Abstract
Background
The comparative effects of acute moderate hyperglycemia and hypoglycemia on in-vivo endothelial function together with pro-inflammatory and pro-atherothrombotic responses in healthy individuals have not been determined.
Methods
To investigate this question 45 healthy subjects were compared during glucose clamp studies consisting of euinsulinemic hyperglycemia and hyperinsulinemic hyperglycemia (plasma glucose 11.1 mmol/L, both with pancreatic clamps) and hyperinsulinemic euglycemia and hyperinsulinemic hypoglycemia (plasma glucose 5.1 and 2.9 mmol/L, respectively). Two D doppler ultrasound was used to determine brachial artery endothelial function.
Results
Insulin levels during euinsulinemia hyperglycemia were 194±23 and (850±49–988±114) pmol/L during all hyperinsulinemic protocols. Responses of VCAM-1, ICAM-1, E-Selectin, P-selectin, PAI-1, and IL-6 were increased (p<0.05-0.0001) during euinsulinemic hyperglycemia or hypoglycemia as compared to hyperinsulinemic euglycemia or hyperinsulinemic hyperglycemia. PAI-1 was increased (p<0.04) during hypoglycemia as compared to euinsulinemic hyperglycemia, TNF-α responses were also increased during hypoglycemia as compared to hyperinsulinemic euglycemia or hyperinsulinemic hyperglycemia (p<0.05). In vivo endothelial function was similarly blunted by acute moderate hyperglycemia or hypoglycemia.
Conclusion
In summary, acute moderate hypoglycemia and euinsulinemic hyperglycemia can result in similar endothelial dysfunction and pro-atherothrombotic responses. Fibrinolytic balance was reduced by a greater extent by hypoglycemia as compared to moderate hyperglycemia. Acutely, hyperinsulinemia can prevent the acute pro-atherothrombotic and pro-inflammatory effects of moderate hyperglycemia but not hypoglycemia.
Keywords: hyperglycemia, hypoglycemia, hyperinsulinemia, endothelial function, inflammation
1. INTRODUCTION
Recent data has suggested an association between severe hypoglycemia and increased serious cardiovascular morbidity and mortality (18, 21, 36, 38). Moreover, several studies have demonstrated or reported that acute hyperglycemia (5, 6, 9, 23, 27) or hypoglycemia (7, 25, 32, 41) can result in increased atherothrombotic mechanisms, reduced fibrinolytic balance, and impaired endothelial function in both nondiabetic and diabetic individuals (6, 10, 25, 32, 37). Despite recent data reporting the acute effects of hyperglycemia and hypoglycemia on pro-atherothrombotic responses, only one study has compared their relative effects in humans (9). Ceriello et al., studying effects of GLP-1 in individuals with type 1 DM, suggested that both hyperglycemia and hypoglycemia could be considered equivalent pro-atherosclerotic risk factors (9). However, there are no data available directly comparing the acute pro-inflammatory and pro-atherothrombotic effects of hypoglycemia and hyperglycemia in non-diabetic individuals. Therefore in this present report we have reanalyzed two of our recent articles (32, 40) in order to compare the acute effects of moderate hyperglycemia (11.1 mmol/L) with and without hyperinsulinemia and moderate hypoglycemia (2.9 mmol/L) on pro-inflammatory, pro-atherothrombotic and pro-coagulant biomarker responses in healthy individuals. The pancreatic clamp technique and various glucose clamp methodologies (euglycemic, hyperglycemic and hypoglycemic) (40) were combined so that the acute independent effects of glycemia and hyperinsulinemia could be determined.
2. METHODS
2.1 Subjects
45 adult volunteers (21M/24F, 38±3yrs, BMI 29±2kg/m2 (23–36kg/m2), HbA1C 5.2±0.2%), were studied. None of the participants smoked, received anticoagulants, clopidogrel, statins, angiotensin converting enzyme inhibitors, angiotensin receptor blockers or oral insulin sensitizers (metformin, thiazolidinediones). Each participant had normal blood count, plasma electrolytes, liver and renal function and no evidence of either impaired fasting glucose or overt diabetes mellitus. Study volunteers participated in four single blind one day protocols consisting of: 1) euinsulinemic/hyperglycemia (n=15) 2) hyperinsulinemic euglycemia (n=16) 3) hyperinsulinemic hyperglycemia (n=14) and 4) hyperinsulinemic hypoglycemia (n=16) Protocols 1 and 3 consisted of an initial pancreatic clamp (40) combined with a single step 4 hour hyperglycemic (11.1 mmol/L) clamp with either euinsulinemia or hyperinsulinemia. Protocol 2 and 4 consisted of a one step hyperinsulinemic euglycemic clamp (5 mmol/L) or hyperinsulinemic hypoglycemic clamp (2.9 mmol/L, both without pancreatic clamps). Data from this manuscript have been included in 2 separate reports (32, 40) examining 1) the independent effects of insulin and hyperglycemia on pro-inflammatory responses (40) and 2) effects of antecedent hypoglycemia on pro-inflammatory and pro-atherothrombotic responses in healthy humans (32). All study participants gave written informed consent. Studies were approved by the Vanderbilt University and University of Maryland Human Subjects Institutional Review Board.
2.2 Experimental Design
All individuals refrained from caffeine, exercise, and alcohol for 24 hours prior to the study. Participants were instructed to not use aspirin, NSAIDs or COX 2 inhibitors for three days prior to a study. Subjects were admitted to the General Clinical Research Center (GCRC) the night prior to the study at 5pm. After an overnight 10 hr fast, two intravenous cannulae were inserted under 1% lidocaine local anesthesia. One cannula was placed in a retrograde fashion into a vein on the back of the hand of the non-dominant arm. This hand was placed in a heated box (55–60°C) during the study so that arterialized blood could be obtained (1). The other cannula was also placed in the non-dominant arm for infusions.
2.3 Pancreatic Clamp and Glucose Clamp Studies
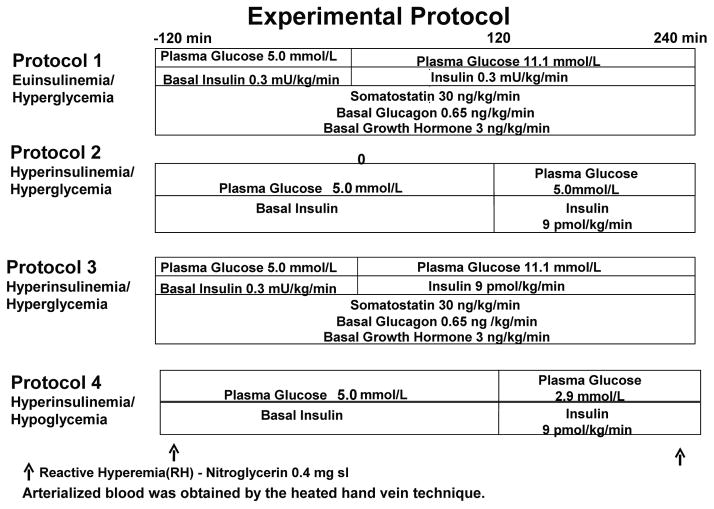
At time −120 min the somatostatin analogue, octreotide, was infused at 30 ng/kg/min to inhibit endogenous insulin, glucagon, and growth hormone secretion. Basal replacement amounts of human regular insulin (1.8 pmol/kg/min) (Lilly USA, Indianapolis, IN), human growth hormone (3 ng/kg/min) (Pharmacia & UpJohn, New York, NY) and a variable basal amount of glucagon (Boehringer Ingelheim, Ridgefield, CT) were infused during the pancreatic clamp to maintain euglycemia of approximately 5 mmol/L.
After stable euglycemia was established during the pancreatic clamp, four hour single-step hyperglycemic clamps at differing insulinemia were performed (protocol 1 and 3). At time 0 min, the insulin infusion was either maintained at 1.8 pmol/kg/min (protocol 1) or increased to 9 pmol/kg/min (protocol 3) and continued until 240 min (figure 1). End of pancreatic clamp glucagon and growth hormone infusion rates were maintained unchanged during the 240 min glucose clamp procedures. Glucose targets of 5 mmol/L or 11.1 mmol/L, were achieved using a modification of the glucose clamp technique (17). Individuals participating in protocols 2 and 4 underwent a 2 hr euglycemic or hypoglycemic clamp at an insulin infusion dose of 9 pmol/kg/min (32). Potassium chloride (20 mmol/l) was infused during hyperinsulinemic clamp studies to reduce insulin-induced hypokalemia. End of clamp blood samples were drawn at the same time of the day during all protocols.
Figure 1.
Experimental protocols.
2.4 Analytical Methods
The collection and processing of blood samples have been described elsewhere (13). Plasma glucose concentrations were measured in triplicate every 5 min using the glucose oxidase method with a glucose analyzer (Beckman, Fullerton, CA). Insulin was measured as previously described (50) with an interassay CV of 9%. Catecholamines were determined by HPLC (8) with an interassay CV of 12% for epinephrine and 8% for norepinephrine. NEFA were measured using the WAKO kit adopted for use on a Packard Instrument (Meriden, CT). Glucagon was measured according to a modification of the method of Aguilar-Parada, et al. with an interassay coefficient of variation (CV) of 12% (2). Cortisol was assayed using the Clinical Assays Gamma Coat Radioimmunoassay (RIA) kit with an interassay CV of 6%. Growth hormone was determined by RIA (30) with a CV of 8.6%.
All vascular adhesion molecules were assayed using LINCO Research Kits (St. Charles, Missouri) with an interassay CV of 8.5% (VCAM-1), CV of 9.7% (ICAM-1), CV of 13.4%, (E-selectin), CV of 9.02% (IL-6), CV of 9.98 (TNF-α), respectively. PAI-1 and tissue plasminogen activator (TPA) were determined by TintElize® Platinum Kit with interassay CV of 3.3%. P-selectin was measured using the Meso Scale Discovery assay kit (Gaithersburg, MD) with a CV of 9.9%. All hormones, pro-inflammatory biomarkers and NEFA included in this report were measured using identical assay methodologies, equipment and personnel (32, 40).
2.5 Endothelial Function
Measurements of endothelial function were conducted at baseline and during the final 30 minutes of each glucose clamp as previously described (32, 40). In brief, endothelial measurements of the dominant brachial artery were measured using 2D Doppler ultrasound during reactive hyperemia and exogenous nitroglycerin administration. Flow mediated dilation was obtained by inflating the sphygmomanometric cuff around the proximal forearm. Brachial artery diameter measurements were taken at time points 30 seconds, 60 seconds, 90 seconds, 120 seconds and after cuff deflation. Then after a 15–20 minute rest period subjects received 0.4 mg sublingual nitroglycerin (as an exogenous nitric oxide donor). Additional scans were performed as above with vessel diameter measurements obtained at 1, 2, 3 and 4 minutes (22). Endothelial function measurements were performed by JP and NJ (32, 40). The coefficient of variation (CV) at baseline and end of clamp measurements was <1%.
2.6 Statistical Analysis
Data are expressed as mean ± SE and were analyzed using standard, parametric, one- and two-way analysis of variance and with repeated measures where appropriate (Graph Pad Software, Inc., San Diego, CA). Tukey’s post hoc analysis was used to delineate statistical significance within each group. Data was also analyzed using paired and unpaired two-tailed t test. In all cases p value of <0.05 was accepted as statistically significant.
3. RESULTS
3.1 Glucose and Insulin
Plasma glucose was maintained at 5.0±0.1 mmol/L during euglycemic, 2.9±0.1 mmol/L during hypoglycemic and 11.1±0.1 mmol/L during the hyperglycemic clamp protocols (figure 1). Insulin levels were 194±23 pmol/L during euinsulinemia and similarly increased (848±49–988± 114 pmol/L) during the hyperinsulinemic hyperglycemic, euglycemic and hypoglycemic studies, respectively. Glucose infusion rates were 29±4.6, 29±5.4 and 71±6 μmol/kg/min during the euinsulinemic hyperglycemic, hyperinsulinemic euglycemic and the hyperinsulinemic hyperglycemic studies, respectively. Glucose infusion rates during hypoglycemia were 5.6 ±1.7 μmol/kg/min.
3.2 Neuroendocrine Counterregulatory Hormones
Baseline epinephrine, norepinephrine, glucagon, growth hormone and cortisol levels were similar at the start of all studies. Glucagon, growth hormone, epinephrine and cortisol remained similar to baseline but norepinephrine increased during the pancreatic clamp studies. As expected glucagon fell during the hyperinsulinemic euglycemic clamps. Epinephrine, norepinephrine, cortisol, growth hormone and glucagon responses were higher (p<0.0001–0.01) during the final 30 min of hypoglycemia as compared to the other groups (table 1).
Table 1.
Baseline (BSL) and End of Clamp (EC) Levels of Neuroendocrine Hormones and NEFA Levels in overnight fasted healthy individuals during euinsulinemic/hyperglycemic, hyperinsulinemic/euglycemic, hyperinsulinemic/hyperglycemic and hyperinsulinemic/hypoglycemic clamps
| SI units | Euins/Hypergly | Hyperins/Eugly | Hyperins/Hypergly | Hyperins/Hypo | ||||
|---|---|---|---|---|---|---|---|---|
| BSL | EC | BSL | EC | BSL | EC | BSL | EC | |
| GH ng/mL | 2.3±0.2 | 2.7±0.2 | 2.5±0.4 | 2.4±0.4 | 2.6±0.3 | 2.2±0.2 | 3.1±0.5 | 28±5*≠ |
|
| ||||||||
| Glucagon ng/L | 56±6 | 46±5 | 65±5 | 43±3* | 65±6 | 49±5 | 57±4 | 164±26*≠ |
|
| ||||||||
| Epinephrine pmol/L | 114±17 | 168±38 | 190±20 | 189±20 | 140±17 | 156±48 | 184±40 | 3741±465*≠ |
|
| ||||||||
| Norepinephrine pmol/L | 946±111 | 1456±172* | 1083±80 | 1159±77 | 780±77 | 1178±208* | 1057±119 | 1966±149*≠ |
|
| ||||||||
| Cortisol nmol/L | 260±50 | 378±83 | 344±31 | 292±30 | 273±27 | 273±55 | 359±34 | 850±70*≠ |
|
| ||||||||
| NEFA mmol/L | 192 ± 53 | 88 ± 28* | 379 ± 48 | 103 ± 40* | 194 ± 31 | 65 ± 16 * | 288±32 | 110±19* |
NEFA= non esterified fatty acids
p<0.03 -0.0001 Significantly different from BSL
p<0.03 -0.0001 Significantly different from all protocols EC
3.3 Intermediary Metabolism
Blood nonesterified fatty acid (NEFA) were similar at the start of all protocols and fell by similar, amounts during hyperglycemic and hyperinsulinemic clamps (p<0.008) (table 1).
3.4 Atherogenic Vascular Adhesion Molecules
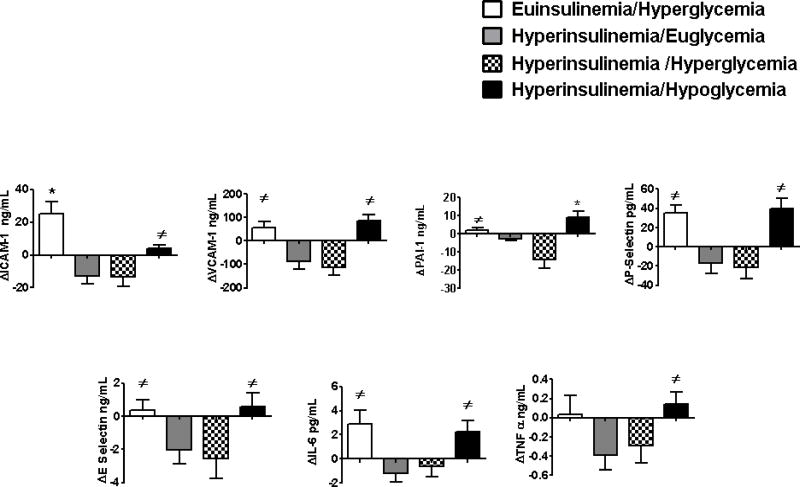
Baseline values of VCAM, ICAM and E-selectin were similar at baseline during all four protocols (table 2). VCAM, ICAM and E-selectin responses increased (p<0.03-0.0001) during both euinsulinemia hyperglycemia and hypoglycemia. Responses of ICAM during euinsulemia/hyperglycemia was greater compared to hypoglycemia P<0.007 (figure 2) VCAM, ICAM and E-selectin responses fell compared to baseline during the hyperinsulinemic euglycemic and hyperinsulinemic hyperglycemic protocols (P<0.05-0.0001).
Table 2.
Baseline (BSL) levels of pro- inflammatory, pro-atherothrombotic and pro-coagulation markers in overnight fasted healthy individuals during euinsulinemic/hyperglycemic, hyperinsulinemic/euglycemic, hyperinsulinemic/hyperglycemic and hyperinsulinemic/hypoglycemic clamps
| SI units | Euinsulinemia/Hyperglycemia | Hyperinsulinemia/Euglycemia | Hyperinsulinemia/Hyperglycemia | Hyperinsulinemia/Hypoglycemia |
|---|---|---|---|---|
| VCAM-1 ng/mL | 737±39 | 838±37 | 752±45 | 819±62 |
| ICAM-1 ng/mL | 76±10 | 90±7 | 81±9 | 88±3 |
| P-Selectin pg/mL | 74±7 | 83±9 | 79±14 | 63±13 |
| E-Selectin ng/mL | 19±2 | 22±2 | 22±3 | 21±2 |
| PAI-1 ng/mL | 15±3 | 18±3 | 24±6 | 13±3 |
| IL-6 ng/mL | 16±5 | 12±7 | 20±9 | 12±3 |
| tPA ng/mL | 5.8±1.2 | 6.1±1 | 5.3±1.3 | 5 ±1 |
Figure 2.
Effects of euinsulinemic/hyperglycemia, hyperinsulinemic/euglycemia, hyperinsulinemic/hyperglycemia, and hyperinsulinemic/hypoglycemia on pro-inflammatory, pro-atherothrombotic, and pro-coagulant responses in overnight fasted, healthy individuals.
*p<0.04-0.0001 Significantly different from all protocols
≠p<0.05-0.0001 Significantly different from hyperinsulinemia/euglycemia and hyperinsulinemia/hyperglycemia
3.5 Platelet Activation and Fibrinolytic Balance
Baseline p-selectin, TPA and PAI-1 were similar at the start of the four glucose clamp protocols (table 2). P-selectin and PAI-1 responses were increased similarly (p<0.0002–0.02) during euinsulinemic hyperglycemia or hypoglycemia compared to the other two protocols (figure 2). PAI-1 responses during hypoglycemia were greater (p<0.04) compared to increases during euinsulinemia hyperglycemia. PAI-1 and P-selectin levels fell from baseline (p<0.02-0.0002) during the hyperinsulinemic euglycemic and hyperglycemic protocols. TPA values remained similar to baseline during all four protocols.
3.6 Pro-inflammatory Markers
Baseline levels of IL-6, and TNF-α were similar at the start of all four protocols. IL-6 increased similarly from baseline during euinsulinemic hyperglycemic and hypoglycemic studies (p<0.02) (figure 2). Responses of IL-6 were reduced during hyperinsulinemic euglycemia and hyperglycemic protocols as compared to both euinsulinemic hyperglycemia and hypoglycemia (p<0.03-0.003). TNF-α responses during hypoglycemia were increased (p<0.05-0.01) compared to hyperinsulinemic euglycemia and hyperinsulinemic hyperglycemic protocols.
3.7 Endothelial Function
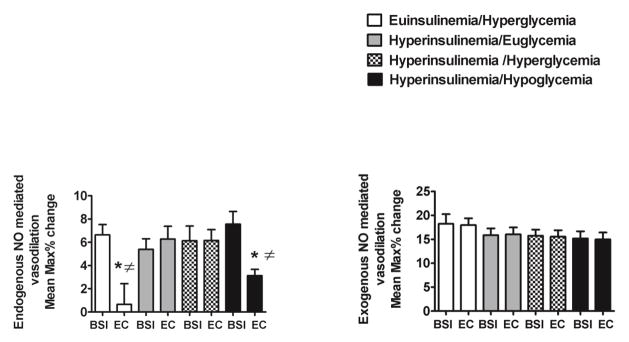
Flow mediated dilation during endogenous nitric oxide (NO) stimulation was significantly decreased during euinsulinemia/hyperglycemia and hypoglycemia as compared to other hyperinsulinemic hyperglycemic and euglycemic protocols (p<0.02-0.01) (figure 3).
Figure 3.
Effects of euinsulinemic/hyperglycemia, hyperinsulinemic/euglycemia, hyperinsulinemic/hyperglycemia, and hyperinsulinemic/hypoglycemia on endogenous and exogenous nitric oxide (NO) mediated brachial artery vasodilation in overnight fasted, healthy individuals.
*p<0.0008-0.0001 Significantly different from baseline
≠p<0.02-0.01 Significantly different from hyperinsulinemia/euglycemia and hyperinsulinemia/hyperglycemia
There were similar changes among all protocols during exogenous NO mediated vasodilation following nitroglycerin administration. Baseline and end of clamp brachial artery diameters were similar (0.43±0.02–0.44±0.02 cm) before flow mediated dilation measurements in all protocols.
4. DISCUSSION
In the present report we have compared the acute effects of euinsulinemic hyperglycemia, hyperinsulinemic hyperglycemia, hyperinsulinemic euglycemia and hyperinsulinemic hypoglycemia on pro-inflammatory, pro-atherothrombotic and in vivo arterial endothelial responses in healthy humans. Euinsulinemic hyperglycemia and hyperinsulinemic hypoglycemia produced similar increases in a wide range of pro-inflammatory (VCAM, E-Selectin, IL-6, TNF-α) and platelet activation (P-selectin) responses combined with similar reductions in NO mediated endothelial function. However, fibrinolytic balance was decreased by a greater extent during hypoglycemia (greater increase in PAI-1, equivalent changes in TPA) as compared to moderate hyperglycemia per se. Hyperinsulinemia prevented the acute effects of hyperglycemia but not hypoglycemia on stimulating pro-inflammatory, pro-atherothrombotic, pro-coagulant and disordered vascular endothelial responses.
Moderate hyperglycemia or hypoglycemia have been demonstrated to acutely induce a wide spectrum of pro-inflammatory, pro-atherothrombotic and pro-coagulant responses in healthy and diabetic individuals (4, 5, 16, 39, 40, 42). Additionally, both stresses can result in reductions in brachial artery flow mediated dilation. To date only one report has compared the effects of hypoglycemia with hyperglycemia on in vivo endothelial function and pro-inflammatory responses (9). This single report by Ceriello et al., performed in type 1 DM focused on two pro-inflammatory markers (IL-6 and ICAM-1) and two markers of oxidative stress. The authors suggested (as there was no formal statistical comparison) that in type 1 DM moderate hypoglycemia (2.9 mmol/L) and more severe hyperglycemia of 15 mmol/L appeared to produce equivalent inflammatory effects. In this present report we have extended the comparison to include the pathophysiologic effects of hyperglycemia or hypoglycemia on a broader group of pro-atherothrombotic, pro-inflammatory and pro-coagulant biomarkers in healthy humans.
We have previously reported in two separate articles that acute hypoglycemia or euinsulinemic hyperglycemia can have widespread systemic pro-inflammatory and pro-atherothrombotic effects (32, 40). In this present article we have compared and re-analyzed the magnitude of the above reported pro-inflammatory effects caused by either moderate hyperglycemia or hypoglycemia and also compared the effects of insulin per se on modulating the acute deleterious actions of differing glycemia on in vivo vascular function.
In order to evaluate the effects of hyperglycemia per se in healthy humans, an experimental design needs to be utilized that can break feedback loops between glucose and pancreatic β-cell insulin secretion. In this study we have utilized the pancreatic clamp technique with octreotide to prevent insulin (and also glucagon, growth hormone) secretion during the two hyperglycemic protocols. Hyperglycemic glucose clamp methodology was then added to the pancreatic clamp to produce stable hyperglycemia. During both protocols, basal amounts of insulin, growth hormone and glucagon were replaced to prevent any acute hormonal deficiencies. Standard glucose clamp methodologies were used during the hyperinsulinemic euglycemic and hypoglycemic protocols. Four hours of moderate hyperglycemia per se (i.e. in the presence of basal euinsulinemia) or 2 hrs of moderate hypoglycemia produced similar increases in the atherogenic adhesion molecules VCAM-1 and E-selectin. Surprisingly, ICAM-1 responses were increased by a greater extent by hyperglycemia as compared to hypoglycemia. The mechanisms responsible for this finding will require further work. Responses of the pro-inflammatory biomarker IL-6 were also similarly increased by hyperglycemia and hypoglycemia.
P-selectin, a marker of platelet aggregation was also increased similarly during hyperglycemia and hypoglycemia. Fibrinolytic balance however was more greatly impaired by hypoglycemia as compared to hyperglycemia. PAI-1 the primary regulator of fibrinolytic balance was selectively increased by a greater extent by hypoglycemia, while TPA remained unchanged during hypoglycemia and hyperglycemia. Thus, taken together moderate hypoglycemia had greater pro-coagulant effects as compared to moderate hyperglycemia.
Insulin initially was considered to have pro-inflammatory effects (6, 19, 23, 44). Recent work from a number of laboratories, including notably, Dandona et al., have reported that acutely insulin has anti-inflammatory effects (11, 12, 16, 45). Our previous reports have also documented that insulin can have acute anti-inflammatory, pro-coagulant and pro-atherothrombotic effects (25, 32, 40). Those properties are more clearly demonstrated in the present report. All of the above biomarker responses were reduced from baseline during the hyperinsulinemic euglycemic studies and were significantly reduced as compared to hyperglycemia per se or hypoglycemia. Similarly, hyperinsulinemia was able to overcome the pro-inflammatory, pro-atherothrombotic and pro-coagulant effects of hyperglycemia leading to similar improvements in inflammatory biomarkers as compared to the hyperinsulinemic euglycemic control studies. However, although it is clear that insulin can overcome the pro-inflammatory effects of moderate hyperglycemia, it is unable to prevent the deleterious inflammatory effects of hypoglycemia. Along similar lines, there was no difference between TNF-α responses during hyperglycemia per se and hyperinulinemic euglycemia and hyperinsulinemic hyperglycemic control studies. In contrast, the effects of hypoglycemia to increase TNF-α were greater compared to the reduction occurring in the hyperinsulinemic studies. Thus, taken together when evaluating overall effects on adhesion molecules and inflammatory biomarkers it would appear that moderate hyperglycemia per se and hyperinsulinemic hypoglycemia have similar pro-inflammatory and pro-atherogenic effects.
We also investigated the effects of hyperglycemia per se, hypoglycemia and hyperinsulinemia on acute nitric oxide mediated brachial artery vasodilator mechanisms. Hyperglycemia per se and hypoglycemia produced similar inhibition of endogenously (i.e. vascular smooth muscle) NO mediated vasodilation. Exogenous NO mediated vasodilation was unaffected by hyperglycemia or hypoglycemia. Hyperinsulinemia was again able to reverse and protect against the deleterious effects of hyperglycemia per se, but not hypoglycemia on the vascular endothelium.
With regard to the potential mechanisms responsible for our findings; clearly insulin can not be implicated as a cause of the observed increased pro-inflammatory, pro-coagulant and pro-atherothrombotic responses. In fact, insulin completely reversed and thus protected the vasculature against the deleterious effects of hyperglycemia. However, importantly hyperinsulinemia could not reverse the widespread pro-inflammatory and pro-atherothrombotic effects of hypoglycemia. Therefore, there must be either direct effects of hypoglycemia (31, 47) or indirect actions consequent on the counter regulatory responses that are causing increased pro-inflammatory and pro-atherothrombotic effects (24, 26, 46).
Cathecholamines and sympathetic nervous system drive were increased during hypoglycemia and have been implicated in causing pro-inflammatory responses in a number of pathophysiologic conditions (24, 26, 46). Growth hormone, glucagon and cortisol were also increased during hypoglycemia. All are considered to have acute anti-inflammatory properties and thus would be unlikely to have contributed to the increased pro-inflammatory response during hypoglycemia (3, 14, 20, 28, 34, 49). NEFA levels were similar during all of the study protocols. Although increased NEFA levels have been reported to have pro-inflammatory properties, it is unlikely that NEFA could have contributed to our present findings (15, 48). In addition, recent reports have also identified that hypoglycemia can directly reduce endothelial function via elevations in mitochondrial superoxide levels, thereby reducing NO bioavailability (47).
In this present study we utilized the pancreatic clamp technique with basal insulin, glucagon and growth hormone replacement. The experimental design to replace growth hormone is unique and relevant as deficiency of GH has been reported to increase PAI-1 levels (33). There are reports that octreotide may have anti-inflammatory effects (35). However, there were clear differences in the effects of euinsulinemic and hyperinsulinemic hyperglycemia on pro-inflammatory, pro-atherothrombotic and endothelial function responses, which both incorporated octreotide in the pancreatic clamp.
We have compared moderate hyperglycemia (11.1 mmol/L) with moderate hypoglycemia (2.9 mmol/L). Therefore, we can not comment on whether more severe hyperglycemia or deeper hypoglycemia would have produced greater overall pro-inflammatory responses from either hyperglycemia of hypoglycemia.
In summary, moderate hyperglycemia and moderate hypoglycemia in healthy humans can acutely induce a wide range of pro-inflammatory, pro-atherothrombotic and pro-coagulant responses coupled with reductions in endogenously NO mediated vasodilation. Fibrinolytic balance appears to be more greatly reduced by moderate hypoglycemia as compared to hyperglycemia. Insulin can prevent the broad pro-inflammatory effects of hyperglycemia but not hypoglycemia.
4.1 Conclusion
In conclusion, both moderate hyperglycemia and hypoglycemia should be considered pro-atherothrombotic stimuli in healthy man. Moderate hypoglycemia’s deleterious vascular effects cannot be prevented by insulin and may have greater pro-coagulant effects as compared to moderate hyperglycemia in healthy humans.
Acknowledgments
We would like to thank Wanda Snead, Eric Allen and the Vanderbilt Hormone Assay Core laboratory for their excellent technical assistance. We would also like to thank the nursing staff of the Vanderbilt Clinical Research Center and the University of Maryland, Baltimore General Clinical Research Center for their excellent care. This work was supported by the following NIH grants: P50 HL081009 NIH/NHLBI, RO1 DK069803 NIH/NIDDK, PO1 HL056693 NIH/NHLBI, Vanderbilt Diabetes Research and Training grant (DRTC) NIH/NIDDK P60 DK020593, Vanderbilt General Clinical Research Center NIH/NCRR TL1 TR000447.
We also thank Takeda Pharmaceuticals for a fellowship award to Nino G. Joy. N. J. performed studies, researched and analyzed data, contributed to writing, reviewing and edited the manuscript. M.M. helped perform studies. L. Y. perform studies, researched data and D. T. helped perform studies, researched data, and reviewed and edited the manuscript. S. D. devised the study, contributed to writing, reviewed and edited data and the manuscript. All are affiliated with the University of Maryland, Baltimore.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Alessi MC, Poggi M, Juhan-Vague I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr Opin Lipidol. 2007;18(3):240–245. doi: 10.1097/MOL.0b013e32814e6d29. [DOI] [PubMed] [Google Scholar]
- 4.Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)-1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J Clin Endocrinol Metab. 2002;87(3):1419–1422. doi: 10.1210/jcem.87.3.8462. [DOI] [PubMed] [Google Scholar]
- 5.Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr. 2004;80(1):51–57. doi: 10.1093/ajcn/80.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 7.Bedenis R, Price AH, Robertson CM, Morling JR, Frier BM, Strachan MW, Price JF. Association between severe hypoglycemia, adverse macrovascular events, and inflammation in the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2014;37(12):3301–3308. doi: 10.2337/dc14-0908. [DOI] [PubMed] [Google Scholar]
- 8.Causon RC, Carruthers ME, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem. 1981;116(1):223–226. doi: 10.1016/0003-2697(81)90347-x. [DOI] [PubMed] [Google Scholar]
- 9.Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Esposito K, Giugliano D, Genovese S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36(8):2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106(10):1211–1218. doi: 10.1161/01.cir.0000027569.76671.a8. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri A, Dandona P, Fonseca V. Cardiovascular benefits of exogenous insulin. J Clin Endocrinol Metab. 2012;97(9):3079–3091. doi: 10.1210/jc.2012-1112. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri A, Janicke D, Wilson MF, Tripathy D, Garg R, Bandyopadhyay A, Calieri J, Hoffmeyer D, Syed T, Ghanim H, Aljada A, Dandona P. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation. 2004;109(7):849–854. doi: 10.1161/01.CIR.0000116762.77804.FC. [DOI] [PubMed] [Google Scholar]
- 13.Cherrington AD, Lacy WW, Chiasson JL. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colao A, Di Somma C, Rota F, Pivonello R, Savanelli MC, Spiezia S, Lombardi G. Short-term effects of growth hormone (GH) treatment or deprivation on cardiovascular risk parameters and intima-media thickness at carotid arteries in patients with severe GH deficiency. J Clin Endocrinol Metab. 2005;90(4):2056–2062. doi: 10.1210/jc.2004-2247. [DOI] [PubMed] [Google Scholar]
- 15.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108(12):1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 16.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Proinflammatory effects of glucose and anti-inflammatory effect of insulin: relevance to cardiovascular disease. Am J Cardiol. 2007;99(4A):15B–26B. doi: 10.1016/j.amjcard.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 18.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26(5):1485–1489. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 19.Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 20.Devin JK, Blevins LS, Jr, Verity DK, Chen Q, Bloodworth JR, Jr, Covington J, Vaughan DE. Markedly impaired fibrinolytic balance contributes to cardiovascular risk in adults with growth hormone deficiency. J Clin Endocrinol Metab. 2007;92(9):3633–3639. doi: 10.1210/jc.2007-0609. [DOI] [PubMed] [Google Scholar]
- 21.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 22.Faulx MD, Wright AT, Hoit BD. Detection of endothelial dysfunction with brachial artery ultrasound scanning. Am Heart J. 2003;145(6):943–951. doi: 10.1016/S0002-8703(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 23.Festa A, D’Agostino R, Jr, Mykkanen L, Tracy RP, Zaccaro DJ, Hales CN, Haffner SM. Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance The Insulin Resistance Atherosclerosis Study (IRAS) Arterioscler Thromb Vasc Biol. 1999;19(3):562–568. doi: 10.1161/01.atv.19.3.562. [DOI] [PubMed] [Google Scholar]
- 24.Frost RA, Nystrom GJ, Lang CH. Epinephrine stimulates IL-6 expression in skeletal muscle and C2C12 myoblasts: role of c-Jun NH2-terminal kinase and histone deacetylase activity. Am J Physiol Endocrinol Metab. 2004;286(5):E809–17. doi: 10.1152/ajpendo.00560.2003. [DOI] [PubMed] [Google Scholar]
- 25.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care. 2010;33(7):1529–1535. doi: 10.2337/dc09-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halleux CM, Declerck PJ, Tran SL, Detry R, Brichard SM. Hormonal control of plasminogen activator inhibitor-1 gene expression and production in human adipose tissue: stimulation by glucocorticoids and inhibition by catecholamines. J Clin Endocrinol Metab. 1999;84(11):4097–4105. doi: 10.1210/jcem.84.11.6127. [DOI] [PubMed] [Google Scholar]
- 27.Haubner F, Lehle K, Munzel D, Schmid C, Birnbaum DE, Preuner JG. Hyperglycemia increases the levels of vascular cellular adhesion molecule-1 and monocyte-chemoattractant-protein-1 in the diabetic endothelial cell. Biochem Biophys Res Commun. 2007;360(3):560–565. doi: 10.1016/j.bbrc.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 28.Henkel E, Gallo S, Siegert G, Koehler C, Hanefeld M. Glucagon as a determinant of fibrinolytic activity in men with different stages of glucose tolerance: impact of glucagon on fibrinolysis. Blood Coagul Fibrinolysis. 2007;18(4):327–334. doi: 10.1097/MBC.0b013e32809cc90b. [DOI] [PubMed] [Google Scholar]
- 29.HUNTER WM, GREENWOOD FC. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 30.Hutton RA, Mikhailidis D, Dormandy KM, Ginsburg J. Platelet aggregation studies during transient hypoglycaemia: a potential method for evaluating platelet function. J Clin Pathol. 1979;32(5):434–438. doi: 10.1136/jcp.32.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joy N, Tate DB, Younk L, Davis SN. Effects of Acute and Antecendent Hypoglycemia on Endothelial Function and Markers of Atherothrombotic Balance in Healthy Humans. Diabetes. 2015;64(7):2571–2580. doi: 10.2337/db14-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kvasnicka J, Marek J, Kvasnicka T, Weiss V, Markova M, Stepan J, Umlaufova A. Increase of adhesion molecules, fibrinogen, type-1 plasminogen activator inhibitor and orosomucoid in growth hormone (GH) deficient adults and their modulation by recombinant human GH replacement. Clin Endocrinol (Oxf) 2000;52(5):543–548. doi: 10.1046/j.1365-2265.2000.01002.x. [DOI] [PubMed] [Google Scholar]
- 33.Laakso M, Edelman SV, Brechtel G, Baron AD. Effects of epinephrine on insulin-mediated glucose uptake in whole body and leg muscle in humans: role of blood flow. Am J Physiol. 1992;263(2 Pt 1):E199–204. doi: 10.1152/ajpendo.1992.263.2.E199. [DOI] [PubMed] [Google Scholar]
- 34.Liu R, Wei N, Guo W, Qiang O, Li X, Ou Y, Huang W, Tang CW. Octreotide alleviates obesity by reducing intestinal glucose absorption and inhibiting low-grade inflammation. Eur J Nutr. 2013;52(3):1067–1075. doi: 10.1007/s00394-012-0413-6. [DOI] [PubMed] [Google Scholar]
- 35.McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive Treatment and Severe Hypoglycemia Among Adults With Type 2 Diabetes. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, Lipinska I, D’Agostino RB, Wilson PW. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA. 2000;283(2):221–228. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 37.Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Simplification of Insulin Regimen in Older Adults and Risk of Hypoglycemia. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.2288. [DOI] [PubMed] [Google Scholar]
- 38.Ohkita M, Takaoka M, Shiota Y, Nojiri R, Matsumura Y. Nitric oxide inhibits endothelin-1 production through the suppression of nuclear factor kappa B. Clin Sci (Lond) 2002;103(Suppl 48):68S–71S. doi: 10.1042/CS103S068S. [DOI] [PubMed] [Google Scholar]
- 39.Perkins JM, Joy NG, Tate DB, Davis SN. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am J Physiol Endocrinol Metab. 2015;309(2):E168–76. doi: 10.1152/ajpendo.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rana O, Byrne CD, Kerr D, Coppini DV, Zouwail S, Senior R, Begley J, Walker JJ, Greaves K. Acute hypoglycemia decreases myocardial blood flow reserve in patients with type 1 diabetes mellitus and in healthy humans. Circulation. 2011;124(14):1548–1556. doi: 10.1161/CIRCULATIONAHA.110.992297. [DOI] [PubMed] [Google Scholar]
- 41.Razavi Nematollahi L, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, Gozashti MH, Omidfar K, Taheri E. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58(4):443–448. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Stegenga ME, van der Crabben SN, Levi M, de Vos AF, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes. 2006;55(6):1807–1812. doi: 10.2337/db05-1543. [DOI] [PubMed] [Google Scholar]
- 43.Trovati M, Anfossi G, Cavalot F, Massucco P, Mularoni E, Emanuelli G. Insulin directly reduces platelet sensitivity to aggregating agents. Studies in vitro and in vivo. Diabetes. 1988;37(6):780–786. doi: 10.2337/diab.37.6.780. [DOI] [PubMed] [Google Scholar]
- 44.van der Poll T, Levi M, Dentener M, Jansen PM, Coyle SM, Braxton CC, Buurman WA, Hack CE, ten Cate JW, Lowry SF. Epinephrine exerts anticoagulant effects during human endotoxemia. J Exp Med. 1997;185(6):1143–1148. doi: 10.1084/jem.185.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol. 2012;32(3):712–720. doi: 10.1161/ATVBAHA.111.227389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 2006;55(8):2301–2310. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 47.Weaver JU, Monson JP, Noonan K, John WG, Edwards A, Evans KA, Cunningham J. The effect of low dose recombinant human growth hormone replacement on regional fat distribution, insulin sensitivity, and cardiovascular risk factors in hypopituitary adults. J Clin Endocrinol Metab. 1995;80(1):153–159. doi: 10.1210/jcem.80.1.7829604. [DOI] [PubMed] [Google Scholar]
- 48.Wide L, Porath J. Radioimmunoassay of proteins with the uses of sephadex-coupled antibodies. Biochem Biophys Acta. 1966;130:257–260. [Google Scholar]
- 49.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev. 2008;24(5):353–363. doi: 10.1002/dmrr.865. [DOI] [PubMed] [Google Scholar]
- 50.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 2010;33(7):1591–1597. doi: 10.2337/dc10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]