Abstract
As they mature into erythrocytes during normal erythropoiesis, reticulocytes lose surface transferrin receptors before or concurrently with reticulin. Exosome release accounts for most of the loss of transferrin receptors from reticulocytes. During erythropoietic stress, reticulocytes are released early from hematopoietic tissues and have increased reticulin staining and transferrin receptors. Flow cytometry of dually stained erythrocytes of mice recovering from phlebotomy demonstrated delayed loss of reticulin and transferrin receptors during in vitro maturation compared to in vivo maturation, indicating that an in vivo process extrinsic to the reticulocytes facilitates their maturation. Splenectomy or macrophage depletion by liposomal clodronate inhibited in vivo maturation of reticulocytes and increased the numbers of reticulin-negative, transferrin receptor-positive cells during and after recovery from phlebotomy. This reticulin-negative, transferrin receptor-positive population was rarely found in normal mice. Transmission electron microscopy demonstrated that the reticulin-negative, transferrin receptor-positive cells were elongated and discoid erythrocytes, but they had intracellular and surface structures that appeared to be partially degraded organelles. The results indicate that maturation of circulating stress reticulocytes is enhanced by an extrinsic process that occurs in the spleen and involves macrophage activity. Complete loss of reticulin with incomplete loss of surface transferrin receptors in this process produces a reticulin-negative, transferrin receptor-positive erythrocyte population that has potential utility for detecting prior erythropoietic stresses including bleeding, hemolysis and erythropoietin administration, even after recovery has been completed.
Keywords: Stress reticulocytes, Transferrin receptors, Anemia
INTRODUCTION
Reticulocytes form in hematopoietic tissues when orthochromatic erythroblasts enucleate. Over the several days of their maturation in the hematopoietic organs and blood, reticulocytes degrade or remove internal organelles, such as ribosomes, mitochondria, endoplasmic reticulum, as well as losing some plasma membrane components expressed during erythroblast stages. These losses of internal organelles and plasma membrane components lead to decreased cell size, conversion from aerobic to anaerobic metabolism, and metamorphosis from the irregularly shaped, motile reticulocyte to the uniform, biconcave, discoid shape that characterizes mature RBCs. During maturation, reticulocytes migrate through the vascular endothelium of venous sinuses in the hematopoietic organs and enter the circulating blood, where they require another one to two days of maturation before becoming erythrocytes, i.e., mature red blood cells (RBCs). Two processes degrade internal organelles in reticulocytes: ubiquitin/proteasomal degradation [1–3] and autophagic vacuole formation and externalization [4–7]. Clinical methods quantify reticulocytes by staining residual RNA, mainly in ribosomes, with either supravital dyes such as new methylene blue or fluorescent dyes such as thiazole orange (TO) [8]. When the last residual RNA in a reticulocyte is degraded, that cell is no longer classified as a reticulocyte but rather is considered a mature RBC. However, when a reticulocyte completes its degradation of RNA, it may have not completed remodeling of the plasma membrane [9].
In addition to the intracellular degradation of internalized plasma membrane components, reticulocytes can externalize plasma membrane components by two processes. In one process, plasma membrane endosomes form intracellular multivesicular bodies which fuse with the plasma membrane, releasing small, membrane-bound exosomes that are enriched in specific membrane components [10, 11]. The most studied plasma membrane component removed from reticulocytes in exosomes is the transferrin receptor (TfR1/CD71), an abundant plasma membrane protein in erythroblasts that imports iron via binding and endocytosis of diferric transferrin. After release of iron from transferrin in the endosome, TfRs are recycled to the erythroblast membrane, but during normal reticulocyte maturation the TfRs are routed to multivesicular bodies and subsequent exosomal release [12, 13]. Other proteins including α4β1 integrin, nucleoside transporters, acetylcholinesterase and aquaporin-1 are also found in reticulocyte exosomes [14]. In addition to forming multivesicular bodies, cytoplasmic vesicles formed by plasma membrane internalization in the late-stage reticulocytes can fuse with autophagosomes, which subsequently exocytose the membrane components along with other organelles in the autophagosome [15]. Membrane proteins eliminated by the autophagosomal pathway in the later stages of reticulocyte maturation do not include TfR and α4β1 integrin, which appear to be lost earlier by the exosomal-multivesicular mechanism [15].
Proteasome and exosome formation are intrinsic to reticulocytes, but a process extrinsic to reticulocytes appears to play a role in their maturation. Indeed, human reticulocytes formed in vitro require circulation in NOD/SCID mice to acquire mature erythrocyte size and shape [16]. Several reports have indicated a splenic role in this extrinsic mechanism of reticulocyte maturation [9, 17–21]. In erythropoietically normal humans, splenectomy delays reticulocyte maturation [18] and increases autophagic vacuoles subjacent to RBC membranes [19]. Splenectomy delays normal loss of reticulocyte membrane cholesterol and phospholipids [9, 20], and delays normal loss of fibronectin binding during maturation of circulating reticulocytes [21]. However, the spleen is not the only organ that mediates the maturation of reticulocytes. Several weeks to months after splenectomy, other organs, presumably the liver and bone marrow, assume the fibronectin-binding and enhancement of reticulocyte maturation functions associated with the spleen [18, 21].
Ultrastructural studies have demonstrated that macrophages are likely mediators of extrinsic membrane remodeling before and after reticulocyte release from the hematopoietic organs. In normal human fetal erythropoiesis, hepatic sinusoidal macrophages surround newly formed reticulocytes in a process, emperipolesis, which precedes reticulocyte entry into hepatic venous sinuses [22]. In normal rat bone marrow, reticulocytes have intimate contact with central macrophages of erythroblastic islands before entering marrow venous sinusoids [23]. In normal erythropoiesis of several different mammalian species, the spleen selectively sequesters reticulocytes that bind to macrophages in the red pulp [24, 25].
Stress erythropoiesis following blood loss, hemolysis, or erythropoietin administration increases the numbers of circulating reticulocytes. Within hours of blood loss, reticulocyte transit across the bone marrow sinusoidal cells into the circulation increases several-fold [26]. This increased reticulocyte transit raises circulating reticulocyte numbers and shifts the circulating reticulocyte population to more immature stages. Reticulocyte immaturity is demonstrated by increases in cell size, reticulin staining, iron uptake [27, 28], mobility and lobulation [29, 30], and membrane ion transporter activities [31, 32]. Circulating, immature reticulocytes produced during erythropoietic stress require the same total amount of time for reticulin degradation as do normal immature reticulocytes that are not released early from the bone marrow [27, 28]. Furthermore, splenectomy does not affect the total time required for reticulin loss in circulating stress reticulocytes [28]. However, erythropoietic stress appears to affect extrinsic remodeling of reticulocyte plasma membrane by delaying and diminishing loss of specific components [21, 33]. In the present study, we examine loss of TfRs by extrinsic plasma membrane remodeling during maturation of stress reticulocytes.
MATERIALS AND METHODS
Mice and induction of erythropoietic stress
The Vanderbilt University Animal Care Committee approved all experiments. All experiments, except for those requiring splenectomized mice, used female CD2F1 mice, aged 8–12 weeks old (Harlan Laboratories, Indianapolis, IN). Experiments with splenectomized mice used similarly aged BALB/c female mice at 2 to 4 weeks after splenectomy (Harlan Laboratories). To induce erythropoietic stress by blood loss, isoflurane-anesthetized mice were phlebotomized 15 – 20 µL blood/gram of body weight with heparinized capillary tubes and given intraperitoneal saline, as described previously [34], on three or five consecutive days. Depending upon the amounts and schedule of phlebotomies, hematocrits on day one of recovery ranged from 15–25%. On various days during their recovery from the phlebotomy, isoflurane-anesthetized mice were phlebotomized one µL blood/gram of body weight for microhematocrits, RBC counts, and flow cytometry analyses. In experiments comparing reticulocyte maturation in vivo and in vitro, the mice were phlebotomized for five days, reducing their hematocrits to the 22–25% range. On the sixth day, a sample of reticulocyte-rich blood was collected for culture. These heparinized samples of peripheral blood that had increased percentages of reticulocytes were washed in PBS and cultured for four days under conditions previously described [35]. Briefly, blood samples were cultured at 37°C in a humidified atmosphere of 5% CO2 in air at 2 × 106 cells/mL. The culture medium was Iscove’s modified Dulbecco’s medium with 30% heat-inactivated fetal bovine serum, 1% deionized bovine albumin, 100 units/mL penicillin-G, 100 µg/mL streptomycin, and 0.1 mM -thioglycerol. Aliquots from cultures of reticulocytes or peripheral blood with increased reticulocytes were collected daily and processed for further analyses.
Immediately after obtaining the peripheral blood sample used for culture, the phebotomized mice were transfused intraperitoneally with 150 µL/gram body weight of packed donor RBCs that had been washed in phosphate buffered saline (PBS). Over the next 16 h, these hypertransfusions raised hematocrits to the 56–58% range, stopping further reticulocyte production. During daily blood sampling for analyses, hematocrits of the phlebotomized-hypertransfused mice remained above the normal baseline hematocrit range of 45 – 49% for four days, permitting the monitoring of circulating reticulocyte maturation in vivo without the influx of new reticulocytes.
In experiments measuring surface TfRs on reticulocytes and RBCs during recovery from blood loss, mice phlebotomized daily for three days, with no intervention other than blood sampling, recovered normally to their baseline hematocrits, RBCs, and reticulocytes. To examine the effects of macrophages on reticulocyte maturation in vivo, one microgram of liposomal clodronate (dichloromethylene diphosphonate) in 0.2 mL of PBS or an equal volume of plain liposomes in 0.2 mL PBS (Encapsula Nanosciences, Nashville, TN) were injected into the lateral tail vein at the beginning of the fourth day of recovery from phlebotomy. Liposomal clondronate disrupts macrophage function by selectively killing those mature macrophages that ingest it [36]. After liposomal clodronate administration, macrophage function remains decreased for several days in vivo until the generation of new mature macrophages.
Analyses of hematocrits, RBC numbers, reticulocytes, and TfR expression
Hematocrits were measured in centrifuged microcapillary tubes. RBC numbers were determined by diluting heparinized blood samples into PBS and counting in a hemocytometer. In fresh and cultured blood samples from phlebotomized mice, reticulocytes and RBCs expressing surface TfRs were analyzed simultaneously by dual color flow cytometry. PBS-washed RBCs were resuspended in PBS plus 2% fetal bovine serum, stained for 20 min on ice with rat monoclonal antibodies to murine TfR/CD71 conjugated with allophycocyanin (APC) (Leinco Technologies, St. Louis, MO), washed in PBS, resuspended in 10 ng/mL thiazole orange (Sigma-Aldrich, St. Louis, MO) in PBS for at least 30 min., and analyzed by two-color flow cytometry. Fresh and cultured blood samples from phlebotomized mice were also analyzed for expression of surface TfRs and α4 integrin (CD49d) using flow cytometry with rat monoclonal antibodies to murine TfR conjugated with APC and murine α4 integrin conjugated to fluoroscein isothiocyanate (FITC) (BD Pharmingen, San Jose, CA). The RBCs were washed in PBS, resuspended in PBS plus 2% fetal bovine serum, stained for 20 min on ice with the antibodies to TfR and α4 integrin, washed in PBS, and examined by flow cytometry. Controls were freshly harvested, PBS-washed RBCs from normal mice. Flow cytometric data were collected with a FACSCantoII equipped with standard 3-laser, 4-2-2 instrument configuration and running FACSDiVa acquisition software (BD Biosciences, San Jose, CA). Listmode files were further analyzed using Winlist Software (Verity Software House, Topsham, ME). Light Scatter (FSC, linear or log and SSC, log) gates to exclude debris, aggregates, platelets, and leukocytes were applied to fluorescent parameters for determination of RBC subset percentages.
Electron Microscopy
To determine the morphological appearance of the circulating RBCs that displayed surface TfRs but had no thiazole orange staining, samples of peripheral blood were obtained from mice on day 5 of recovery from phlebotomy. These blood samples were washed with PBS, stained for TfR and thiazole orange and separated by fluorescence-activated cell sorting. Cells were sorted for electron microscopy using a FACSAriaII (BD Biosciences, San Jose, CA) with standard filter sets. Gates and sorting regions were similar to those used for analysis, and aliquots of sorted cells were evaluated for purity using the FACSCantoII. RBCs with surface TfRs (thiazole orange negative, TfR positive) were fixed in glutaraldehyde and processed for transmission electron microscopy as previously described [35].
RESULTS
Transferrin receptor expression on reticulocytes during recovery from blood loss
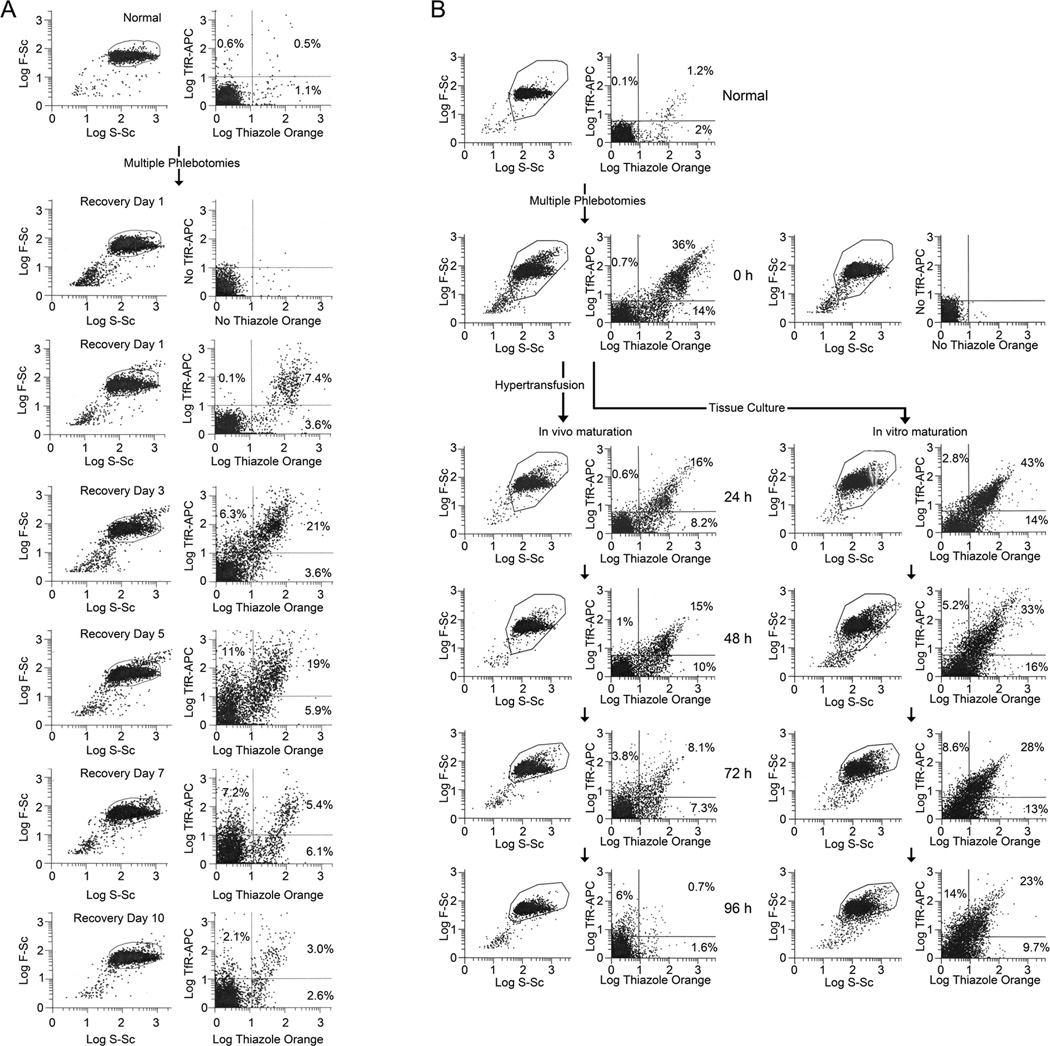
In order to determine the pattern of TfR expression in circulating reticulocytes during recovery after blood loss, mice were made anemic by phlebotomies on three consecutive days, and their blood was analyzed on the subsequent 10 days (recovery days 1 to 10) by flow cytometry for thiazole orange (TO) staining and surface TfR/CD71 expression. Fig. 1A shows paired flow cytometry histograms for forward-scattering (F-Sc) versus side-scattering (S-Sc) of light and TO versus TfR-allophycocyanin (TfR-APC) fluorescence of the RBCs of a representative mouse on recovery days 1, 3, 5, 7, and 10. In Fig. 1A, compared to an unbled, control (Normal) mouse, the percentages of TO+ and TFR+ cells were increased on recovery day 1. TO+ and TfR+ percentages increased further on recovery days 3 and 5 before decreasing on recovery day 7 (Fig. 1A). On recovery day 10, one day after fully recovering from their anemias, the mouse had persistently increased TO+ and TfR+ cells, including TO−,TfR+ cells, which were always less than 1% in normal control mice.
Figure 1. Pattern of reticulin staining and transferrin receptor (TfR) expression of RBCs during recovery from blood loss.
(A) Histograms of flow cytometry analyses of RBCs for reticulin with thiazole orange (TO) staining and transferrin receptor expression with APC-conjugated TfR/CD71 antibodies in mice recovering from phlebotomy. Mice phlebotomized on three consecutive days had reduction of mean baseline hematocrit of 46% and to a mean of 15% on the fourth day (recovery day 1). During recovery to baseline hematocrits over 10 days, blood samples were analyzed by flow cytometry. In the pair of histograms shown at each time point, the forward-scattering versus side-scattering of light in the left histogram shows the outlines of the gates used to select the cells for flow cytometric analyses of TfR-APC versus TO fluorescence shown in the right histogram. Results for an unbled control mouse (Normal) that was also analyzed on recovery day 1 and for the unstained recovery day 1 cells (No TfR-APC and No TO staining) are shown above the recovery day 1 histograms. The paired histograms for F-Sc/S-Sc and TfR-APC/TO of a representative mouse are shown on recovery days 1, 3, 5, 7, and 10. Percentages of total analyzed RBCs are shown in the respective quadrants for TO−,TfR+, TO+,TfR+, and TO+,TfR− cells. (B) Flow cytometry analyses of TO staining and TfR expression during in vivo and vitro maturation of reticulocytes. Mice were phlebotomized on five consecutive days with reduction in mean hematocrit from 48% to 23%. On the sixth day (time = 0 hours), samples of blood were obtained for flow cytometry analyses and initiation of tissue culture, and the mice were then hypertransfused as described in Methods. At each time point the forward-scattering versus side-scattering of light is shown in the left histogram with outlines of the gates used to select cells for flow cytometric analyses of TfR-APC versus TO fluorescence that are shown in the right histogram. Results for an unbled control mouse (Normal) that was also analyzed at 0 h and for the unstained 0 h cells (No TfR-APC and No TO staining) are shown above and to the right of 0 h histograms, respectively. The paired histograms for F-Sc/S-Sc and TfR-APC/TO of RBCs of a representative mouse are shown at 24 h, 48 h, 72 h and 96 h for in vivo maturation in the left-hand column and for in vitro maturation in the corresponding right-hand column. Percentages of total analyzed RBCs are shown in the respective quadrants for TO−,TfR+, TO+,TfR+, and TO+,TfR− cells.
To determine whether mechanisms extrinsic to reticulocytes may play a role in the patterns of TO staining and surface TfR expression during recovery from blood loss, cohorts of RBC populations with increased percentages of stress reticulocytes were examined during maturation for 96 h in vitro or in vivo. Blood samples of mice made anemic by phlebotomy were placed in tissue culture where the reticulocytes matured in vitro, while their cohort reticulocytes remaining in vivo matured in the phlebotomized mice that had new reticulocyte production suppressed by hypertransfusion. Fig. 1B shows paired flow cytometry histograms for F-Sc versus S-Sc of light and TO versus TfR-allophycocyanin (TfR-APC) fluorescence of RBCs from a representative mouse that was made anemic by five consecutive days of phlebotomies, with the 0 h of recovery on the sixth day. At recovery time 0 h, a sample of blood was cultured in vitro. The flow cytometry histograms are shown in Fig. 1B for this 0 h blood sample and for the subsequent 24 h, 48 h, 72 h and 96 h times of maturation in vivo (left-hand column) and in vitro (right-hand column). In Fig. 1B, 50% of the RBCs from the phlebotmized mouse at recovery time 0 h stained with TO, indicating that they were reticulocytes, whereas 3.2% of the RBCs of an unbled, control (Normal) mouse blood analyzed at the same time were reticulocytes. The majority of reticulocytes from the anemic mouse at 0 h expressed TfRs with 36% of all RBCs staining TO+,TfR+, while only 14% of all RBCs stained TO+,TfR−. Conversely, Fig. 1B shows that among the few reticulocytes in a normal mouse 1.2% of RBCs stained TO+,TfR+, while 2% stained TO+,TfR−. Only 0.1% of the RBCs were TO−,TfR+ RBCs, indicating the rarity of these cells in the blood, except under conditions of erythropoietic stress.
In Fig. 1B, hypertransfusion of the phlebotomized mouse reduced the reticulocyte percentage of RBCs from 50% at 0 h of recovery to 24.2% at 24 hours later (left-hand column). In vivo, the percentages of reticulocytes (total TO+ RBCs) steadily decreased from 24.2% at 24 h to only 2.3% of the cells at 96 h (Fig. 1B, left-hand column). In vitro, the loss of TO staining was much less complete, as shown in Fig. 1B right-hand column, in which 52% of cells were TO+ at 24 h and 32.7% remained TO+ at 96 h. Furthermore, among those reticulocytes that became TO− RBCs in vitro, many retained TfR expression as shown in the TO−,TfR+ population that was only 2.8% at 24 h of culture became 14% of the cells by 96 h of culture. Unexpectedly, the reticulocytes that matured in vivo also demonstrated a persistence of TfR expression after the loss of TO staining, as the percentages of TO−,TfR+ RBCs increased ten-fold from 0.6% at 24 h to 6% at 96 h (Fig. 1B, right-hand column).
Like TfR, α4 integrin is a component of the plasma membrane of nucleated erythroid cells that is normally lost by exocytosis in the late stages of erythroblast maturation and continues in the early stages of reticulocyte maturation [14, 15]. In Suppl. Fig. 1, flow cytometry histograms of TfR and α4 integrin expression on RBCs from a phlebotomized, anemic mouse are shown at 0 h and daily from 24 to 96 h during reticulocyte maturation in vitro (upper row) and, after hypertransfusion, in vivo (lower row). In the anemic phlebotomized mouse, the percentages of cells expressing α4 integrin on the plasma membrane was always less than those expressing TfR. However, the loss of α4 integrin during in vitro maturation (Suppl. Fig. 1, upper row of panels) was delayed compared to in vivo maturation, a pattern similar to the delayed loss of TfR during maturation in vitro compared to in vivo.
The percentages of TfR+ cells maturing in vivo showed essentially no change from 16.6% at 24 h to 16.0% at 48 h in Fig. 1B and increased slightly from 18.6% to 22.8% between 24 and 48 h in Suppl. Fig. 1. After 48 h, percentages of TfR+ cells declined greatly in vivo (Figs. 1B and Suppl. Fig. 1). These results indicate that maximal suppression of new reticulocyte production in vivo required about 2 days following the abrupt decrease in EPO production by hypertransfusion. In the case of reticulocytes maturing in vitro, the plating, harvesting, and washing of cultured cells were associated with about 15% loss of cells, with relatively greater percentages of mature RBCs lost. As a result, TfR+ cell percentages were slightly increased at 24 h compared to their respective percentages in anemic blood (Fig. 1B and Suppl. Fig. 1). TfR+ cells declined in vitro from 45.8% at 24 h to 38.5% at 48 h in Fig. 1B, whereas TfR+ cells increased slightly in vitro from 33.1% at 24 h to 38.8% at 48 h in Supplemental Fig. 1. After 48 h, the percentages of TfR+ cells declined slightly in vitro (Fig. 1B and Suppl. Fig. 1).
Splenic function and reticulocyte maturation
The delayed maturation rate of reticulocytes maturing in vitro versus in vivo in Fig. 1B and Suppl. Fig.1 suggested that an extrinsic process in vivo enhanced loss of reticulin, TfR and α4 integrin in reticulocytes that were produced and released into the blood during erythropoietic stress. The results in Fig. 1B and Suppl. Fig. 1 indicated that the extrinsic process that enhanced maturation of stress reticulocytes in vivo resulted in complete loss of reticulin and α4 integrin. However, this extrinsic process did not achieve complete loss of TfRs, as the percentages of TO−,TfR+ RBCs, which are relatively rare cells in normal blood, accumulated in the blood of phlebotomized mice that had been hypertransfused (Fig. 1B). Therefore, we designed experiments to determine the roles played by the spleen and/or macrophages in the loss of TfRs from circulating stress reticulocytes in mice that were recovering normally after phlebotomies.
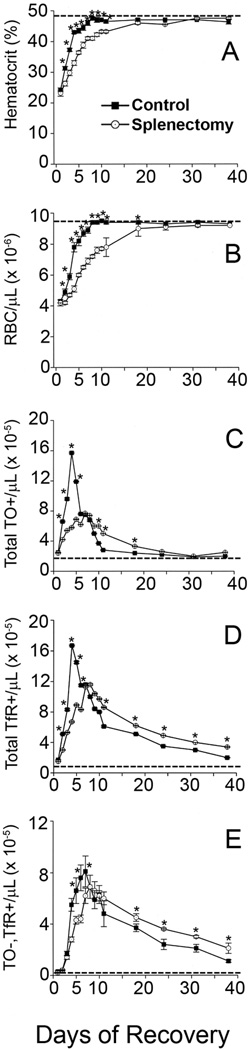
Fig. 2 shows changes in hematocrits, RBC numbers, and flow cytometry results for TO staining and TfR expression of circulating RBCs from splenectomized mice and control, non-splenectomized littermates during recovery from anemia induced by three consecutive daily phlebotomies similar to the experiment shown in Fig. 1A, except that the anemia was slightly less severe. Before phlebotomy, the splenectomized mice had similar hematocrits, total RBCs, and reticulocyte counts as the non-splenectomized normal controls. After phlebotomy, the splenectomized mice eventually recovered to similar hematocrits and reticulocyte counts as did control mice. However, recovery from phlebotomy-induced anemia was delayed in splenectomized mice compared to non-splenectomized controls (Fig. 2A, 2B), because the murine spleen is the primary organ responsible for stress erythropoiesis. The absence of the spleen resulted in blunted and delayed reticulocyte increases in response to phlebotomies (Fig. 2C). However, in both splenectomized and normal control mice, hematocrits returned to pre-phlebotomy values by recovery day 17 (Fig. 2A), and reticulocytes (total TO+ RBCs) returned to pre-phlebotomy values by recovery day 24 (Fig. 2C). On the other hand, the total TfR+ RBCs (Fig. 2D) and TO−,TfR+ RBCs (Fig. 2E) remained increased above pre-phlebotomy values for an additional two weeks in both splenectomized and normal control mice. Most importantly, during the last three weeks, the splenectomized mice had significantly greater increases in both total TfR+ RBCs (Fig. 2D) and TO−,TfR+ RBCs (Fig. 2E) than did control mice. On day 3 of recovery, both normal and splenectomized mice had less than 1% of the total RBC populations stained with annexin V, indicating an absence of apoptotic or dead cells in RBC populations. The development and persistence of the TO−,TfR+ RBC population after phlebotomy in normal mice demonstrated that loss of reticulocyte surface TfRs was compromised during stress erythropoiesis. The increased TO−,TfR+ RBC population in the splenectomized mice after phlebotomy compared to control mice after phlebotomy indicated that the spleen plays a role in the loss of TfRs from the maturing reticulocytes.
Figure 2. Effects of splenectomy on stress reticulocyte maturation.
Control (■) or splenectomized (O) mice were phlebotomized on days −2 to 0 to induce anemia and allowed to recover without any intervention other than blood sampling as described in Methods. Samples of blood were analyzed as described in Methods for (A) Hematocrit and (B) Total erythrocyte (RBC) numbers as well as by flow cytometry for (C) Total reticulocyte numbers (TO+ RBCs), (D) Total number of RBCs displaying surface TfR (Total TfR+ RBCs), and (E) Total number of RBCs without reticulin that express TfR (TO−,TfR+ RBCs). Pre-phlebotomy mean values are shown by dashed lines. Results are means ± SEM of five individual mice. *Significantly different than control at p < 0.05.
Macrophage activity in reticulocyte maturation
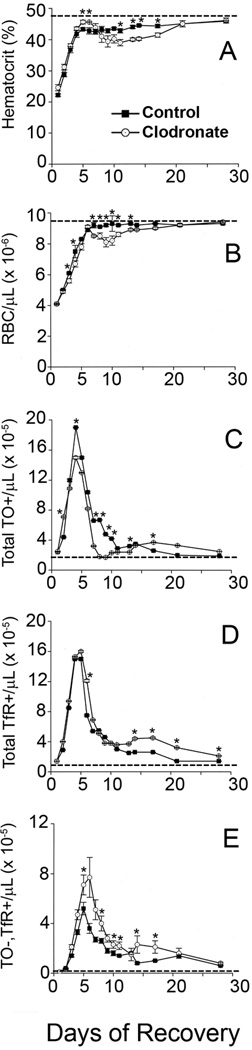
Macrophages are among the most likely candidate cell types mediating the loss of reticulocyte TfRs in the spleen [22–25]. Therefore, hematocrits, RBC numbers, and flow cytometry results for TO staining and surface TfR expression of circulating RBCs were examined in mice treated with liposomal clodronate and control mice treated with plain liposomes on day 4 of recovery from anemia induced by three consecutive daily phlebotomies (Fig. 3). As previously reported [37, 38], liposomal clodronate reduced new reticulocyte formation due to its disruption of erythroblastic island function such that the blood of liposomal clodronate-treated mice had fewer absolute numbers of reticulocytes (total TO+ RBCs) that reached statistical significance during the period of recovery days 7–13 compared to control mice that received plain liposomes (Fig. 3C). This reduced production of reticulocytes resulted in a statistically significant decline in total numbers of RBCs in the same post-phlebotomy period of days 7–13 compared to controls (Fig. 3B) and a decline in hematocrit that reached statistical significance on recovery days 11–17 (Fig. 3A). After day 14 in clodronate-treated mice, these lower hematocrits from decreased reticulocyte production on days 7–13 and newly regenerated macrophages in erythroblastic islands helped to produce more reticulocytes than controls (Fig. 3C). Despite fewer absolute numbers of reticulocytes than controls in the period following liposomal clodronate, the liposomal clodronate-treated mice had similar absolute numbers of TfR+ RBCs (Fig. 3D) and increased absolute numbers of TO−,TfR+ RBCs on these days (Fig. 3E). These results demonstrated that liposomal clodronate-treated mice had significantly impaired loss of TfR from newly formed RBCs compared to controls, indicating that macrophages mediated loss of TfRs from the surface of maturing reticulocytes.
Figure 3. Effects of liposomal chlodronate on stress reticulocyte maturation.
Mice were phlebotomized on days −2 to 0 to induce anemia, allowed to recover without intervention other than blood sampling, and given a single administration of liposomal clodronate as described in Methods. On recovery day 4, control mice (■) received plain, empty liposomes, and liposomal clodronate-treated mice (O) received one microgram of clodronate in liposomes as described in Methods. Samples of blood were analyzed as described in Methods for (A) Hematocrit, (B) Total erythrocyte (RBC) numbers, as well as by flow cytometry for (C) Total reticulocyte numbers (TO+ RBCs), (D) Total number of RBCs displaying surface TfR (Total TfR+ RBCs), and (E) Total number of RBCs without reticulin that express TfR (TO−,TfR+ RBCs). Pre-phlebotomy mean values are shown by dashed lines. Results are means ± SEM of five individual mice. *Significantly different than control at p < 0.05.
Morphological appearance of TO−,TfR+ RBCs
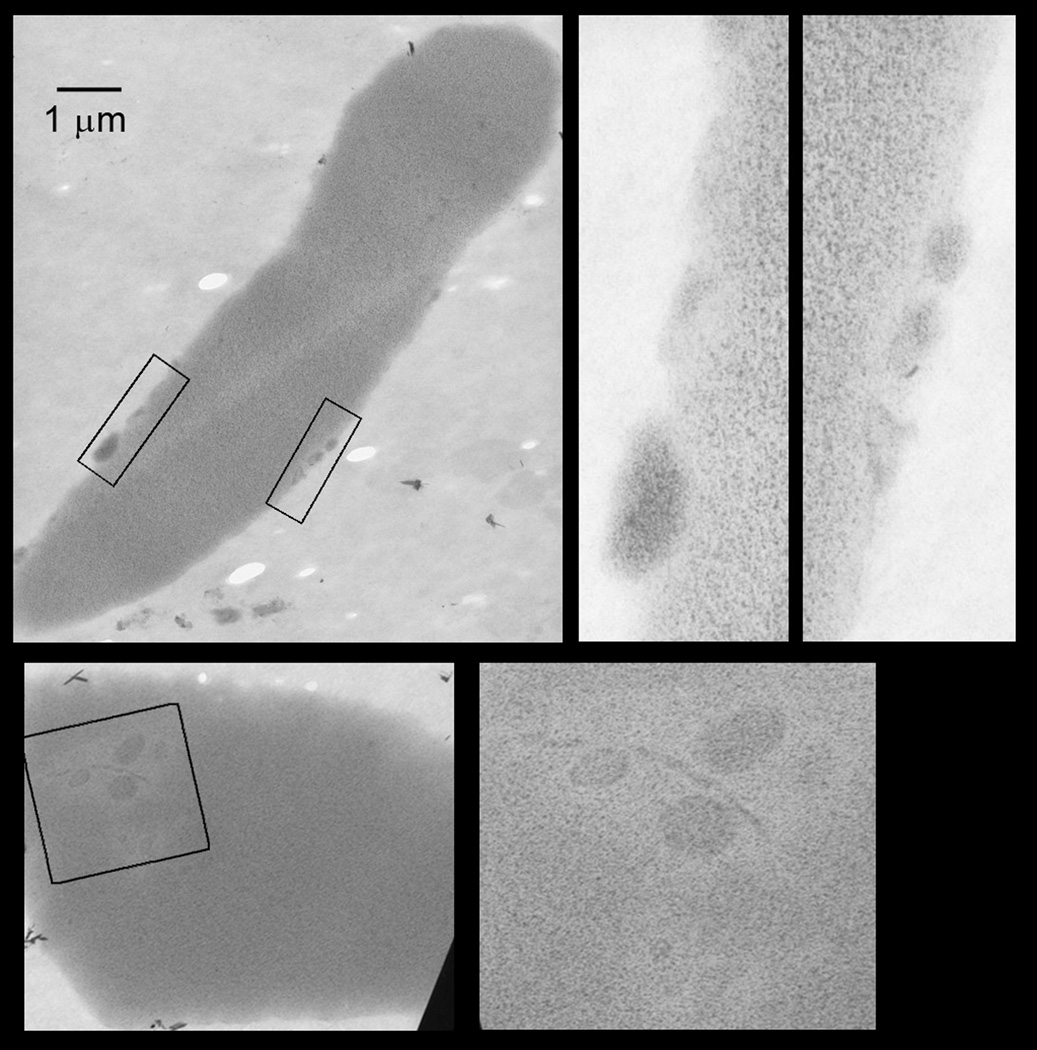
To determine whether the TO−,TfR+ RBCs were similar to mature biconcave RBCs, we sorted TO−,TfR+ RBCs from mice on recovery day 5 after three daily phlebotomies and examined them by transmission electron microscopy (Fig. 4). Supplemental Fig. 2 shows flow cytometry histograms of the RBCs prior to cell sorting and the post-sorting fraction of TO−,TfR+ RBCs. Although all of the TO−,TfR+ RBCs had smooth contours and some had achieved biconcavity and elongation, they also had remnant structures that appeared to be degraded organelles in the cytoplasm or displayed on the cell surface (Fig. 4). Therefore, TO−,TfR+ RBCs circulating in the blood of the post–phlebotomy mice represent a stage of maturation in which they are no longer reticulocytes because they have lost their reticulin staining with TO. However, despite their regular, symmetrical, and frequently biconcave appearance, TO−,TfR+ RBCs are also unlike mature erythrocytes because they have residual small structures in the cytoplasm and on the surface of the cells.
Figure 4. Transmission electron micrographs of TO−,TfR+ RBCs.
TO−,TfR+ RBCs were isolated by fluorescence activated cell sorting from the blood of a mouse on the fifth day of recovery from three consecutive days of phlebotomy. Pre-sorting and post-sorting analyses of forward and side light-scattering (shown in Supplemental Fig. 2) indicated that the TO−,TfR+ RBCs were intact and unchanged in these properties after sorting procedure. The micrographs show individual cells at similar magnifications (bar equals 1 micrometer). The rectangles in each micrograph designate those areas shown at higher magnification in the adjacent micrographs. These selected areas demonstrate internal or surface structures consistent with degraded organelles.
DISCUSSION
Most reticulocytes in normal mice did not express TfRs, i.e., they were TO+,TfR− RBCs as shown in histograms Figs. 1A and 1B. Similar populations of TO+,TfR− RBCs were found in healthy humans using dual reticulin and TfR staining, when TO [39–41] or auramine-O [42] were used to detect reticulin. In these healthy individuals, all circulating RBCs that expressed surface TfRs also stained for reticulin, i.e., all were TO+,TfR+ RBCs. TO−,TfR+ RBCs were not detected in normal individuals. In normal human umbilical cord blood, reticulocyte percentages have a slightly higher range of up to 5% of all RBCs, and about 87% of reticulocytes as measured by TO staining do not express TfRs [43]. Furthermore, a histogram of dually stained RBCs showed that TO−,TfR+ RBCs are rare in human cord blood [43], similar to their rarity in normal mice. Thus, under normal conditions, the majority of circulating reticulocytes have lost all surface TfRs, and those that enter the circulation expressing surface TfRs will completely lose those TfRs prior to completing loss of reticulin.
During erythropoietic stress, reticulocytes exit early from the hematopoietic tissues, increasing circulating reticulocyte numbers and the proportion of those reticulocytes that express surface TfRs. The recovery day 1 cells in Fig. 1A and 0 h recovery cells in Fig. 1B from phlebotomized mice show that the majority of circulating reticulocytes produced by the erythropoietic stress of phlebotomy had surface TfRs, i.e., they were TO+,TfR+ RBCs. As these TO+,TfR+ RBCs matured in vivo or in vitro, some lost all reticulin staining before they lost all surface TfRs, giving rise to a population of TO−,TfR+ RBCs (Figs. 1A, 1B). Therefore, stress reticulocytes lost surface TfRs after entering the circulation, but their TfR loss was sufficiently delayed that some completely degraded their reticulin before loss of TfRs was completed. As a result, a population of TO−,TfR+ RBCs circulated during and after resolution of erythropoietic stress as demonstrated in Figs. 2 and 3. TO−,TfR+ RBCs occur in erythropoietically stressed humans as a patient with sickle cell disease had more RBCs expressing TfR than staining with TO, but a histogram of dually stained RBCs was not provided [39]. Two human studies suggest delayed loss of TfR in reticulocytes produced during erythropoietic stress, but did not show a specific population of TO−,TfR+ RBCs [41, 44]. Hemodialysis patients receiving combined EPO and IV iron therapy had increased percentages of reticulocytes expressing TfR [41]. Also, EPO-treated hemodialysis patients demonstrate increased TfR expression in later stages of reticulocyte maturation compared to untreated patients [44].
Our results demonstrate that an extrinsic process involving the spleen and macrophages facilitates loss of surface TfRs during maturation of circulating reticulocytes. The role of the spleen in this extrinsic facilitation of circulating reticulocyte maturation is unclear. In rats, the spleen sequestered exchange-transfused reticulocytes, and erythropoietic stress in donors and recipients contributed to the degree of sequestration and RBC survival [45]. Splenectomy, however, did not affect reticulocyte sequestration in rats that were hypertransfused with blood from phlebotomized rats [28]. We avoided transfusion of reticulocyte populations by studying TfRs on reticulocytes produced endogenously in mice during recovery from phlebotomy. The results for TfRs in Fig. 2 suggest that the spleen plays a role during erythropoietic stress in the loss of reticulocyte surface proteins that are highly expressed in erythroblasts and normally lost earlier in maturation when the reticulocyte is in the marrow. These surface proteins are more highly expressed on circulating stress reticulocytes because of their relative immaturity when released early from hematopoietic tissues. In addition to TfRs, several plasma membrane proteins expressed prominently in erythroblasts are selectively removed from maturing reticulocytes via exosomes [14]. One of these proteins, α4β1 integrin, mediates erythroid cell adhesion to central macrophages in erythroblastic islands via its macrophage counter receptor, vascular adhesion molecule 1 (VCAM-1) [46, 47] as well as being the erythroid cell receptor for fibronectin, a component of hematopoietic stroma [48]. The persistence of α4 integrin in Suppl. Fig. 1 and splenectomy effects on TfR expressing cells in Fig. 2 are consistent with the persistent fibronectin binding of RBCs in post-phlebotomy rabbits and in splenectomized mice [21].
Residual TfRs on the plasma membrane and residual cytoplasmic inclusions and surface deposits of reticulin-negative RBCs in Fig. 4 suggests that plasma membrane remodeling and the loss of residual intracellular or surface structures may be functionally associated. The spleen has a “pitting” function that removes cytoplasmic inclusions without disrupting the RBC integrity [17]. During normal erythropoiesis, cytoplasmic inclusions are more prevalent in splenectomized individuals [19]. Also, reticulocytes in splenectomized individuals have more inclusions than mature RBCs, but some ‘mature’ RBCs, presumably those recently completing reticulin degradation, have inclusions [19]. The inclusions are mostly autophagic vacuoles located in the cellular periphery, immediately beneath the cell surface, or protruding from the cell surface [19]. The close association of autophagic vacuoles with the plasma membrane may allow splenic pitting to remove simultaneously a vacuole and the overlying surface membrane.
Fig. 3 demonstrates a role for macrophages in the maturation of circulating stress reticulocytes. Reticulocytes interact with macrophages at several times during their maturation: a) with central macrophages during their residence in erythroblastic islands [47], b) with sinusoidal macrophages during their emperipoletic passage into the sinusoidal blood [21], and c) with macrophages of the spleen, liver or bone marrow during their sequestration in these organs [18, 21]. This last interaction appears to be most important for circulating stress reticulocytes. Macrophages have a proposed role in the exosomal process through their phagocytic clearance of exosomes [49]. If interactions with macrophages induce reticulocyte exosome formation or release, decreased loss of TfRs in mice treated with liposomal clodronate, as shown in Fig. 3, may be attributed to prevention of these interactions. However, exosomes account for removal of about 50% of TfRs on reticulocytes [11, 50] indicating that another mechanism plays a significant role in reticulocyte loss of TfRs. If exosome formation requires functional endocytic activity, then more mature stress reticulocytes that no longer form endocytic vesicles may not lose TfRs through exosomal release, but rather through another mechanism like macrophage-mediated removal of TfR-containing membrane in exocytosed autophagic vacuoles [15]. Electron microscopic images including those in Fig.4 did not show intact organelles including mitochondria, which are normally removed from reticulcoytes in autophagic vacuoles. Reactive oxygen species are increased in stress reticulocytes [51], and they may play a role in the incomplete loss of TfR. However, we did not examine ROS status of the reticulocytes during their maturation. If these TfR losses are not achieved when the other aspects of reticulocyte maturation are completed, small amounts of residual surface TfRs may persist resulting in TO−,TfR+ RBCs.
Control mice in Figs. 3 and 4 show that TO−,TfR+ RBCs produced during erythropoietic stress persist for weeks after standard hematological determinations such as hematocrit, hemoglobin and reticulocytes have returned to baseline values. Persistent expression of TfRs on RBCs that have lost their reticulocyte staining provides a potential marker for recent stress erythropoiesis. If human RBCs produced during stress erythropoiesis express surface TfRs for weeks after the loss of reticulin from reticulocytes, then circulating TO−,TfR+ RBCs may be used clinically to detect recent episodes of bleeding, hemolysis, or EPO administration, even when hematocrit, hemoglobin and reticulocytes have returned to normal levels.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant K08DK077056 (M.M.R.) and the Vanderbilt Physician Scientist Development Program.
Footnotes
Contributions: Melissa Rhodes designed and performed experiments, analyzed data, wrote manuscript; Stephen Koury performed experiments, helped write the manuscript; Prapaporn Kopsombut performed experiments, helped write manuscript; Catherine Alford performed experiments, helped write manuscript; James Price interpreted data, helped write manuscript; Mark Koury designed and performed experiments, interpreted data, wrote manuscript.
Conflicts of interest: All of the authors declare no conflict of interest.
REFERENCES
- 1.Rapoport S, Dubiel W, Müller M. Proteolysis of mitochondria in reticulocytes during maturation is ubiquitin-dependent and is accompanied by a high rate of ATP hydrolysis. FEBS Lett. 1985;180:249–252. doi: 10.1016/0014-5793(85)81080-2. [DOI] [PubMed] [Google Scholar]
- 2.Raviv O, Heller H, Hershko A. Alterations in components of the ubiquitin-protein ligase system following maturation of reticulocytes to erythrocytes. Biochem Biophys Res Commun. 1987;145:658–665. doi: 10.1016/0006-291x(87)91015-1. [DOI] [PubMed] [Google Scholar]
- 3.Wefes I, Mastrandrea LD, Haldeman M, et al. Induction of ubiquitin-conjugating enzymes during terminal erythroid differentiation. Proc Natl Acad Sci U S A. 1995;92:4982–4986. doi: 10.1073/pnas.92.11.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasko O, Danon D. Deterioration and disappearance of mitochondria during reticulocyte maturation. Exp Cell Res. 1972;75:159–169. doi: 10.1016/0014-4827(72)90532-0. [DOI] [PubMed] [Google Scholar]
- 5.Heynen MJ, Tricot G, Verwilghen RL. Autophagy of mitochondria in rat bone marrow erythroid cells. Relation to nuclear extrusion. Cell Tissue Res. 1985;239:235–239. doi: 10.1007/BF00214924. [DOI] [PubMed] [Google Scholar]
- 6.Schweers RL, Zhang J, Randall MS, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval H, Thiagarajan P, Dasgupta SK, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piva E, Brugnara C, Spolaore F, Plebani M. Clinical utility of reticulocyte parameters. Clin Lab Med. 2015;35:133–163. doi: 10.1016/j.cll.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Shattil SJ, Cooper RA. Maturation of macroreticulocyte membranes in vivo. J Lab Clin Med. 1972;79:215–227. [PubMed] [Google Scholar]
- 10.Gasko O, Danon D. Endocytosis and exocytosis in membrane remodelling during reticulocyte maturation. Br J Haematol. 1974;28:463–470. doi: 10.1111/j.1365-2141.1974.tb06665.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone RM. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70:179–190. doi: 10.1139/o92-028. [DOI] [PubMed] [Google Scholar]
- 12.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 13.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanc L, Vidal M. Reticulocyte membrane remodeling: contribution of the exosome pathway. Curr Opin Hematol. 2010;17:177–183. doi: 10.1097/MOH.0b013e328337b4e3. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths RE, Kupzig S, Cogan N, et al. Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis. Blood. 2012;119:6296–6306. doi: 10.1182/blood-2011-09-376475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giarratana MC, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 17.Crosby WH. Normal functions of the spleen relative to red blood cells: a review. Blood. 1959;14:399–408. [PubMed] [Google Scholar]
- 18.De Haan LD, Werre JM, Ruben AM, et al. Reticulocyte crisis after splenectomy: evidence for delayed red cell maturation? Eur J Haematol. 1988;41:74–79. doi: 10.1111/j.1600-0609.1988.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 19.Kent G, Minick OT, Volini FI, Orfei E. Autophagic vacuoles in human red cells. Am J Pathol. 1966;48:831–857. [PMC free article] [PubMed] [Google Scholar]
- 20.De Haan LD, Werre JM, Ruben AM, et al. Alterations in size, shape and osmotic behaviour of red cells after splenectomy: a study of their age dependence. Br J Haematol. 1988;69:71–80. doi: 10.1111/j.1365-2141.1988.tb07605.x. [DOI] [PubMed] [Google Scholar]
- 21.Patel VP, Ciechanover A, Platt O, Lodish HF. Mammalian reticulocytes lose adhesion to fibronectin during maturation to erythrocytes. Proc Natl Acad Sci U S A. 1985;82:440–444. doi: 10.1073/pnas.82.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WB, Erm SK, Kim KY, Becker RP. Emperipolesis of erythroblasts within Kupffer cells during hepatic hemopoiesis in human fetus. Anat Rec. 1999;256:158–164. doi: 10.1002/(SICI)1097-0185(19991001)256:2<158::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama T, Kitagawa H, Takeuchi T, et al. No apoptotic cell death of erythroid cells of erythroblastic islands in bone marrow of healthy rats. J Vet Med Sci. 2002;64:913–919. doi: 10.1292/jvms.64.913. [DOI] [PubMed] [Google Scholar]
- 24.Song SH, Groom AC. Sequestration and possible maturation of reticulocytes in the normal spleen. Can J Physiol Pharmacol. 1972;50:400–406. doi: 10.1139/y72-062. [DOI] [PubMed] [Google Scholar]
- 25.Song SH, Groom AC. Scanning electron microscope study of the splenic red pulp in relation to the sequestration of immature and abnormal red cells. J Morphol. 1974;144:439–451. doi: 10.1002/jmor.1051440405. [DOI] [PubMed] [Google Scholar]
- 26.Aoki M, Tavassoli M. Dynamics of red cell egress from bone marrow after blood letting. Br J Haematol. 1981;49:337–347. doi: 10.1111/j.1365-2141.1981.tb07235.x. [DOI] [PubMed] [Google Scholar]
- 27.Hillman RS. Characteristics of marrow production and reticulocyte maturation in normal man in response to anemia. J Clin Invest. 1969;48:443–453. doi: 10.1172/JCI106001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganzoni A, Hillman RS, Finch CA. Maturation of the macroreticulocyte. Br J Haematol. 1969;16:119–135. doi: 10.1111/j.1365-2141.1969.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 29.Mel HC, Prenant M, Mohandas N. Reticulocyte motility and form: studies on maturation and classification. Blood. 1977;49:1001–1009. [PubMed] [Google Scholar]
- 30.Coulombel L, Tchernia G, Mohandas N. Human reticulocyte maturation and its relevance to erythropoietic stress. J Lab Clin Med. 1979;94:467–474. [PubMed] [Google Scholar]
- 31.Brugnara C, Kruskall MS, Johnstone RM. Membrane properties of erythrocytes in subjects undergoing multiple blood donations with or without recombinant erythropoietin. Br J Haematol. 1993;84:118–130. doi: 10.1111/j.1365-2141.1993.tb03034.x. [DOI] [PubMed] [Google Scholar]
- 32.Mairbaurl H, Schulz S, Hoffman JF. Cation transportand cell volume changes in maturing rat reticulocytes. Am J Physiol. 2000;279:C1621–C1630. doi: 10.1152/ajpcell.2000.279.5.C1621. [DOI] [PubMed] [Google Scholar]
- 33.Come SE, Shohet SB, Robinson SH. Surface remodelling of reticulocytes produced in response to erythroid stress. Nat New Biol. 1972;236:157–158. doi: 10.1038/newbio236157a0. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes MM, Kopsombut P, Bondurant MC, et al. Adherence to macrophages in erythroblastic islands enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood. 2008;111:1700–1708. doi: 10.1182/blood-2007-06-098178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koury MJ, Koury ST, Kopsombut P, Bondurant MC. In vitro maturation of nascent reticulocytes to erythrocytes. Blood. 2005;105:2168–2174. doi: 10.1182/blood-2004-02-0616. [DOI] [PubMed] [Google Scholar]
- 36.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 37.Sadahira Y, Yasuda T, Yoshino T, et al. Impaired splenic erythropoiesis in phlebotomized mice injected with CL2MDP-liposome: an experimental model for studying the role of stromal macrophages in erythropoiesis. J Leukoc Biol. 2000;68:464–470. [PubMed] [Google Scholar]
- 38.Giuliani AL, Wiener E, Lee MJ, et al. Changes in murine bone marrow macrophages and erythroid burst-forming cells following the intravenous injection of liposome-encapsulated dichloromethylene diphosphonate (Cl2MDP) Eur J Haematol. 2001;66:221–229. doi: 10.1034/j.1600-0609.2001.066004221.x. [DOI] [PubMed] [Google Scholar]
- 39.Serke S, Huhn D. Identification of CD71 (transferrin receptor) expressing erythrocytes by multiparameter-flow-cytometry (MP-FCM): correlation to the quantitation of reticulocytes as determined by conventional microscopy and by MP-FCM using a RNA-staining dye. Br J Haematol. 1992;81:432–439. doi: 10.1111/j.1365-2141.1992.tb08252.x. [DOI] [PubMed] [Google Scholar]
- 40.Ervasti M, Matinlauri I, Punnonen K. Quantitative flow cytometric analysis of transferrin receptor expression on reticulocytes. Clin Chim Acta. 2007;383:153–157. doi: 10.1016/j.cca.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Borrione P, Spaccamiglio A, Parisi A, et al. A biparametric flow cytometry analysis for the study of reticulocyte patterns of maturation. Int J Lab Hematol. 2010;32:65–73. doi: 10.1111/j.1751-553X.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 42.Kono M, Kondo T, Takagi Y, et al. Morphological definition of CD71 positive reticulocytes by various staining techniques and electron microscopy compared to reticulocytes detected by an automated hematology analyzer. Clin Chim Acta. 2009;404:105–110. doi: 10.1016/j.cca.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Malleret B, Xu F, Mohandas N, et al. Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PLoS One. 2013;8:e76062. doi: 10.1371/journal.pone.0076062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borrione P, Parisi A, Salvo RA, et al. A peculiar pattern of expression of the transferrin receptor (CD71) by reticulocytes in patients given recombinant human erythropoietin (rHuEPO): a novel marker for abuse in sport? J Biol Regul Homeost Agents. 2007;21:79–88. [PubMed] [Google Scholar]
- 45.Noble NA, Xu QP, Hoge LL. Reticulocytes II: Reexamination of the in vivo survival of stress reticulocytes. Blood. 1990;75:1877–1882. [PubMed] [Google Scholar]
- 46.Sadahira Y, Yoshino T, Monobe Y. Very late activation antigen 4-vascular cell adhesion molecule 1 interaction is involved in the formation of erythroblastic islands. J Exp Med. 1995;181:411–415. doi: 10.1084/jem.181.1.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eshghi S, Vogelezang MG, Hynes RO, et al. Alpha4beta1 integrin and erythropoietin mediate temporally distinct steps in erythropoiesis: integrins in red cell development. J Cell Biol. 2007;177:871–880. doi: 10.1083/jcb.200702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanc L, De Gassart A, Géminard C, et al. Exosome release by reticulocytes--an integral part of the red blood cell differentiation system. Blood Cells Mol Dis. 2005;35:21–26. doi: 10.1016/j.bcmd.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 51.Blanc L, Barres C, Bette-Bobillo P, Vidal M. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood. 2007;110:3407–3416. doi: 10.1182/blood-2007-04-085845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.