than raise questions about their involvement in fear and extinction memory, have the potential to inform us about the neurobiology of fear memory, novel ways fear extinction can be disrupted, and neural plasticity that modulates fear and extinction memory.
2. Definition of fear and extinction memory
Fear memory is almost always measured in relatively simple behavioral paradigms where a neutral stimulus such as a tone or a light is paired with an aversive event (Davis and Whalen, 2001; Fanselow, 2000; Lang, Davis, and Ohman, 2000; LeDoux, 2000; Maren, 2001). In the majority of experiments, the neutral stimulus, referred to as the conditioned stimulus (CS) is paired once or several times with an unconditioned aversive stimulus (UCS, typically a footshock) in order to induce a fear memory. If the CS co-terminates with the UCS this is referred to as cued or delayed fear conditioning (Maren, 2001). If there is an interval between the offset of the CS and onset of the UCS, this is referred to as trace fear conditioning (Marchand, Luck, and Di Scala, 2004). In many experiments that examine the neurobiology of fear and extinction memory, the CS can also be a distinct space or arrangement of objects (e.g. type of background noise, odor, or texture places into a distinct space) that form a context (but for a detailed review and complete definition of a context see (Bouton, Westbrook, Corcoran, and Maren, 2006; Maren, Phan, and Liberzon, 2013)). When the CS is a context, fear conditioning is referred to as contextual fear conditioning (Bouton et al., 2006; Fanselow, 2000; Maren, 2001; Maren et al., 2013; Rudy, Huff, and Matus-Amat, 2004).
Behavioral responses indicative of fear-like states (e.g. freezing, potentiated startle) are used to measure fear learning. The change in fear-like behavior induced by the CS after pairing with the UCS is used as an index of fear memory, which can be conceptualized as a CS-UCS association. Thus, for virtually all behavioral paradigms, pairing the CS and UCS comprises the fear learning phase. Fear memory is usually tested a day later by presenting the CS alone. High levels of conditioned responding during this test are indicative of a robust fear memory. Three common variables used to measure fear memory are freezing, potentiated startle, and avoidance (Cahill and McGaugh, 1998; Davis and Whalen, 2001; Lang et al., 2000; Maren, 2001; 2005; McGaugh, 2004; Williams and Clayton, 2001). In the freezing paradigm, which we will refer to as Pavlovian fear conditioning, freezing behavior induced by CS presentation is used to measure fear learning and memory (Blanchard and Blanchard, 1972; Fanselow, 1994). In the fear potentiated startle (FPS) paradigm a brief white noise (i.e. startle probe) is used to elicit a startle reflex in the absence or presence of the CS. After fear conditioning, the startle reflex in the presence of the CS is enhanced, and this enhancement is used as the measure of fear learning and memory (Davis, 1992a; b). Another behavioral paradigm is inhibitory avoidance (IA), which is comprised of a well-lit chamber and a dark chamber joined together and connected by a doorway. Typically, when the animal is placed in the light chamber, it avoids the brightly lit chamber by entering the dark chamber. Upon entering the dark chamber, animals experience a footshock. On the following day, animals are again placed in the well-lit chamber, but typically take a longer time to enter the dark chamber, because the dark chamber was paired with a footshock on the previous day. This increase in avoidance is used to index fear memory (for examples see Gold, van Buskirk, and McGaugh, 1975; Introini-Collison, Saghafi, Novack, and McGaugh, 1992; Power and McGaugh, 2002b; Williams and McGaugh, 1993). Another paradigm used to study fear memory is the suppression of operant responses (for examples see Amorapanth, Nader, and LeDoux, 1999; Estes and Skinner, 1941; Killcross, Robbins, and Everitt, 1997; Milad, Rauch, Pitman, and Quirk, 2006; Sierra-Mercado, Corcoran, Lebron-Milad, and Quirk, 2006), though this is less common than the other variables mentioned above. In this paradigm, animals are first trained to press a bar for a reward. After training they are then presented with the CS-UCS stimulus set (i.e. fear conditioning). After conditioning, cessation in bar pressing occurs with CS presentation. This change in bar pressing is used to index fear memory.
After fear conditioning, presentation of the CS in the absence of the UCS results in a decrease in conditioned responding (Bouton et al., 2006; Estes and Skinner, 1941; Milad et al., 2006; Orsini and Maren, 2012; Pavlov, 1927; Quirk, 2002; Quirk, Garcia, and Gonzalez-Lima, 2006; Rescorla, 2004; Rothbaum and Davis, 2003). This inhibition in conditioned fear responding is referred to as fear extinction. This inhibition in conditioned fear responding does not only represent suppression of behavior, forgetting, or erasure of the fear memory, but also represents acquisition of a new memory (i.e. CS predicts no UCS) (Bouton et al., 2006; Milad et al., 2006; Orsini and Maren, 2012; Pavlov, 1927; Quirk, 2002; Quirk et al., 2006; Rescorla, 2004; Rothbaum and Davis, 2003). This new extinction memory can be examined in all of the behavioral fear paradigms mentioned above. Extinction memory is typically tested one day after induction of fear extinction by presenting the CS alone. Low levels of conditioned fear responding during the extinction test are indicative of robust extinction memory. In this review, extinction training refers to the repeated presentation of CSs without reinforcement, acquisition of extinction refers to the decrease in conditioned fear responding that typically occurs with non-reinforced CS presentation, and the ability to inhibit conditioned fear responding during an extinction test is indicative of robust extinction memory.
Cued and contextual fear and extinction memory can be examined in Pavlovian fear conditioning, FPS, and conditioned suppression, but only contextual fear and extinction memory can be examined in IA. These behavioral paradigms and how they are used to measure fear and extinction memory are illustrated in Figure 1.
Figure 1.
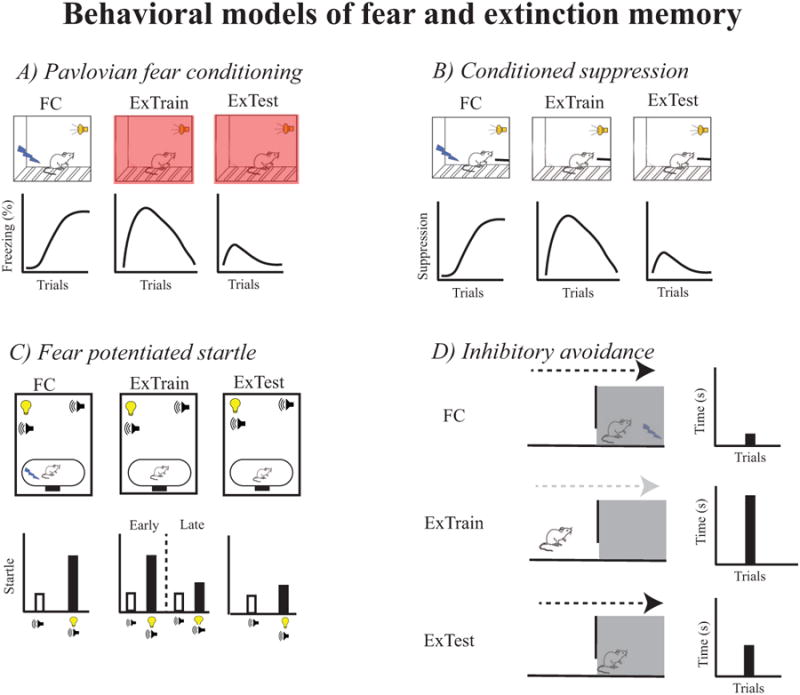
Behavioral paradigms used to examine emotional memory (fear and extinction) in neuroscience experiments. Cartoons illustrate the paradigm and graphs adjacent to the cartoons illustrate the change in fear behavior during fear conditioning (FC), extinction training (ExTrain), and extinction testing (ExTest). A) Pavlovian fear conditioning. Freezing is used as the measure of fear in this paradigm. Often the conditioning and extinction contexts are different in this paradigm (represented by change in color), which can allow for separate measurement of cued and contextual fear and extinction memory. B) Conditioned fear suppression. In this paradigm, changes in operant performance are used as the measure of fear behavior, but a context shift is not typically used. C) Fear potentiated startle. This paradigm involves presentation of a white noise burst to elicit a startle reflex, which is typically measured by an accelerometer that transduces movement into an electrical signal. During conditioning and in the presence of the conditioned stimulus (CS) the startle reflex is enhanced. D) Inhibitory avoidance. This paradigm involves placing the animal in a light compartment that is connected to a dark compartment. Upon entering the dark compartment the animal then receives a footshock. Avoidance of the dark compartment after conditioning is the measure of fear behavior.
3. BF Cholinergic Neurons
There are excellent reviews and empirical studies about BF cholinergic neurons (Jones, 2004; Mesulam et al., 1983a; Mesulam et al., 1983b; Semba, 1991; Woolf and Butcher, 1982; Woolf et al., 1983; 1984; Zaborszky et al., 1999). This section is not meant to be a comprehensive description of BF cholinergic neurons or cholinergic receptor pharmacology. Instead, the goal of this section is to provide the reader with brief definitions of different BF cholinergic clusters and summarize the cholinergic synapse within fear and extinction circuits.
BF cholinergic neurons are located on the base of the brain and can be found in clusters from the olfactory tubercle to the rostral end of the lateral geniculate bodies. At the anterior pole of the BF are cholinergic neurons in the medial septum and diagonal band of Broca (MS/DBB). These cholinergic neurons project to the Hipp (Mesulam et al., 1983b; Woolf et al., 1984) and midline cortical structures such as the posterior anterior cingulate cortex, retrosplenial cortex (RSC), and mPFC (Mesulam et al., 1983b; Woolf et al., 1984), including the infralimbic cortex (IL) (Knox and Keller, 2015). Thus, in the MS/DBB there are septohippocampal as well as corticopetal cholinergic neurons. At the posterior pole of the BF are cholinergic neurons in the nucleus basalis of Meynert (NBM, including substantia innominata). NBM cholinergic neurons project to dorsolateral surfaces of the cortical mantle, PFC, and BLA (Mesulam et al., 1983b; Woolf et al., 1984). Thus, in the NBM there are corticopetal and amygdalopetal cholinergic neurons. Other BF corticopetal cholinergic neurons that project predominantly to anterior medial cortical regions (e.g. mPFC) and the olfactory tubercle can be found in the medial preoptic area, lateral preoptic area, and the horizontal limb of the diagonal band of Broca (hDBB) (Mesulam et al., 1983b; Woolf et al., 1984).
Upon membrane depolarization and Ca2+ influx into the axon terminal, acetylcholine (ACh) is released from the axon terminal (Birks and Fitch, 1974; Collier, Tandon, Prado, and Bachoo, 1993). Termination of ACh actions on receptors is accomplished by metabolism of ACh by acetylcholinesterase (AChE) (Chatonnet and Lockridge, 1989). Choline is then taken back up into the axon terminal via the high affinity choline transporter (Bazalakova and Blakely, 2006; Ferguson, Savchenko, Apparsundaram, Zwick, Wright, Heilman, Yi, Levey, and Blakely, 2003), though there is low affinity choline and ACh transport as well (Cooper, Bloom, and Roth, 1996). This choline is then used to synthesize ACh at the axon terminal (Bazalakova and Blakely, 2006; Ferguson et al., 2003); a reaction catalyzed by choline acetyltransferase (ChAT) (Benishin and Carroll, 1983). ACh is then packaged into synaptic vesicles via the vesicular acetylcholine transporter (VAChT) (Prado, Roy, Kolisnyk, Gros, and Prado, 2013).
Cholinergic neurons signal through metabotropic muscarinic receptors (mAChRs) and ionotropic nicotinic receptors (nAChRs) (Cooper et al., 1996). There are five broad types of mAChRs with m1AChR and m3-5AChRs being post synaptic and m2AChR being presynaptic (Cooper et al., 1996; Levey, 1993; Quirion, 1993). Activation of mAChRs lead to stimulation of phosphoinositol synthesis and or inhibition of cAMP synthesis (Bonner, 1989) and changes in the permeability of the neuronal membrane to K+, Ca2+, and Cl- channels (Schimerlik, 1989). NAChRs are pentameric ion channels comprised of α and β subunits in different configurations (Cooper et al., 1996). There are at least 11 variants of the α subunit and eight variants of the β subunit (Decker, Brioni, Bannon, and Arneric, 1995). The two most common types of nAChRs in the brain comprise of α4β2 subunits and α7 subunits exclusively (Davis and Gould, 2007; Decker et al., 1995). Furthermore, even though nAChRs form a non-specific cation pore, receptors with different subunits can have selectivity for different types of cation species (McGehee, 1999).
In addition to expression Trk class of receptors that bind neurotrophic factors (Steininger, Wainer, Klein, Barbacid, and Palfrey, 1993), BF cholinergic neurons also express the p75 receptor (Heckers, Ohtake, Wiley, Lappi, Geula, and Mesulam, 1994) and are the only known neuronal group in the BF that express this receptor (Frick, Kim, and Baxter, 2004; Heckers et al., 1994). Figure 2 illustrates BF cholinergic neurons and summarizes the cholinergic synapse in the central nervous system.
Figure 2.
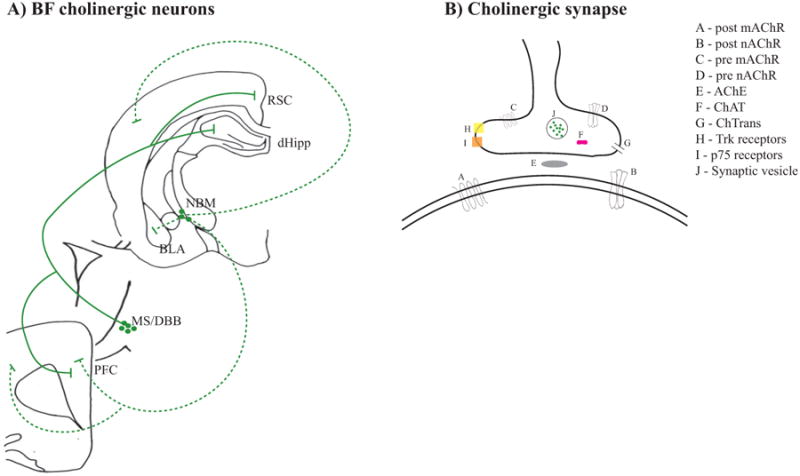
A) Schematic of the basal forebrain (BF) cholinergic neurons that project to fear and extinction circuits. BF neurons in the preoptic area are not being shown, because these neurons are rarely manipulated in studies that examine the effects of BF cholinergic manipulation on fear and extinction memory. NBM neurons are showed in dashed lines while MS/DBB neurons are showed in solid lines. B) Summarized schematic of the cholinergic synapse in fear and extinction circuits. Post and pre prefixes refer to whether receptors are pre or post synaptic. ChTrans – choline transporter,
4. BF Cholinergic Neurons In Fear and Extinction Memory
4.1 NBM amygdalopetal cholinergic neurons
4.1.1 Fear Memory
By infusing excitotoxins in the NBM that preferentially destroy BF amygdalopetal cholinergic neurons (Mallet, Beninger, Flesher, Jhamandas, and Boegman, 1995), a previous study has shown that BF amygdalopetal cholinergic lesions disrupt IA (Power and McGaugh, 2002b). Furthermore, memory deficits induced by BF amygdalopetal cholinergic lesions are attenuated by mAChR agonism in the BLA immediately after IA (Power and McGaugh, 2002b), which suggests BF amygdalopetal cholinergic neurons are critical for contextual fear memory consolidation in IA. BF amygdalopetal cholinergic lesions also disrupt Pavlovian contextual fear conditioning (Power and McGaugh, 2002a) and mAChR agonism in the BLA enhances consolidation of Pavlovian contextual fear conditioning (Power and McGaugh, 2002a). Systemic administration of histaminergic agents that decrease ACh levels in the BLA disrupt Pavlovian contextual fear conditioning (Passani, Cangioli, Baldi, Bucherelli, Mannaioni, and Blandina, 2001). The complementary effects that BF amygdalopetal excitotoxic lesions, direct BLA mAChR manipulation, and decreasing BLA ACh levels have on IA and Pavlovian contextual fear conditioning suggest that BF amygdalopetal cholinergic input to mAChRs in the BLA are critical for acquisition and consolidation of contextual fear memory.
Non-specific excitotoxic lesions in the NBM have no effects on cued FPS (Schauz and Koch, 1999), but studies using optogenetic and pharmacological methods in the Pavlovian fear conditioning paradigm suggest that BF amygdalopetal cholinergic neurons are critical for cued fear memory (Jiang, Kundu, Lederman, Lopez-Hernandez, Ballinger, Wang, Talmage, and Role, 2016). Optogenetic-induced inhibition of BF amygdalopetal cholinergic input to the BLA has no effect on acquisition, but disrupts expression, of cued fear memory (Jiang et al., 2016). MAChR antagonism in the BLA has no effect on cued Pavlovian fear conditioning (Baysinger, Kent, and Brown, 2012; Jiang et al., 2016), but simultaneously antagonizing mAChRs and nAChRs during fear conditioning disrupts expression of cued fear memory (Jiang et al., 2016). Interestingly, mAChR antagonism in the lateral amygdala (LA) disrupts trace fear conditioning (Baysinger et al., 2012), which does raise the possibility that BF amygdalopetal cholinergic input to mAChRs in the BLA are selectively critical for trace fear memory.
4.1.2. Extinction memory
MAChR agonism in the BLA enhances contextual extinction memory consolidation in the Pavlovian fear conditioning paradigm (Boccia, Blake, Baratti, and McGaugh, 2009), which raises the possibility that BF amygdalopetal cholinergic neurons may modulate consolidation of contextual extinction memory. While a previous study has shown that activation of BF amygdalopetal cholinergic input to the BLA during fear conditioning has effects on acquisition and maintenance of fear extinction (Jiang et al., 2016), no study to date has selectively examined the role of BF amygdalopetal cholinergic neurons in cued extinction memory.
4.1.3. Neurobiological mechanism
Neural plasticity in the LA and BLA is critical for acquisition, consolidation, and retrieval of cued and contextual fear memory (Davis and Whalen, 2001; Maren, 1998; 2001; 2005; Pare, Quirk, and Ledoux, 2004; Phillips and LeDoux, 1992). Specifically, a fairly large amount of empirical data suggests that convergence of neural activity representing the CS and UCS onto BLA neurons increase the responsivity of BLA neurons to the CS via LTP-like mechanisms, which helps drive fear expression with CS presentation (Fanselow and LeDoux, 1999; Isoardi, Martijena, Carrer, and Molina, 2004; Maren, 2001; Pare et al., 2004). The BLA is also critical for the memory modulating effects adrenal hormones have on fear memory (Cahill and Alkire, 2003; Cahill and McGaugh, 1998; Cahill, Prins, Weber, and McGaugh, 1994; Gold et al., 1975; Introini-Collison et al., 1992; McGaugh, 1966; 2004; Williams and Clayton, 2001). Based on these observations one would expect BF amygdalopetal cholinergic neurons to be critical for cued and contextual fear memory. Previous findings support this assertion, though it remains unknown why BF amygdalopetal cholinergic neurons are critical for cued fear memory in Pavlovian fear conditioning, but not FPS (Baysinger et al., 2012; Boccia et al., 2009; Jiang et al., 2016; Power and McGaugh, 2002a; b; Schauz and Koch, 1999).
Through what neurobiological mechanisms might BF amygdalopetal cholinergic neurons facilitate contextual and trace fear memory? A previous study has shown that optogenetic-induced stimulation of NBM cholinergic axons in the BLA has two specific types of effects on principal neurons. When principal neurons fire at a low frequency, stimulation of NBM cholinergic axons inhibit the activity of principal neurons via m1AChRs. However, when principal neurons fire at a high frequency, stimulation of BF cholinergic axons enhance the after depolarization of principal cells, which enhances the excitability of these neurons (Unal, Pare, and Zaborszky, 2015). This excitability may have a role in facilitating types of learning where occurrence of conditioned and unconditioned stimuli are not optimal for learning (Unal et al., 2015). In trace fear conditioning, presentation of the CS is removed in time from presentation of the UCS. During the course of trace fear conditioning, BF amygdalopetal cholinergic input to BLA neurons could help sustain excitability of weak CS-responses in BLA neurons, and by doing this, allow synaptic plasticity critical for trace fear memory. Similarly, in contextual fear conditioning it is thought that binding of elements of an environment into a context occurs in the hippocampus, while association of that context with an aversive outcome occurs in the BLA (Bouton et al., 2006; LeDoux, 2000; Maren, 1998; 2001; Maren et al., 2013; Phillips and LeDoux, 1992). BF amygdalaopetal cholinergic input to BLA neurons could help sustain weak BLA neuron responses to the emerging context early in contextual fear learning, which would facilitate synaptic plasticity critical for associating a context with an aversive outcome. However, it should be noted that one study has observed that BF amygdalopetal cholinergic input to the BLA excites principal neurons in the BLA and this excitation is driven by nAChRs (Jiang et al., 2016). Furthermore, the results of this same study observed that BF amygdalopetal cholinergic input to the BLA potentiates glutamatergic neural transmission in the BLA and cortical input onto BLA neurons. The authors propose that these effects go on to facilitate cued fear memory formation in the Pavlovian fear conditioning paradigm (Jiang et al., 2016).
BF amygdalopetal cholinergic neurons are critical for contextual fear memory consolidation (see above) and this occurs via mAChRs. While molecular mechanisms remain undefined, there are a number of possibilities. M1AChR expression is enhanced after fear conditioning while m2AChR expression is downregulated (Ortega, del Guante, Prado-Alcala, and Aleman, 1996). Activation of m2AChRs tend to lead to inhibition of ACh release (Quirion, Richard, and Wilson, 1994). Thus, if upregulation of m1AChRs coupled with downregulation of m2AChRs occur after fear conditioning, this could lead to potentiation of m1AChR activation and signaling cascades coupled to this receptor. These include increased intracellular Ca2+ levels driven by inositol triphosphate (IP3) binding to receptors on the endoplasmic reticulum (Abe, Sugihara, Nawa, Shigemoto, Mizuno, and Nakanishi, 1992; Power and Sah, 2002), increased protein kinase C (PKC) activity driven by diacylglycerol (DAG) synthesis and enhanced intracellular Ca2+ levels (Newton, 2001; Sun and Alkon, 2010), or inhibition of K+ channels driven by decreased phosphoinositol diphosphate levels in the neuronal membrane upon IP3 synthesis (Brown, 2010; Brown and Adams, 1980). Further research is needed to determine which of the above processes, if any, are relevant for the role of BF amygdalopetal cholinergic neurons in contextual fear memory consolidation. BF amygdalopetal cholinergic input to BLA nAChRs, in conjunction with mAChRs, are critical for cued fear memory in Pavlovian fear conditioning (Jiang et al., 2016). How might activation of BLA nAChRs facilitate cued fear memory? BF amygdalopetal cholinergic input to nAChRs during fear memory formation could enhance Ca2+ conductance in BLA principal neurons, which drives plasticity critical for fear memory (Bauer, Schafe, and LeDoux, 2002; Davis, 1992b; Davis and Whalen, 2001; Pare et al., 2004). This hypothesis is consistent with the observation that the subunit composition of nAChRs in fear and extinction circuits (i.e. α7 and β4 subunits) allow for relatively specific conductance of Ca2+ currents (Marks, Stitzel, and Collins, 1989; Seguela, Wadiche, Dineley-Miller, Dani, and Patrick, 1993)
The BLA is also critical for acquisition and consolidation of cued and contextual extinction memory (Berlau and McGaugh, 2006; Boccia et al., 2009; Chhatwal, Myers, Ressler, and Davis, 2005; Herry, Trifilieff, Micheau, Luthi, and Mons, 2006; Laurent and Westbrook, 2008). Specifically, ventromedial prefrontal cortex (vmPFC) input to the intercalated cell clusters of the amygdala are critical for inhibiting neural activity in the BLA (Cho, Deisseroth, and Bolshakov, 2013; Likhtik, Popa, Apergis-Schoute, Fidacaro, and Pare, 2008; Milad and Quirk, 2002; Royer and Pare, 2002). The finding that the BLA is critical for acquisition and consolidation of extinction memory raises the possibility that BF amygdalopetal cholinergic neurons would be critical for extinction memory. While, BF amygdalopetal cholinergic input to mAChR receptors in the BLA may be critical for contextual extinction memory (see above), no study to date has examined the role of these neurons in cued extinction memory.
How might NBM amygdalopetal cholinergic input to mAChRs in the BLA be important for both contextual fear and extinction memory? Many molecular processes critical for fear memory are also critical for extinction memory (Orsini and Maren, 2012), so it is not surprising that BLA mAChRs modulate contextual fear and extinction memory. However, an important difference between fear conditioning and extinction training is levels of arousal and/or stress (Maren and Chang, 2006; Orsini and Maren, 2012). Indeed, if levels of arousal/stress are high prior to extinction training, extinction memory is impaired (Maren and Chang, 2006). It is possible that BLA mAChR function changes with levels of arousal/stress, where if arousal/stress is high, then these receptors facilitate neural plasticity critical for fear memory, but when arousal/stress levels are low, mAChRs help formation of the new extinction memory. Of course, there are a number of neurobiological processes through which NBM amygdalopetal cholinergic neurons could modulate consolidation of contextual extinction memory (Bouton et al., 2006; Chhatwal et al., 2005; Herry et al., 2006; Lee and Kim, 1998; Maren et al., 2013; Orsini and Maren, 2012), and thus, more research is needed to identify the molecular processes through which BF amygdalopetal cholinergic input to BLA mAChRs modulate contextual fear and extinction memory.
In addition to receiving cholinergic input from the NBM, the BLA also receives cholinergic input from the lateral parabrachial nucleus (LPN) (Mesulam et al., 1983b; Woolf et al., 1984), which means care has to be taken when interpreting studies that have only employed cholinergic pharmacological manipulation in the BLA. This applies to trace fear memory and contextual extinction memory (Baysinger et al., 2012; Boccia et al., 2009). While LPN input to the central nucleus of the amygdala does modulate cued fear memory (Watabe, Ochiai, Nagase, Takahashi, Sato, and Kato, 2013), no study to date has implicated LPN input to the BLA as critical for fear or extinction memory. As a result, it is likely that BF amygdalopetal cholinergic neurons facilitate trace fear memory and contextual extinction memory, though further studies empirically demonstrating this are needed. Neurobiological mechanisms through which BF amygdalopetal cholinergic neurons could facilitate emotional memory are illustrated in Figure 3.
Figure 3.
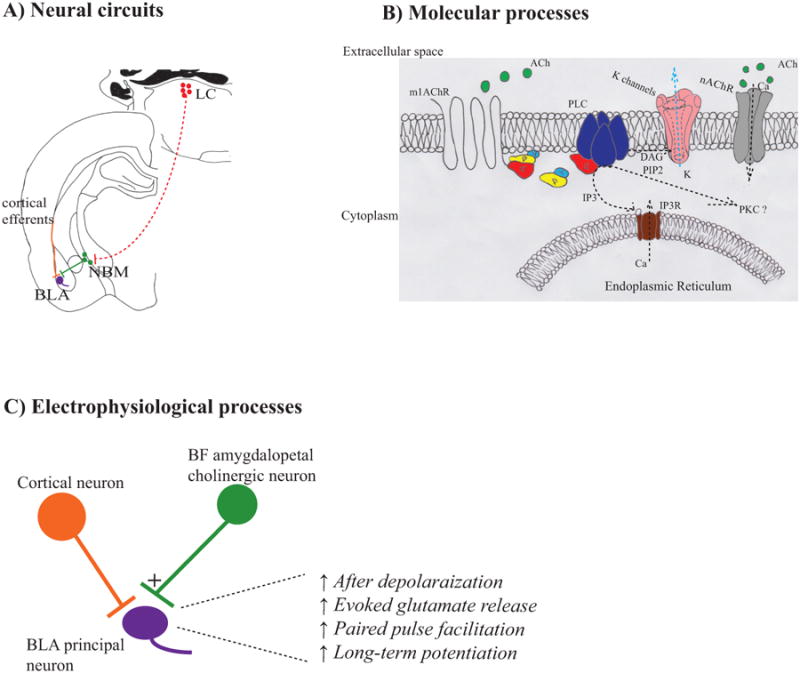
Neurobiological processes via which BF amygdalopetal cholinergic neurons modulate emotional memory. A) Hypothetical circuit. Circles in red illustrate locus coeruleus neurons, green represent BF amygdalopetal cholinergic neurons, purple represent BLA principal neurons, and orange represent cortical input to BLA principal neurons. Components of the circuit that need verification are shown in dashed lines. B) Molecular processes by which BF amygdalopetal cholinergic neurons modulate emotional memory. Unknown mechanisms are shown as question marks or dashed lines. Decreased signaling is indicated by light blue arrows. C) Fear relevant electrophysiological processes by which BF amygdalopetal cholinergic neurons modulate BLA principal neurons. LC – locus coeruleus. m1AChR – muscarinic receptor type 1, nAChR – nicotinic receptor, IP3 – inositol triphosphate, IP3R – IP3 receptor, DAG – diacylglycerol, PIP2 – phosphatidylinositol biphosphate, PKC – protein kinase C.
4.2. NBM corticopetal cholinergic neurons
Many studies investigating the role of NBM corticopetal cholinergic neurons in mediating fear and extinction memory have utilized the selective cholinergic immunotoxin 192 IgG saporin to induce permanent NBM corticopetal cholinergic lesions. This toxin does not affect NBM amygdalopetal cholinergic neurons and has minimal effects on non-cholinergic neurons (Conner et al., 2003; Frick et al., 2004; Heckers et al., 1994). Thus, when this toxin is infused into the NBM, selective corticopetal cholinergic neuronal loss can be induced. However, with this treatment residual cholinergic input to the mPFC remains and cholinergic input to posterior midline cortical systems (e.g. RSC) is unaffected (Knox and Berntson, 2008; Stowell, Berntson, and Sarter, 2000; Torres, Perry, Blockland, Wilkinson, Wiley, Lappi, and Dunnet, 1994).
4.2.1. Fear memory
NBM corticopetal cholinergic lesions attenuate cued and contextual fear conditioned suppression (Knox and Berntson, 2006; 2008; Stowell et al., 2000), though operant performance is not affected, and animals with NBM corticopetal cholinergic lesions can still display operant suppression to footshocks (Knox and Berntson, 2008; McGaughy et al., 1996; Stowell et al., 2000). These findings strongly suggest that NBM corticopetal cholinergic neurons are critical for fear memory. Surprisingly, complete BF or NBM corticopetal cholinergic lesions have no effect on cued or contextual Pavlovian fear conditioning (Conner et al., 2003; Frick et al., 2004; Knox and Berntson, 2006; Knox and Keller, 2015; Tronson, Schrick, Guzman, Huh, Srivastava, Penzes, Guedea, Gao, and Radulovic, 2009) and non-specific excitatory lesions in the NBM have no effects on FPS (Schauz and Koch, 1999).
There are a number of reasons why NBM corticopetal cholinergic lesions have robust effects on fear memory in the conditioned fear suppression paradigm only. In Pavlovian fear conditioning and FPS, animals show fear behaviors to CS presentation after the CS is paired with a UCS (i.e. CS-UCS memory). In the conditioned suppression paradigm, animals change their learned operant responses based on acquisition of a fear memory (i.e. CS-UCS). This parameter of the conditioned suppression paradigm could be critical in making fear memory in this paradigm sensitive to NBM corticopetal cholinergic manipulation. It should be noted that previous studies have observed differences between the neurobiology of conditioned freezing and suppression (Amorapanth et al., 1999; Killcross et al., 1997), which supports the hypothesis that the neurobiology of fear memory is different in the conditioned suppression paradigm when compared to other paradigms.
One study has reported that NBM cholinergic input to the auditory cortex is critical for auditory Pavlovian fear conditioning (Letzkus, Wolff, Meyer, Tovote, Courtin, Herry, and Luthi, 2011). The results of this study suggest that cholinergic input (via nAChRs) to the auditory cortex inhibit GABAergic neurons in layers I and II, which lead to activation of neurons in the layers 2/3 of the auditory cortex and via this mechanism, conditioning to the auditory CS. It is important to note that other studies have shown that complete BF cholinergic deafferentation of the neocortex has no effect on auditory fear conditioning (Conner et al., 2003; Frick et al., 2004; Knox and Berntson, 2006). The discrepancy between this study and several others could be due to a number of reasons. Letzkus et al. (2011), used complex auditory stimuli, but most other studies used single frequency tones (Conner et al., 2003; Frick et al., 2004; Knox and Berntson, 2006). Letzkus et al., used mice as experimental subjects, but in other studies subjects were rats (Conner et al., 2003; Frick et al., 2004; Knox and Berntson, 2006). Finally, rodents have interneurons in the neocortex that secrete ACh (Bayraktar, Staiger, Acsady, Cozzari, Freund, and Zilles, 1997; von Engelhardt, Eliava, Meyer, Rozov, and Monyer, 2007), and it is possible that these neurons, not NBM corticopetal cholinergic neurons, are critical for complex auditory Pavlovian fear conditioning.
Two previous studies have reported that contextual fear learning in IA is sensitive to NBM corticopetal cholinergic lesions. However, instead of disrupting IA, NBM corticopetal cholinergic lesions enhance IA (Pizzo, Thal, and Winkler, 2002; Torres et al., 1994). This is quite perplexing as it would appear the same experimental manipulation (i.e. NBM corticopetal cholinergic lesions) can disrupt contextual fear memory in one behavioral paradigm (i.e. conditioned suppression), have no effect in another behavioral paradigm (i.e. Pavlovian fear conditioning), and inhibit contextual fear memory formation in yet another paradigm (i.e. IA). It should be noted that another study has reported that NBM corticopetal cholinergic lesions have no effects on IA, but instead is critical for the enhancing effects norepinephrine release in the BLA has on contextual fear memory in IA (Power, Thal, and McGaugh, 2002). Norepinephrine release in the BLA is critical for the enhancing effects adrenal hormones have on contextual fear memory consolidation (Cahill and Alkire, 2003; Cahill and McGaugh, 1998; Power et al., 2002; Williams and Clayton, 2001). Thus, NBM corticopetal cholinergic neurons may be critical for mediating the enhancing effects adrenal hormones have on contextual fear memory consolidation, but not contextual fear memory per se (Power et al., 2002). To resolve the discrepancies observed with NBM corticopetal cholinergic manipulation on contextual fear memory in IA, further research is needed.
4.2.2. Extinction memory
One study to date has investigated the effects of NBM corticopetal cholinergic lesions on cued extinction memory in the Pavlovian fear conditioning paradigm and found that these lesions have no effects on extinction memory (i.e. CS-no UCS memory) (Knox and Keller, 2015). MAChR antagonism in the IL attenuates extinction memory consolidation (Santini, Sepulveda-Orengo, and Porter, 2012), which suggests that ACh in the IL is critical for extinction memory consolidation. NBM cholinergic neurons project to the mPFC, but these projections may specifically terminate in the ACC and PL (Knox and Keller, 2015). Cholinergic neurons in the MS/DBB project to the mPFC as well, and may specifically project to the IL (Knox and Keller, 2015). Given that MS/DBB cholinergic neurons are critical for extinction memory (see below) it is possible that MS/DBB cholinergic input to the IL is critical for extinction memory formation.
4.2.3. Neurobiological mechanisms
Unlike NBM amygdalopetal cholinergic neurons, NBM cholinergic neurons project to a wide range of neocortical systems, some of which have been implicated in fear memory. The prelimbic cortex (PL) modulates expression of cued and contextual fear memory (Corcoran and Quirk, 2007), the anterior cingulate cortex (ACC) is critical for the formation and expression of weak cued fear memory (Bissiere, Plachta, Hoyer, McAllister, Olpe, Grace, and Cryan, 2008) and contextual fear memory consolidation (Einarsson and Nader, 2012; Frankland, Bontempi, Talton, Kaczmarek, and Silva, 2004). Given these findings, one would expect NBM corticopetal cholinergic neurons to be critical for fear memory. NBM corticopetal cholinergic neurons are critical for fear memory, but this only applies to the conditioned fear suppression paradigm (Baysinger et al., 2012; Boccia et al., 2009; Conner et al., 2003; Frick et al., 2004; Knox and Berntson, 2006; 2008; Power and McGaugh, 2002a; b; Schauz and Koch, 1999; Stowell et al., 2000), which is somewhat surprising.
How might NBM corticopetal cholinergic neurons facilitate fear memory in the conditioned suppression paradigm? During acquisition of contextual fear conditioned suppression there is a decrease in slow wave components (1-4Hz) of the PFC local field potential (LFP), and this decrease appears critical for contextual fear conditioned suppression (Knox and Berntson, 2008). NBM corticopetal cholinergic lesions increase slow wave PFC LFP at baseline and attenuate decreases in slow wave PFC LFP that occur during acquisition of contextual fear conditioned suppression (Knox and Berntson, 2008). These findings suggest that NBM cholinergic input to the PFC is critical for mediating fear conditioned suppression, though exact regions of the PFC and electrophysiological mechanisms that underlie changes in slow wave PFC LFP remain unknown. The PL and ACC are critical for fear memory (Bissiere et al., 2008; Corcoran and Quirk, 2007; Einarsson and Nader, 2012; Frankland et al., 2004), which raises the possibility that NBM corticopetal cholinergic input to these regions of the PFC are critical for fear memory in the conditioned suppression paradigm.
During fear conditioning enhanced arousal results in the release of adrenal hormones, which can modulate fear memory formation (Cahill and McGaugh, 1998; Cahill et al., 1994; McGaugh, 2004). NBM corticopetal cholinergic neurons could facilitate the modulatory effect adrenal hormones have on the PFC LFP during acquisition of fear memory and via this mechanism modulate fear memory. Pharmacologically enhancing arousal by systemic epinephrine administration enhances sensory evoked LFPs in the PFC and RSC (Berntson, Shafi, Knox, and Sarter, 2003b; Knox, Sarter, and Berntson, 2004) and both NBM cholinergic lesions and blockade of α1 noradrenergic receptors in the NBM attenuate these effects (Berntson et al., 2003b; Knox et al., 2004). Locus coeruleus neurons could secrete norepinephrine onto NBM corticopetal cholinergic neurons during acquisition of fear memory, which would cause an increase in ACh release in the PFC that is critical for fear memory formation in the conditioned suppression paradigm (LC➔NBM➔PFC). This hypothetical model has been previously proposed as critical for anxiety (Berntson, Sarter, and Cacioppo, 1998; 2003a), but could be important for fear memory as well.
Neurons in the central nucleus of the amygdala (CeA) project to the NBM (Jolkkonen, Miettinen, Pikkarainen, and Pitkanen, 2002; McDonald, 1991; Petrovich and Swanson, 1997; Price and Amaral, 1981), and given the role of the CeA in conditioned fear memory (Goosens and Maren, 2001; Wilensky, Schafe, Kristensen, and LeDoux, 2006), CeA input to NBM cholinergic neurons could stimulate ACh release in the PFC and induce plasticity critical for fear memory in the conditioned suppression paradigm (i.e. CeA➔NBM➔PFC) (Kapp, Supple, and Whalen, 1994; Weinberger, 1998). CeA neurons predominantly terminate on GABAergic neurons in the NBM (Jolkkonen et al., 2002), which suggests for the CeA➔NBM➔PFC circuit to be valid, CeA input to the NBM would have to act via GABAergic NBM neurons (Jolkkonen et al., 2002). Neurobiological mechanisms through which NBM corticopetal cholinergic neurons could facilitate fear memory in the conditioned suppression paradigm are illustrated in Figure 4.
Figure 4.
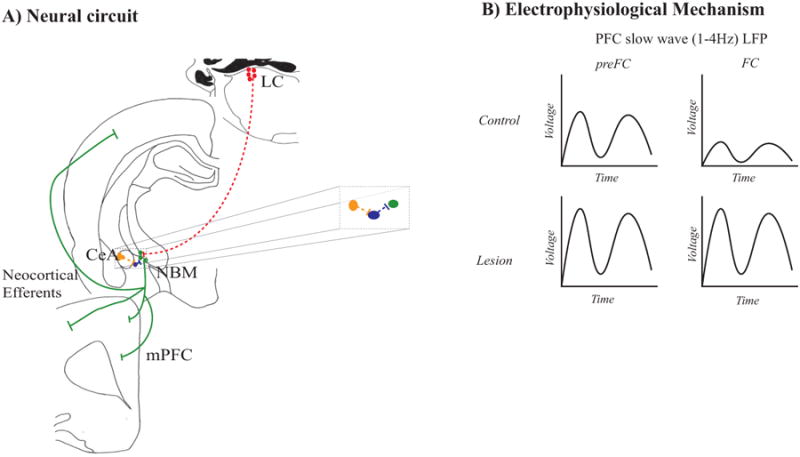
Neurobiological processes via which NBM corticopetal cholinergic neurons modulate emotional memory. A) Neural circuits. NBM cholinergic neurons are in green, LC neurons are in red, central amygdala (CeA) neurons are in yellow, and NBM GABAergic neurons are in purple. Components of the neural circuit that need verification are shown in dashed lines. B) Electrophysiological mechanisms. NBM corticoptal cholinergic input to the PFC increases the slow-wave components (1-4Hz) of the PFC local field potential (LFP) and may disrupt decreases in slow wave PFC LFP during fear conditioning. These effects may mediate the disruptive effect NBM corticopetal cholinergic lesions have on fear memory in the conditioned suppression paradigm. preFC – pre fear conditioning.
Unfortunately, no previous study has examined the role of BF cholinergic neurons in mediating extinction memory in the conditioned suppression paradigm, even though these neurons are critical for fear memory (see above), which raises the possibility that they may be critical for extinction memory. More research is needed to identify neural circuitry, pharmacological mechanisms, and molecular mechanisms through which NBM corticopetal cholinergic neurons mediate emotional memory in the conditioned suppression paradigm, and address why the neurobiology of fear memory seems to be different in this paradigm when compared to other paradigms.
4.3 MS/DBB Cholinergic Neurons
MS/DBB contains cholinergic neurons project to the Hipp, but there are neurons in the MS/DBB that also project to the mPFC as well (Knox and Keller, 2015; Woolf et al., 1983). While much of the studies covered in this section focus on MS/DBB hippocampal cholinergic neurons, we do try to identify where MS/DBB corticopetal cholinergic neurons may be critical for emotional memory.
4.3.1. Fear memory
Research measuring ACh levels in the dHipp, pharmacologically manipulating cholinergic receptors in the dHipp, or optogenetic activation of MS/DBB cholinergic neurons suggest that MS/DBB hippocampal cholinergic neurons are critical for mediating Pavlovian contextual fear conditioning. It should be noted that MS/DBB cholinergic input to the Hipp is virtually the exclusive source of ACh in the Hipp (Mesulam et al., 1983a; Mesulam et al., 1983b; Woolf et al., 1983; 1984). As a result, pharmacological manipulation of cholinergic receptors in the Hipp directly manipulates MS/DBB cholinergic input to the Hipp.
Hipp ACh levels are enhanced during Pavlovian contextual fear conditioning (Calandreau, Trifilieff, Mons, Costes, Marien, Marighetto, Micheau, Jaffard, and Desmedt, 2006; Nail-Boucherie, Dourmap, Jaffard, and Costentin, 2000) and blockade of mAChRs in the dHipp disrupts acquisition (Rogers and Kesner, 2004; Wallenstein and Vago, 2001) and consolidation (Izquierdo, da Cunha, Rosat, Jerusalinsky, Ferreira, and Medina, 1992; Wallenstein and Vago, 2001) of Pavlovian contextual fear conditioning. Physiostigmine is an AChE inhibitor, and local AChE inhibition in distinct brain regions increases ACh levels (Messamore, Warpman, (Rogers and Kesner, 2004). nAChR agonism in the dHipp enhances Pavlovian contextual fear conditioning (Davis, Kenney, and Gould, 2007; Kenney, Raybuck, and Gould, 2012), although nAChR antagonism in the dHipp has no effect on Pavlovian contextual fear conditioning (Raybuck and Gould, 2010). Interestingly, nAChR agonism in the vHipp also disrupts Pavlovian contextual fear conditioning (Kenney et al., 2012), which suggests MS/DBB cholinergic input to nAChRs in the dHipp and vHipp are critical for Pavlovian contextual fear conditioning, though they may have different roles. Finally, optogenetic stimulation of MS/DBB cholinergic neurons enhances contextual fear conditioning in mice (Hersman and Fanselow, 2015). Together, these findings suggest that MS/DBB hippocampal cholinergic neurons are critical for contextual fear memory.
Research using direct measurement of ACh in the dHipp and direct pharmacological methods to manipulate AChRs in the dHipp suggests that MS/DBB hippocampal cholinergic neurons are critical for contextual fear memory in IA. ACh levels in the dHipp are enhanced during IA and drug treatments that enhance IA also enhance ACh levels in the dHipp (Kart, Jocham, Muller, Schlomer, Brandao, Huston, and de Souza Silva, 2004; Nail-Boucherie, Dourmap, Jaffard, and Costentin, 1998; Qi and Gold, 2009). Disrupting IA by inhibiting MS neural activity is reversed by enhancing ACh release in the dHipp (Degroot and Parent, 2001). MAChR antagonism disrupts acquisition (Khakpai, Nasehi, Haeri-Rohani, Eidi, and Zarrindast, 2012; Nail-Boucherie et al., 1998; Qi and Gold, 2009) and consolidation (Zarrindast, Ardjmand, Ahmadi, and Rezayof, 2012) of IA, as well as long-term contextual fear memory in IA (Parfitt, Campos, Barbosa, Koth, and Barros, 2012).
NAChRs have also been implicated in contextual fear memory in IA. Non-selective nAChR antagonism in the dHipp disrupts acquisition, consolidation, and retrieval of contextual fear memory (Marti Barros, Ramirez, Dos Reis, and Izquierdo, 2004) and nAChR agonism in the dHipp enhances IA (Marti Barros et al., 2004). NAChRs are also critical for long-term contextual fear memory in the IA paradigm (Parfitt et al., 2012) and the homomeric α7 nAChR is critical for reconsolidation of IA (Boccia, Blake, Krawczyk, and Baratti, 2010).
Cholinergic manipulation in the dHipp modulates trace fear conditioning. Nicotine administration in the dHipp enhances trace fear conditioning, and β2 containing nAChRs appear critical for this effect (Davis and Gould, 2007; Raybuck and Gould, 2010). MAChR antagonism in the dHipp also disrupts trace fear conditioning (Pang, Kim, Kim, Kim, Kim, and Choi, 2010).
While the studies using pharmacological and optogenetic manipulation of MS/DBB hippocampal cholinergic neurons suggest these neurons are critical for contextual fear memory, results of studies employing selective MS/DBB cholinergic lesions suggest otherwise. MS/DBB cholinergic lesions have no effects on contextual Pavlovian fear conditioning (Craig, Hong, Kopp, and McDonald, 2009; Frick et al., 2004; Pizzo et al., 2002; Tronson et al., 2009) or IA (Pizzo et al., 2002; Torres et al., 1994). However, MS/DBB cholinergic lesions disrupt contextual fear memory discrimination (Knox and Keller, 2015). If animals are fear conditioned in one context, then tested for fear memory in a novel context, contextual fear expression is observed in this novel context in rats with MS/DBB cholinergic lesions, even though this context was never paired with a UCS (Knox and Keller, 2015).
There are several possible explanations for the discrepancies observed when using pharmacological manipulation in the dHipp vs. MS/DBB cholinergic lesion with regard to contextual fear memory. One reason could be due to cholinergic regeneration that occurs after cholinergic deafferentation of the dHipp (Gage, Buzsaki, and Armstrong, 1990). The ACh content could be somewhat normalized if cholinergic terminal regeneration were to occur after cholinergic lesions in the dHipp, which may render MS/DBB cholinergic lesions ineffective in disrupting contextual fear memory. A previous study has shown that MS/DBB cholinergic lesions attenuate ACh levels in the dHipp by 60% relative to controls (Chang and Gold, 2004). This finding suggests that residual ACh levels may be sufficiently high in the dHipp to mediate contextual fear memory after MS/DBB cholinergic lesions.
In spite of discrepancies observed with pharmacological and optogentic manipulation vs. permanent selection lesions, it is very likely that MS/DBB hippocampal cholinergic neurons modulate contextual fear memory, given the extensive data set supporting this hypothesis. The finding that MS/DBB cholinergic lesions disrupt contextual fear memory discrimination is consistent with this hypothesis.
4.3.2. Extinction memory
MS/DBB cholinergic lesions disrupt acquisition of contextual fear extinction in the Pavlovian fear conditioning paradigm (Tronson et al., 2009) and induce deficits in acquisition of cued extinction memory (Knox and Keller, 2015). These two findings suggest that MS/DBB cholinergic neurons are critical for extinction memory.
4.3.3. Neurobiological mechanisms
MS/DBB cholinergic neurons project to the dHipp, vHipp, RSC, and IL and these neural substrates have been implicated in fear and extinction memory. The RSC has been implicated in recent and long-term contextual fear memory (Corcoran, Donnan, Tronson, Guzman, Gao, Jovasevic, Guedea, and Radulovic, 2011), while the dHipp is critical for contextual fear memory (for reviews see Jadhav, Kemere, German, and Frank, 2012; Maren et al., 2013; Phillips and LeDoux, 1992; Quinn, Loya, Ma, and Fanselow, 2005), as well as trace fear memory (Beeman, Bauer, Pierson, and Quinn, 2013; Kent and Brown, 2012; Raybuck and Lattal, 2011), and contextual control of extinction memory retrieval (Bouton et al., 2006; Corcoran and Maren, 2004). The IL and vHipp are critical for extinction memory (Lebron, Milad, and Quirk, 2004; Milad et al., 2006; Quirk et al., 2006; Quirk, Pare, Richardson, Herry, Monfils, Schiller, and Vicentic, 2010; Sierra-Mercado et al., 2006; Sierra-Mercado, Padilla-Coreano, and Quirk, 2011; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, and Quirk, 2006). Given these observations, it is not surprising that MS/DBB cholinergic neurons are critical for contextual fear memory, trace fear memory, and extinction memory.
How might MS/DBB cholinergic input to the dHipp facilitate contextual and trace fear memory? During IA, mAChR antagonism disrupts ERK phosphorylation (Giovannini, Pazzagli, Malmberg-Aiello, Della Corte, Rakovska, Cerbai, Casamenti, and Pepeu, 2005), which is the last kinase in the MAPK pathway (RAF➔MEK➔ERK). Given that MAPK signaling is critical for fear memory consolidation (Cammarota, Bevilaqua, Ardenghi, Paratcha, Levi de Stein, Izquierdo, and Medina, 2000; Schafe, Atkins, Swank, Bauer, Sweatt, and LeDoux, 2000; Schafe and LeDoux, 2000; Schafe, Nadel, Sullivan, Harris, and LeDoux, 1999; Walz, Roesler, Quevedo, Sant'Anna, Madruga, Rodrigues, Gottfried, Medina, and Izquierdo, 2000) these findings raise the possibility that during contextual and trace fear conditioning, mAChR activation in the dHipp leads to enhanced MAPK signaling, which then contributes to neural plasticity in the dHipp critical for contextual and trace fear memory. However, mechanisms by which mAChR activation leads to enhanced MAPK signaling during contextual (and possibly trace) fear memory formation remain undefined.
MS/DBB cholinergic input to dHipp nAChRs also contribute to contextual and trace fear memory (Davis and Gould, 2007; Davis et al., 2007; Kenney et al., 2012; Marti Barros et al., 2004; Parfitt et al., 2012; Raybuck and Gould, 2010) and this could occur via increased conductance of Ca2+ currents upon nAChR activation. Sustained depolarization of the neuronal membrane leading to enhanced Ca2+ entry into the neuron is believed to trigger a number of events that are critical for neural plasticity that mediates fear memory (Bauer et al., 2002; Davis, 1992a; Davis and Whalen, 2001; Pare et al., 2004). MS/DBB cholinergic input to nAChRs during contextual and trace fear memory formation could enhance Ca2+ conductance in dHipp neurons, which drives plasticity critical for both types of fear memory. This hypothesis is consistent with the observation that the subunit composition of nAChRs in the dHipp (α7 and β4 subunits) allows for relatively specific conductance of Ca2+ currents (Marks et al., 1989; Seguela et al., 1993).
Tronson et al., observed that MS/DBB cholinergic lesions disrupted increases in ERK phosphorylation in CA1 during contextual fear extinction training, which raises the possibility that cholinergic input to CA1 mediates contextual extinction memory via MAPK signaling, though it is not clear if this is initiated by mAChR or nAChR activation. Similar to what was observed for BF amygdalopetal cholinergic neurons, it would appear that the same signaling pathway initiated by AChR activation facilitates contextual fear and extinction memory. As mentioned previously, a difference between fear conditioning and extinction training is levels of arousal/stress, and this could serve as a discriminating signal for cholinergic input to the dHipp. High levels of arousal/stress could bias AChRs in the dHipp to facilitate plasticity critical for contextual and trace fear memory, but low levels of arousal/stress could then bias these receptors to facilitate plasticity critical for contextual extinction memory. Of course, further research is needed to test this hypothesis.
MS/DBB cholinergic lesions disrupt contextual fear memory discrimination (Knox and Keller, 2015), and this effect may be linked to cued extinction memory deficits induced by MS/DBB cholinergic lesions. Formation of distinct ensemble of neurons that respond to and represent specific contexts within the dHipp is critical for pattern separation and discriminating one spatial context from another (Frankland, Cestari, Filipkowski, McDonald, and Silva, 1998; Guzowski, Knierim, and Moser, 2004; McHugh, Jones, Quinn, Balthasar, Coppari, Elmquist, Lowell, Fanselow, Wilson, and Tonegawa, 2007). MS/DBB cholinergic lesions have no effect on place field formation in the dHipp, but inhibit place field re-organization when a rat is moved from a familiar context to a novel context (Ikonen et al., 2002). This raises the possibility that MS/DBB cholinergic neurons may facilitate contextual fear memory discrimination by facilitating the formation of ensembles of neurons in the dHipp that represent distinct contexts. Because fear conditioning always precedes extinction training, deficits in discriminating the fear and extinction contexts could lead to enhanced representation of the fear context. If the fear generated from this contextual representation was resistant to extinction (i.e. deficit in contextual fear memory discrimination and contextual fear extinction), then persistent contextual fear expression would occur, which would then prime cued fear expression (i.e. disrupt cued extinction memory) (Bouton et al., 2006).
Another mechanism via which MS/DBB cholinergic neurons may facilitate extinction memory is via a feedforward positive loop with IL neurons. Previous studies suggest that PFC input to BF cholinergic neurons can drive increased ACh release in the PFC and other neocortical regions (Nelson, Sarter, and Bruno, 2005; Sarter, Hasselmo, Bruno, and Givens, 2005) and this mechanism is believed to be important for attentional processing (Nelson et al., 2005; Parikh, Kozak, Martinez, and Sarter, 2007; Sarter et al., 2005; Zaborszky et al., 1999). Thus, PFC neurons (in particular in the IL and possibly PL) could drive MS/DBB cholinergic neurons to secrete ACh in the dHipp, RSC, and mPFC. In turn, this enhancement in ACh may facilitate formation of contextual memory and neural plasticity critical for extinction memory. Consistent with this hypothesis, mAChR antagonism in the IL disrupts extinction memory formation (Santini et al., 2012). However, more research is needed to explicitly test the hypothesis that MS/DBB cholinergic neurons facilitate extinction memory via a feedforward excitatory loop with neurons in the mPFC. This is particularly so, because mPFC stimulation results in complex single unit responses in BF cholinergic neurons and mPFC neurons do not make direct synapses with BF cholinergic neurons (Gyengesi, Zaborszky, and Detari, 2008). Neurobiological mechanisms via which MS/DBB cholinergic neurons could facilitate fear and extinction memory are illustrated in Figure 5.
Figure 5.
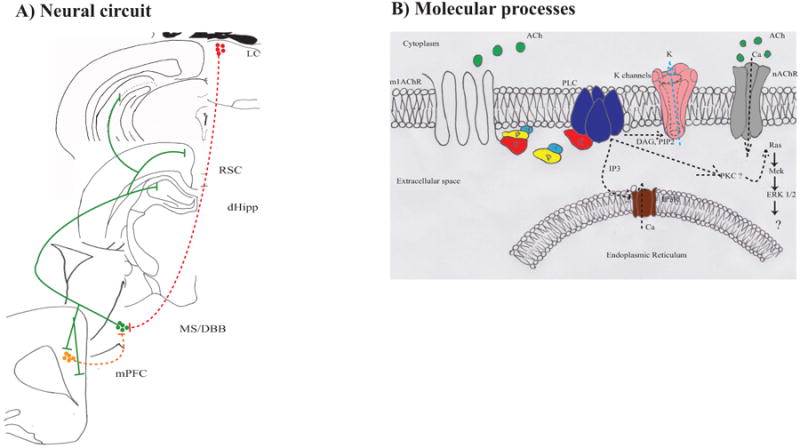
Neurobiological processes via which MS/DBB cholinergic neurons modulate emotional memory. A) Hypothetical circuit. Components of the circuit that need verification are shown in dashed lines. B) Molecular processes by which MS/DBB cholinergic neurons modulate emotional memory. Unknown mechanisms are shown as question marks or dashed lines. Decreased signaling is indicated by light blue arrows.
5. Clinical Relevance
The finding that BF cholinergic neurons are critical for fear and extinction memory may have relevance to anxiety disorders in humans. Changes in fear and extinction memory have been implicated in post traumatic stress disorder (PTSD) and specific phobia (Milad et al., 2006; Pitman, Sanders, Zusman, Healy, Cheema, Lasko, Cahill, and Orr, 2002; Quirk et al., 2006; Rothbaum and Davis, 2003). Neuroscience theories posit that PTSD can stem from enhanced fear conditionability (Rothbaum and Davis, 2003) and/or deficits in extinction memory (Milad et al., 2006; Pitman et al., 2002; Quirk et al., 2006; Quirk et al., 2010), while persistent fear in specific phobia may stem from deficits in extinction memory (Hofmann, Pollack, and Otto, 2006). It may be possible to enhance extinction-based therapies (e.g. exposure therapy, cognitive behavioral therapy) by targeting BF cholinergic systems. Because a number of drugs that increase ACh levels already exist and are in current clinical use, this line of research has high translational value and could have a significant impact on how excessive fear is treated in anxiety disorders such as specific phobia and PTSD.
6. Conclusion
Previous studies that have used a variety of experimental methods (e.g. pharmacological manipulation, selection lesion, direct measurements of acetylcholine in restricted brain regions, optogenetic stimulation) in a number of different behavioral paradigms (e.g. Pavlovian fear conditioning and IA) suggest that BF cholinergic neurons can facilitate fear and extinction memory. However, we still do not have a sufficiently comprehensive understanding of how BF cholinergic neurons modulate these mnemonic processes. In particular, identification of neural circuitry by which clusters of BF cholinergic neurons modulate fear and extinction memory remains undefined. Teasing apart the neural circuitry via which BF cholinergic neurons contribute to fear and extinction memory can be greatly facilitated by optogenetics, since the technology to use light to selectively stimulate or inhibit BF cholinergic efferents in target brain regions (e.g. dHipp vs. RSC) exist (Brown, Tan, O'Connor, Nikonenko, Muller, and Luscher, 2012; Hersman and Fanselow, 2015; Jiang et al., 2016; Unal et al., 2015). In spite of these gaps in knowledge, research conducted to date has demonstrated that BF cholinergic neurons are critical for mediating fear and extinction memory.
Highlights.
BF cholinergic neurons are critical for emotional memory
Different BF cholinergic neuronal clusters have different roles in emotional memory
BF cholinergic function in emotional memory changes with behavioral paradigm
BF cholinergic neurons are a critical component of emotional memory systems
Acknowledgments
I would like to thank all the individuals who helped make my empirical contribution to this review a possibility. My empirical contribution to this review was made possible by NHLBI grant (HL54428) awarded to Gary G. Berntson. I would also like to thank Pauline V. C. David, for her help with the illustrations in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- Amorapanth P, Nader K, LeDoux JE. Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behavior in rats. Learn Mem. 1999;6:491–499. doi: 10.1101/lm.6.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe JH, McKenna TM, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: II. Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse. 1989;4:44–54. doi: 10.1002/syn.890040106. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bayraktar T, Staiger JF, Acsady L, Cozzari C, Freund TF, Zilles K. Colocalization of vasoactive intestinal polypeptide, gamma-aminobutyric acid and choline acetyltransferase in neocortical interneurons of the adult rat. Brain Res. 1997;757:209–217. doi: 10.1016/s0006-8993(97)00218-7. [DOI] [PubMed] [Google Scholar]
- Baysinger AN, Kent BA, Brown TH. Muscarinic receptors in amygdala control trace fear conditioning. PLoS One. 2012;7:e45720. doi: 10.1371/journal.pone.0045720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazalakova MH, Blakely RD. The high-affinity choline transporter: a critical protein for sustaining cholinergic signaling as revealed in studies of genetically altered mice. Handb Exp Pharmacol. 2006:525–544. doi: 10.1007/3-540-29784-7_21. [DOI] [PubMed] [Google Scholar]
- Beeman CL, Bauer PS, Pierson JL, Quinn JJ. Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learn Mem. 2013;20:336–343. doi: 10.1101/lm.031161.113. [DOI] [PubMed] [Google Scholar]
- Benishin CG, Carroll PT. Multiple forms of choline-O-acetyltransferase in mouse and rat brain: solubilization and characterization. J Neurochem. 1983;41:1030–1039. doi: 10.1111/j.1471-4159.1983.tb09047.x. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci. 2003a;18:2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Shafi R, Knox D, Sarter M. Blockade of epinephrine priming of the cerebral auditory evoked response by cortical cholinergic deafferentation. Neuroscience. 2003b;116:179–186. doi: 10.1016/s0306-4522(02)00702-9. [DOI] [PubMed] [Google Scholar]
- Birks RI, Fitch JG. Storage and release of acetylcholine in a sympathetic ganglion. J Physiol. 1974;240:125–134. doi: 10.1113/jphysiol.1974.sp010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, Cryan JF. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol Psychiatry. 2008;63:821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Baratti CM, McGaugh JL. Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiol Learn Mem. 2009;91:93–97. doi: 10.1016/j.nlm.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Krawczyk MC, Baratti CM. Hippocampal alpha7 nicotinic receptors modulate memory reconsolidation of an inhibitory avoidance task in mice. Neuroscience. 2010;171:531–543. doi: 10.1016/j.neuroscience.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Bonner TI. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989;12:148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci. 2010;41:340–346. doi: 10.1007/s12031-010-9377-2. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O'Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Calandreau L, Trifilieff P, Mons N, Costes L, Marien M, Marighetto A, Micheau J, Jaffard R, Desmedt A. Extracellular hippocampal acetylcholine level controls amygdala function and promotes adaptive conditioned emotional response. J Neurosci. 2006;26:13556–13566. doi: 10.1523/JNEUROSCI.3713-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, Medina JH. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: abolition by NMDA receptor blockade. Brain Res Mol Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Impaired and spared cholinergic functions in the hippocampus after lesions of the medial septum/vertical limb of the diagonal band with 192 IgG-saporin. Hippocampus. 2004;14:170–179. doi: 10.1002/hipo.10160. [DOI] [PubMed] [Google Scholar]
- Chatonnet A, Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem J. 1989;260:625–634. doi: 10.1042/bj2600625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron. 2013;80:1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B, Tandon A, Prado MA, Bachoo M. Storage and release of acetylcholine in a sympathetic ganglion. Prog Brain Res. 1993;98:183–189. doi: 10.1016/s0079-6123(08)62397-3. [DOI] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Cooper J, Bloom F, Roth RH. Biochemical Basis of Neuropharmacology. 7th. Oxford University Press, USA; 1996. [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Guedea AL, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig LA, Hong NS, Kopp J, McDonald RJ. Cholinergic depletion of the medial septum followed by phase shifting does not impair memory or rest-activity rhythms measured under standard light/dark conditions in rats. Brain Res Bull. 2009;79:53–62. doi: 10.1016/j.brainresbull.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992a;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992b;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Bannon AW, Arneric SP. Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci. 1995;56:545–570. doi: 10.1016/0024-3205(94)00488-e. [DOI] [PubMed] [Google Scholar]
- Degroot A, Parent MB. Infusions of physostigmine into the hippocampus or the entorhinal cortex attenuate avoidance retention deficits produced by intra-septal infusions of the GABA agonist muscimol. Brain Res. 2001;920:10–18. doi: 10.1016/s0006-8993(01)02798-6. [DOI] [PubMed] [Google Scholar]
- Einarsson EO, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem. 2012;19:449–452. doi: 10.1101/lm.027227.112. [DOI] [PubMed] [Google Scholar]
- Estes W, Skinner B. Some quantitative properties of anxiety. J Exp Psychol. 1941;29:390. [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23:9697–9709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier GN, Materi LM, Semba K, Rasmusson DD. Cortical acetylcholine release and electroencephalogram activation evoked by ionotropic glutamate receptor agonists in the rat basal forebrain. Neuroscience. 2004;123:785–792. doi: 10.1016/j.neuroscience.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim JJ, Baxter MG. Effects of complete immunotoxin lesions of the cholinergic basal forebrain on fear conditioning and spatial learning. Hippocampus. 2004;14:244–254. doi: 10.1002/hipo.10169. [DOI] [PubMed] [Google Scholar]
- Gage FH, Buzsaki G, Armstrong DM. NGF-dependent sprouting and regeneration in the hippocampus. Prog Brain Res. 1990;83:357–370. doi: 10.1016/s0079-6123(08)61262-5. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Pazzagli M, Malmberg-Aiello P, Della Corte L, Rakovska AD, Cerbai F, Casamenti F, Pepeu G. Inhibition of acetylcholine-induced activation of extracellular regulated protein kinase prevents the encoding of an inhibitory avoidance response in the rat. Neuroscience. 2005;136:15–32. doi: 10.1016/j.neuroscience.2005.07.046. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk RB, McGaugh JL. Effects of hormones on time-dependent memory storage processes. Prog Brain Res. 1975;42:210–211. doi: 10.1016/s0079-6123(08)63665-1. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gyengesi E, Zaborszky L, Detari L. The effect of prefrontal stimulation on the firing of basal forebrain neurons in urethane anesthetized rat. Brain Res Bull. 2008;75:570–580. doi: 10.1016/j.brainresbull.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Ohtake T, Wiley RG, Lappi DA, Geula C, Mesulam MM. Complete and selective cholinergic denervation of rat neocortex and hippocampus but not amygdala by an immunotoxin against the p75 NGF receptor. J Neurosci. 1994;14:1271–1289. doi: 10.1523/JNEUROSCI.14-03-01271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hersman S, Fanselow MS. Optogenetic activaiton of medial septum cholinergic neurons improves contextual fea learning and alters choline levels in hippocampus. Society for Neuroscience; Chicago IL: 2015. [Google Scholar]
- Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev. 2006;12:208–217. doi: 10.1111/j.1527-3458.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002;12:386–397. doi: 10.1002/hipo.1109. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I, Saghafi D, Novack GD, McGaugh JL. Memory-enhancing effects of post-training dipivefrin and epinephrine: involvement of peripheral and central adrenergic receptors. Brain Res. 1992;572:81–86. doi: 10.1016/0006-8993(92)90454-h. [DOI] [PubMed] [Google Scholar]
- Isoardi NA, Martijena ID, Carrer HF, Molina VA. Increased fear learning coincides with neuronal dysinhibition and facilitated LTP in the basolateral amygdala following benzodiazepine withdrawal in rats. Neuropsychopharmacology. 2004;29:1852–1864. doi: 10.1038/sj.npp.1300478. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Kundu S, Lederman JD, Lopez-Hernandez GY, Ballinger EC, Wang S, Talmage DA, Role LW. Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits. Neuron. 2016 doi: 10.1016/j.neuron.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Miettinen R, Pikkarainen M, Pitkanen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience. 2002;111:133–149. doi: 10.1016/s0306-4522(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci. 2003;8:s438–451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Supple WF, Jr, Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behav Neurosci. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kart E, Jocham G, Muller CP, Schlomer C, Brandao ML, Huston JP, de Souza Silva MA. Neurokinin-1 receptor antagonism by SR140333: enhanced in vivo ACh in the hippocampus and promnestic post-trial effects. Peptides. 2004;25:1959–1969. doi: 10.1016/j.peptides.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012;22:1681–1690. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent BA, Brown TH. Dual functions of perirhinal cortex in fear conditioning. Hippocampus. 2012;22:2068–2079. doi: 10.1002/hipo.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpai F, Nasehi M, Haeri-Rohani A, Eidi A, Zarrindast MR. Scopolamine induced memory impairment; possible involvement of NMDA receptor mechanisms of dorsal hippocampus and/or septum. Behav Brain Res. 2012;231:1–10. doi: 10.1016/j.bbr.2012.02.049. [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Knox D, Berntson GG. Effect of nucleus basalis magnocellularis cholinergic lesions on fear-like and anxiety-like behavior. Behavioral Neuroscience. 2006;120:307–312. doi: 10.1037/0735-7044.120.2.307. [DOI] [PubMed] [Google Scholar]
- Knox D, Berntson GG. Cortical modulation by nucleus basalis magnocellularis corticopetal cholinergic neurons during anxiety-like states is reflected by decreases in delta. Brain Res. 2008;1227:142–152. doi: 10.1016/j.brainres.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Keller SM. Cholinergic neuronal lesions in the medial septum and vertical limb of the Diagonal Bands of Broca induce contextual fear memory generalization and mpair acquisition of fear extinction. Hippocampus. 2015;26:718–726. doi: 10.1002/hipo.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Sarter M, Berntson GG. Visceral afferent bias on cortical processing: role of adrenergic afferents to the basal forebrain cholinergic system. Behav Neurosci. 2004;118:1455–1459. doi: 10.1037/0735-7044.118.6.1455. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ, Flesher SN, Jhamandas K, Boegman RJ. Nucleus basalis lesions: implication of basoamygdaloid cholinergic pathways in memory. Brain Res Bull. 1995;36:51–56. doi: 10.1016/0361-9230(94)00162-t. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000a;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000b;20:9252–9263. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- Marchand AR, Luck D, Di Scala G. Trace fear conditioning: a role for context? Arch Ital Biol. 2004;142:251–263. [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- Marti Barros D, Ramirez MR, Dos Reis EA, Izquierdo I. Participation of hippocampal nicotinic receptors in acquisition, consolidation and retrieval of memory for one trial inhibitory avoidance in rats. Neuroscience. 2004;126:651–656. doi: 10.1016/j.neuroscience.2004.03.010. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Koene RA, Eichenbaum H, Hasselmo ME. Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatch-to-sample task. J Neurosci. 2005;25:10273–10281. doi: 10.1523/JNEUROSCI.2386-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS. Molecular diversity of neuronal nicotinic acetylcholine receptors. Ann N Y Acad Sci. 1999;868:565–577. doi: 10.1111/j.1749-6632.1999.tb11330.x. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Messamore E, Warpman U, Williams E, Giacobini E. Muscarinic receptors mediate attenuation of extracellular acetylcholine levels in rat cerebral cortex after cholinesterase inhibition. Neurosci Lett. 1993;158:205–208. doi: 10.1016/0304-3940(93)90265-m. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983b;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K, Dourmap N, Jaffard R, Costentin J. The specific dopamine uptake inhibitor GBR 12783 improves learning of inhibitory avoidance and increases hippocampal acetylcholine release. Brain Res Cogn Brain Res. 1998;7:203–205. doi: 10.1016/s0926-6410(98)00023-8. [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K, Dourmap N, Jaffard R, Costentin J. Contextual fear conditioning is associated with an increase of acetylcholine release in the hippocampus of rat. Brain Res Cogn Brain Res. 2000;9:193–197. doi: 10.1016/s0926-6410(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A, del Guante MA, Prado-Alcala RA, Aleman V. Changes in rat brain muscarinic receptors after inhibitory avoidance learning. Life Sci. 1996;58:799–809. doi: 10.1016/0024-3205(95)02358-5. [DOI] [PubMed] [Google Scholar]
- Pang MH, Kim NS, Kim IH, Kim H, Kim HT, Choi JS. Cholinergic transmission in the dorsal hippocampus modulates trace but not delay fear conditioning. Neurobiol Learn Mem. 2010;94:206–213. doi: 10.1016/j.nlm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parfitt GM, Campos RC, Barbosa AK, Koth AP, Barros DM. Participation of hippocampal cholinergic system in memory persistence for inhibitory avoidance in rats. Neurobiol Learn Mem. 2012;97:183–188. doi: 10.1016/j.nlm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passani MB, Cangioli I, Baldi E, Bucherelli C, Mannaioni PF, Blandina P. Histamine H3 receptor-mediated impairment of contextual fear conditioning and in-vivo inhibition of cholinergic transmission in the rat basolateral amygdala. Eur J Neurosci. 2001;14:1522–1532. doi: 10.1046/j.0953-816x.2001.01780.x. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned refelexes. London: Oxford University Press; 1927. [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Pizzo DP, Thal LJ, Winkler J. Mnemonic deficits in animals depend upon the degree of cholinergic deficit and task complexity. Exp Neurol. 2002;177:292–305. doi: 10.1006/exnr.2002.7993. [DOI] [PubMed] [Google Scholar]
- Power AE, McGaugh JL. Cholinergic activation of the basolateral amygdale regulates unlearned freezing behavior in rats. Behav Brain Res. 2002a;134:307–315. doi: 10.1016/s0166-4328(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Power AE, McGaugh JL. Phthalic acid amygdalopetal lesion of the nucleus basalis magnocellularis induces reversible memory deficits in rats. Neurobiol Learn Mem. 2002b;77:372–388. doi: 10.1006/nlme.2001.4030. [DOI] [PubMed] [Google Scholar]
- Power AE, Thal LJ, McGaugh JL. Lesions of the nucleus basalis magnocellularis induced by 192 IgG-saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala. Proc Natl Acad Sci U S A. 2002;99:2315–2319. doi: 10.1073/pnas.022627799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Sah P. Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci. 2002;22:3454–3462. doi: 10.1523/JNEUROSCI.22-09-03454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado VF, Roy A, Kolisnyk B, Gros R, Prado MA. Regulation of cholinergic activity by the vesicular acetylcholine transporter. Biochem J. 2013;450:265–274. doi: 10.1042/BJ20121662. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Gold PE. Intrahippocampal infusions of anisomycin produce amnesia: contribution of increased release of norepinephrine, dopamine, and acetylcholine. Learn Mem. 2009;16:308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quirion R. Cholinergic markers in Alzheimer disease and the autoregulation of acetylcholine release. J Psychiatry Neurosci. 1993;18:226–234. [PMC free article] [PubMed] [Google Scholar]
- Quirion R, Richard J, Wilson A. Muscarinic and nicotinic modulation of cortical acetylcholine release monitored by in vivo microdialysis in freely moving adult rats. Synapse. 1994;17:92–100. doi: 10.1002/syn.890170205. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Pare D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D, Tuszynski MH, Conner JM. The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. J Neurosci. 2009;29:5992–6000. doi: 10.1523/JNEUROSCI.0230-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One. 2011;6:e15982. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shock-induced fear conditioning. Learn Mem. 2004;11:102–107. doi: 10.1101/lm.64604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology. 2012;37:2047–2056. doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Schauz C, Koch M. Lesions of the nucleus basalis magnocellularis do not impair prepulse inhibition and latent inhibition of fear-potentiated startle in the rat. Brain Res. 1999;815:98–105. doi: 10.1016/s0006-8993(98)01134-2. [DOI] [PubMed] [Google Scholar]
- Schimerlik MI. Structure and regulation of muscarinic receptors. Annu Rev Physiol. 1989;51:217–227. doi: 10.1146/annurev.ph.51.030189.001245. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K. The cholinergic basal forebrain: a critical role in cortical arousal. Adv Exp Med Biol. 1991;295:197–218. doi: 10.1007/978-1-4757-0145-6_10. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011;31:9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Wainer BH, Klein R, Barbacid M, Palfrey HC. High-affinity nerve growth factor receptor (Trk) immunoreactivity is localized in cholinergic neurons of the basal forebrain and striatum in the adult rat brain. Brain Res. 1993;612:330–335. doi: 10.1016/0006-8993(93)91681-h. [DOI] [PubMed] [Google Scholar]
- Stowell JR, Berntson GG, Sarter M. Attenuation of the bidirectional effects of chlordiazepoxide and FG 7142 on conditioned response suppression and associated cardiovascular reactivity by loss of cortical cholinergic inputs. Psychopharmacology (Berl) 2000;150:141–149. doi: 10.1007/s002130000443. [DOI] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. Pharmacology of protein kinase C activators: cognition-enhancing and antidementic therapeutics. Pharmacol Ther. 2010;127:66–77. doi: 10.1016/j.pharmthera.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Torres EM, Perry TA, Blockland A, Wilkinson LS, Wiley RG, Lappi DA, Dunnet SB. Behavioural, histochemical and biochemical consequences of selective immunolesions in discrete regions of the basal forebrain cholinergic system. Neuroscience. 1994;63:95–122. doi: 10.1016/0306-4522(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J Neurosci. 2009;29:3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal CT, Pare D, Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci. 2015;35:853–863. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75:245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Walz R, Roesler R, Quevedo J, Sant'Anna MK, Madruga M, Rodrigues C, Gottfried C, Medina JH, Izquierdo I. Time-dependent impairment of inhibitory avoidance retention in rats by posttraining infusion of a mitogen-activated protein kinase kinase inhibitor into cortical and limbic structures. Neurobiol Learn Mem. 2000;73:11–20. doi: 10.1006/nlme.1999.3913. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain. 2013;6:11. doi: 10.1186/1756-6606-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol Learn Mem. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: receptive fields plasticity, model, and mechanisms. Audiol Neurootol. 1998;3:145–167. doi: 10.1159/000013787. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci. 1993;107:955–962. [PubMed] [Google Scholar]
- Williams EC, Clayton EC. Contribution of brainstem structures in modulating memory storage processes. In: G PE, Greenough WT, editors. Memory consolidation: Essays in honor of James L McGaugh. Washington: American Psychological Association; 2001. pp. 141–163. [Google Scholar]
- Winters BD, Bussey TJ. Removal of cholinergic input to perirhinal cortex disrupts object recognition but not spatial working memory in the rat. Eur J Neurosci. 2005;21:2263–2270. doi: 10.1111/j.1460-9568.2005.04055.x. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic projections to the basolateral amygdala: a combined Evans Blue and acetylcholinesterase analysis. Brain Res Bull. 1982;8:751–763. doi: 10.1016/0361-9230(82)90102-2. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic projections from the basal forebrain to the frontal cortex: a combined fluorescent tracer and immunohistochemical analysis in the rat. Neurosci Lett. 1983;40:93–98. doi: 10.1016/0304-3940(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Research Bulletin. 1984;13:751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann N Y Acad Sci. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Ardjmand A, Ahmadi S, Rezayof A. Activation of dopamine D1 receptors in the medial septum improves scopolamine-induced amnesia in the dorsal hippocampus. Behav Brain Res. 2012;229:68–73. doi: 10.1016/j.bbr.2011.12.033. [DOI] [PubMed] [Google Scholar]


