Abstract
Objective
During critical illness, impaired endothelial vascular reactivity predicts prolonged acute brain dysfunction, but relationships between endothelial activation, blood-brain barrier (BBB)/neurological injury, and acute brain dysfunction, including delirium, remain unexamined. We tested the hypothesis that elevated plasma markers of endothelial activation and BBB/neurological injury are associated with delirium duration during critical illness.
Design
Prospective cohort study.
Setting
Medical and surgical intensive care units in an academic medical center.
Patients
Adults in acute respiratory failure and/or shock.
Interventions
None.
Measurements and Main Results
No more than 72 hours after organ failure was diagnosed in the ICU, we enrolled subjects and measured plasma concentrations of plasminogen activator inhibitor-1 (PAI-1), E-selectin, and angiopoietin-2 (Ang-2) as markers of endothelial activation and S100B as a marker of BBB/neurological injury in blood collected at enrollment. We assessed patients for delirium and coma twice daily after enrollment using the Confusion Assessment Method for the ICU and the Richmond Agitation-Sedation Scale. Among 134 patients with a median (interquartile) age of 57 years (46–66 years) and Acute Physiology and Chronic Health Evaluation II of 26 (19–31), delirium occurred in 94 (70%) patients with a median duration of 2 days (0–4 days). Higher PAI-1 (p = 0.002), E-selectin (p = 0.02), and S100B (p < 0.001) concentrations were associated with fewer delirium/coma-free days after adjusting for age, Charlson comorbidity index, modified Sequential Organ Failure Assessment score, and severe sepsis. Similarly, higher PAI-1 (p = 0.007) and S100B (p = 0.01) concentrations were associated with longer delirium duration in survivors. Adjusting for S100B did not alter PAI-1 and E-selectin associations with delirium, suggesting that these associations were not mediated by BBB/neurological injury.
Conclusions
Elevated plasma markers of endothelial activation and BBB/neurological injury during critical illness are associated with prolonged delirium after biomarker measurement. Future research is needed to determine whether these processes have pathophysiologic roles in delirium and whether therapies targeted at the endothelium or BBB can prevent and/or treat delirium during critical illness.
Keywords: Delirium, coma, endothelium, blood-brain barrier, critical illness, sepsis
INTRODUCTION
Delirium is common during critical illness and is associated with increased cost, length of stay, functional dependence, long-term cognitive impairment, and mortality (1–7). An incomplete understanding of the pathophysiology of delirium, however, currently hinders its prevention and treatment. Because endothelial function—a major determinant of microvascular blood flow and a key component of the blood-brain barrier (BBB)—is likely essential to brain function during critical illness, endothelial dysfunction may lead to delirium via perturbations in blood flow, release of biochemical mediators, and increased permeability of the BBB. Yet, few studies have examined whether markers of endothelial dysfunction and/or injury are associated with delirium.
We recently examined endothelial vascular reactivity in critically ill patients and found that impaired vascular reactivity independently predicted acute brain dysfunction (8). In addition to vasomotor impairment, the endothelium becomes activated in response to acute illness, releasing mediators that modulate the body’s response to disease states. When exaggerated, this mediator release creates pathophysiologic changes in the microvasculature and activates coagulation (9). In the brain specifically, injury to BBB endothelial cells has been associated with increased microvascular permeability and impaired cerebral microcirculation in animal and in vitro studies (10–13), and endothelial cell structural changes have been shown to correlate with neurological injury in human cortical biopsy specimens (14). Whether endothelial activation and BBB/neurological injury are associated with delirium during critical illness, however, remains unclear.
We therefore conducted a prospective cohort study whose objective was to test the hypothesis that elevated plasma markers of endothelial activation and BBB/neurological injury are associated with delirium duration during critical illness.
MATERIALS AND METHODS
Study Design and Population
We conducted this single-center, prospective cohort study concurrently within the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study (7) conducted at Vanderbilt University Medical Center, which examined critically ill patients for delirium and long-term cognitive impairment. From April 2009 to September 2010, we assessed BRAIN-ICU participants for inclusion in the current study. Adults admitted to a Vanderbilt University medical or surgical ICU with respiratory failure and/or shock were eligible for the BRAIN-ICU study unless they met exclusion criteria: severe neurologic disease that prevented independent living prior to critical illness; cardiac surgery within the previous 3 months; suspected acute brain injury due to overt neurologic insult or cardiopulmonary arrest; active substance abuse, psychotic disorder, or residence > 200 miles from Nashville, which would impede long-term follow-up; blindness, deafness, or inability to speak English, which would prevent delirium assessments by study staff; life expectancy <24 hours; substantial recent critical illness (as described in detail previously) (7); or lack of informed consent from the participant or an authorized surrogate within 72 hours of onset of organ failure. Patients who were comatose or delirious at the time of screening were eligible for inclusion. We excluded BRAIN-ICU participants from the current study if endothelial vascular reactivity could not be reliably measured due to upper extremity injury, arteriovenous fistula, prone positioning, or unavailability of equipment/technician within 24 hours of enrollment. The Vanderbilt University Institutional Review Board approved the study protocol, and we obtained informed consent from patients or authorized surrogates.
Exposures
As shown in Figure S1 (see Supplemental Digital Content), we collected blood immediately upon study enrollment, centrifuged it at 3000 × g, removed the supernatants, and then labeled and stored the samples in 500 µL aliquots at −80°C until a late date, at which time we performed batched analyses in duplicate of plasma marker concentrations using commercially available enzyme-linked immunosorbent assays. We measured plasma concentrations of plasminogen activator inhibitor-1 (PAI-1), E-selectin, and angiopoietin-2 (Ang-2) as markers of endothelial activation leading to microvascular thrombosis, cell adhesion, and vascular permeability and remodeling, respectively (15). We also measured S100B as a specific plasma marker of BBB/neurological injury (16–18).
We assessed endothelial vascular reactivity within 24 hours of study enrollment (Figure S1) with a reactive hyperemia method using peripheral artery tonometry to measure the reactive hyperemia index, as described previously (8). Specifically, we used the Endo_PAT device standardized protocol (Itamar Medical Ltd; Franklin, Massachusetts, USA), which has been validated against coronary angiography (19, 20). In an operator-independent manner, the Endo_PAT calculates the reactive hyperemia index as the ratio between the magnitude of the average post-occlusive pulse wave amplitude and the average baseline pulse wave amplitude, corrected to systemic changes. A lower reactive hyperemia index indicates worse endothelial vascular reactivity, with values <1.67 considered to represent endothelial dysfunction (19, 20). The Endo_PAT technician was blinded to the results of research evaluations for delirium and coma.
Outcomes
After enrollment, trained research personnel who were blinded to vascular reactivity results assessed patients for delirium and coma using the Confusion Assessment Method for the ICU (CAM-ICU) (21, 22) and the Richmond Agitation-Sedation Scale (RASS) (23, 24) twice daily in the ICU and once daily after ICU discharge (Figure S1). We considered a patient delirious if they were not comatose (i.e., had a RASS of −3 or more awake) and were CAM-ICU positive on either of the CAM-ICU assessments. Patients with a RASS of −4 (responsive to physical stimulus only) or −5 (completely unresponsive) were considered comatose.
Statistical Analysis
To analyze the associations between plasma markers of endothelial activation and BBB/neurological injury measured at enrollment and duration of delirium measured after enrollment, we used two different, complimentary outcome measures. First, to avoid confounding by coma and death—both of which can truncate delirium duration and which we hypothesized would be associated with the exposures—we used the number of days alive without delirium or coma (i.e., delirium/coma-free days) during the first 14 days after study enrollment, a period of analysis chosen because almost all delirium and coma in our cohort occurred within 14 days of enrollment. We considered patients who were discharged from the hospital prior to study day 14 to be delirium/coma-free. In addition, we used days of delirium among survivors over the same 14-day period to focus more specifically on delirium. Patients who died in hospital were excluded from this analysis because early death curtails delirium duration.
To determine whether PAI-1, E-selectin, Ang-2, or S100B were associated with delirium/coma-free days or days of delirium among survivors, we used multiple linear regression with Huber-White sandwich estimation (25) applied to adjust standard errors of estimates to take into account the correlation between data from the same patient. We selected covariates a priori based on prior research and clinical judgment and included age, Charlson comorbidity index (26), modified (excluding the neurologic component) Sequential Organ Failure Assessment (SOFA) at enrollment (27), and severe sepsis at enrollment defined as known or suspected infection with two or more systemic inflammatory response syndrome criteria and presence of organ dysfunction (mechanical ventilation and/or vasopressor requirement).
We limited the number of covariates included to avoid overfitting. Given the sample size of 134 subjects, which was determined by the resources available for endothelial vascular reactivity measurements (8), we determined that the minimum degrees of freedom required for each regression model was 9 by assuming each degree of freedom required 15 patients to reliably fit the model. I.e., a multivariable model with a complexity of 9 degrees of freedom requires an effective sample size of 135 (=9 × 15) subjects (28). For all models, biomarkers were log transformed to reduce the influence of outliers and improve model fit. We used restricted cubic splines to allow continuous variables to have nonlinear relationships with outcomes unless there was no evidence that an association was nonlinear (specifically, if the p value for nonlinearity was >0.20), in which case we removed the nonlinear terms for parsimony.
Because the endothelial biomarkers we measured reflect distinct but related aspects of endothelial function, we not only sought to assess their associations with delirium but also with each other. Thus, we used Spearman’s rank correlation to determine whether markers of endothelial dysfunction (including activation and impaired vascular reactivity) correlated with BBB/neurological injury. Further, we included S100B as a covariate in other linear regression models to assess whether BBB/neurological injury mediated any associations observed between markers of endothelial activation or vascular reactivity and delirium/coma-free days.
Lastly, because some relationships between microvascular injury and outcomes have been shown to be modified by infection in acute lung injury patients (29), we performed post hoc analyses to determine whether severe sepsis modified the associations between PAI-1, E-selectin, Ang-2, S100B, or vascular reactivity and delirium/coma-free days. We examined potential interactions by including cross product terms (e.g., sepsis*PAI-1) in the aforementioned linear regression models. We used R version 2.15.1 for all statistical analyses and considered p<0.05 as statistical significance for independent variables.
RESULTS
Patient characteristics and outcomes from the 134-patient cohort are presented in Table 1; details regarding patients screened and excluded were previously reported (8). In general, patients had high severity of illness and frequent organ dysfunctions. Delirium was common, with 70% of patients being delirious at some point after enrollment during the 14-day study period. The median duration of delirium for the entire cohort was 2 days, and the median duration of coma was 1 day.
Table 1.
Clinical Characteristics of the Study Population
| Variablea | N=134 |
|---|---|
| Age, years | 57 (46, 66) |
| Male sex (%) | 57 |
| Charlson comorbidity index | 2 (1,4) |
| Medical ICU/Surgical ICU (%) | 54/46 |
| APACHE II at ICU admission | 26 (19, 31) |
| SOFA score at enrollment | 10 (8, 12) |
| Mechanically ventilated at enrollment (%) | 85 |
| Reactive hyperemia index at enrollment | 1.51 (1.31, 1.81) |
| PAI-1 at enrollment, ng/mL | 126 (74, 171) |
| E-selectin at enrollment, ng/mL | 18 (8.8, 29.8) |
| Ang-2 at enrollment, pg/mL | 18474 (9639, 26370) |
| S100B at enrollment, pg/mL | 59 (38, 129) |
| Duration of mechanical ventilation, days | 3 (0.9, 7.1) |
| ICU length of stay, days | 4.8 (2.0, 10.8) |
| Hospital length of stay, days | 9 (5.5, 17.0) |
| Delirium present during study period (%) | 70 |
| Delirium duration, days | 2 (0, 4) |
| Coma duration, days | 1 (0, 4) |
| Delirium/coma-free daysb | 10.5 (3, 13) |
| Died within study period (%) | 16 |
Median (Interquartile range) unless specified.
The number of days alive without delirium or coma during the 14 days after study enrollment.
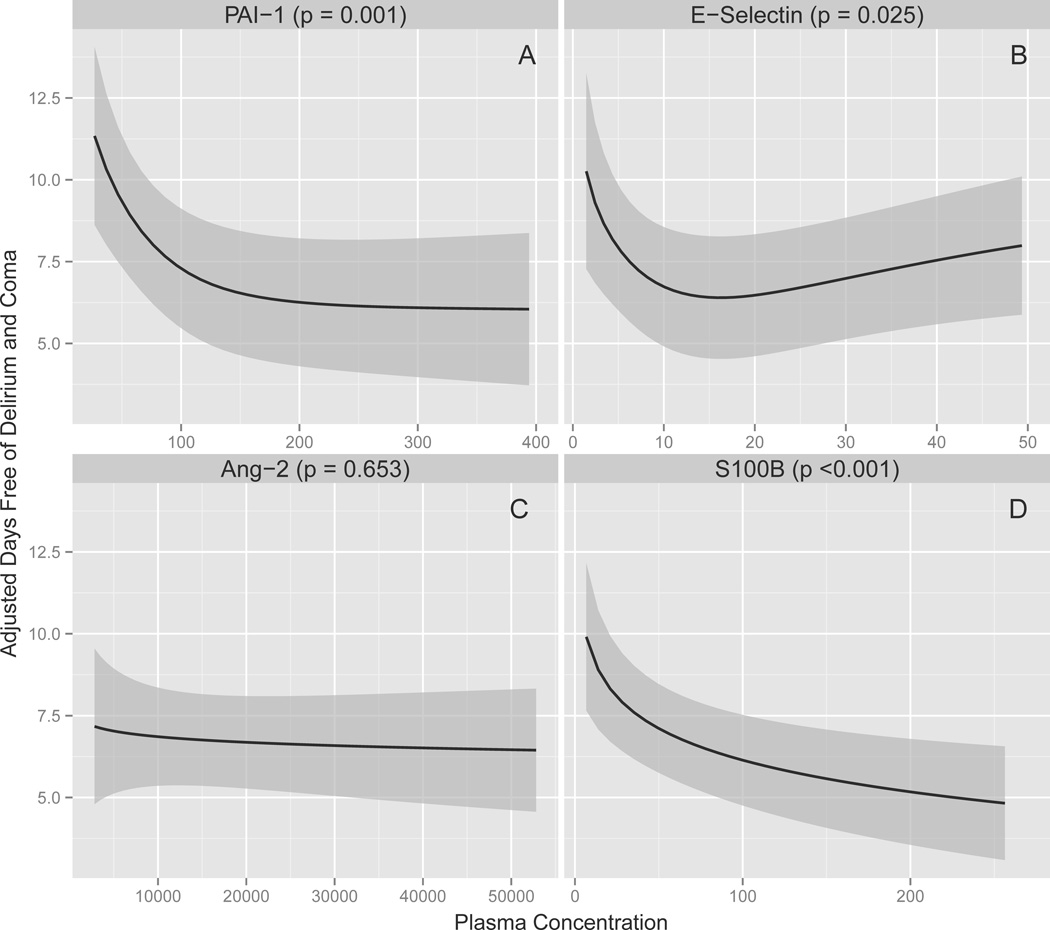
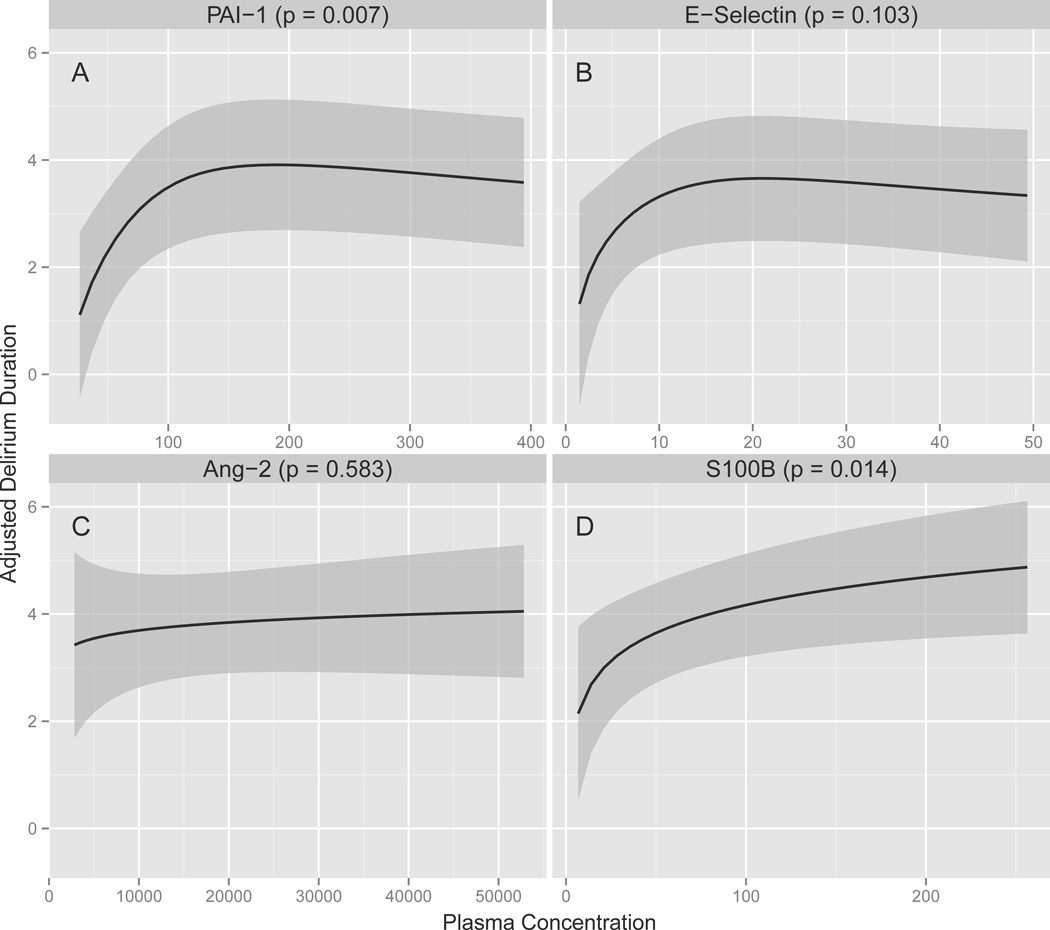
As shown in Table 2, Figure 1, and Figure 2, after adjusting for age, Charlson comorbidity index, modified SOFA score, and severe sepsis, baseline plasma concentrations of PAI-1 were consistently associated with delirium, with higher PAI-1 concentrations associated with fewer delirium/coma-free days in the full cohort (p = 0.002) and a longer duration of delirium among survivors (p = 0.007). Similarly, S100B concentrations were consistently associated with delirium; higher S100B concentrations predicted fewer delirium/coma-free days in the full cohort (p < 0.007) and more delirium days among survivors (p = 0.01). Severe sepsis marginally modified the associations that PAI-1 (interaction p = 0.10) and S100B (interaction p = 0.06) had with delirium/coma-free days such that the magnitude of the associations were slightly larger in severely septic patients but the direction of the associations were unchanged (see Supplemental Digital Content).
Table 2.
Associations between Plasma Markers of Endothelial Activation and Blood-Brain Barrier Injury and Delirium
| Percentile | Adjusted Differencea | |||
|---|---|---|---|---|
| Biomarker | 25th | 75th | Estimate (95% CI) | P Value |
| Associations with delirium/coma-free days among all patients (N=134) | ||||
| PAI-1(ng/mL) | 74.3 | 171.5 | −1.72 (−2.65, −0.79)b | 0.002 |
| E-selectin(ng/mL) | 9.8 | 29.8 | 0.24 (−0.62, 1.10)b | 0.02 |
| Ang-2(pg/mL) | 9639 | 26369 | −0.25 (−1.32, 0.82) | 0.64 |
| S100B(pg/mL) | 38.4 | 128.7 | −1.70 (−2.66, −0.74) | <0.001 |
| RHI | 1.21 | 1.76 | 1.09 (0.50, 1.68) | <0.001 |
| Associations with delirium days among survivors (N=113) | ||||
| PAI-1(ng/mL) | 74.3 | 171.5 | 0.83 (0.25, 1.40)b | 0.007 |
| E-selectin(ng/mL) | 9.8 | 29.8 | 0.28 (−0.26, 0.83)b | 0.12 |
| Ang-2(pg/mL) | 9639 | 26369 | 0.22 (−0.54, 0.99) | 0.57 |
| S100B(pg/mL) | 38.4 | 128.7 | 0.93 (0.23, 1.63) | 0.01 |
| RHI | 1.21 | 1.76 | −0.63 (−1.15, −0.12) | 0.02 |
Results, which were determined using multiple linear regression, show the adjusted difference (and 95% confidence intervals) in delirium/coma-free days or delirium days when the specified biomarker of endothelial activation or blood-brain barrier injury increased from the 25th percentile value to the 75th percentile value. In the delirium/coma-free days models, a negative adjusted difference indicates that higher biomarker concentrations were associated with worse cognitive outcomes, i.e., fewer days without delirium or coma. Alternatively, in the delirium duration model, a positive adjusted difference indicates that higher biomarker concentrations were associated with worse cognitive outcomes, i.e., more days of delirium. Adjusted differences, however, may vary depending on comparators in nonlinear associations and are best represented graphically (Figure 1 and 2).
Indicates the association was nonlinear.
Abbreviations: Ang-2, angiopoietin-2; PAI-1, plasminogen activator inhibitor-1; RHI, reactive hyperemia index
Figure 1. Associations between Markers of Endothelial Activation and Blood-Brain Barrier Injury and Delirium/Coma-Free Days.
Higher PAI-1 (P = 0.002), E-selectin (P = 0.02), and S100B (P < 0.001) plasma concentrations were associated with fewer delirium/coma-free days after adjusting for age, Charlson comorbidity index (45), modified SOFA score (27), and severe sepsis. The solid lines demonstrate the point estimates of the associations between PAI-1, E-selectin, and Ang-2 as markers of endothelial activation and S100B as a marker of blood-brain barrier injury versus delirium/coma-free days, with the gray ribbons indicating the 95% confidence intervals. Abbreviations: Ang-2, angiopoietin-2; PAI-1, plasminogen activator inhibitor-1
Figure 2. Associations between Markers of Endothelial Activation and Blood-Brain Barrier Injury and Delirium Duration.
Higher PAI-1 (P = 0.007) and S100B (P = 0.01) plasma concentrations were associated with longer duration of delirium in survivors after adjusting for age, Charlson comorbidity index (45), modified SOFA score (27), and severe sepsis. The solid lines demonstrate the point estimates of the associations between PAI-1, E-selectin, and Ang-2 as markers of endothelial activation and S100B as a marker of blood-brain barrier injury versus delirium duration in survivors, with the gray ribbons indicating the 95% confidence intervals. Abbreviations: Ang-2, angiopoietin-2; PAI-1, plasminogen activator inhibitor-1
In contrast to PAI-1 and S100B, Ang-2 was consistently not associated with delirium measures after adjusting for covariates (p = 0.64 and 0.57 for delirium/coma-free days and delirium duration, respectively; Table 2, Figure 1, and Figure 2). Analyses of E-selectin yielded inconsistent results; high E-selectin concentrations were independently associated with fewer delirium/coma-free days in the full cohort (p = 0.02) but were not associated with delirium duration among survivors (p = 0.12). No significant interactions were found between severe sepsis and E-selectin (interaction p = 0.57) or Ang-2 (interaction p = 0.51).
As shown in Table 3, several of the plasma markers studied were significantly but weakly correlated with each other, including S100B and PAI-1, and the majority were not collinear. None of the plasma markers correlated with vascular reactivity as measured by the reactive hyperemia index. When we sought to determine whether S100B mediated the association between PAI-1, E-selectin, and vascular reactivity and acute brain dysfunction by including S100B as a covariate in models, we found that adjusting for S100B did not alter the independent associations between PAI-1, E-selectin, or endothelial vascular reactivity with either delirium/coma-free days among all patients or delirium duration among survivors (Table 4). These findings indicate that BBB/neurological injury does not mediate the associations of endothelial dysfunction (activation and impaired vascular reactivity) with acute brain dysfunction outcomes.
Table 3.
Correlations between Plasma Markers of Endothelial Activation, Blood-Brain Barrier Injury, and Reactive Hyperemia Index at Enrollmenta
| Marker | S100B | Ang-2 | E-selectin | PAI-1 | |
|---|---|---|---|---|---|
| Ang-2 | rho | 0.40 | |||
| P Value | <0.001 | ||||
| E-selectin | rho | 0.13 | 0.31 | ||
| P Value | 0.14 | <0.001 | |||
| PAI-1 | rho | 0.22 | 0.13 | 0.09 | |
| P Value | 0.01 | 0.15 | 0.33 | ||
| RHI | rho | −0.06 | −0.13 | −0.13 | 0.05 |
| P Value | 0.48 | 0.14 | 0.13 | 0.58 |
Correlations were assessed using Spearman’s rank correlation.
Abbreviations: Ang-2, angiopoietin-2; PAI-1, plasminogen activator inhibitor-1; RHI, reactive hyperemia index
Table 4.
Associations between Endothelial Dysfunction and Acute Brain Dysfunction by Blood-Brain Barrier Injury
| Percentile | Without S100B | With S100B | ||||
|---|---|---|---|---|---|---|
| Variable | 25th | 75th | Adj Difference* | P value | Adj Difference* | P value |
| Associations with delirium/coma-free days among all patients (N=134) | ||||||
| PAI-1(ng/mL) | 74.3 | 171.5 | −1.72 (−2.65, −0.79)† | 0.002 | −1.54 (−2.46, −0.61)† | 0.005 |
| E-selectin(ng/mL) | 9.8 | 29.8 | 0.24 (−0.62, 1.10)† | 0.02 | 0.41 (−0.49, 1.31)† | 0.07 |
| Ang-2(pg/mL) | 9639 | 26369 | −0.25 (−1.32, 0.82) | 0.64 | Did not perform | |
| RHI | 1.21 | 1.76 | 1.09 (0.50, 1.68) | <0.001 | 0.97 (0.38, 1.56) | 0.001 |
| Associations with delirium days among survivors (N=113) | ||||||
| PAI-1(ng/mL) | 74.3 | 171.5 | 0.83 (0.25, 1.40)† | 0.007 | 0.74 (0.18, 1.31)† | 0.01 |
| E-selectin(ng/mL) | 9.8 | 29.8 | 0.28 (−0.26, 0.83)† | 0.12 | Did not perform | |
| Ang-2(pg/mL) | 9639 | 26369 | 0.22 (−0.54, 0.99) | 0.57 | Did not perform | |
| RHI | 1.21 | 1.76 | −0.63 (−1.15, −0.12) | 0.02 | −0.61 (−1.11, −0.11) | 0.02 |
Results, which were determined using multiple linear regression, show the adjusted difference (and 95% confidence intervals) in delirium/coma-free days or delirium days when the variable increased from the 25th percentile value to the 75th percentile value. In the delirium/coma-free days models, a negative adjusted difference indicates that higher biomarker concentrations were associated with worse cognitive outcomes, i.e., fewer days without brain dysfunction. Alternatively, in the delirium duration model, a positive adjusted difference indicates that higher biomarker concentrations were associated with worse cognitive outcomes, i.e., more days of delirium.
Indicates the association was nonlinear.
Abbreviations: Adj, adjusted; Ang-2, angiopoietin-2; PAI-1, plasminogen activator inhibitor-1; RHI, reactive hyperemia index
We included S100B as a covariate in linear regression models to assess whether BBB/neurological injury mediated any significant associations observed between markers of endothelial activation or vascular reactivity and delirium/coma-free days. Higher PAI-1 plasma concentrations at enrollment remained significantly associated with fewer delirium/coma-free days among all patients and with longer delirium duration among survivors. The association between E-selectin at enrollment and delirium/coma-free days, while no longer statistically significant, was very similar in size and direction to the association without adjustment for S100B; therefore, the nonsignificance was likely due to having more terms and less power in the model. Improved vascular reactivity (higher RHI) remained significantly associated with increased delirium/coma-free days among all patients and with shorter delirium duration among survivors.
DISCUSSION
In this study, we found that higher plasma markers of endothelial activation and BBB/neurological injury at baseline were associated with increased duration of delirium in critically ill patients. Though this observational study cannot prove causation, our results are consistent with the hypothesis that perturbations of the endothelium—including activation and vascular functional impairment—and the BBB during critical illness are associated with prolongation of delirium. Thus, this investigation, the first to our knowledge to examine associations between markers of endothelial activation and delirium during critical illness, suggests that future research is needed to determine whether endothelial dysfunction and BBB/neurological injury have a causal role in the pathogenesis of delirium during critical illness.
During critical illness, inflammatory mediators bind to receptors on the endothelium, causing alterations in adhesion molecules, signaling pathways, and nitric oxide production (30, 31), changes that contribute to endothelial cell detachment and death, mediator release, altered vasomotor function and permeability, microvascular injury, and altered organ perfusion (9, 32). The various biochemical activities of the endothelium, however, may be differentially affected by critical illness. In this heterogeneous cohort with multiple diagnoses, elevated markers of microvascular thrombosis (PAI-1) and cell adhesion (E-selectin) were associated with brain dysfunction, whereas Ang-2, which is involved in cell death, permeability, and vascular remodeling, was not associated with outcomes. Overall, our results, which support that biochemical and blood flow alterations from endothelial dysfunction may have a pathogenic role in delirium, are complemented by those of previous studies showing that endothelial activation is associated with organ dysfunction and mortality during sepsis (30, 31, 33). We previously found that impaired endothelial vascular reactivity was significantly associated with acute brain dysfunction during critical illness (8). In addition, Pfister et al. used transcranial Doppler to measure cerebrovascular autoregulation in adults with sepsis and found disturbed cerebral autoregulation was associated with sepsis-associated delirium (34). Taken together, these data support that microcirculatory blood flow abnormalities from endothelial dysfunction contribute to significant organ dysfunction, including brain dysfunction, during critical illness.
In addition to altered blood flow to the brain, endothelial dysfunction may contribute to increased BBB permeability and neurological injury given that the BBB is composed of an endothelial layer with tight junctions and astrocyte foot processes. Structural and functional alterations of BBB endothelial cells have been associated with increased microvascular permeability and impaired microcirculation in inflammatory and infectious states, including animal models of sepsis (10–12). In animal and in vitro models, endothelial activation was associated with BBB leukocyte adhesion and BBB dysfunction (35). Furthermore, plasma markers of neurologic injury in traumatic brain injury have been shown to correlate with endothelial cell structural changes in cortical biopsy specimens (14). Indeed, we found that higher circulating concentrations of S100B, a validated measure of BBB injury (16–18), was associated with prolonged periods of delirium. Notably, S100B did not correlate with endothelial vascular reactivity nor did it mediate the association between endothelial activation or vascular reactivity and delirium, despite being moderately correlated with PAI-1. This implies that if BBB injury is involved in delirium during critical illness, functional changes in the BBB are not the sole contributor. Instead, endothelial activation and changes in the microcirculation are implicated as additional contributors with regard to delirium during critical illness.
The gold standards for measuring BBB injury include dynamic contrast-enhanced magnetic resonance imaging and CSF-to-serum albumin quotient, both of which are impractical in the study of ICU patients. Markers of neurologic injury, which are released into the plasma when neurons or the BBB are damaged, provide a more feasible measure of BBB injury. Of the markers previously studied, S100B is the one most correlated with BBB injury measured by other methods (16–18). S100B is expressed and secreted by astrocytes after CNS injury or ischemia and cell death (36), and extracranial sources of S100B do not affect circulating concentrations even after trauma (37, 38). Levels of S100B have been associated with the development of delirium and cognitive changes after cardiac surgery, neurological complications after aortic aneurysm surgery, low consciousness septic encephalopathy, and now delirium during critical illness (36, 39). Thus, current evidence suggests that BBB injury might contribute to the pathogenesis of brain dysfunction from acute surgical or medical insults.
Our investigation was strengthened by enrollment of a heterogeneous cohort that included medical and surgical ICU patients with a broad range of diagnoses, which increases generalizability of our findings. Additionally, we examined a complimentary array of plasma markers selected to reflect multiple components of endothelial function, and trained research personnel carried out twice-daily delirium and coma assessments after blood samples were collected. Biomarker measurement, therefore, preceded our assessments of delirium. Finally, we used robust multivariable regression analyses designed to minimize the possibility of confounding and overfitting.
Notable limitations are that we performed a single measurement of biomarkers at enrollment, did not follow biomarkers trends, and did not precisely identify the time of delirium onset for all subjects. Though our approach allowed us to determine whether biomarkers of endothelial activation and BBB/neurological injury were associated with subsequent duration of delirium, we could not assess whether these markers predicted delirium onset. An ideal design for future investigations would involve measuring biomarkers prior to delirium onset, the timing of which would be clearly defined, and then repeating biomarker measurements over time to confirm that the marker(s) in question remain elevated during delirium and then resolve in parallel with delirium resolution. Future studies are also needed to determine whether relationships between markers of endothelial or BBB injury and delirium change over time in response to disease progression and/or medical therapy (29). We did not directly assess endothelial activation and BBB injury in the brain, but we did rely on well-validated circulating measures of endothelial activation and BBB/neurological injury, which previous studies have shown reflect processes in the brain (16, 17, 35). Finally, unlike an animal model or an interventional trial (e.g., one examining endothelium-directed therapies) that might prove that endothelial injury is causally related to delirium, the observational design in this investigation cannot prove causation.
Because we did not coordinate delirium assessments with interruption of sedation, we could not distinguish rapidly reversible sedation-related delirium (previously reported to affect 12% of patients similar to those in our cohort) (40) from other forms of delirium, an approach that may have reduced the magnitude of the observed associations if rapidly reversible sedation-related delirium is not promoted by endothelial injury. Furthermore, some drug-induced forms of delirium are not rapidly reversible, and individual sedatives have differential effects on inflammation and bacterial clearance (41–44), potentially leading to varied effects on endothelial function. Given that prior studies have shown that delirium identified by the CAM-ICU predicts long-term cognitive impairment after critical illness (6, 7), associations between biomarkers of endothelial injury and long-term cognitive impairment should be studied. However, such analyses would be underpowered in the current cohort given the high observed mortality during long-term follow-up. Finally, we chose a priori not to adjust for multiple comparisons, an approach that may increase the likelihood of a type I error but avoid an unnecessary inflation of type II errors. If we had used Bonferroni corrections, of note, PAI-1 and S100B would have remained significant predictors of delirium.
CONCLUSIONS
In this prospective cohort study of critically ill medical and surgical ICU patients, we found that plasma markers of endothelial activation and BBB/neurological injury were independently associated with delirium duration. These findings support the hypothesis that dysfunction of the endothelium and BBB is involved in the pathophysiology of delirium during critical illness. However, subsequent investigations (e.g., studies examining biomarkers serially both before and after delirium onset and trials examining whether modulation of endothelial/BBB dysfunction prevents and/or treats delirium) are needed before the observed relationships can be fully understood.
Supplementary Material
Acknowledgments
Dr. Hughes received support for article research from the National Institutes of Health (NIH) and other (This project was supported by CTSA award TR000445 from the National Center for Advancing Translational Sciences and by the National Institutes on Aging [AG027472]. In addition, Dr. Hughes received support from a Foundation for Anesthesia Education and Research Mentored Research Training Grant, American Geriatrics Society Jahnigen Career Development Award, and the National Institutes of Health [HL111111, R03AG045085], Dr. Pandharipande received support from the NIH [AG027472, HL111111], Dr. Ely received support from the VA Clinical Science Research and Development Service and the NIH [AG027472, AG035117, HL111111], Dr. Ware received support from the NIH [HL103836 and HL112656], and Dr. Girard received support from the NIH [AG034257, AG035117]. Drs. Ely and Girard also received support from the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center). Dr. Pandharipande received support for article research from the NIH. His institution received funding from a grant from Hospira Inc in collaboration with NIH. Dr. Chandrasekhar received support for article research from the NIH. Her institution received funding from the NIH. Dr. Ware received support for article research from the NIH and received funding from Glaxo Smith Kline and Abbot. Dr. Ely received support for article research from the NIH and VA funding and Merit Awards; received funding from Orion, Abbott, and Hospira; and disclosed other support (NIH support for several ongoing studies. BRAIN-ICU, EXD Study, MENDS2 Study and MIND-USA study. VA support for MIND-ICU, PTSD and Predictors of cognitive impairment in survivors of critical illness). His institution received funding from the NIH and VA Funding and Merit Awards. Dr. Girard received support for article research from the NIH and received funding from Hospira.
Footnotes
Attribution: Vanderbilt University Medical Center
Copyright form disclosures: Dr. Thompson disclosed that she does not have any potential conflicts of interest.
REFERENCES
- 1.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 4.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 6.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes CG, Morandi A, Girard TD, et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. 2013;118:631–639. doi: 10.1097/ALN.0b013e31827bd193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ait-Oufella H, Maury E, Lehoux S, et al. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36:1286–1298. doi: 10.1007/s00134-010-1893-6. [DOI] [PubMed] [Google Scholar]
- 10.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 11.Gavins F, Yilmaz G, Granger DN. The evolving paradigm for blood cell-endothelial cell interactions in the cerebral microcirculation. Microcirculation. 2007;14:667–681. doi: 10.1080/10739680701404903. [DOI] [PubMed] [Google Scholar]
- 12.Taccone FS, Su F, Pierrakos C, et al. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14:R140. doi: 10.1186/cc9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel C, Bauer A, Wiesnet M, et al. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-K/Akt pathway. J Cell Physiol. 2007;212:236–243. doi: 10.1002/jcp.21022. [DOI] [PubMed] [Google Scholar]
- 14.Vajtr D, Benada O, Kukacka J, et al. Correlation of ultrastructural changes of endothelial cells and astrocytes occurring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol Res. 2009;58:263–268. doi: 10.33549/physiolres.931253. [DOI] [PubMed] [Google Scholar]
- 15.Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers. 2011;16(Suppl 1):S11–S21. doi: 10.3109/1354750X.2011.587893. [DOI] [PubMed] [Google Scholar]
- 16.Blyth BJ, Farhavar A, Gee C, et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma. 2009;26:1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanner AA, Marchi N, Fazio V, et al. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchi N, Rasmussen P, Kapural M, et al. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci. 2003;21:109–121. [PMC free article] [PubMed] [Google Scholar]
- 19.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 20.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 22.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 25.White H. A heteroskedasticity-consistent covariance matrix and a direct test for heteroskedasticity. Econometrica. 1980;48:22. [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 29.Calfee CS, Gallagher D, Abbott J, et al. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med. 2012;40:1731–1737. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro NI, Schuetz P, Yano K, et al. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care. 2010;14:R182. doi: 10.1186/cc9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 33.Ricciuto DR, dos Santos CC, Hawkes M, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39:702–710. doi: 10.1097/CCM.0b013e318206d285. [DOI] [PubMed] [Google Scholar]
- 34.Pfister D, Siegemund M, Dell-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vachharajani V, Cunningham C, Yoza B, et al. Adiponectin-deficiency exaggerates sepsis-induced microvascular dysfunction in the mouse brain. Obesity (Silver Spring) 2012;20:498–504. doi: 10.1038/oby.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cata JP, Abdelmalak B, Farag E. Neurological biomarkers in the perioperative period. Br J Anaesth. 2011;107:844–858. doi: 10.1093/bja/aer338. [DOI] [PubMed] [Google Scholar]
- 37.Pham N, Fazio V, Cucullo L, et al. Extracranial sources of S100B do not affect serum levels. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blyth BJ, Farahvar A, He H, et al. Elevated serum ubiquitin carboxy-terminal hydrolase L1 is associated with abnormal blood-brain barrier function after traumatic brain injury. J Neurotrauma. 2011;28:2453–2462. doi: 10.1089/neu.2010.1653. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen DN, Spapen H, Su F, et al. Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34:1967–1974. doi: 10.1097/01.CCM.0000217218.51381.49. [DOI] [PubMed] [Google Scholar]
- 40.Patel SB, Poston JT, Pohlman A, et al. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189:658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 41.Memis D, Hekimoglu S, Vatan I, et al. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98:550–552. doi: 10.1093/bja/aem017. [DOI] [PubMed] [Google Scholar]
- 42.Sanders RD, Godlee A, Fujimori T, et al. Benzodiazepine augmented gamma-amino-butyric acid signaling increases mortality from pneumonia in mice. Crit Care Med. 2013;41:1627–1636. doi: 10.1097/CCM.0b013e31827c0c8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venn RM, Bryant A, Hall GM, et al. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth. 2001;86:650–656. doi: 10.1093/bja/86.5.650. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Cheng Y, Liu X, et al. Unexpected pro-injury effect of propofol on vascular smooth muscle cells with increased oxidative stress. Crit Care Med. 2011;39:738–745. doi: 10.1097/CCM.0b013e318206bd86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.