Abstract
This review summarizes a prospective, longitudinal 10-year study in Rochester NY with virtually every clinically diagnosed acute otitis media (AOM) confirmed by bacterial culture of middle ear fluid. Children experiencing 3 episodes within 6 months or 4 episodes in 12 months were considered stringently-defined otitis prone (sOP). We found stringent diagnosis compared with clinical diagnosis reduced the frequency of children meeting the OP definition from 27% to 6% resulting in 14.8% and 2.4% receiving tympanostomy tubes, respectively. Significantly more often RSV infection led to AOM in sOP than non-otitis prone (NOP) children that correlated with diminished total RSV-specific serum IgG. sOP children produced low levels of antibody to Streptococcus pneumoniae and Haemophilus influenzae candidate vaccine protein antigens and to routine pediatric vaccines. sOP children generated significantly fewer memory B cells, functional and memory T cells to otopathogens following NP colonization and AOM than NOP children and they had defects in antigen presenting cells.
Keywords: Acute Otitis Media, otitis prone: Stringently-defined
Introduction
Acute otitis media (AOM) often recurs, and the impact of AOM on health costs is significant due to direct medical costs including doctor visits, antibiotic prescriptions and referral to otolaryngologists for surgery as well as indirect medical costs such as lost job productivity for the parents and lost time at school or daycare for the children.1 In the United States alone, the economic burden of otitis media rose to $6 billion annually during1998–2008 in medical treatment, surgical management, and loss of income for working parents.2
During the time frame 2006–2016 our group in Rochester NY conducted investigations of the otitis prone child. We applied a challenging and unprecedented study design wherein every attempt was made to confirm each episode of clinically diagnosed AOM by tympanocentesis. To distinguish our study population we proposed a new term for these children - “stringently-defined” otitis prone (sOP) when these children experienced 3 AOM episodes in 6 months or 4 AOM episodes in 12 months.3
Study Design
We prospectively enrolled and examined every child for nearly every illness and confirmed nearly every clinical diagnosis of AOM by culture of middle ear fluid (MEF) using tympanocentesis from 6 months to 36 months of age. In this manner we prospectively identified children who became otitis prone (stringently-defined; sOP), who had infrequent otitis, or who remained otitis free.
Subjects came from a middle class, suburban socio-demographic private pediatric practice population. Children were seen for illness visits by one of two physician investigators, both of whom are validated otoscopists.3 Demographic data collected included family history of AOM, day-care attendance, breastfeeding history, number and ages of siblings and tobacco smoke exposure.4 This review describes results from 760 of 840 primary care patients evaluated at Legacy Pediatrics including >4,000 visits of which 970 were AOM episodes where tympanocentesis was performed. The 80 excluded subjects were the result of no samples collected after the enrollment visit due to parental refusal and subject withdrawal, family relocation, lack of child cooperation or difficult blood draw at multiple visits. 14 episodes of possible AOM occurred that were diagnosed by physicians in Urgent Care or Emergency Room settings and not examined and tapped the next day; these illnesses were not included as AOM episodes. Tympanocentesis without obtaining fluid occurred in a single ear during episodes when bilateral infection was suspected in 2% of cases but there were no cases where a unilateral AOM yielded a “dry tap” or where both tympanic membranes were tapped and neither yielded middle ear pus. Subject samples from all types of episodes were treated and analyzed the same. This study was approved by the National Institute of Health, National Institute on Deafness and Other Communication Disorders along with approval of Rochester General Hospital Institutional Review Board.
Incidence of Otitis Proneness and Effect of Tympanocentesis
In many prior studies the definition of OP was based exclusively on clinical diagnoses made by primary care providers and about 30% of children in the US became classified as OP in the first years of life5–7 We compared the frequency with which children met the definition of OP based on clinical diagnoses made by community-based pediatricians (community control group) with the frequency observed when the clinical diagnosis was made by validated otoscopists but without tympanocentesis and the frequency where the sOP definition was applied.3 In the community control group by age 2 years old the cumulative frequency of meeting the OP definition was 27% compared to 14% of children diagnosed by validated otoscopists in the Legacy group and 6% in the sOP (intervention) group diagnosed by the same two validated otoscopists (Figure 1).3 We attribute the differences between the intervention group that received tympanocentesis and that of the Legacy group that did not receive tympanocentesis to 2 possible factors: (1) tympanocentesis helped remove pus, bacteria and provided aeration of the middle ear space thereby removing tissue damaging pro-inflammatory cytokine8 and reducing the likelihood of biofilm formation9; and (2) modification of the therapy based on MEF cultures. These factors contributed to faster recovery and health of the child and reduction in AOM treatment failure. The data provide a reference for frequency of over-diagnosis of AOM by primary care pediatricians compared to validated otoscopists and the benefits of tympanocentesis drainage of MEF. Figure 2 shows the percentage of children that received tympanostomy tube surgery: 14.8% of community control children, 6.3% when diagnosed by validated otoscopists and 2.4% of sOP children.3 The data provide a reference for frequency of tympanostomy tube surgery based on referrals to otolaryngologists by primary care pediatricians compared to validated otoscopists and sOP children.
Figure 1. AOM-Free Rates.
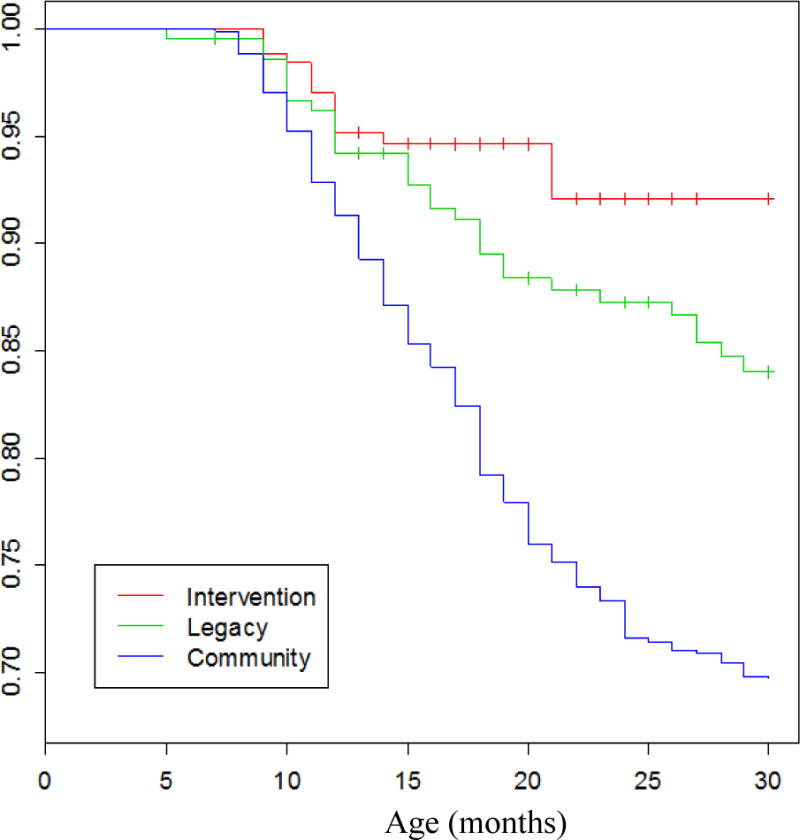
Kaplan–Meier estimates of proportion children with AOM as they age in the Intervention, Legacy and Community groups.3
Figure 2. PET-Free Rates.
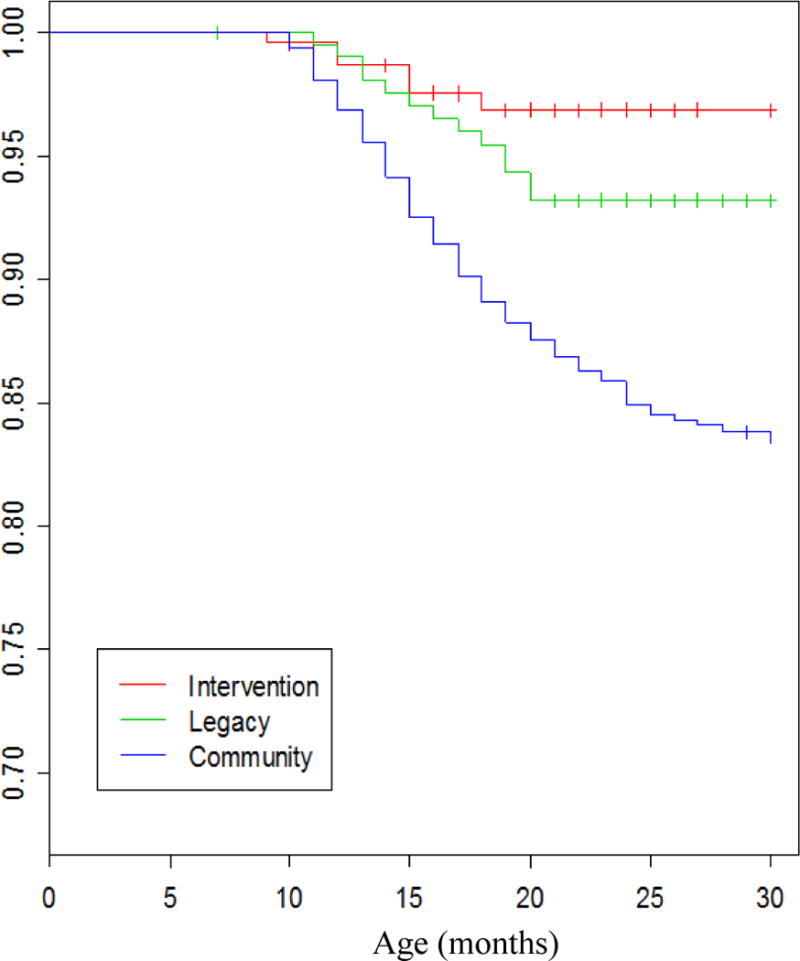
Kaplan–Meier estimates of proportion children receiving tympanostomy tubes as they age in the Intervention, Legacy and Community groups.3
Differences in Pathogenesis During Viral URI Leading to AOM
A risk factor known to be associated with AOM is a preceding or concurrent viral upper respiratory tract infection (URI).10 In our studies, at onset of AOM, 93% of the children had clinical signs of a viral URI.11 We examined the differential impact of respiratory syncytial virus (RSV) and parainfluenza virus (PIV) URIs on the frequency of AOM caused by Spn and NTHi in sOP and non-otitis prone (NOP) children as a potential mechanism to explain increased susceptibility to AOM.12 A significant increase was found in frequency of AOM events caused by Spn and NTHi, with a concurrent RSV infection in sOP children but not with PIV. These results correlated with diminished total RSV-specific serum IgG, higher viral nasal burdens and lower serum IgG neutralizing capacity. To investigate the interface of a diminished neutralizing antibody response to virus and correlative heightened viral replication in relation to neutrophil phagocytic function during AOM, an ex vivo phagocytic assay was developed. We found neutrophils isolated from the blood of children infected with RSV or PIV had a reduced capacity to ingest Spn in both groups of children with greater significant interference caused by RSV. Hence, the lower innate and adaptive immune responses to RSV in sOP children resulted in slower kinetics of viral clearance from the NP and allowed for viral interference with innate antibacterial immune responses, thus contributing to increased frequency of AOMs.
Immunological Features of Stringently Defined Otitis Prone Children
There are many factors that may contribute to children with recurrent AOM who we define as sOP3 such as environmental, epigenetics, anatomical and inheritable genetics. The focus of our work has been to study differences in pathogenesis and the immunological causes for otitis proneness and point towards possible immunotherapeutic treatments for recurrent AOM. The approach we have taken is to dissect the immune response elements of antibody response, B and T cells and professional antigen presenting cells (APCs) is depicted in Figure 3.
Figure 3. B-cell, T-cell and APC Responses.
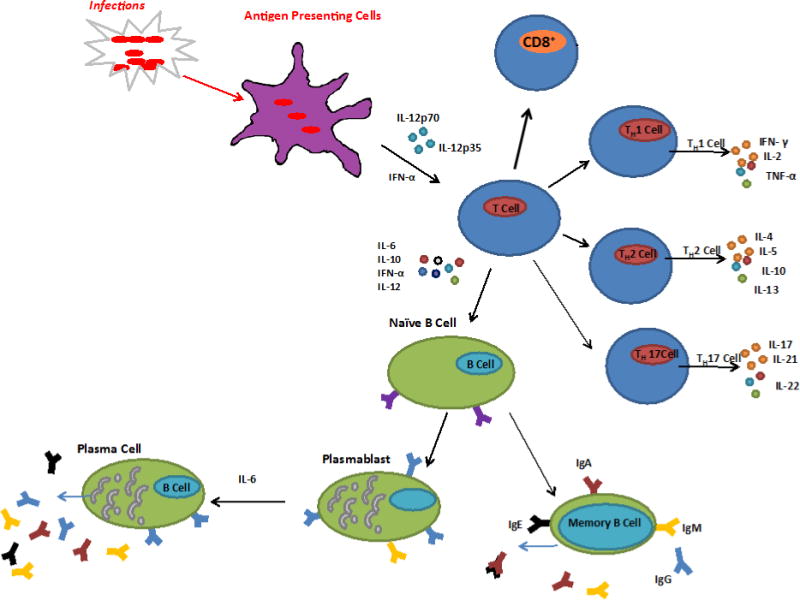
Recognition of infecting agent by antigen presenting cells (APC) and subsequent interaction with T-cells. Activation of B-cells result in antibody production and long-lived memory B-cells. Activation of CD4+ T-cells via MHCII pathway produces Th1, Th2 or Th17 depending on the cytokine stimulation. Recognition of infecting agent via the MHCI pathway activates cytotoxic CD8+ T-cells that directly attack the infecting cell.
Serum Antibody Immune Responses to Otopathogens After Nasopharyngeal Colonization and AOM
Nasopharyngeal (NP) colonization is a natural immunization event and most children have been colonized once or multiple times with Spn, NTHi or Mcat at some point during early childhood. Therefore, we undertook studies to determine the serum antibody levels in sOP and NOP children to Spn proteins, PhtD, LytB, PcpA, PhtE and Ply, and to NTHi proteins D, P6 and OMP26. Specifically, we investigated antibody levels as children transitioned from 6 to 30–36 months old, following colonization and AOM and during convalescence from AOM. All the proteins selected were candidates for inclusion in multi-component vaccines targeting the respective organisms.
Spn proteins
We evaluated the Spn antigen-specific serum IgG titers in sOP and NOP children associated with NP colonization. Among the Spn antigens, IgG titers to PhtD, LytB, PhtE, and PlyD1 were significantly lower in sOP than in NOP children, whereas PcpA levels were not significantly different between the groups as shown in Figure 4.11 At their acute AOM visit, serum antibody titers to PhtD, LytB, PhtE and Ply in sOP children were significantly lower compared to NOP children (p <0.05) and children with AOM treatment failure (AOMTF) (p <0.05).13 Comparing acute to convalescent titers after AOM we found that sOP and NOP children had no significant change in geometric mean IgG titers against the five proteins, but detailed analysis showed that about one-third of the children in each cohort had a 2-fold rise in antibody to the studied antigens.11 We concluded that sOP and AOMTF children mount less of an IgG serum antibody response than NOP children to Spn proteins following AOM and NP colonization.
Figure 4.
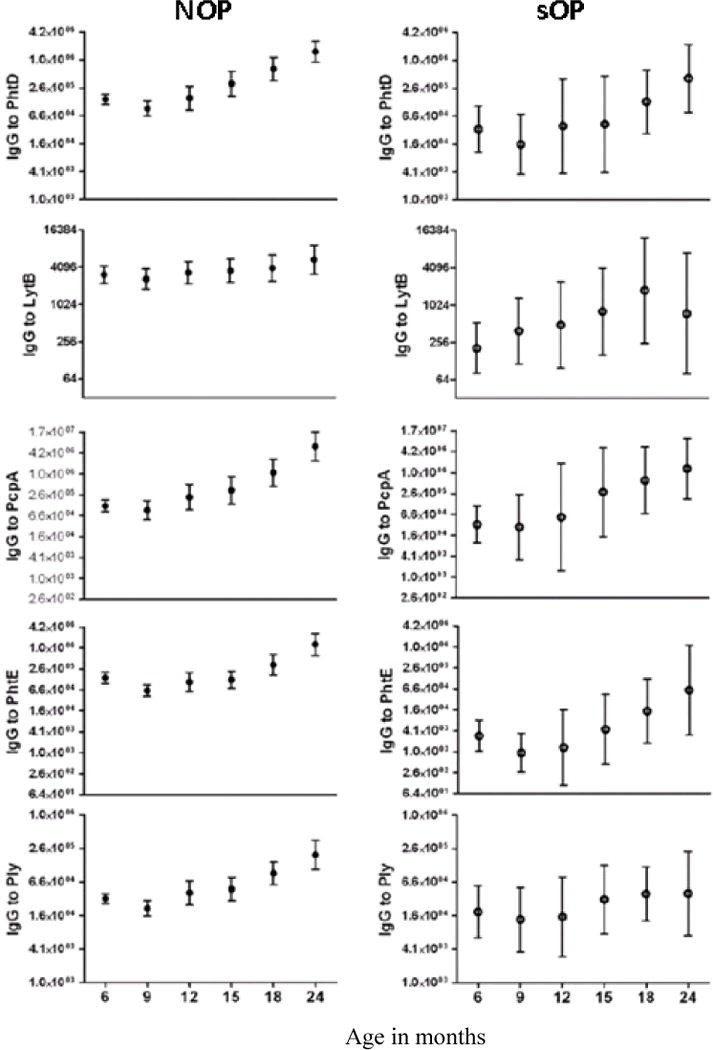
Antibody levels to Spn antigens in non-otitis prone (NOP) and otitis prone (sOP) children, age 6–24 months. Significant difference for all five antigens except for LytB (p<0.07), comparing relative rise in IgG serum antibody between 6 to 24 months was found in NOP children while the difference was not significant in sOP children. Therefore must confirm potential effectiveness of candidate vaccine proteins in this immunologically different and vulnerable population.11
NTHi proteins
We compared the serum antibody responses to NTHi proteins P6, protein D and OMP26 in sOP and NOP children during NP colonization and AOM.14 The IgG titers following NP colonization against P6, protein and OMP26 in sOP children were significantly lower compared to NOP children. Comparing acute to convalescent titers after AOM, sOP children had no significant change in total IgG against all three proteins, while NOP children had significant increases to protein D.
Since bactericidal activity is one of the main host mechanisms in bacterial clearance, we tested the functionality of the serum antibodies in a bactericidal assay. Antibodies to protein D and P6 but not OMP26 were bactericidal to the homologous infecting AOM NTHi strain.15 Levels of protein D and P6 but not OMP26 antibodies were higher in bactericidal sera compared with non-bactericidal sera. For five (24%) and 16 (76%) of 21 bactericidal sera tested, removal of anti-protein D and P6 antibody, respectively, resulted in a two- to fourfold drop in bactericidal antibody. As observed in the previous study, antibodies to OMP26 did not make any contribution to the overall bactericidal activity in any serum samples.
Mcat proteins
We selected 5 Mcat surface proteins to study as candidates for an AOM vaccine in children 6–30 months of age.16 Thus far, all 5 Mcat antigens evaluated showed a significant rise in serum IgG levels over time in healthy children from 6–30 months of age (p<0.001), with a rank order as follows: Msp22 = OppA > OMPCD = Hag = PilA2. We have recently commenced study of these antigens in sOP children.
Antibody Responses Following Routine Vaccinations in otitis prone children
Our results of differing antibody responses in sOP versus NOP children highlight an important immunological dysfunction in sOP children. This phenomenon we describe as neonatal-like because subsequent studies demonstrate immunologic immaturity as the mechanism.17 A question arose as to whether sOP children would also elicit lower antibody responses to pediatric vaccine immunizations. We analyzed sera collected from sOP children for IgG concentrations to diphtheria toxoid, tetanus toxoid, pertussis toxoid, filamentous hemagglutinin, pertactin (DTaP), polio, hepatitis B, H. influenzae type b capsule polyribosyl-ribitol-phosphate (PRP) and Spn capsular polysaccharide conjugate vaccine. IgG protective titers to diphtheria toxoid, tetanus toxoid, pertussis toxoid, filamentous hemagglutinin, pertactin, hepatitis B, polio 3 and Spn 23F but not polio 1,2, PRP or Spn 6B, and 14 were decreased in sOP versus NOP children.17 A high percentage of sOP children had non-protective antibody titers to current pediatric vaccines that persisted until 24 months of age despite routine vaccination boosters. Vulnerable sOP children likely do not contract vaccine-preventable infections because they are protected by herd immunity.
Adaptive Immune Cell Responses
Memory B Cells
Antigen specific memory B cells act as reservoirs for serum antibody maintenance that, on antigen reencounter and with adequate help from CD4 T cells, can proliferate into plasma cells and lead to an increase in the serum antibody levels. We determined the percentages of memory B cells to the Spn antigens, PhtD, LytB, PcpA, PhtE, and Ply, in sOP and NOP children at the time of AOM or NP colonization with Spn.18 We found significantly lower percentages of memory B cells in sOP children to 3 Spn protein antigens (PhtD, PhtE, and Ply) compared to NOP children Figure 5.16 The percentage of circulating PhtD-specific memory B cells correlated with serum IgG levels to PhtD.
Figure 5.
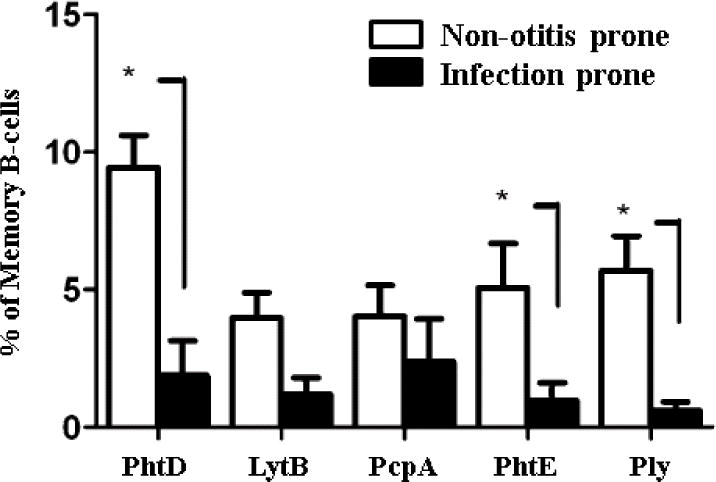
During nasopharyngeal colonization with Spn or Spn-caused AOM, infection prone (sOP) children develop a lower percentage of memory B-cells to Spn antigens PhtD, LytB, PcpA, PhtE and Ply than NOP children. *(p<0.05)16
Knowing that a high percentage of sOP children also fail to elicit protective antibody levels to standard pediatric vaccines17, we studied memory B cell responses to DTaP vaccine antigens diphtheria toxoid (DT), tetanus toxoid (TT) and pertussis toxoid (PT) of sOP and NOP children.19 We found similar proportions of CD19+ B cells in the lymphocyte population in both groups of children but significantly lower percentages of memory CD19+ CD27+ B cells in sOP children. The frequencies of DT, TT or PT-specific memory B cells in sOP children were significantly lower compared to NOP children. We also found a significant correlation between IgG levels and memory B cell frequencies for DT, TT and PT antigens in both sOP and NOP children. This is in agreement with earlier reports that antigen-specific memory B cells persist years after vaccination and correlate with humoral immunity.19
The overall results suggested that the antigen-specific B cell memory populations in sOP children have a mechanistic dysfunction, interfering with proliferation or differentiation into plasma cells. The end result of this dysfunction is that sOP children have lower immune responses to vaccine antigens administered parenterally.
Memory CD4 T Cells
We studied the generation of different subsets of memory CD4 T-helper cells (Th-1, Th-2, and Th-17) to the Spn and NTHi protein antigens discussed above.20 sOP and NOP children were compared for pathogen-specific CD4 T-helper memory responses by stimulating PBMCs with 6 Spn or 3 NTHi protein antigens. Table 1 shows cytokine response level comparison of sOP versus NOP CD4 T-helper memory cells to the antigens. To determine whether there was an intrinsic T-cell defect in sOP children, we stimulated the PBMCs with Staphylococcal Enterotoxin B (SEB) and observed no difference in the percentage of memory CD45RALow CD4 T cells producing IFN-γ, IL-4, IL-2 or IL-17a in sOP vs. NOP children (see Figure, SDC 1).21 The results from this study showed that sOP children have suboptimal circulating functional T-helper memory cells after colonization and after AOM and this immune dysfunction contributes to susceptibility to recurrent AOM infections.
Table 1.
Relative Cytokine-specific CD4 Memory (CD69+) T-cell responses of sOP vs. NOP
| Stimulus | INF-γ | IL-2 | IL-4 | IL-17a |
|---|---|---|---|---|
| Spn-PhtD | ↓(p<0.02) | ↓(p<0.05) | ↓(p<0.02) | ↓(p<0.05) |
| Spn-PcpA | ↓(p<0.02) | ↓(p<0.005) | NS | ↓(p<0.05) |
| Spn-PhtE | ND | ↓(p<0.05) | NS | ↓(p<0.05) |
| Spn-PlyD1 | ND | ↓(p<0.005) | NS | NS |
| Spn-PspA | ↓(p<0.02) | ↓(p=0.02) | NS | NS |
| Spn-LytB | ND | NS | ↓(p<0.02) | NS |
| NTHi-P6 | ND | ↓(p<0.05) | ↓(p<0.05) | NR |
| NTHi-D | ND | ND | NS | NS |
| NTHi-Omp26 | ND | ↓(p<0.05) | NS | ↓(p<0.05) |
ND = Not detected in sOP; NS = No significant difference; NR = No response in sOP and NOP
Innate Immune Responses
Professional Antigen Presenting Cells
Activation of antigen presenting cells (APCs), as part of the innate immunity response, plays a significant role in the quantity and quality of the adaptive immune response. Single-stranded viral RNAs are recognized by TLR7 and 8 in the endosomal membrane and triggering of these leads to release of type 1 IFNs and cytokines that modulate immune responses.22 We found that APCs isolated from PBMCs of sOP children have lower expression of TLR7 during RSV infections compared to NOP children.12 No difference was observed in TLR8 expression from either population during RSV infections.
We investigated whether APCs of sOP infants elicited any defects that might account for the dysfunction we detected in otopathogen-specific antibody, B-cell memory and T-cell memory responses.23 APC phenotypic counts, MHC II expression and intracellular cytokine levels were determined in response to TLR 7/8 (R848) stimulation.24 No significant differences in APC activation or function were observed. Innate immune mRNA levels of various TLRs, intracellular signaling molecules as well as downstream cytokines measured from whole PBMCs were also not found to be significantly different between sOP and NOP infants. However, statistically significant increases in the phenotypic counts of monocytes and conventional DCs but not plasmacytoid DCs were observed in sOP compared to NOP age-matched infants. Higher numbers of APCs in sOP infants suggest the possibility of a persistent mucosal inflammatory status. Overall, the data suggests that among sOP infants, their systemic innate responses are not different compared to NOP infants.
Future Directions
We have accumulated sufficient immunologic evidence to hypothesize that sOP children might be divided into subgroups with different immunologic and non-immunologic explanations of otitis proneness. We discovered that analysis of antibody responses to routine vaccines was a useful tool to identify sub-groups. We would have preferred to apply antibody responses following NP colonization but the variability in age when colonization occurred, frequency with which it occurred and silent colonization events missed by our sampling intervals precluded that approach. Using differences in antibody levels to vaccines administered by injection at standard times to all of the children in the study cohort we identified about 30% of our sOP population that responded with very low quantities of antibody. We propose that this cohort suffers from frequent AOM due to immunologic deficiencies relating to delayed maturation of their immune response (prolonged neonatal-like immunity). 50% of the sOP children have normal responses to vaccinations and we propose that this cohort suffers from frequent AOM due to anatomic problems (e.g. Eustachian tube dysfunction) and/or high prevalence of risk factors for AOM (e.g. day care attendance and excessively frequent viral URI). About 10% of the sOP children have remarkably high immune responses to vaccination and infection and we propose that this cohort may experience more frequent AOM because of a hyper-inflammatory response that damages the NP and/or middle ear sufficiently to allow recurrent infections to occur.
We have begun and plan to expand our studies of specific mechanistic defects in B cells, T cells and professional APCs of sOP children. In preliminary work we have identified sub-optimal B cell receptor activation and altered activation of intracellular pathways in both B and T cells in the cohort of sOP children who display deficiencies in immune responses. APCs of those same sOP children have variable and diminished expression of TLRs that may play a role in downstream activation of both B and T cells. We plan to use results gathered regarding mechanistic defects in the immune cells of sOP children to begin experiments to improve immune responses.
Acknowledgments
Contributors of this work from Rochester General Hospital Research Institute: Drs. Qingfu Xu, Ravinder Kaur, Nadeem Khan, Naveen Surendran, Saleem Basha, Sharad Sharma, David Verhoeven, Dabin Ren, Matthew Morris and Robert Zagursky, and administrative assistant, Kari Pedreira; from Legacy Pediatrics: Dr. Janet Casey and her staff along with the families who have participated in this research; from University of Rochester: Drs. Tim Mosmann, Alexandra Livingstone and Anthony Almudevar.
Funding: This study was supported by NIH NIDCD R01 DC08671.
References
- 1.Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7(4):e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey JR, Pichichero ME. Payment analysis of two diagnosis and management approaches of acute otitis media. Clin Pediatr (Phila) 2014;53(9):865–873. doi: 10.1177/0009922814533592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichichero ME, Casey JR, Almudevar A. Reducing the Frequency of Acute Otitis Media by Individualized Care. Pediatr Infect Dis J. 2013;32(5):473–478. doi: 10.1097/INF.0b013e3182862b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32(8):805–809. doi: 10.1097/INF.0b013e31828d9acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson D, Oster G, McGarry LJ, Klein JO. Management of otitis media among children in a large health insurance plan. Pediatr Infect Dis J. 1999;18(3):239–244. doi: 10.1097/00006454-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Alho OP, Laara E, Oja H. What is the natural history of recurrent acute otitis media in infancy? J Fam Pract. 1996;43(3):258–264. [PubMed] [Google Scholar]
- 7.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119(4):707–715. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 8.Kaur R, Casey J, Pichichero M. Cytokine, chemokine, and toll-like receptor expression in middle ear fluids of children with acute otitis media. Laryngoscope. 2015;125(1):E39–44. doi: 10.1002/lary.24920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osgood R, Salamone F, Diaz A, Casey JR, Bajorski P, Pichichero ME. Effect of pH and oxygen on biofilm formation in acute otitis media associated NTHi clinical isolates. Laryngoscope. 2015;125(9):2204–2208. doi: 10.1002/lary.25102. [DOI] [PubMed] [Google Scholar]
- 10.Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol. 2010;87(2):213–222. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Almudervar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerg Infect Dis. 2012;18(11):1738–1745. doi: 10.3201/eid1811.111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhoeven D, Xu Q, Pichichero ME. Differential Impact of Respiratory Syncytial Virus and Parainfluenza Virus on the Frequency of Acute Otitis Media Is Explained by Lower Adaptive and Innate Immune Responses in Otitis-Prone Children. Clin Infect Dis. 2014;59(3):376–383. doi: 10.1093/cid/ciu303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J. 2011;30(8):645–650. doi: 10.1097/INF.0b013e31821c2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29(5):1023–1028. doi: 10.1016/j.vaccine.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS Immunol Med Microbiol. 2012;65(3):439–447. doi: 10.1111/j.1574-695X.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 16.Ren D, Almudevar AL, Murphy TF, et al. Serum antibody response to Moraxella catarrhalis proteins OMP CD, OppA, Msp22, Hag, and PilA2 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2015;33:5809–5814. doi: 10.1016/j.vaccine.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichichero ME, Casey JR, Almudervar A. Federation of Clinical Immunology Societies. Boston, Massachusetts: Jun 27–30, 2013. Non- Protective Responses to Pediatric Vaccines Occur in Children Who are Otitis Prone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis. 2012;205(8):1225–1229. doi: 10.1093/infdis/jis179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basha S, Pichichero ME. Poor memory B cell generation contributes to non-protective responses to DTaP vaccine antigens in otitis-prone children. Clin Exp Immunol. 2015;182(3):314–322. doi: 10.1111/cei.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204(4):645–653. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhoeven D, Pichichero ME. Divergent mucosal and systemic responses in children in response to acute otitis media. Clin Exp Immunol. 2014;178(1):94–101. doi: 10.1111/cei.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 23.Sharma SK, Pichichero ME. Cellular immune response in young children accounts for recurrent acute otitis media. Curr Allergy Asthma Rep. 2013;13(5):495–500. doi: 10.1007/s11882-013-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surendran N, Nicolosi T, Kaur R, Pichichero M. Peripheral blood antigen presenting cell responses in otitis-prone and non-otitis prone infants. Innate Immunity (Formerly: Journal of Endotoxin Research) doi: 10.1177/1753425915616267. Accepted. [DOI] [PubMed] [Google Scholar]


